ABSTRACT
Background
The aim of this study is to evaluate the sex differential effect in the COVID-19 mortality by different age groups and polymerase chain reaction (PCR) test results.
Research design
In a multicenter cross-sectional study from 55 hospitals in Tehran, Iran, patients were categorized as positive, negative, and suspected cases.
Results
A total of 25,481 cases (14,791 males) were included in the study with a mortality rate of 12.0%. The mortality rates in positive, negative, and suspected cases were 20.55%, 9.97%, and 7.31%, respectively. Using a Cox regression model, sex had a significant effect on the hazard of death due to COVID-19 in adult and senior male patients having positive and suspected PCR test results. However, sex was not found as significant factor for mortality in patients with a negative PCR test in different age groups.
Conclusions
Regardless of other risk factors, we found that the effect of sex on COVID-19 mortality varied significantly in different age groups. Therefore, appropriate strategies should be designed to protect adult and senior males from this deadly infectious disease. Furthermore, owing to the considerable death rate of COVID-19 patients with negative test results, new policies should be launched to increase the accuracy of diagnosis tests.
KEYWORDS: COVID-19, death rate, RT-PCR testing, Iran
1. Background
Currently, coronavirus disease 2019 (COVID-19) has seriously affected people’s lives worldwide [1]. This deadly novel human infectious disease is known as the fifth pandemic after the 1918 flu [2]. The first cases of COVID-19 were reported in Wuhan, China, in late December 2019 and subsequently have spread all over the world [3]. Up to now, over 4.5 million people infected by COVID-19 have died from the disease (31 August 2021) [4].
The highest number of Covid-19 cases have occurred in the USA and India [5]. The COVID-19 outbreak in Iran started on 19 February 2020 in the city of Qom and over 4 million cases and 100 thousand deaths have been reported, as of 31 August 2021 [4]. On the basis of the latest online global statistics, Iran ranks first in the Middle East area in terms of number of cases and deaths due to COVID-19 [5].
Among all critical risk factors, demographic considerations including sex and age are recognized as having the most influence on COVID-19 disease outcomes [6]. Although there are various unanswered questions regarding these risk factors, they are likely to have influence on the different clinical outcomes across the world [7,8]. According to recent studies, men are more susceptible to COVID-19 compared to women. The results of different studies have demonstrated that the mortality rate among older men with COVID-19 is higher than women [9,10]. Furthermore, according to a study assessing the mortality ratio in several European countries, the male to female sex ratio was different in various age groups [11]. Consequently, evaluation of sex-specific data in different age groups is of importance to identify early changes in health between the different sexes infected by COVID-19 [12]. In order to assess the impact of sex among COVID-19 patients, essential health policies should be undertaken to implement targeted treatment plans including early recognition and aggressive testing, and to avoid treatment bias among males and females [12].
In order to screen and manage patients with COVID-19, the use of an accurate and rapid diagnostic test such as reverse transcriptase polymerase chain reaction (RT-PCR) was recommended by the World Health Organization [13]. Although RT-PCR is a molecular test currently used as the gold standard for diagnosis of COVID-19, many studies have reported that it might fail to identify positive cases, thus leading to false-negative results [14–16]. Hence, it is critical to evaluate the prevalence and incidence among patients with different test results in order to improve disease management for better patient outcomes.
This study aimed to assess the effect of sex in different age groups on mortality rates in a large Iranian sample of COVID-19 patients with different PCR test outcomes, including suspected, negative, and positive results. In addition, we compared the death rate of COVID-19 patients with suspected, negative, and positive results to determine if PCR test outcome could be taken as a moderator variable for the effect of sex on death outcomes in different age groups.
2. Material and methods
2.1. PCR testing and patient classification
PCR tests amplify portions of viral RNA to detect viral infection. The procedure begins by taking a sample from the nose or mouth of a potentially infected person, where the virus may be found [17]. In our study, tested patients were classified as positive if SARS-CoV-2 RNA was detected by RT-PCR. Patients with a negative RT-PCR result were classed in the negative group. The diagnosis of COVID-19 virus in all patients was evaluated only once. In addition, false-negative RT-PCT tests from the upper respiratory tract specimens were possible. Patients were classified as suspected when only one of the two or more genes that the RT-PCT test targets was identified. The high case fatality rate in this group was shown in a previous study in Iran [18].
2.2. Participants
This is multicenter cross-sectional study from 55 hospitals and conducted in the city of Tehran metropolitan area between 20 February 2020 and 8 June 2020. According to the protocols established in Iran, RT-PCR was considered as the only ‘gold standard’ test for diagnosing COVID-19. Patients included in the study were categorized as either positive, negative, or suspected cases based on the RT-PCT test results. In this study, all cases were hospitalized at different times. Patients were admitted as necessary, according to their clinical condition and PCR tests of their samples conducted by trained laboratory assistants or nurses. Those who had positive PCR results and did not have good clinical conditions were admitted to hospital. However, mild-cases who were not considered high risk were usually advised to receive medical care at home and only hospitalized if their condition worsened.
2.3. Data collection
All detailed information of patients was obtained using the Health Information System (HIS). This system has been used in hospitals to improve the quality, effectiveness, and efficiency of health services [19]. In this study, we used data regarding demographics (sex and age), laboratory findings (PCR), CT scans, outcome (deceased or survivor), date of admission and discharge or death. Age was categorized to four groups of children (0–14 years), youth (15–24 years), adults (25–64 years), and seniors (>65 years) [20]. Survival time was defined as the time interval between hospital admission and discharge or death. Patients who did not experience a death event or were discharged or excluded from the study were considered censored. The study was approved by Research Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.RETECH.REC.1399.087). In addition, participatory consent was obtained from all patients. For patients who could not provide consent, a relative or care person provided consent on their behalf.
2.4. Statistical analysis
Descriptive statistics were expressed by mean (SD) or median (first quartile – third quartile) and frequency (percentage) for numeric and categorical variables. The independent t-test and Mann-Whitney U test were used to compare difference in numeric variables between two independent groups (deceased and survivors). The Chi-square independence test was used to determine whether there is an association between categorical variable and outcome. Logistic regression was applied to evaluate the impact of sex on the death due to COVID-19 by age group. The Cox regression model was used to investigate the effect of variables upon the time the death takes to happen due to COVID-19. Statistical assumptions were considered and checked before performing any methods. Statistical analyzes were done using R 3.6.2 and p-values less than 0.05 were considered as statistically significant.
3. Results
A total of 25,481 cases (14,791 males, 10,690 females) were included in the study. Of these, 21,791 cases with the mean age of 52.8 years survived and 3057 with a mean age of 67.5 years died due to COVID-19 (deceased cases). The status of 633 cases was unknown. Based on patient test results, the study data was divided into the three groups of positive (n = 8800), negative (n = 3489) and suspected (n = 13,192) patients of having COVID-19 disease. A significant association between age and hospital length with patient status (deceased vs. survivor) was observed among the positive, negative, and suspected cases. Although hospital ward [intensive care unit (ICU), critical care unit (CCU) and others] was identified as a significant risk factor for death outcome in suspected and confirmed cases of COVID-19, the association with patient status was not significant in cases with negative PCR test results. Among the positive COVID-19 patients, 1781 (20.6%) died, whereas 928 (7.3%) of the suspected cases died. In addition, 348 deaths (10.0%) were reported among individuals with negative COVID-19 test results and a large number of deaths occurred in patients with negative PCR test results compared to suspected cases for both sexes (Table 1). The death rate of COVID-19 patients with negative test results was approximately the same among males and females (Figure 1).
Table 1.
Descriptive Statistics by sex and mortality status classified by COVID-19 Status
| |
|
Male |
Female |
Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivor | Deceased | P-value | Survivor | Deceased | P-value | Survivor | Deceased | P-value | ||||||
| Total | ||||||||||||||
| No. of cases | 12,492 | 1901 | 9299 | 1156 | 21,791 | 3057 | ||||||||
| Age | Mean (SD) | 51.8 (18.6) | 66.7 (16.3) | <0.001 | 54.2 (18.6) | 68.8 (15.7) | <0.001 | 52.8 (16.1) | 67.5 (18.7) | <0.001 | ||||
| Median (Range) | 52.0 (38.0–65.0) |
69.0 (57.0–79.0) |
<0.001 | 55.0 (40.0–68.0) |
71.0 (60.3–80.0) |
<0.001 | 53.0 (38.0–67.0) |
70.0 (58.0–80.0) |
<0.001 | |||||
| Children | 194 (1.6) | 14 (0.7) | <0.001 | 115 (1.2) | 8 (0.7) | <0.001 | 309 (1.4) | 22 (0.7) | <0.001 | |||||
| Youth | 563 (4.5) | 20 (1.1) | 329 (3.5) | 12 (1.0) | 892 (4.1) | 32 (1.0) | ||||||||
| Adult | 8418 (67.4) | 712 (37.5) | 5945 (63.9) | 364 (31.5) | 14,363 (65.9) | 1076 (35.2) | ||||||||
| Seniors | 3317 (26.6) | 1155 (60.8) | 2910 (31.3) | 772 (66.8) | 6227 (28.6) | 1927 (63.0) | ||||||||
| Wards | <0.001 | <0.001 | <0.001 | |||||||||||
| ICU & CCU | 597 (4.8) | 614 (32.3) | 438 (4.7) | 354 (30.6) | 1035 (4.7) | 968 (31.7) | ||||||||
| Others | 11,895 (95.2) | 1287 (67.7) | 8861 (95.3) | 802 (69.4) | 20,756 (95.3) | 2089 (68.3) | ||||||||
| Hospital length | Median (Range) | 3.0 (1.0–7.0) |
6.0 (3.0–11.0) |
<0.001 | 3.0 (1.0–7.0) |
6.0 (3.0–11.0) |
<0.001 | 3.0 (1.0–7.0) |
6.0 (3.0–11.0) |
<0.001 | ||||
| Positive | ||||||||||||||
| No. of cases | 3996 | 1127 | 2888 | 654 | 6884 | 1781 | ||||||||
| Age | Mean (SD) | 53.6 (16.9) | 67.2 (15.3) | <0.001 | 55.6 (16.3) | 68.0 (15.3) | <0.001 | 54.5 (16.7) | 67.5 (15.3) | <0.001 | ||||
| Median (Range) | 53.0 (41.0–66.0) |
69.0 (58.0–79.0) |
<0.001 | 56.0 (44.0–67.0) |
70.0 (60.0–80.0) |
<0.001 | 55.0 (42.0–66.0) |
70.0 (58.0–79.0) |
<0.001 | |||||
| Children | 36 (0.9) | 2 (0.2) | <0.001 | 13 (0.5) | 4 (0.6) | <0.001 | 49 (0.7) | 6 (0.3) | <0.001 | |||||
| Youth | 89 (2.2) | 12 (1.1) | 57 (2.0) | 6 (0.9) | 146 (2.1) | 18 (1.0) | ||||||||
| Adult | 2795 (69.9) | 415 (36.8) | 1931 (66.9) | 216 (33.0) | 4726 (68.7) | 631 (35.4) | ||||||||
| Seniors | 1076 (26.9) | 698 (61.9) | 887 (30.7) | 428 (65.4) | 1963 (28.5) | 1126 (63.2) | ||||||||
| Wards | <0.001 | <0.001 | <0.001 | |||||||||||
| ICU & CCU | 118 (3.0) | 381 (33.8) | 109 (3.8) | 212 (32.4) | 227 (3.3) | 593 (33.3) | ||||||||
| Others | 3878 (97.0) | 746 (66.2) | 2779 (96.2) | 442 (67.6) | 6657 (96.7) | 1188 (66.7) | ||||||||
| Hospital length | Median (Range) | 6.0 (3.0–9.0) |
7.0 (4.0–11.0) |
<0.001 | 6.0 (3.0–9.0) |
7.0 (3.8–12.0) |
<0.001 | 6.0 (3.0–9.0) |
7.0 (4.0–11.0) |
<0.001 | ||||
| Suspected | ||||||||||||||
| No. of cases | 6791 | 572 | 4975 | 356 | 11,766 | 928 | ||||||||
| Age | Mean (SD) | 49.0 (18.8) | 65.5 (18.6) | <0.001 | 51.1 (19.1) | 69.4 (17.0) | <0.001 | 49.9 (19.0) | 67.0 (18.1) | <0.001 | ||||
| Median (Range) | 48.0 (35.0–63.0) |
68.5 (56.0–80.0) |
<0.001 | 51.0 (36.0–65.0) |
72.0 (61.0–81.0) |
<0.001 | 49.0 (35.0–64.0) |
70.0 (57.0–80.0) |
<0.001 | |||||
| Children | 148 (2.2) | 12 (2.1) | <0.001 | 89 (1.8) | 4 (1.1) | <0.001 | 237 (2.0) | 16 (1.7) | <0.001 | |||||
| Youth | 395 (5.8) | 7 (1.2) | 229 (4.6) | 4 (1.1) | 624 (5.3) | 11 (1.2) | ||||||||
| Adult | 4733 (69.7) | 218 (38.1) | 3348 (67.3) | 110 (30.9) | 8081 (68.7) | 328 (35.3) | ||||||||
| Seniors | 1515 (22.3) | 335 (58.6) | 1309 (26.3) | 238 (66.9) | 2824 (24.0) | 573 (61.7) | ||||||||
| Wards | <0.001 | <0.001 | <0.001 | |||||||||||
| ICU & CCU | 473 (7.0) | 230 (40.2) | 315 (6.3) | 140 (39.3) | 788 (6.7) | 370 (39.9) | ||||||||
| Others | 6318 (93.0) | 342 (59.8) | 4660 (93.7) | 216 (60.7) | 10,978 (93.3) | 558 (60.1) | ||||||||
| Hospital length | Median (Range) | 2.0 (1.0–4.0) |
4.0 (2.0–8.0) |
<0.001 | 1.0 (1.0–4.0) |
4.0 (2.0–8.0) |
<0.001 | 1.0 (1.0–4.0) |
4.0 (2.0–8.0) |
<0.001 | ||||
| Negative | ||||||||||||||
| No. of cases | 1705 | 202 | 1436 | 146 | 3141 | 348 | ||||||||
| Age | Mean (SD) | 58.8 (19.1) | 67.7 (14.2) | <0.001 | 61.7 (19.0) | 70.5 (14.6) | <0.001 | 60.1 (19.1) | 68.9 (14.4) | <0.001 | ||||
| Median (Range) | 61.0 (46.0–73.0) |
69.0 (58.8–80.0) |
<0.001 | 64.0 (48.0–77.0) |
73.0 (63.0–82.0) |
<0.001 | 62.0 (47.0–75.0) |
71.0 (60.3–81.0) |
<0.001 | |||||
| Children | 10 (0.6) | 0 (0.0) | <0.001 | 13 (0.9) | 0 (0.0) | <0.001 | 23 (0.7) | 0 (0.0) | <0.001 | |||||
| Youth | 79 (4.6) | 1 (0.5) | 43 (3.0) | 2 (1.4) | 122 (3.9) | 3 (0.9) | ||||||||
| Adult | 890 (52.2) | 79 (39.1) | 666 (46.4) | 38 (26.0) | 1556 (49.5) | 117 (33.6) | ||||||||
| Seniors | 726 (42.6) | 122 (60.4) | 714 (49.7) | 106 (72.6) | 1440 (45.8) | 228 (65.5) | ||||||||
| Wards | 0.061 | 0.654 | 0.096 | |||||||||||
| ICU & CCU | 6 (0.4) | 3 (1.5) | 14 (1.0) | 2 (1.4) | 20 (0.6) | 5 (1.4) | ||||||||
| Others | 1699 (99.6) | 199 (98.5) | 1422 (99.0) | 144 (98.6) | 3121 (99.4) | 343 (98.6) | ||||||||
| Hospital length | Median (Range) | 5.0 (2.0–9.0) |
9.0 (4.0–15.0) |
<0.001 | 5.0 (2.0–8.0) |
7.5 (3.0–14.0) |
<0.001 | 5.0 (2.0–9.0) |
8.0 (4.0–14.8) |
<0.001 | ||||
Figure 1.
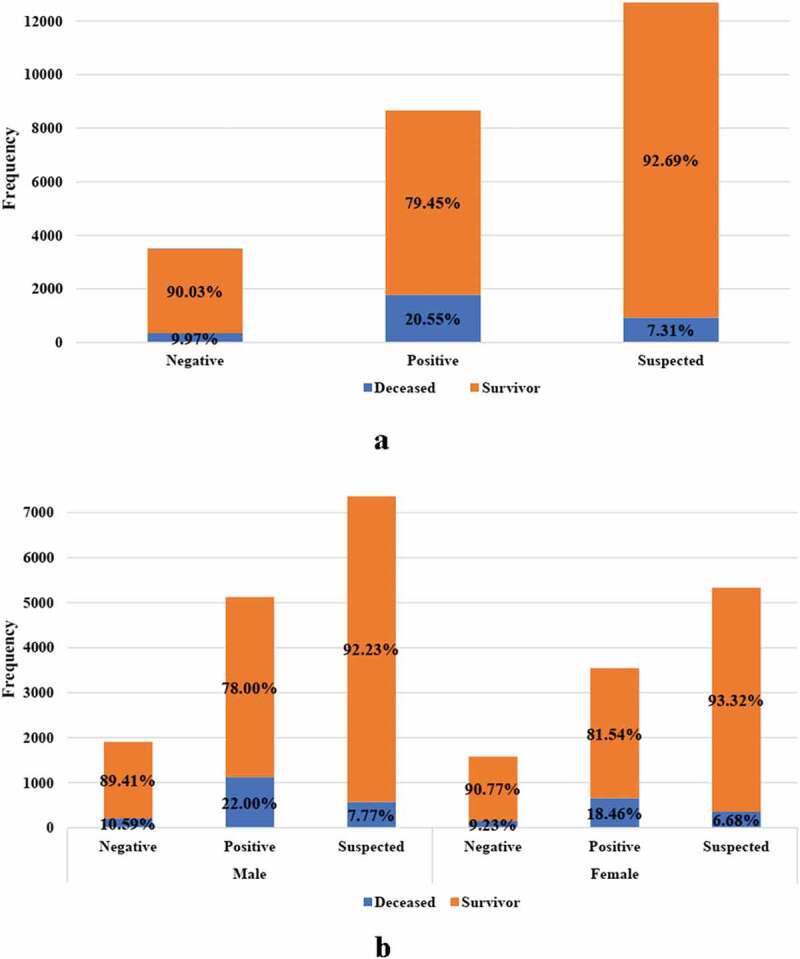
The proportion of deceased cases among negative, positive and suspected patients with COVID-19 in (a) total patients, (b) by gender (the proportion of deceased patients in negative group was significantly higher than in suspected group)
Death frequency distribution was indicated for total cases, and for those all three test result categories (Figure 2). The results showed a constant trend over time followed by a gradual decrease in death frequency of patients with negative PCR test results. Moreover, death frequency followed an approximate bell-shaped curve over time in patients with positive and suspected PCR test results (Figure 2). Kaplan Meier curve was employed to evaluate the hazard of death from COVID-19 between different PCR test results groups. The survival probability of cases with negative test results remained almost zero two months after admission (Supplementary 1).
Figure 2.
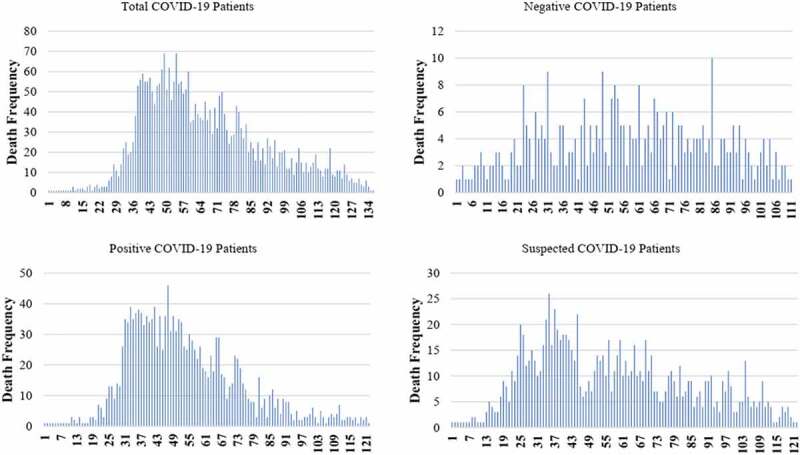
The death frequency per day among total COVID-19 patients, negative COVID-19 patients, positive COVID-19 patients and suspected COVID-19 patients (the values displayed in the axis indicated the days of the study from the start date (20 February 2020) to the end (8 June 2020) date of the study
Logistic regression model was applied to assess the effect of sex on the outcome of interest by age groups. As can be seen, the overall sex effect on mortality was not significant in the younger patient groups. However, male patients were more likely to experienced death if they were adults (OR = 1.38, 95% CI: 1.21– 1.57) or seniors (OR = 1.31, 95% CI: 1.18– 1.46). Also, we observed similar results of a strong association between male sex and outcome among patients with positive and suspected PCR test results in the adult (OR = 1.33, 95% CI: 1.11– 1.58; OR = 1.40, 95% CI: 1.11– 1.77) and senior (OR = 1.34, 95% CI: 1.16– 1.56; OR = 1.22, 95% CI = 1.01– 1.46) groups. In addition, adult male patients with negative PCR test results (OR = 1.56, 95% CI: 1.04– 2.32) were more likely to die due to COVID-19. We also observed that the effect of sex between senior patients in ICU/CCU wards was significant on the outcome of interest (OR = 1.49, 95% CI: 1.15– 1.94) (Table 2).
Table 2.
Sex differential effect on COVID-19 mortality between different age and wards using logistic regression
| Variables | Levels | Age group | Sex | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Wards | ICU.CCU | ||||
| Children (0–14) | Male vs. Female | 0.62 (0.10–3.66) | .593 | ||
| Youth (15–24) | Male vs. Female | 0.92 (0.22–3.92) | .911 | ||
| Adults (25–64) | Male vs. Female | 1.30 (0.99–1.70) | .062 | ||
| Seniors (>65) | Male vs. Female | 1.49 (1.15–1.94) | .003 | ||
| Others | |||||
| Children (0–14) | Male vs. Female | 1.20 (0.40–3.62) | .740 | ||
| Youth (15–24) | Male vs. Female | 0.95 (0.39–2.32) | .916 | ||
| Adults (25–64) | Male vs. Female | 1.37 (1.17–1.61) | <0.001 | ||
| Seniors (>65) | Male vs. Female | 1.27 (1.13–1.43) | <0.001 | ||
| COVID-19 status | Negative | ||||
| Children (0–14) | Male vs. Female | – – | – – | ||
| Youth (15–24) | Male vs. Female | 0.27 (0.02–3.09) | 0.294 | ||
| Adults (25–64) | Male vs. Female | 1.56 (1.04–2.32) | 0.030 | ||
| Seniors (>65) | Male vs. Female | 1.13 (0.86–1.50) | 0.386 | ||
| Positive | |||||
| Children (0–14) | Male vs. Female | 0.18 (0.03–1.11) | 0.064 | ||
| Youth (15–24) | Male vs. Female | 1.28 (0.46–3.61) | 0.639 | ||
| Adults (25–64) | Male vs. Female | 1.33 (1.11–1.58) | 0.001 | ||
| Seniors (>65) | Male vs. Female | 1.34 (1.16–1.56) | 0.000 | ||
| Suspected | |||||
| Children (0–14) | Male vs. Female | 1.80 (0.56–5.76) | 0.320 | ||
| Youth (15–24) | Male vs. Female | 1.01 (0.29–3.50) | 0.982 | ||
| Adults (25–64) | Male vs. Female | 1.40 (1.11–1.77) | 0.005 | ||
| Seniors (>65) | Male vs. Female | 1.22 (1.01–1.46) | 0.035 | ||
| Total | |||||
| Children (0–14) | Male vs. Female | 1.04 (0.42–2.55) | 0.936 | ||
| Youth (15–24) | Male vs. Female | 0.97 (0.47–2.02) | 0.943 | ||
| Adults (25–64) | Male vs. Female | 1.38 (1.21–1.57) | <0.001 | ||
| Seniors (>65) | Male vs. Female | 1.31 (1.18–1.46) | <0.001 |
Cox regression model analysis showed that sex had a significant effect on the hazard of death due to COVID-19 in adults (HR = 1.28, 95% CI: 1.13– 1.46) and seniors (HR = 1.27, 95% CI: 1.15– 1.39). Similar results were found regarding the effect of sex on the death outcome in adults and senior patients having positive (HR = 1.34, 95% CI: 1.14–1.58; HR = 1.23, 95% CI: 1.09–1.39) and suspected (HR = 1.26, 95% CI: 1.00–1.59; HR = 1.22, 95% CI: 1.03–1.44) PCR test results. However, sex was not found as a significant factor for the death outcome in patients with negative PCR test results (Table 3). Using the multiple model controlling for age category and hospital ward, male sex was found to be associated with a 26% higher hazard of death (adjusted HR = 1.26, 95% CI: 1.17– 1.35). Relative to children, seniors had a significant hazard of death (adjusted HR = 2.57, 95% CI: 1.68– 3.91). In addition, the hazard of death among patients in ICU/CCU wards was 2.49 times higher than that of other hospital wards (adjusted HR = 3.49, 95% CI: 3.23– 3.77) (Figure 3).
Table 3.
Sex differential effect on COVID-19 survival time between different age and wards using Cox regression
| Ward | Age Group | Sex | HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Wards | ICU.CCU | ||||
| Children (0–14) | Male vs. Female | 0.67 (0.13–3.34) | 0.620 | ||
| Youth (15–24) | Male vs. Female | 0.46 (0.12–1.76) | 0.258 | ||
| Adults (25–64) | Male vs. Female | 1.24 (1.00–1.55) | 0.051 | ||
| Seniors (>65) | Male vs. Female | 1.25 (1.06–1.48) | 0.009 | ||
| Others | |||||
| Children (0–14) | Male vs. Female | 1.40 (0.47–4.17) | 0.541 | ||
| Youth (15–24) | Male vs. Female | 1.04 (0.43–2.51) | 0.935 | ||
| Adults (25–64) | Male vs. Female | 1.27 (1.09–1.48) | 0.003 | ||
| Seniors (>65) | Male vs. Female | 1.25 (1.12–1.39) | <0.001 | ||
| COVID-19 status | Negative | ||||
| Children (0–14) | Male vs. Female | – - | – - | ||
| Youth (15–24) | Male vs. Female | 0.23 (0.02–2.57) | 0.234 | ||
| Adults (25–64) | Male vs. Female | 1.22 (0.82–1.80) | 0.323 | ||
| Seniors (>65) | Male vs. Female | 1.21 (0.93–1.57) | 0.152 | ||
| Positive | |||||
| Children (0–14) | Male vs. Female | 0.18 (0.02–1.71) | 0.135 | ||
| Youth (15–24) | Male vs. Female | 1.15 (0.43–3.08) | 0.774 | ||
| Adults (25–64) | Male vs. Female | 1.34 (1.14–1.58) | 0.001 | ||
| Seniors (>65) | Male vs. Female | 1.23 (1.09–1.39) | 0.001 | ||
| Suspected | |||||
| Children (0–14) | Male vs. Female | 1.53 (0.49–4.84) | 0.466 | ||
| Youth (15–24) | Male vs. Female | 1.11 (0.33–3.80) | 0.865 | ||
| Adults (25–64) | Male vs. Female | 1.26 (1.00–1.59) | 0.046 | ||
| Seniors (>65) | Male vs. Female | 1.22 (1.03–1.44) | 0.021 | ||
| Total | |||||
| Children (0–14) | Male vs. Female | 1.05 (0.43–2.55) | 0.917 | ||
| Youth (15–24) | Male vs. Female | 0.96 (0.47–1.97) | 0.911 | ||
| Adults (25–64) | Male vs. Female | 1.28 (1.13–1.46) | 0.000 | ||
| Seniors (>65) | Male vs. Female | 1.27 (1.15–1.39) | 0.000 |
Figure 3.
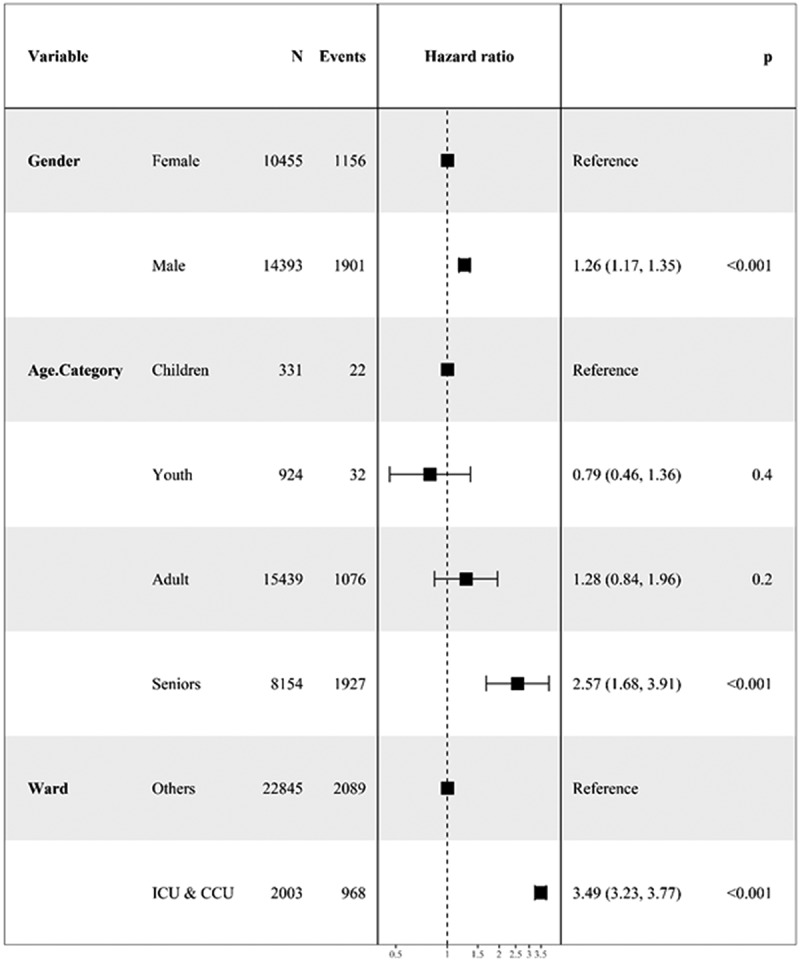
The forest plot for showing the adjusted effect of categorical variables on survival time of patients with COVID-19
4. Discussion
Our study showed that the sex differential effect in COVID-19 mortality varied significantly by age in the categories of test result and hospital ward. Although the highest death rate was observed for patients with confirmed positive test results, it is of note that it was higher in patients with a negative test result compared to suspected ones for both sexes. In addition, our study showed that most of patients in negative group and suspected cases with inconclusive results might be COVID-19 cases. This argues for repeat testing whenever possible.
The official diagnosis of COVID-19 disease in Iran is based on a PCR test. This means that patients with COVID-19 are only confirmed by a positive test. However, the PCR test used in Iran and several other countries have been found to suffer from false-negative results, and a negative test does not necessarily mean that the person does not have COVID-19 [21]. A multicenter study performed on patients with COVID-19 in 19 hospitals in Tehran showed that PCR test results were negative in almost half of the hospitalized patients and the CFR in this group was reported to be about 6.5%. According to the same study, even patients with acute COVID-19 disease who had negative PCR test were not included in the COVID-19 statistics of Iran and were only classified as suspected cases [18]. In the current study, tested patients were classified positive if the SARS-CoV-2 RNA was detected by RT-PCR. Patients were classified as suspected when only one of the two or more genes was identified and cases with negative PCR test were considered as negative group.
We found that almost 12% of the cases with at least one positive PCR test died due to COVID-19 in the total data. Among positive PCR test cases, the CFR was 20%. In the first multicenter study in Tehran conducted by Zali et al, this figure was 13.5% among patients with positive test results [18]. An updated multicenter study in Tehran examining 14 hospitals found that the CFR had risen to 23% [22]. The results differed in a systematic review of 33 studies of other countries, which showed a CFR of 17.1% among hospitalized patients [23]. In Brazil, the CFR trend even showed a decline in the overall hospital CFR of COVID-19 with a minimum value of 20% [24]. A similar downward trend was observed in studies of hospitalized patients in Spain and United States [25,26]. These latter findings may be indicative of improved treatments and patient management with time in these countries. Thus, differences in CFRs in local and regional reports might be due to factors such as the stage of the pandemic, the presence of higher incidence of recognized comorbidity factors including lifestyle and culture, and differences in health-care facilities in hospitals and ICUs [27,28].
We found that the mortality rate of COVID-19 is high even if the PCR results are negative. This finding might be due to multiple reasons. Firstly, the limited and variable sensitivity of the test (reportedly almost 70%) [29] and laboratory equipment might result in false-negative cases. The guidelines by the World Health Organization (WHO) recommended chest imaging as the first diagnostic phase to scan patients who need prompt hospitalization in Iran in the absence of other tests and some inefficiency of existing RT-PCR test kits [30,31]. For example, although there has been a strong association among throat swab and viral loads in sputum samples, investigating the bio-distribution of COVID-19 in various body tissues recorded positive PCR tests in only 72% of these [32,33]. Secondly, it is likely that individuals with a negative result might have been tested when COVID-19 was no longer present [34]. Hyam et al. evaluated the impact of risk factors on positive and negative COVID-19. They investigated demographic, social, health, medical, and environmental characteristics. They found that health risk factors and comorbidities were not associated with the outcome of the test. They also showed that male sex, lower educational attainment, and ethnicity are potential predictors of a positive/negative test outcome [34]. Thirdly, negative PCR results might be due to lower viral load in some patient specimens. Xie et al. conducted a study to find the association between chest CT and negative RT-PCR test results and they showed that negative PCR tests can occur due to laboratory error or insufficient viral material in the specimen [35]. Liu et al. also assessed the indispensable role of chest CT in the detection of COVID-19 and reported that a negative PCR test might be caused by inadequate amounts of virus extracted for testing or incorrect extraction approaches [36]. Finally, some studies found that some cases with positive PCR tests, including some severe cases, originally had normal chest X-ray or CT findings [29,37].
Ignoring the impact of age on the association between sex and COVID-19 outcome, it has been well established that males are more likely to die from COVID-19 than females. The literature has associated this effect with a potential protective influence of female sex steroids, sex-specific expression of pro- and anti-inflammatory cytokines, higher density of ACE2 receptors in childbearing age women, as well as higher numbers of CD4 + T cells, CD8 + T cell cytotoxic functioning, more type 1 interferon, and B cell production of immunoglobulin [38–42]. We addressed the sex difference by age categories. However, reporting the total death rates can be misleading regarding the potential hazards and dangers influenced by age. The mortality proportions in one country might not apply to countries with older or younger age structures or with different methods of confirming a COVID-19 diagnosis. It has been suggested that data should be published according to age groups to provide much more informative estimate of mortality [43]. We reported that the odds of death from COVID-19 is significantly higher in males (compared to females) with a negative PCR test and aged 25–64 years. However, being older than 25 is strongly associated with a higher mortality rate for men compared to women among suspected and positive cases. Moreover, regardless of the COVID-19 status, the likelihood of death is significantly higher among males over 25 years compared to the same age group of females. It has been argued that the COVID-19 mortality sex-differential is not the same at different age. Bhopal et al. showed that the ratio increases with patient age up to 80 years old and decreases thereafter. Their analysis showed that sex differences rose from <60 to 60–69 years but eventually declined, with the lowest sex difference observed at 80+ years of age. Nonetheless, if estrogen protects women against COVID-19, females might experience the greatest protection before the menopause because of the larger blood levels of estrogen [11]. In addition, the importance of progesterone in the sex disparity of COVID-19 was recently demonstrated by Jakovac [44]. Accordingly, there is ample evidence of a protective role of progesterone during COVID-19 disease, suggesting that it could be used as a treatment [45]. Yanez et al. evaluated the COVID-19 mortality risk for older men and women among 16 countries with a relatively high number of cases. Again, this study revealed that men have a higher risk of death from COVID-19 [46]. Ahrenfeldt et al. reported the results of sex and age differences in COVID-19 mortality in Europe and they found that the rate of death for men was higher than for women and the relative rate was the same for cases younger than 60 and older than 80 years [28]. Moreover, among those hospitalized in ICU-CCU, males over 65 years-old are 50% more likely to die than females. In the same manner, studies have found an association between ICU admission and age such that cases younger than 20 years-old are less prone to experience hospitalization, ICU admission, or death from COVID-19 disease [47]. It has been argued that some chronic conditions such as diabetes and cardiovascular diseases are more common among males than in females, especially in older ages [48].
The higher risk of mortality in male patients with COVID-19 than in females was also examined from other aspects. Accordingly, biological, psychological, behavioral, and social factors may put men at higher risk of death. In a study by Griffith et al., the role of these factors on men’s health was reported [49]. In other studies, the effect of behavioral and social differences between men and women as contributing to the disparities in COVID-19 mortality by sex was investigated [50,51]. However, due to insufficient available data in our study, the effect of these factors on the difference in mortality between men and women was not investigated.
We used the Kaplan-Meyer survival curve to visualize the survival probability of cases with suspected, positive, and negative PCR test results. As expected, we observed lower probability of survival for patients with a positive PCR result. The results of the survival analysis showed that the higher risk of death is associated with male sex, senior age, and being hospitalized in ICU-CCU wards. The survival of cases with negative PCR results was not associated with age and sex. However, among those with suspected or positive tests, the hazard ratio was significant for cases older than 25 years-old. Williamson et al. modeled the association between COVID-19 risk factors and mortality using cox regression and they showed that the hazard of death was higher among men than women and older age [52]. Also, the risk factors of COVID-19 outcome was investigated by Chen et al. among hospitalized patients. It was revealed that the hazard of death increased with age so that cases younger than 65 years-old had an almost constant and higher survival probability than older patients [53]. The impact of sex on clinical outcomes in cases with COVID-19 was also evaluated by Cho et al. Their subgroup analysis revealed that the adverse effect of male sex and older age is almost the same in different wards [54]. Another study by Pan et al. assessed the time course of lung changes at chest CT during recovery from coronavirus disease 2019. In their study, women had 5% more probability of discharge and male sex was associated with longer recovery times and a higher average morbidity rate [55]. In a systematic review, Quah et al. investigated mortality rates of patients with COVID-19 in the ICU setting and found that almost 25% of cases died and only 18% were discharged. This finding might be due to the fact that only the more severe cases are hospitalized in ICU-CCU wards. They also revealed that 29% of the ICU patients who died in the Chinese studies did not receive mechanical ventilation and some other countries were struggling with resource constraints so that rationing of ventilators and ICU beds may have postponed the intubation [56]. This could also be influenced by the policy of physicians in transferring new cases to the ICU-CCU wards. Usually, new patients are transferred into the ICU after a previous patient has died and this might delay the golden time of hospitalization. In addition, decision makers in ICU-CCU wards prefer to delegate ICU treatment to the younger patients due to their greater chances of survival.
The major strength of this multicenter study lies in its large sample size, collected from 55 hospitals in the city of Tehran. The study is limited by the fact that we could not consider the interaction of age or sex with the risk factors such as demographics, co-morbidities, clinical signs and symptoms, and drugs on death outcomes in different age categories. This was due to the poor accounting of such data across studies.
5. Conclusion
In this study, we found that the sex differential effect on COVID-19 mortality varied significantly in different age groups with males being more affected only in the higher age groups. Therefore, appropriate strategies should be designed to protect adult and senior males from this deadly infectious disease. Furthermore, owing to the considerable death rate of COVID-19 patients with negative test results, new policies should be launched so as to increase the accuracy of diagnostic tests or implement repeat testing whenever possible.
Supplementary Material
Funding Statement
This study was supported by a grant (number: 23443) from Proteomics Research Center, Faculty of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author contributions
MAL, SJ, EZ, PA, FM, MK, AVA, MRT, GM, MRN, and MAP contributed to project conception; GM prepared data cleaning; MAL and FM did the statistical analysis; AVA did the literature review; MAL, PA, and FM wrote the manuscript and MAP critically revised the successive drafts. All authors read and approved the final manuscript.
Data availability
Data would be available by contacting corresponding author and after excluding the personal information of patients.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical approval
The study was approved by Research Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.RETECH.REC.1399.087).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary Material
Supplemental data for this article can be accessed here.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Clark A, Jit M, and Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]; “ This paper shows differences in COVID-19 mortality between males and females by age groups.
- 2.Liu YC, Kuo RL, Shih SR., et al. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng Y, Wu P, Lu W, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worldometer . COVID-19 CORONAVIRUS PANDEMIC. 2021. [cited 2021 Aug 31]. Available from: https://www.worldometers.info/coronavirus/
- 5.JOHNS HOPKINS UNIVERSITY & MEDICINE/Coronavirus Resource Center . COVID-19 dashboard by the center for systems science and engineering at Johns Hopkins University. 2021. [cited 2021 Aug 31]. Available from: https://coronavirus.jhu.edu/map.html
- 6.Falahi S, Kenarkoohi A.. Sex and gender differences in the outcome of patients with COVID‐19. J Med Virol. 2021;93(1):151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu R, Han H, Liu F, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Chowdhry M, Kahn A., et al. Gender-based disparities in COVID-19: clinical characteristics and propensity-matched analysis of outcomes. medRxiv. ID: ppmedrxiv-20079046 (preprint).
- 9.Wenham C, Smith J, Morgan R., et al. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GlobalHealth5050 . COVID‐19 sex‐disaggregated data tracker. Sex, gender, and COVID‐19 Project; 2020. [cited 2020 Sept 29]. Available from: https://globalhealth5050.org/wp-content/uploads/January-2021-the-covid-19-sex-disaggregated-data-tracker-update.pdf
- 11.Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma G, Volgman AS, and Michos ED., et al. Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC Case Rep. 2020;2(9):1407–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valent F, Doimo A, Mazzilis G, et al. RT-PCR tests for SARS-CoV-2 processed at a large Italian Hospital and false negative results among COVID-19 confirmed cases. Infect Control Hosp Epidemiol. 2021;42(4):498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Yao L, Li J, et al. Stability issues of RT‐PCR testing of SARS‐CoV‐2 for hospitalized patients clinically diagnosed with COVID‐19. J Med Virol. 2020;92(7):903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao AT, Tong YX, Zhang S., et al. False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.JOHNS HOPKINS BLOOMBERG SCHOOL of PUBLIC HEALTH/Center for Health Security . Antigen and molecular tests types of COVID-19 tests. COVID-19 Testing Toolkit 2021; [cited 2021 Mar 26]. Available from: https://www.centerforhealthsecurity.org/covid-19TestingToolkit/testing-basics/types-of-COVID-19-tests/antigen-and-molecular-tests.html
- 18.Zali A, Gholamzadeh S, Mohammadi G, et al. Baseline characteristics and associated factors of mortality in COVID-19 patients; an analysis of 16000 cases in Tehran, Iran. Arch Acad Emerg Med. 2020;8(1):e70. [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadian L, Nejad SS, Khajouei R., et al. Evaluation methods used on health information systems (HISs) in Iran and the effects of HISs on Iranian healthcare: a systematic review. Int J Med Inform. 2015;84(6):444–453. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Canada . Age categories, life cycle groupings. 2021. [cited 2021 Jan 21]. Available from: https://www.statcan.gc.ca/eng/concepts/definitions/age2
- 21.Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vali B. Clinical and epidemiological features of hospitalized patients with COVID-19 in hospitals of Tehran University of Medical Sciences. Frontiers in Emergency Medicine. 2020;5(2 e20). [Google Scholar]
- 23.Macedo A, Gonçalves N, Febra C., et al. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol. 2021;57:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann IR, Sanchez MN, Frio GS, et al. Trends in COVID-19 case-fatality rates in Brazilian public hospitals: a longitudinal cohort of 398,063 hospital admissions from 1st March to 3rd October 2020. PLoS One. 2021;16(7):e0254633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Vidal C, Cózar-Llistó A, and Meira F, et al. Trends in mortality of hospitalised COVID-19 patients: a single centre observational cohort study from Spain. Lancet Reg Health Eur. 2021; 3: 100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16(2):90–92. [DOI] [PubMed] [Google Scholar]
- 27.Sorci G, Faivre B, Morand S., et al. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10(1):18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann C, Wolf E. Older age groups and country-specific case fatality rates of COVID-19 in Europe, USA and Canada. Infection. 2021;49(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y, Zhang H, and Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020. . 296(2): E115–E117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Oragnization . Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization; 2020. [cited 2021 Jan 21]. Available from: https://apps.who.int/iris/handle/10665/331329
- 32.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadeau-Hyam M. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK biobank data. Int J Epidemiol. 2020;49(5):1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;296(2):E41–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Yu H, Zhang S., et al. The indispensable role of chest CT in the detection of coronavirus disease 2019 (COVID-19). Eur J Nucl Med Mol Imaging. 2020;47(7):1638–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frink M, Pape HC, van Griensven M, et al. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27(2):151–156. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakiani S, Olsen NJ, Kovacs WJ., et al. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. [DOI] [PubMed] [Google Scholar]
- 41.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alkhouli M, Nanjundappa A, Annie F, et al. Sex differences in COVID-19 case fatality rate: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhopal R. Covid-19 worldwide: we need precise data by age group and sex urgently. BMJ 2020. . Apr 3;369:m1366. [DOI] [PubMed] [Google Scholar]
- 44.Jakovac H. Sex differences in COVID-19 course and outcome: progesterone should not be neglected. J Appl Physiol (1985). 2020;129(5):1007–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanez ND, Weiss NS, Romand JA, et al. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien J, Du KY, Peng C., et al. Incidence, clinical features, and outcomes of COVID-19 in Canada: impact of sex and age. J Ovarian Res. 2020;13(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamm NC, Pelletier L, Ellison J, et al. Original quantitative research trends in chronic disease incidence rates from the Canadian chronic disease surveillance system. Health Promot Chronic Dis Prev Can. 2019;39(6–7):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffith DM, Sharma G, Holliday CS, et al. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev Chronic Dis. 2020;17:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galasso V, Pons V, Profeta P, et al. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc Natl Acad Sci U S A. 2020;117(44):27285–27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu JJ, Chen JT, Belin TR, et al. Male-female disparities in years of potential life lost attributable to COVID-19 in the United States: a State-by-State analysis. medRxiv. 2021:2021.05.02.21256495. DOI: 10.1101/2021.05.02.21256495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho KH, Kim SW, Park JW, et al. Effect of sex on clinical outcomes in patients with coronavirus disease: a population-based study. J Clin Med. 2021;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quah P, Li A, Phua J., et al. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data would be available by contacting corresponding author and after excluding the personal information of patients.


