Abstract
PCR-based methods to amplify the 3′ untranslated region (3′-UTR) of the heat shock protein 70 (type I) gene (HSP70-I) have previously been used for typing of Leishmania but not with Leishmania (Mundinia) martiniquensis and L. (Mundinia) orientalis, newly identified human pathogens. Here, the 3′-UTRs of HSP70-I of L. martiniquensis, L. orientalis, and 10 other species were sequenced and analyzed. PCR-Restriction Fragment Length Polymorphism (RFLP) analysis targeting the 3′-UTR of HSP70-I was developed. Also, the detection limit of HSP70-I-3′-UTR PCR methods was compared with two other commonly used targets: the 18S small subunit ribosomal RNA (SSU-rRNA) gene and the internal transcribed spacer 1 region of the rRNA (ITS1-rRNA) gene. Results showed that HSP70-I-3′-UTR PCR methods could be used to identify and differentiate between L. martiniquensis (480–2 bp) and L. orientalis (674 bp) and distinguished them from parasites of the subgenus Viannia and of the subgenus Leishmania. PCR-RFLP patterns of the 3′-UTR of HSP70-I fragments digested with BsuRI restriction enzyme successfully differentiated L. martiniquensis, L. orientalis, L. braziliensis, L. guyanensis = L. panamensis, L. mexicana = L. aethiopica = L. tropica, L. amazonensis, L. major, and L. donovani = L. infantum. For the detection limit, the HSP70-I-3′-UTR PCR method could detect the DNA of L. martiniquensis and L. orientalis at the same concentration, 1 pg/μL, at a similar level to the SSU-rRNA PCR. The PCR that amplified ITS1-rRNA was more sensitive (0.01 pg/μL) than that of the HSP70-I-3′-UTR PCR. However, the sizes of both SSU-rRNA and ITS1-rRNA PCR amplicons could not differentiate between L. martiniquensis and L. orientalis. This is the first report of using HSP70-I-3′-UTR PCR based methods to identify the parasites causing leishmaniasis in Thailand. Also, the BsuRI-PCR-RFLP method can be used for differentiating some species within other subgenera.
Author summary
L. martiniquensis and L. orientalis, newly identified human pathogens, cause visceral leishmaniasis and cutaneous leishmaniasis in HIV-negative patients, respectively. However, both parasite species cause disseminated cutaneous leishmaniasis accompanying visceral leishmaniasis in HIV-positive patients. Species typing in leishmaniasis is important in diagnostics, epidemiology, and clinical studies. We show here that the 3′-UTR of HSP70-I region is a suitable target for PCR-based identification and discrimination between L. martiniquensis and L. orientalis. The technique is simple to perform and can be implemented in all settings where PCR is available. In species with similar PCR product size, the BsuRI-PCR-RFLP patterns of the 3′-UTR of HSP70-I fragments can be used for differentiating some species within other subgenera. However, where identification of species is essential or there is a travel history outside Thailand, sequencing of the HSP70-I-3′-UTR product or a similar discriminating target sequence is recommended. The PCR-based methods used in this study can also be applicable to the identification of Leishmania species obtained from vectors and reservoirs.
Introduction
Leishmaniasis is a newly emerging disease in Thailand. To date two species of Leishmania have been reported from Thailand: L. (Mundinia) martiniquensis [1] and L. (Mundinia) orientalis [2]. There has been one report of L. infantum but this may have been an imported case [3]. The clinical signs of L. martiniquensis infection include both cutaneous and visceral leishmaniasis in both HIV-infected and immunocompetent patients [1,4]. One case of L. orientalis presented as simple cutaneous leishmaniasis in an immunocompetent patient, and two others as disseminated cutaneous/visceral leishmaniasis in HIV-infected patients [2].
Identification of the species responsible for a Leishmania infection is important for clinical management, treatment, and epidemiology. Although there can be substantial overlap in symptoms, each species will cause its own spectrum of disease manifestations that require their own supportive measures and have different prognoses. For example, L. major and L. tropica both cause cutaneous leishmaniasis in the majority of patients and can occur sympatrically in many regions of North Africa and the Middle East but have different epidemiology [5]. L. major is exclusively zoonotic in origin and skin lesions infection will typically resolve, whereas L. tropica tends to occur in epidemics driven by anthroponotic transmission, with lesions that are more persistent or reactivate (leishmaniasis recidivans). Another example is cutaneous leishmaniasis in Central and South America where infections caused by L. amazonensis and L. braziliensis can occur in the same areas, but the consequences of infection are potentially very different. L. amazonensis mostly causes simple cutaneous leishmaniasis but can also progress to disseminated cutaneous leishmaniasis with multiple lesions, whereas L. braziliensis will sometimes progress to destructive mucocutaneous disease [6,7].
Although multilocus enzyme electrophoresis (MLEE) of cultured parasites was the best approach for Leishmania species identification for many years [8], it is a time-consuming procedure and requires mass parasite culturing. However, more critically, it has now become apparent that strains with apparently the same enzyme phenotype can have distinct amino acid sequences, and putative heterozygous phenotypes are also difficult to interpret [9]. Therefore, MLEE has become superseded by molecular approaches such as PCR and DNA sequencing, which have the potential to be more sensitive, rapid and easier to perform. Results are available within days versus weeks or months, and in the case of sequencing provide a permanent record available to all researchers. So far, several target genes have been used in PCR and sequencing approaches for Leishmania species identification, such as the SSU-rRNA gene [10], the RNA polymerase II gene [11], the DNA polymerase α gene [12], gp63 genes [13], the cytochrome oxidase II gene [14,15], spliced leader mini-exon genes [16], the glucose-6-phosphate dehydrogenase (G6PD) gene [17], the heat shock protein 70 (HSP70) gene [18], 18S ribosomal RNA [19], the cysteine protease b (cpb) gene [20], the N-acetylglucosamine-1-phosphate transferase gene [21], the internal transcribed spacer 1 (ITS1) region of the SSU-rRNA gene [22,23], the cytochrome b (cyt b) gene [24], 7SL RNA genes [25], and triose-phosphate isomerase (tim) genes [26].
For the species found in Thailand, so far only one PCR-based method, using a pair of primers for minicircle kinetoplast DNA [27], has been used to discriminate L. martiniquensis from ‘L. siamensis’ (MHOM/TH/2010/TR) (syn. L. orientalis), but the size of the PCR products could not differentiate these parasites from other subgenera [28]. In 2012, Requena and colleagues analyzed the 3′untranslated region (3′UTR) of Leishmania HSP70-type I (HSP70-I) genes from 24 strains representing eleven Leishmania species following PCR amplification [29]. It was observed that there was sufficient sequence conservation in the primer targets to enable amplification of DNA from species of both the subgenus Leishmania and Viannia. This particular region of the HSP70-I gene appears to have the potential to discriminate between Leishmania subgenera by direct visualization of different sizes of PCR amplification products [29]. The main objective of this study was to use the 3′UTR region of the HSP70-I gene to identify and diagnose L. martiniquensis and L. orientalis, thereby expanding the species range to the subgenus Mundinia [30] and including the species found in Thailand. In addition to direct PCR, HSP70-I-3′-UTR restriction fragment length polymorphism (HSP70-I-3′-UTR PCR-RFLP) was also performed to investigate the potential of this method to identify Leishmania species. In addition, the detection limit of the HSP70-I-3′-UTR PCR was compared with that of two other widely used targets: the SSU-rRNA gene and the ITS1 of the rRNA gene.
Materials and methods
Ethics statement
The study was approved by the ethics committee of the Faculty of Medicine, Chulalongkorn University (IRB number: 051/64). No patient information is presented in this study.
Parasite isolates and culture
The following species isolates were grown as promastigotes in vitro as previously described [1]: L. aethiopica (LV546); L. amazonensis (M2269); L. braziliensis (U1096); L. donovani (LV9); L. guyanensis (M4147); L. infantum (JPC); L. major (FV1); L. martiniquensis (LSCM1, LSCM2, LSCM3, LSCM5, LEM2494); L. mexicana (M379); L. orientalis (LSCM4); L. panamensis (LS94); L. tropica (LV357).
Isolation of DNA
Leishmania species and human blood DNAs were extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA concentration was measured using a Nano Drop spectrophotometer (ND-1000 model, Fisher Scientific, Loughborough, UK).
PCR and sequencing
The HSP70-I-3′-UTR amplification using primers 70-IR-D (5′- CCAAGGTCGAGGAGGTCGACTA -3′) and 70-IR-M (5′- ACGGGTAGGGGGAGGAAAGA -3′) [29] was performed with proof-reading DNA polymerase (Qiagen HotStar HiFidelity Polymerase, Qiagen, USA). PCR was performed in a final volume of 50 μL, containing 50 pmol of each primer, 10 μL of 5X Qiagen PCR buffer, 1 μL of DNA polymerase and 1 μL (40 pg) of each DNA template, using the following amplification cycle: 95°C for 2 min followed by 30 cycles of 95°C for 30 sec, 62.5°C for 30 sec, and 72°C for 1 min and 20 sec, and a final extension at 72°C for 5 min [29]. Distilled water was used as the negative control. Expected amplicons of PCR products were separated on 1.5% agarose gels, stained with GelRed (Thermo Fisher Scientific, Loughborough, UK), and visualized using a GelDoc imaging system (Ultra-Violet Products Ltd., Cambridge, UK). PCR products were purified using a PCR purification kit (Thermo Fisher Scientific, Loughborough, UK) and directly sequenced using commercial services (Source Bioscience Sequencing, Cambridge, UK), and checked for quality using Chromas Lite 2.1.1 (http://technelysium.com.au/). Sequence alignments were performed using Clustal Omega (http://www.ebi.ac.uk/tools/msa/clustalo/).
In silico restriction analysis
In silico analyses of predicted BsuRI restriction fragments of the 3′-UTR sequences of HSP70-I genes were performed using RestrictionMapper version 3 (www.restrictionmapper.org/) and theoretical fragment sizes were determined manually. Microsatellite distribution in the 3′-UTR of HSP70-I genes was analyzed manually.
PCR-RFLP analysis
PCR amplifications targeting the 3′-UTR sequences of HSP70-I genes were performed as described above. The PCR products were digested with a restriction enzyme, BsuRI, (Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania) according to the manufacturer’s instruction. BsuRI is an isoschizomer of HaeIII, which has been previously used to analyze Leishmania by PCR-RFLP [16,18], both cutting the target sequence GGCC. Patterns of digestion products were analyzed by 3% agarose gel electrophoresis. The GeneRuler 100 bp DNA ladder (Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania) was used as a DNA size marker. Gels were stained with GelRed (Thermo Fisher Scientific, Loughborough, UK), and visualized using a GelDoc imaging system (Ultra-Violet Products Ltd., Cambridge, UK).
Detection limits of the PCR method
Ten-fold serial dilutions of the extracted Leishmania DNAs were prepared to generate standard concentrations at 100,000; 10,000; 1,000; 100; 10; 1; 0.1; 0.01; 0.001; and 0.0001 pg/μL. To mimic a real situation of the detection of DNA of parasites extracted from blood or tissue samples, human DNA extracted from blood at a concentration of 18 ng/μL was used as a background DNA and diluent in the 10-fold dilution series of the Leishmania DNA. One microliter of each concentration was used as a DNA template for PCR amplification in a final volume of 25 μL. Each PCR reaction contained 100,000 to 0.0001 pg of Leishmania DNA and 19.8 ng of human DNA. PCR was performed on the 3′-UTR sequences of HSP70-I genes as described above and these results compared with two other commonly used identification targets as follows. Leishmania-specific primers R221 (5′-GGTTCCTTTCCTTGATTTAGC-3′) and R332, (5′-GGCCGGTAAAGGCCGAATAG-3′) were used to amplify a region of the 18S rRNA gene, generating a product of 603 bp [31,32]. Primers LeR (5′-CCAAGTCATCCATCGCGACACG-3′) and LeF (5′-TCCGCCCGAAAGTTCACCGATA-3′) were used to amplify an internal transcribed spacer 1 region (ITS1) of the rRNA gene, generating a product of 379 bp [22]. To confirm the presence of a standard concentration of background human DNA universal primers UNFOR403 (5’-TGAGGACAAATATCATTCTGAGG-3’) and UNREV1025 (5’-GGTTGTCCTCCAATTCATGTTA-3’) were used to amplify the human DNA [33]. PCR products were run on 1.5% agarose gels, stained with GelRed (Thermo Fisher Scientific, Loughborough, UK), and visualized using a GelDoc imaging system (Ultra-Violet Products Ltd., Cambridge, UK).
Evaluation of the assay on DNA from clinical samples
To test this PCR assay on DNA extracted directly from clinical samples, six DNA samples extracted from saliva, blood, and skin of L. martiniquensis cases [34,35] were used.
Results
PCR amplification and sequencing of 3′-UTR regions of Leishmania HSP70-I genes
The 70-IR-D and 70-IR-M primers were used to amplify the HSP70-I-3′-UTR DNA from five previously characterized isolates of Leishmania from northern Thailand. Four of them were L. martiniquensis (LSCM1, LSCM2, LSCM3, LSCM5) and all of these produced a band of ~480 bp on agarose gel electrophoresis, similar to the reference isolate LEM2494 (S1 Fig). Another isolate was L. orientalis (LSCM4; reference isolate). It produced a band of ~670 bp. The PCR products of the five Thai isolates were sequenced. The sequences of the four isolates of L. martiniquensis were aligned and showed a high degree of similarity with each other and the reference isolate LEM2494 (99.5–100% identity) (S2 Fig).
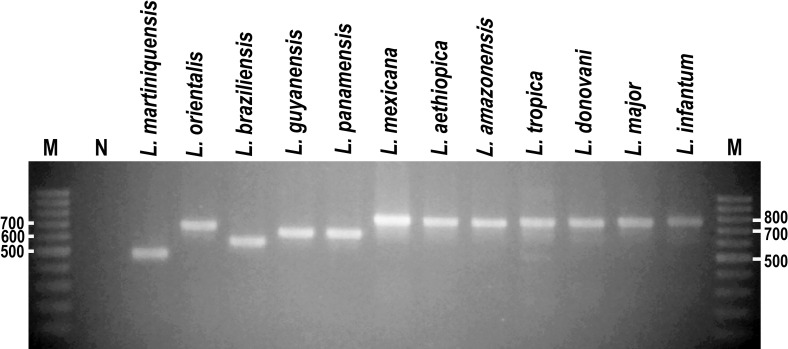
In addition to these, HSP70-I-3′-UTR PCR products were generated from 10 other species of Leishmania: L. aethiopica, L. amazonensis, L. braziliensis, L. donovani, L. guyanensis, L. infantum, L. major, L. mexicana, L. panamensis, and L. tropica. These PCR products were variable in size, but all could be clearly discriminated from the small ~480 bp product of L. martiniquensis using gel electrophoresis (Fig 1). The ~670 bp product of L. orientalis was also a different size to those of the other species but would be quite difficult to discriminate by electrophoresis alone. Parasites of the subgenus Viannia (L. braziliensis, L. guyanensis, and L. panamensis) produced products of ~550–630 bp, whereas the remaining species all in subgenus Leishmania produced larger products of ~750–780 bp (Fig 1). To confirm these results, these HSP70-I-3′-UTR PCR products were all sequenced, and the exact sizes of these sequences are shown in Table 1, together with their GenBank accession numbers.
Fig 1. Agarose gel electrophoresis of HSP70-I-3′-UTR PCR products of 12 Leishmania species.
M = Molecular markers and N = Negative control.
Table 1. In silico prediction of the HSP70-I-3′-UTR-BsuRI restriction fragments and fragment sizes in PCR-RFLP.
| Leishmania species | Isolates | Accession number | PCR product size (bp) | BsuRI restriction fragments | Fragment sizes (bp) by in silico prediction | Fragment sizes (bp) on 3% agarose gel |
|---|---|---|---|---|---|---|
| L. martiniquensis | MHOM/MQ/1992/MAR1;LEM2494 | MK607435 | 482 | 3 | 343, 131, 8 | ND |
| MHOM/TH/2012/LSCM1 | MK607436 | 480 | 3 | 341, 131, 8 | 341, 131 | |
| MHOM/TH/2013/LSCM2 | MK60737 | 482 | 3 | 343, 131, 8 | ND | |
| MHOM/TH/2013/LSCM3 | MK60738 | 480 | 3 | 341, 131, 8 | ND | |
| MHOM/TH/2015/LSCM5 | MK60739 | 480 | 3 | 341, 131, 8 | ND | |
| L. orientalis | MHOM/TH/2014/LSCM4 | MK607444 | 674 | 4 | 300, 286, 82, 6 | 300–286a, 82 |
| L. braziliensis | MHOM/GT/2001/U1096 | MK607440 | 562 | 4 | 330, 104, 81, 47 | 330, 104–81a |
| L. guyanensis | MHOM/BR/1975/M4147 | MK607441 | 627 | 3 | 442, 104, 81 | >500, 442, 104–81a |
| L. panamensis | MHOM/PA/1971/LS94 | MK607442 | 629 | 3 | 444, 104, 81 | >500, 442, 104–81a |
| L. mexicana | MNYC/BZ/1962/M379 | MK607446 | 738 | 3 | 325, 237, 176 | 325, 237, 176 |
| L. aethiopica | MHOM/ET/1972/L100;LV546 | HE575327 | 742 | 3 | 321, 243, 178 | 321, 243, 178 |
| L. amazonensis | MHOM/BR/1997/M2269 | MK607447 | 740 | 3 | 413, 216, 111 | 413, 216, 111 |
| L. tropica | MHOM/IR/1960/LV357 | MK607448 | 746 | 3 | 321, 243, 182 | 321, 243, 182 |
| L. donovani | MHOM/ET/1967/HU3;LV9 | HE575332 | 753 | 4 | 437, 165, 124, 27 | 437, 165, 124 |
| L. major | MHOM/IL/1980/FV1 | MK607449 | 759 | 4 | 323, 188, 178, 70 | 323, 188–178a, 70 |
| L. infantum | MCAN/ES/1998/JPCM5 | MK607450 | 773 | 4 | 436, 182, 128, 27 | 436, 182, 128 |
ND = not done
aMerged band from 2 DNA fragments that were different in size approximately 20 bp or less.
The HSP70-I-3′-UTR PCR assay on DNA extracted from clinical samples
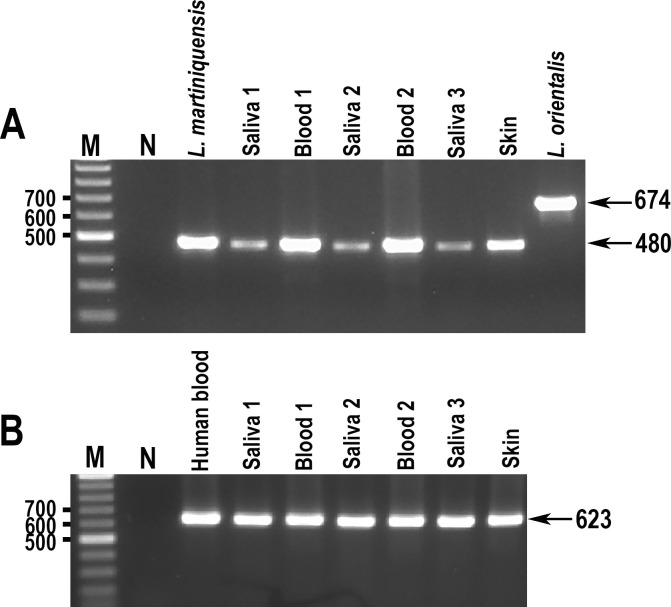
The HSP70-I-3′-UTR PCR was applied to six DNA samples extracted from clinical specimens: three from saliva; two from blood; and one from skin of L. martiniquensis cases [34,35]. PCR products were successfully generated in each case and, as expected, the size of these products from saliva, blood, and skin specimens completely matched with the size of the L. martiniquensis PCR product (Fig 2).
Fig 2. Agarose gel electrophoresis of HSP70-I-3′-UTR PCR products of DNA extracted from clinical samples.
A. HSP70-I-3′-UTR PCR products B. PCR products amplified using UNFOR403 and UNREV1025 primers. M = Molecular markers and N = Negative control.
The 3′-UTRs of HSP70-I genes contain various microsatellites
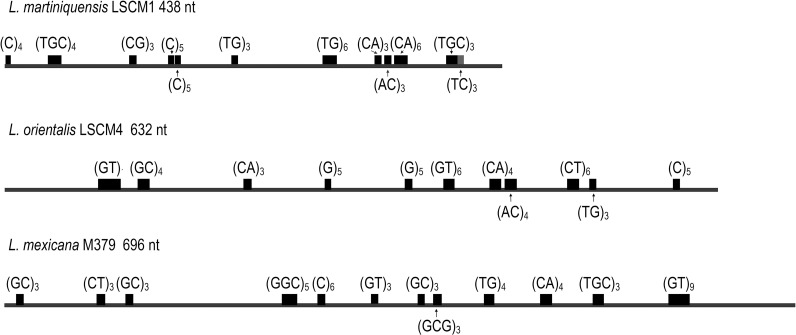
One source of size variation in untranslated regions is the occurrence of microsatellites. Since the 3′-UTRs of HSP70-I genes in L. martiniquensis, L. orientalis, and L. mexicana have not been analyzed for microsatellites, a search for structural motifs along the sequenced fragments of the three species was performed. The results show that the 3′-UTR of HSP70-I fragments contained many microsatellites. The most common microsatellite was CA repeats (as TG or GT on the complementary strand). However, TGC-microsatellites were not found in L. orientalis (Fig 3).
Fig 3. Microsatellite distribution in 3’-UTR of HSP70-I genes of L. martiniquensis, L. orientalis, and L. mexicana.
The repeated motifs correspond to those found in the sense strand. Note that the drawing scale is not proportional for the different Leishmania species.
In silico prediction of BsuRI restriction fragments of 3′-UTR sequences of HSP70-I genes and PCR-RFLP analysis
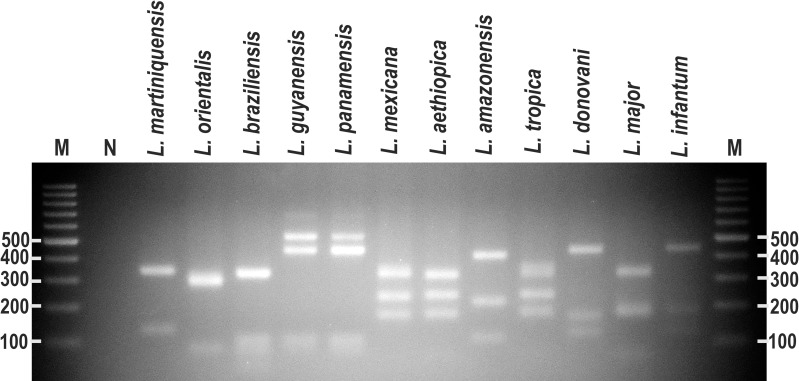
To explore the potential for enhanced discrimination between species by PCR-RFLP, in silico analyses of BsuRI restriction fragments of the 3′-UTR sequences of HSP70-I genes of L. martiniquensis, L. orientalis and the 10 other species were performed. This revealed a variety of different predicted patterns and fragment sizes (Table 1). We also analyzed L. mexicana for the first time, and the in silico analysis showed different fragment sizes from most other species, but in this respect was similar to L. aethiopica and L. tropica.
To test the in silico prediction, PCR-RFLP analysis targeting the 3′-UTR of HSP70-I following by digesting with BsuRI restriction enzyme was performed for the 12 Leishmania species. Different PCR-RFLP patterns of digested 3′-UTR of HSP70-I amplicons with BsuRI were obtained for L. martiniquensis, L. orientalis, L. braziliensis, L. guyanensis = L. panamensis, L. mexicana = L. aethiopica = L. tropica, L. amazonensis, L. donovani = L. infantum, and L. major (Fig 4 and Table 1). The PCR-RFLP patterns of L. guyanensis (M4147) and L. panamensis (LS94) were similar and one extra band (>500 bp) was observed differentially from the in silico predicted patterns. Results also confirm that L. mexicana, L. aethiopica and L. tropica had similar PCR-RFLP patterns (Fig 4 and Table 1). Likewise, L. donovani and L. infantum had similar banding patterns. Some of the fragments that were similar in size were below the resolution limit and could not be discriminated by gel electrophoresis, appearing as merged bands. These are indicated in Table 1.
Fig 4. PCR-RFLP analyses of HSP70-I-3′-UTR fragments of 12 Leishmania species.
PCR amplification was performed with the HSP70-I-3′-UTR-specific primers, and PCR products were digested with BsuRI. M = Molecular markers and N = Negative control.
Of these results perhaps the most surprising is the similarity in banding pattern of L. mexicana to L. aethiopica/L. tropica and difference to L. amazonensis. To confirm this was a genuine result and not due to a mix up of cultures further sequence analysis was performed on three L. amazonensis and two L. mexicana isolates (S3 Fig). This analysis confirmed the close sequence similarity between all five of these isolates and the reason for the banding patterns observed. The two L. mexicana isolate sequences were identical to each other and the three L. amazonensis isolates showed 95–99% identity. All five isolates had a conserved BsuRI site, but due to single nucleotide polymorphisms and insertions, an additional site found in all three L. amazonensis isolates was absent in both L. mexicana isolates, and conversely an additional site in both L. mexicana isolates was absent from all three L. amazonensis isolates. These account for the difference in banding pattern between L. amazonensis and L. mexicana. The analysis shows that the similar sized bands observed in L. mexicana and L. aethiopica/L. tropica is a result of co-incidence rather than overall sequence similarity.
Detection limits of three PCR methods to differentiate between L. martiniquensis and L. orientalis
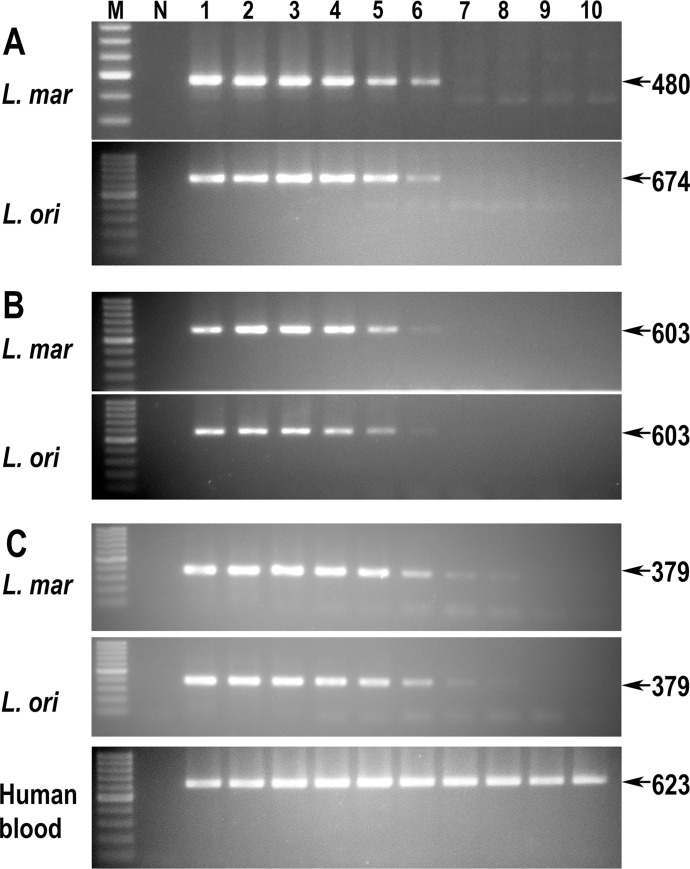
Finally, for L. martiniquensis and L. orientalis we investigated the detection limit of the HSP70-I-3′-UTR PCR method and compared this target with two other widely used sequences, SSU-rRNA and ITS1-rRNA. The HSP70-I-3′-UTR method could detect L. martiniquensis and L. orientalis at the same DNA concentration which was 1 pg/μL (Fig 5A). Compared to this, the PCR amplified SSU-rRNA regions showed similar detection limits in both Leishmania species (Fig 5B). The ITS1-rRNA PCR method could detect the DNA of both Leishmania species at a lower concentration of 0.01 pg/μL (Fig 5C). However, note that the sizes of both SSU-rRNA and ITS1-rRNA PCR amplicons could not differentiate between L. martiniquensis and L. orientalis.
Fig 5. Agarose gel electrophoresis of PCR products of L. martiniquensis and L. orientalis.
A. HSP70-I-3′-UTR PCR products B. SSU-rRNA PCR products C. ITS1-rRNA PCR products. Human blood DNA at 19.8 ng per reaction was used as a background control. N = negative control and lanes 1–10 indicate amounts of 100,000; 10,000; 1,000; 100; 10; 1; 0.1; 0.01; 0.001; and 0.0001 pg of Leishmania DNA in each reaction, respectively.
Discussion
Species identification in leishmaniasis is important in diagnostics, epidemiology, and clinical studies. To that end, DNA sequencing is an effective method used to identify Leishmania species. However, in some remote areas of Thailand and other low income or developing countries in Southeast and East Asia or elsewhere, it is not a cost-effective method and is not feasible for routine diagnosis, and sending samples elsewhere for sequencing has other problems, especially in transportation. Simple PCR methods would be preferred for diagnosis and identification of the pathogenic parasites and also medical follow-up. The current study demonstrated that PCR of the HSP70-I-3′-UTR region was able to generate products that could readily distinguish between L. martiniquensis (480–2 bp) and L. orientalis (674 bp), the two major causative agents of leishmaniasis in Thailand, on the basis of their size alone, providing this is accurately measured. To confirm the application of the HSP70-I-3′-UTR PCR method to clinical samples, DNA extracted from saliva, blood, and skin, were used. The positive results indicate that the PCR method can be applied to DNA extracted from clinical samples in general. Although we did not test with DNA extracts from clinical samples of L. orientalis because no materials were available, the sensitivity testing shows that the PCR was able to identify DNA down to 1 pg/uL, which in practice is certainly useful for diagnosis. Such information is clinically useful, as from the cases known to date L. martiniquensis appears capable of causing more serious disease than L. orientalis. However, information on possible intra-specific diversity of L. orientalis HSP70-I-3′-UTR is limited as only one L. orientalis isolate [2] was cultured and available for this study. If more L. orientalis isolates are obtained in the future, intra-specific diversity of the region can be investigated. Therefore, PCR alone can be used to confirm a suspected autochthonous case of leishmaniasis in Thailand and could provisionally identify the species.
Analysis of the HSP70-I sequences generated in this and previous studies has shown that the 3′-UTR region in Leishmania species of the subgenus Leishmania was clearly the longest. For the subgenus Mundinia, the UTR regions of all species except L. martiniquensis were longer than species of the subgenus Viannia. The UTR region of L. martiniquensis was the shortest. The biological significance of this size variation, if any, is unclear, but it might influence adaptation of the parasites in different environments/hosts as the 3′-UTR contains various sequences of regulatory regions that are involved in post-transcription of HSP70 expression. The HSP70 protein assists the Leishmania parasites in survival within host macrophages and adaptation to the new environment following infection and tolerance against different stresses during their life cycle [36]. Further study on the regulation of the HSP70-I expression in the new species in the subgenus Mundinia should be performed, especially L. martiniquensis, as it can be a pathogen in various hosts in several regions of the world [1,37–39].
Microsatellites are repeated motifs of about one to six non-coding nucleotides ubiquitously distributed in all eukaryotic genomes [40]. Microsatellites of the HSP70-I-3′-UTR fragments of L. martiniquensis, L. orientalis, and L. mexicana were reported here for the first time. The most common microsatellite found, CA repeats, was observed in the 3′-UTR of all studied species. This is consistent with the reports in L. infantum, L. major and L. braziliensis genomes [29,41]. However, another abundant repeat in Leishmania genomes, TGC-microsatellite [41], was absent in L. orientalis, as also reported in the species of the subgenus Viannia [29]. Many other microsatellites such as C, GC, GTG, GGC, and GCG that might be categorized as uncommon for Leishmania [29] were noted in all samples of Leishmania species studied. In this study, size variants of the 3′-UTR fragments were partially due to a consequence of variations in the repeat number of the microsatellite sequences, the longer UTRs having a greater number of microsatellites. Although not seen within the isolate sequences examined in this study, such variations could also contribute to infraspecific variations in PCR product sizes.
Regarding species found outside Thailand, the HSP70-I-3′-UTR PCR generated products for L. martiniquensis and L. orientalis that were different in size from other tropical Leishmania as confirmed by sequencing. However, by gel electrophoresis alone the sizes of these PCR products could not reliably identify them, in particular L. orientalis. Therefore, PCR-RFLP analyses were carried out to attempt an improved level of discrimination. PCR-RFLP methods have been reported to be sometimes useful in providing improved Leishmania species discrimination compared to PCR alone, for example, using HaeIII restriction fragments of a conserved region of the Leishmania minicircle kinetoplast DNA to distinguish L. braziliensis from L. amazonensis species [42]. Similarly, PCR-RFLP assays of ITS1 genes were used for direct identification of L. major and L. tropica [43]. In the current study the results of PCR-RFLP analyses in general corresponded to the prediction by in silico analyses of BsuRI restriction fragments of the HSP70-I-3′-UTR amplified region. The PCR-RFLP patterns could clearly distinguish L. martiniquensis, L. orientalis, L. braziliensis, L. amazonensis and L. major species. Although different to the other species, the PCR-RFLP still could not distinguish two pairs of closely related species: L. guyanensis and L. panamensis; and L. donovani and L. infantum. However, previous studies have reported that PCR-RFLP patterns of the HSP70 gene fragments digested with BccI restriction enzyme can differentiate between L. guyanensis and L. panamensis if required [44,45]. In this study, an unexpected band of >500 bp in both L. guyanensis and L. panamensis PCR-RFLP patterns was noted. The origin of this band is unclear but may have resulted from sequence variation in the HSP70-I-3′-UTR from these species. For L. mexicana, its pattern was similar to L. aethiopica and L. tropica but different from other species in the subgenus Viannia and Leishmania, including L. amazonensis, which is otherwise generally considered closely related to L. mexicana. However, in this case the results are due to co-incidence rather than any underlying relationship between L. mexicana and L. aethiopica or L. tropica. Overall, the combination of the PCR and PCR-RFLP analysis enabled clear identification of the two species that cause leishmaniasis in Thailand and discrimination of these from species found elsewhere in the world.
In Thailand, Sriworarat et al. (2015) have demonstrated a colorimetric loop-mediated isothermal amplification (LAMP) technique for the direct detection of Leishmania DNA. The LAMP assay could also detect DNA from multiple Leishmania species other than ‘L. siamensis (MHOM/TH/2010/TR)’ and L. martiniquensis, including L. aethiopica, L. braziliensis, L. donovani and L. tropica [46]. However, LAMP is prone to contamination due to the large amount of DNA that it can generate, and its capability to amplify minute amounts of DNA [46]. One PCR-based method using a pair of primers for minicircle kinetoplast DNA gene [27] can be used to discriminate L. martiniquensis from ‘L. siamensis’ (MHOM/TH/2010/TR) but the size of PCR products could not differentiate parasites in other subgenera [28]. For the ITS1-PCR method, it is not suitable for the discrimination of ‘L. siamensis’ (syn L. orientalis) and L. martiniquensis infection as it generates the same size of products [1,28,47].
The results of the detection limits in this study showed that the ITS1-rRNA PCR was the most sensitive method which could detect DNA of L. martiniquensis and L. orientalis at a concentration of 0.01 pg/μL compared to the HSP70-I-3′-UTR PCR and SSU-rRNA PCR methods which could detect DNAs of both species at the same DNA concentration (1 pg/μL). This can be explained in that the HSP70 genes in Leishmania species studied so far are all arranged in a single genomic cluster that contains five or six HSP70-I copies followed by one HSP70-II copy [48], whereas copies of the SSU rRNA gene and the ITS1 region of the rRNA gene have been observed at between 20–40 copies per cell in Leishmania species [31,49].
The PCR-based methods used in this study can now be applied to the identification of Leishmania species obtained from vectors and reservoirs in Thailand to investigate their epidemiological significance. The technique is simple to perform and can be implemented in all settings where PCR-RFLP is available. For definite autochthonous cases, the PCR product alone may be sufficient for identification. However, where an imported case is suspected or cannot be eliminated, the L. orientalis PCR product is quite similar in size to that of L. panamensis and L. guyanensis, in which case the BsuRI-PCR-RFLP method can be used for differentiating these species and some other species in other subgenera. However, where identification of species is essential or the infection is likely to have been acquired outside Thailand, sequencing of the HSP70-I-3′-UTR product or a similar discriminating target sequence is recommended. In conclusion, our data show that the 3′-UTR of HSP70-I region is a suitable target for PCR-based identification of and discrimination between L. martinquensis and L. orientalis.
Supporting information
M = Molecular markers and N = Negative control.
(TIF)
(TIF)
The positions of the BsuRI (HaeIII) restriction sites (GGCC) are highlighted.
(TIF)
Acknowledgments
We thank Prof. Dr. Padet Siriyasatien and Dr. Atchara Phumee for the clinical samples and Dr. Sriwatraporn Sor-suwan and Dr. Benjarat Phatanawiboon for their technical help.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
NJ was financially supported by the Program Management Unit for Human Resources & Institutional Development, Research and Innovation – Chulalongkorn University [grant number B16F630071] and Thailand Science Research and Innovation (TSRI) Fund [grant number CU_FRB640001_01_30_1], https://www.chula.ac.th/en/ and https://www.tsri.or.th/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, et al. First isolation of Leishmania from Northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014; 8(12):e3339. doi: 10.1371/journal.pntd.0003339 ; PMCID: PMC4256172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jariyapan N, Daroontum T, Jaiwong K, Chanmol W, Intakhan N, Sor-Suwan S, et al. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous leishmaniasis. Parasit Vectors. 2018; 11(1):351. doi: 10.1186/s13071-018-2908-3 ; PMCID: PMC6006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, et al. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008; 39(6):988–90. . [PubMed] [Google Scholar]
- 4.Chiewchanvit S, Tovanabutra N, Jariyapan N, Bates MD, Mahanupab P, Chuamanochan M, et al. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br J Dermatol. 2015; 173(3):663–70. doi: 10.1111/bjd.13812 Epub 2015 Jun 1. . [DOI] [PubMed] [Google Scholar]
- 5.Wolf Nassif P, DE Mello TFP, Navasconi TR, Mota CA, Demarchi IG, Aristides SMA, et al. Safety and efficacy of current alternatives in the topical treatment of cutaneous leishmaniasis: a systematic review. Parasitology. 2017; 144(8):995–1004. doi: 10.1017/S0031182017000385 Epub 2017 Apr 3. . [DOI] [PubMed] [Google Scholar]
- 6.de Vries HJ, Reedijk SH, Schallig HD. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015; 16(2):99–109. doi: 10.1007/s40257-015-0114-z ; PMCID: PMC4363483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. 2017; 6:750. doi: 10.12688/f1000research.11120.1 ; PMCID: PMC5464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rioux JA, Lanotte G, Serres E, Pratlong F, Bastien P, Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990; 65(3):111–25. doi: 10.1051/parasite/1990653111 . [DOI] [PubMed] [Google Scholar]
- 9.Mauricio IL, Yeo M, Baghaei M, Doto D, Pratlong F, Zemanova E, et al. Towards multilocus sequence typing of the Leishmania donovani complex: resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD). Int J Parasitol. 2006; 36(7):757–69. doi: 10.1016/j.ijpara.2006.03.006 Epub 2006 Apr 25. . [DOI] [PubMed] [Google Scholar]
- 10.Uliana SR, Nelson K, Beverley SM, Camargo EP, Floeter-Winter LM. Discrimination amongst Leishmania by polymerase chain reaction and hybridization with small subunit ribosomal DNA derived oligonucleotides. J Eukaryot Microbiol. 1994; 41(4):324–30. doi: 10.1111/j.1550-7408.1994.tb06085.x . [DOI] [PubMed] [Google Scholar]
- 11.Croan D, Ellis J. Phylogenetic relationships between Leishmania, Viannia and Sauroleishmania inferred from comparison of a variable domain within the RNA polymerase II largest subunit gene. Mol Biochem Parasitol. 1996; 79(1):97–102. doi: 10.1016/0166-6851(96)02629-1 . [DOI] [PubMed] [Google Scholar]
- 12.Croan DG, Morrison DA, Ellis JT. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol Biochem Parasitol. 1997; 89(2):149–59. doi: 10.1016/s0166-6851(97)00111-4 . [DOI] [PubMed] [Google Scholar]
- 13.Mauricio IL, Howard MK, Stothard JR, Miles MA. Genomic diversity in the Leishmania donovani complex. Parasitology. 1999; 119 (Pt 3):237–46. doi: 10.1017/s0031182099004710 . [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim ME, Barker DC. The origin and evolution of the Leishmania donovani complex as inferred from a mitochondrial cytochrome oxidase II gene sequence. Infect Genet Evol. 2001; 1(1):61–8. doi: 10.1016/s1567-1348(01)00009-0 . [DOI] [PubMed] [Google Scholar]
- 15.Cao DP, Guo XG, Chen DL, Chen JP. Species delimitation and phylogenetic relationships of Chinese Leishmania isolates reexamined using kinetoplast cytochrome oxidase II gene sequences. Parasitol Res. 2011; 109(1):163–73. doi: 10.1007/s00436-010-2239-6 Epub 2011 Jan 11. . [DOI] [PubMed] [Google Scholar]
- 16.Marfurt J, Nasereddin A, Niederwieser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J Clin Microbiol. 2003; 41(7):3147–53. doi: 10.1128/JCM.41.7.3147-3153.2003 ; PMCID: PMC165364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castilho TM, Shaw JJ, Floeter-Winter LM. New PCR assay using glucose-6-phosphate dehydrogenase for identification of Leishmania species. J Clin Microbiol. 2003; 41(2):540–6. doi: 10.1128/JCM.41.2.540-546.2003 ; PMCID: PMC149696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Microbiol. 2004; 42(5):2294–7. doi: 10.1128/JCM.42.5.2294-2297.2004 ; PMCID: PMC404633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chargui N, Bastien P, Kallel K, Haouas N, Akrout FM, Masmoudi A, et al. Usefulness of PCR in the diagnosis of cutaneous leishmaniasis in Tunisia. Trans R Soc Trop Med Hyg. 2005; 99(10):762–8. doi: 10.1016/j.trstmh.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Hide M, Bras-Gonçalves R, Bañuls AL. Specific cpb copies within the Leishmania donovani complex: evolutionary interpretations and potential clinical implications in humans. Parasitology. 2007; 134(Pt 3):379–89. doi: 10.1017/S0031182006001600 Epub 2006 Nov 28. . [DOI] [PubMed] [Google Scholar]
- 21.Waki K, Dutta S, Ray D, Kolli BK, Akman L, Kawazu S, et al. Transmembrane molecules for phylogenetic analyses of pathogenic protists: Leishmania-specific informative sites in hydrophilic loops of trans- endoplasmic reticulum N-acetylglucosamine-1-phosphate transferase. Eukaryot Cell. 2007; 6(2):198–210. doi: 10.1128/EC.00282-06 Epub 2006 Dec 1. ; PMCID: PMC1797956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanakos G, Piperaki ET, Menounos PG, Tegos N, Flemetakis A, Vakalis NC. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg. 2008; 102(1):46–53. doi: 10.1016/j.trstmh.2007.05.019 Epub 2007 Jul 31. . [DOI] [PubMed] [Google Scholar]
- 23.de Lima AC, Zampieri RA, Tomokane TY, Laurenti MD, Silveira FT, Corbett CE, et al. Leishmania sp. identification by PCR associated with sequencing of target SSU rDNA in paraffin-embedded skin samples stored for more than 30 years. Parasitol Res. 2011; 108(6):1525–31. doi: 10.1007/s00436-010-2208-0 Epub 2010 Dec 15. . [DOI] [PubMed] [Google Scholar]
- 24.Martínez LP, Rebollo JA, Luna AL, Cochero S, Bejarano EE. Molecular identification of the parasites causing cutaneous leishmaniasis on the Caribbean coast of Colombia. Parasitol Res. 2010; 106(3):647–52. doi: 10.1007/s00436-009-1712-6 . [DOI] [PubMed] [Google Scholar]
- 25.Stevenson LG, Fedorko DP, Zelazny AM. An enhanced method for the identification of Leishmania spp. using real-time polymerase chain reaction and sequence analysis of the 7SL RNA gene region. Diagn Microbiol Infect Dis. 2010; 66(4):432–5. doi: 10.1016/j.diagmicrobio.2009.11.005 ; PMCID: PMC2856081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushawaha PK, Gupta R, Tripathi CD, Khare P, Jaiswal AK, Sundar S, et al. Leishmania donovani triose phosphate isomerase: a potential vaccine target against visceral leishmaniasis. PLoS One. 2012; 7(9):e45766. doi: 10.1371/journal.pone.0045766 Epub 2012 Sep 24. ; PMCID: PMC3454378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H, Uezato H, Gomez EA, Terayama Y, Calvopiña M, Iwata H, et al. Establishment of a mass screening method of sand fly vectors for Leishmania infection by molecular biological methods. Am J Trop Med Hyg. 2007; 77(2):324–9. . [PubMed] [Google Scholar]
- 28.Tiwananthagorn S, Kato H, Yeewa R, Muengpan A, Polseela R, Leelayoova S. Comparison of LAMP and PCR for molecular mass screening of sand flies for Leishmania martiniquensis infection. Mem Inst Oswaldo Cruz. 2017; 112(2):100–7. doi: 10.1590/0074-02760160254 ; PMCID: PMC5293119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Requena JM, Chicharro C, García L, Parrado R, Puerta CJ, Cañavate C. Sequence analysis of the 3’-untranslated region of HSP70 (type I) genes in the genus Leishmania: its usefulness as a molecular marker for species identification. Parasit Vectors. 2012; 5:87. doi: 10.1186/1756-3305-5-87 ; PMCID: PMC3425316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2018; 145(4):430–42. doi: 10.1017/S0031182016002092 Epub 2016 Dec 15. . [DOI] [PubMed] [Google Scholar]
- 31.van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992; 51(1):133–42. doi: 10.1016/0166-6851(92)90208-2 . [DOI] [PubMed] [Google Scholar]
- 32.Meredith SE, Zijlstra EE, Schoone GJ, Kroon CC, van Eys GJ, Schaeffer KU, et al. Development and application of the polymerase chain reaction for the detection and identification of Leishmania parasites in clinical material. Arch Inst Pasteur Tunis. 1993; 70(3–4):419–31. . [PubMed] [Google Scholar]
- 33.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005; 73(2):336–42. ; PMCID: PMC4147110. [PMC free article] [PubMed] [Google Scholar]
- 34.Phumee A, Kraivichian K, Chusri S, Noppakun N, Vibhagool A, Sanprasert V, et al. Detection of Leishmania siamensis DNA in saliva by polymerase chain reaction. Am J Trop Med Hyg. 2013; 89(5):899–905. doi: 10.4269/ajtmh.12-0612 Epub 2013 Sep 23. ; PMCID: PMC3820333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siriyasatien P, Chusri S, Kraivichian K, Jariyapan N, Hortiwakul T, Silpapojakul K, et al. Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients. BMC Infect Dis. 2016; 16:89. doi: 10.1186/s12879-016-1433-2 ; PMCID: PMC4793580.2016; 16:89. PMCID: PMC4793580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Codonho BS, Costa Sdos S, Peloso Ede F, Joazeiro PP, Gadelha FR, Giorgio S. HSP70 of Leishmania amazonensis alters resistance to different stresses and mitochondrial bioenergetics. Mem Inst Oswaldo Cruz. 2016; 111(7):460–8. doi: 10.1590/0074-02760160087 ; PMCID: PMC4957499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, et al. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009; 166(3–4):346–51. doi: 10.1016/j.vetpar.2009.09.001 Epub 2009 Sep 15. . [DOI] [PubMed] [Google Scholar]
- 38.Lobsiger L, Müller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, et al. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010; 169(3–4):408–14. doi: 10.1016/j.vetpar.2010.01.022 Epub 2010 Jan 25. . [DOI] [PubMed] [Google Scholar]
- 39.Desbois N, Pratlong F, Quist D, Dedet JP. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite. 2014; 21:12. doi: 10.1051/parasite/2014011 Epub 2014 Mar 14. ; PMCID: PMC3952653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarne P, Lagoda PJ. Microsatellites, from molecules to populations and back. Trends Ecol Evol. 1996; 11(10):424–9. doi: 10.1016/0169-5347(96)10049-5 . [DOI] [PubMed] [Google Scholar]
- 41.Fakhar M, Motazedian MH, Daly D, Lowe CD, Kemp SJ, Noyes HA. An integrated pipeline for the development of novel panels of mapped microsatellite markers for Leishmania donovani complex, Leishmania braziliensis and Leishmania major. Parasitology. 2008; 135(5):567–74. doi: 10.1017/S0031182008004186 Epub 2008 Mar 27. . [DOI] [PubMed] [Google Scholar]
- 42.Volpini AC, Passos VM, Oliveira GC, Romanha AJ. PCR-RFLP to identify Leishmania (Viannia) braziliensis and L. (Leishmania) amazonensis causing American cutaneous leishmaniasis. Acta Trop. 2004; 90(1):31–7. doi: 10.1016/j.actatropica.2003.10.008 . [DOI] [PubMed] [Google Scholar]
- 43.Hashemi N, Mohaghegh MA, Hashemi M, Azami M, Mortazavidehkordi N, Hashemi C, et al. PCR-RFLP diagnosis and characterization of Leishmania species causing human cutaneous leishmaniasis and evaluation of treatment times with glucantime in these patients. Trop Biomed. 2016; 33(4):689–96. . [PubMed] [Google Scholar]
- 44.Montalvo Alvarez AM, Nodarse JF, Goodridge IM, Fidalgo LM, Marin M, Van Der Auwera G, et al. Differentiation of Leishmania (Viannia) panamensis and Leishmania (V.) guyanensis using BccI for hsp70 PCR-RFLP. Trans R Soc Trop Med Hyg. 2010; 104(5):364–7. doi: 10.1016/j.trstmh.2009.12.002 Epub 2010 Mar 15. . [DOI] [PubMed] [Google Scholar]
- 45.Kato H, Gomez EA, Seki C, Furumoto H, Martini-Robles L, Muzzio J, et al. PCR-RFLP analyses of Leishmania species causing cutaneous and mucocutaneous leishmaniasis revealed distribution of genetically complex strains with hybrid and mito-nuclear discordance in Ecuador. PLoS Negl Trop Dis. 2019; 13(5):e0007403. doi: 10.1371/journal.pntd.0007403 ; PMCID: PMC6522058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriworarat C, Phumee A, Mungthin M, Leelayoova S, Siriyasatien P. Development of loop-mediated isothermal amplification (LAMP) for simple detection of Leishmania infection. Parasit Vectors. 2015; 8:591. doi: 10.1186/s13071-015-1202-x ; PMCID: PMC4650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hitakarun A, Tan-ariya P, Siripattanapipong S, Mungthin M, Piyaraj P, Naaglor T, et al. Comparison of PCR methods for detection of Leishmania siamensis infection. Parasit Vectors. 2014; 7:458. doi: 10.1186/s13071-014-0458-x ; PMCID: PMC4188918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramírez CA, Requena JM, Puerta CJ. Identification of the HSP70-II gene in Leishmania braziliensis HSP70 locus: genomic organization and UTRs characterization. Parasit Vectors. 2011; 4:166. doi: 10.1186/1756-3305-4-166 ; PMCID: PMC3185273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inga R, De Doncker S, Gomez J, Lopez M, Garcia R, Le Ray D, et al. Relation between variation in copy number of ribosomal RNA encoding genes and size of harbouring chromosomes in Leishmania of subgenus Viannia. Mol Biochem Parasitol. 1998; 92(2):219–28. doi: 10.1016/s0166-6851(98)00009-7 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
M = Molecular markers and N = Negative control.
(TIF)
(TIF)
The positions of the BsuRI (HaeIII) restriction sites (GGCC) are highlighted.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.