Abstract
Introduction
In low- and middle-income nations, acute respiratory infection (ARI) is the primary cause of morbidity and mortality. According to some studies, Ethiopia has a higher prevalence of childhood acute respiratory infection, ranging from 16 to 33.5%. The goal of this study was to determine the risk factors for acute respiratory infection in children under the age of five in rural Ethiopia.
Methods
A cross-sectional study involving 7911 children under the age of five from rural Ethiopia was carried out from January 18 to June 27, 2016. A two stage cluster sampling technique was used recruit study subjects and SPSS version 20 was used to extract and analyze data. A binary logistic regression model was used to identify factors associated with a childhood acute respiratory infection. The multivariable logistic regression analysis includes variables with a p-value less than 0.2 during the bivariate logistic regression analysis. Adjusted odds ratios were used as measures of effect with a 95% confidence interval (CI) and variables with a p-value less than 0.05 were considered as significantly associated with an acute respiratory infection.
Results
The total ARI prevalence rate among 7911 under-five children from rural Ethiopia was 7.8%, according to the findings of the study. The highest prevalence of ARI was found in Oromia (12.8%), followed by Tigray (12.7%), with the lowest frequency found in Benishangul Gumuz (2.4%). A multivariable logistic regression model revealed that child from Poor household (AOR = 2.170, 95% CI: 1.631–2.887), mother’s no education (AOR = 2.050,95% CI: 1.017–4.133), mother’s Primary education (AOR = 2.387, 95% CI:1.176–4.845), child had not received vitamin A (AOR = 1.926, 95% CI:1.578–2.351), child had no diarrhea (AOR = 0.257, 95% CI: 0.210–0.314), mothers not working (AOR = 0.773, 95% CI:0.630–0.948), not stunted (AOR = 0.663, 95% CI: 0.552–0.796), and not improved water source (AOR = 1.715, 95% CI: 1.395–2.109). Similarly, among under-five children, the age of the child, the month of data collection, anemia status, and the province were all substantially linked to ARI.
Conclusions
Childhood ARI morbidity is a serious health challenge in rural Ethiopia, according to this study, with demographic, socioeconomic, nutritional, health, and environmental factors all having a role. As a result, regional governments, healthcare staff, and concerned groups should place a priority on reducing ARI, and attempts to solve the issue should take these variables into account.
Keywords: Acute respiratory infections, Under-five children, Logistic Regression, EDHS
Introduction
ARI is an infection that makes it difficult to breathe normally. Acute Upper Respiratory Infections and Acute Lower Respiratory Infections are the two forms of Acute Respiratory Infections based on the site of infection [1]. The airways from the nose to the voice cords within the larynx make up the upper tract.
The continuation of the airways from the trachea and bronchi to the bronchioles, as well as the alveoli, is part of the lower tract [2, 3]. In low- and middle-income nations, respiratory illness is the major cause of morbidity and mortality [4] across all age groups and sexes [5]. In 2016, lower respiratory infections were responsible for 652, 572 deaths in children under the age of five globally [6]. Acute lower respiratory tract infection in the form of pneumonia, in particular, is regarded as the leading cause of childhood death worldwide, accounting for 16 percent of all deaths in 2015 [7].
Acute respiratory tract infection is one of the most common childhood illnesses, and it almost invariably results in major health problems and death in children under the age of five [8]. ARIs were blamed for 73 percent of the estimated 10.4 million deaths of children under the age of five that occurred over the world [9]. In the WHO African Region, the under-five death rate is 74/1000 live births, over eight times higher than in the WHO European Region (9/1000 live births) [8]. According to the 2016 Ethiopian Demographic and Health Surveys, the under-five mortality rate in Ethiopia is 67/1000 live births [10]. Each year, four to five bouts of ARI affect children under the age of five. ARI is responsible for 30–50% of visits to health institutions and 20–40% of hospital admissions for children under the age of five [11].
Acute Lower respiratory infections (ALRI) were the leading cause of premature mortality across all ages in Ethiopia [12] and pneumonia, in particular, was the second most common cause of mortality in Northeast Ethiopia [13]. Numerous studies have found that socioeconomic and other characteristics, such as the child's age, household income, environmental conditions, parental education, maternal age, and other factors, are linked to ARI [14–18]. In addition, many cross-sectional studies conducted in different parts of the country reported an even higher prevalence of childhood ARI in Ethiopia which ranges from 16% up to 33.5% [19–23].
The majority of factors of ARI occur in rural areas and among children under one year of age (Mirji et al. 2014). Several studies showed that Children from rural setups were more prone to develop ARI [24, 25]. The probable explanation for the greater ARI symptoms proportion for rural children may be due to lack of access to medical treatment, low socio-economic standards in rural regions [26]. According to the Ethiopian demographic and health survey (EDHS), the prevalence of ARI among under-five children was 13% in 2005, and therefore the magnitude reduced to 7% in EDHS 2011, however, the prevalence remained 7% in EDHS 2016. So far, no study had been done on determinants of ARI in different rural communities of Ethiopia and contributing factors for the disease were not well explored. Therefore, ARI cannot be tackled without understanding its causes that is why the study is crucial to investigate the major socio-economic, demographic, health, environmental and nutritional related determinate of ARI among under-five children in rural Ethiopia.
Methods and materials
Study design and setting
A cross-sectional study was conducted from January 18 to June 27, 2016. The study was conducted in Ethiopia, located in the Horn of Africa between 3 and 15 degrees’ north latitude and 33 and 48 degrees’ east longitude. Ethiopia is bordered by Eritrea to the North, Djibouti, and Somalia to the East, Sudan and South Sudan to the West, and Kenya to the South. Ethiopia has eleven geographic or administrative regions; it includes nine regional states and two city administrations. The dataset in this study was obtained from the Demographic and Health Survey conducted in Ethiopia in 2016. The 2016 EDHS was the fourth survey conducted in Ethiopia as part of the worldwide Demographic and Health Surveys project. It was conducted by the Central Statistical Agency (CSA) at the request of the Federal Ministry of Health (FMoH).
Population and sampling procedures
The 2016 EDHS sample was selected by considering two-stage cluster design and census enumeration areas (EAs) were the sampling units for the first stage. A typical two-level stratification involves first stratifying the population by region at the first level, next by urban–rural within each region. The sample included 645 EAs (202 in urban areas and 443 in rural areas). In the sampling procedure, households comprised the second stage of sampling. A complete listing of households was carried out in each of the 645 selected enumeration areas by equal probability systematic sampling according to proportional to EAs measure of size from January 18, 2016, to June 27, 2016. National representatives of 18,008 households were selected based on a nationally representative sample and 16,583 eligible women were included. In this study, 443 EAs from rural Ethiopia were used. All women were aged 15–49 years who had at least one child in the five years before the survey were eligible for participation. The children’s record data and their mother’s record data were merged to obtain a working dataset. The sample for this study would consist of 9254 under-five children from rural Ethiopia, from which only 7911 of them would be considered in this study. The detailed sampling procedure was presented in the measure EDHS report [10].
Inclusion and exclusion criteria
Any child aged 0–59 months who lived with their family in rural Ethiopia and whose parents were willing to participate in the study met the study's inclusion criteria. Children whose mothers or caregivers refused to participate in the study, mothers/caregivers of children who were seriously ill during data collection and/or children on treatment for a confirmed severe respiratory illness, and children whose mothers provided incomplete information were all excluded.
Data collection instrument and quality control
A structured and pre-tested questionnaire was utilized to collect data. Interviewers used tablet computers to record responses during the 2016 EDHS interviews. Bluetooth technology was installed on the tablets to enable distant electronic file transfer (transfer assignment sheets from team supervisors to interviewees and transfer completed copies from interviewers to supervisors) To ensure that the data was of good quality, data collectors were given training. Data quality control and fieldwork coordination were also taught to regional coordinators, field supervisors, and CAPI (computer-assisted personal interview) supervisors. The data collection was overseen by the research investigators on a daily basis. A protocol was written and given to data collectors that govern the survey's design, execution, and administration. Interviews were conducted in quiet, pleasant places at convenient times after data collectors were briefed. Furthermore, participants were encouraged to provide honest responses by describing the study's purpose and significance, as well as ensuring the confidentiality of the data they would provide. Completed surveys were checked for completeness and consistency on a daily basis.
Measurement of variables
The response variable
In this study, the interest variable (y) was constructed as the occurrence of cough accompanied by short, rapid breathing in the 2 weeks preceding the survey. The result was measured as a binary variable, with a code of 1 if a child had ARI and 0 otherwise.
Independent variables
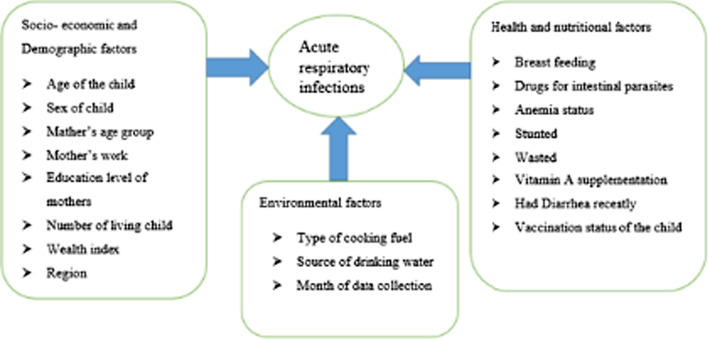
All the explanatory variables were chosen based on existing literature and summarized in a conceptual framework (Fig. 1). Major explanatory variables included the nutritional index of the child, demographic and socio-economic factors, comorbidity disease, and environmental factors. Some of the variables were re-coded. The re-coding was done to make the results more understandable and to keep the sample size for the study at a manageable level. The nutritional status of children was determined by calculating z-scores for "height-for-age (stunting)" and "weight-for-height (wasting)" using WHO-recommended child physical growth indices [10, 27]. If the z-score for each nutritional status was two standard deviations below the median of the WHO reference population, children were declared stunted or wasted, according to WHO criteria [10, 27]. The age of the child was categorized as, less than 6 months, 6–11 months, 12–23 months, 24–35 months, 36–47 months, and 48–59 months; Sex of child; Mother’s age was defined as 15–29, 20–34 and 35 years and above; Household wealth was measured by a wealth index calculated using household assets data via principal component analysis as per the DHS guideline and categorized into five equal categories as poorest (1), poorer (2), middle (3), richer (4), and richest (5) and it is recorded as poor, middle and rich; Maternal education was divided into three categories: no education, primary and secondary education, and higher. Mothers' work was grouped into working and not working; Region; Number of a living child was expressed as 1–3 child, 4–6 child, and six and above [10, 25, 28].
Fig. 1.
Conceptual framework to assess risk factors of acute respiratory infections among under-five children
Receipt of vitamin A in the last 6 months (Yes or No); Vaccination status was measured as ‘No’ for children who never had vaccine and ‘Yes’ for those who were vaccinated. Breast-feeding was assessed by asking mothers whether the child was exclusively breastfed for the first 6 months by classifying into never breastfeeding, ever breastfeeding, not currently, and still breastfeeding. Anemia status of the child (anemic/not anemic), the child had diarrhea (Yes or No) and children received no drugs for intestinal parasites in the last 6 months (Yes or No). Other categorizations of the explanatory variables were the source of drinking water, classified as “improved” or “not improved”. In the case of water piped into the home, piped to the yard/plot, public tap/standpipe, tube-well or borehole, protected well, rainwater, and bottled water, the source of drinking water is designated as "improved." If a drinking water source comprised of an unprotected well, unprotected spring, tanker truck/cart with drum, or surface water, it was classified as "not-improved." Whether the household uses improved cooking fuel (electricity, liquid petroleum gas, natural gas, biogas, kerosene, coal, lignite, charcoal) or unimproved (wood, animal dung, straw/shrubs/grass) fuel, and month of data collection was made from January 18 to June 27 [10, 29].
Statistical analysis
The analysis was done using SPSS version 20 software. Descriptive statistics and a multivariable logistic regression model were used to evaluate the data. To describe our study sample and to determine the prevalence of ARI in our study sample, descriptive statistics were used. The link between the response variable and the explanatory variables was investigated using a multivariable logistic regression model. Both bivariate and multivariable logistic regression analyses were performed. To choose candidate variables for multivariable logistic regression, bivariate logistic regression was used. In bivariate logistic regression, a p-value of less than 0.2 was used as a cut-off point. Before fitting the final model, the variance inflation factor (VIF) was employed to check for multi-collinearities amongst candidate variables. VIF values larger than 10 are typically assumed to indicate multi-collinearity, while values greater than 2.5 in weaker models, such as logistic regression, may be cause for concern [30]]. Based on the findings, all sets of variables in the current study have VIF values of less than 2.5, indicating that multi-collinearity is not an issue. Multivariable logistic regression model was employed to find the predictors of ARI in rural Ethiopian children under the age of five. To analyze the relationship between independent variables considered in the model and ARI, adjusted odds ratios (AOR) with 95% confidence intervals (CI) were generated. Finally, Hosmer and Lemeshow's test was employed to determine whether the fitted model was adequate. A p-value of less than 0.05 was judged to be statistically significant in this study's final model.
Operational definitions
Acute respiratory infection was defined as a child will be considered as having experience of ARI if the mother reported that the child had a cough in the last two weeks before the survey date, accompanied by short rapid breathing. The symptoms are compatible with ARIs [10].
Co-morbidity was defined as the existence of one or more additional diseases co-occurring with primary disease, in this study comorbid diseases were drugs for intestinal parasites, anemia status, malnutrition, and diarrhea in under-five children.
Results
The total number of children included in this study was 7911. From the sampled children, the two weeks’ prevalence of ARI among under-five children was about 7.8% in rural Ethiopia. Considering the ARI distribution by region, rural Oromia and rural Tigray account for the highest proportion of ARIs 12.8% and 12.7%, respectively, and lowest in rural Benishangul Gumuz 2.4% (Table 1).
Table 1.
Socio-economic and demographic characteristics of respondents in rural Ethiopia from January 18 to June 27, 2016 (n = 7911)
| Variables | Categories | Presence of ARI | Total | P-value | |
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| Sex | Male | 3728 (92.2) | 314 (7.8) | 4042 | 0.816 |
| Female | 3563 (92.1) | 306 (7.9) | 3869 | ||
| Age of the child | Less than 6 months | 814 (92.0) | 71 (8) | 885 | 0.000 |
| 6–11 months | 694 (88.4) | 91 (11.6) | 785 | ||
| 12–23 months | 1326 (89) | 164 (11) | 1490 | ||
| 24–35 months | 1421 (91.8) | 127 (8.2) | 1548 | ||
| 36–47 months | 1443 (93.2) | 106 (6.8) | 1549 | ||
| 48–59 months | 1593 (96.3) | 61 (3.7) | 1654 | ||
| Maternal age | 15–19 | 290 (91.8) | 26 (8.2) | 316 | 0.818 |
| 20–34 | 5193 (92.1) | 447 (7.9) | 5640 | ||
| 35–49 | 1808 (92.5) | 147 (7.5) | 1955 | ||
| Wealth index | Poor | 4670 (91.1) | 458 (8.9) | 5128 | 0.000 |
| Middle | 1211 (92.9) | 92 (7.1) | 1303 | ||
| Rich | 1410 (95.3) | 70 (4.7) | 1480 | ||
| Mothers education level | No education | 5262 (92.1) | 451 (8.5) | 5713 | 0.002 |
| Primary | 1721 (91.5) | 160 (7.9) | 1881 | ||
| Secondary and above | 308 (97.2) | 9 (2.8) | 317 | ||
| Mothers currently working | Not working | 5508 (92.4) | 456 (7.6) | 5964 | 0.19 |
| Working | 1783 (91.6) | 164 (8.4) | 1947 | ||
| Number of living child | 1–3 child | 3264 (92.4) | 267 (7.6) | 3531 | 0.972 |
| 4–6 child | 2828 (91.8) | 251 (8.2) | 3079 | ||
| 6 and above | 1199 (92.2) | 102 (7.8) | 1301 | ||
| Region | Rural Tigray | 716 (87.3) | 104 (12.7) | 820 | 0.000 |
| Rural Afar | 804 (92.9) | 61 (7.1) | 865 | ||
| Rural Amhara | 733 (90.3) | 79 (9.7) | 812 | ||
| Rural Oromia | 1217 (87.2) | 179 (12.8) | 1396 | ||
| Rural Somali | 1042 (95.2) | 53 (4.8) | 1095 | ||
| Rural Benishangul Gumuz | 725 (97.6) | 18 (2.4) | 743 | ||
| Rural SNNPR | 987 (92) | 86 (8) | 1073 | ||
| Rural Gambela | 473 (96.3) | 18 (3.7) | 491 | ||
| Rural Harari | 351 (96.7) | 12 (3.3) | 363 | ||
| Rural Dire Dawa | 243 (96) | 10 (4) | 253 | ||
| Total | 7261 (92.2) | 620 (7.8) | 7911 | ||
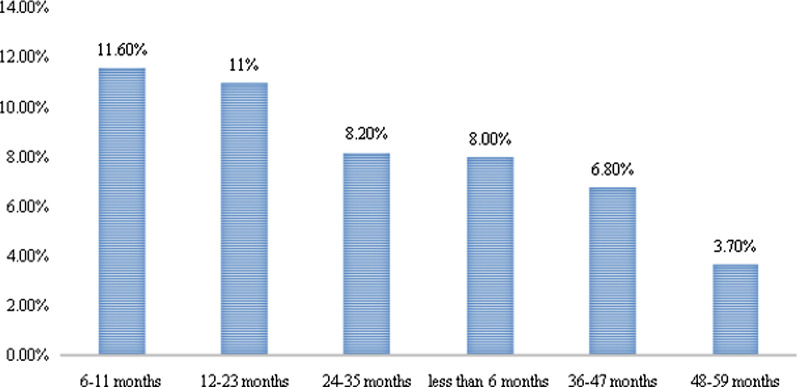
Out of 7911 under-five children the proportion of males and females were 4042 (51.1%) and 3869 (48.9%) with the prevalence of ARI 7.8% and 7.9% respectively. The prevalence of ARI was highest in the age group 6–11 months (11.6%), followed by 12–23 months (11%), and comparatively lower in the 48–59 months’ age group (3.7%) (Fig. 2).
Fig. 2.
Distribution of ARIs according to the age of the child in rural Ethiopia
Regarding maternal educational status, 5713(72.2%) had no education. In terms of the wealth index, 5128 (64.8%) of the participants were from poor families. Poor families have a larger percentage of under-five children with ARI (8.9%) (Table 1.
The child whose source of drinking water was unprotected/unimproved had the highest rate of ARI (9. 2%). In addition, the highest frequency of ARI was documented in January (12.5%), and the highest prevalence of ARI was recorded in households that utilized Unsafe/unclean cooking fuel (8.3%) (Table 2).
Table 2.
Environmental characteristics of the respondents in rural Ethiopia from January 18 to June 27, 2016 (n = 7911)
| Variables | Categories | Presence of ARI | Total | P-value | |
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| Type of cooking fuel | Unsafe/unclean | 7247 (92.2) | 616 (8.3) | 7863 | 0.898 |
| Safe/clean | 44 (91.7) | 4 (7.8) | 48 | ||
| Type of water source | Not improved | 4714 (90.8) | 476 (9.2) | 5190 | 0.000 |
| Improved | 2577 (94.7) | 144 (5.3) | 2721 | ||
| Month of data collection | January | 496 (87.5) | 71 (12.5) | 567 | 0.000 |
| February | 1647 (91.2) | 158 (8.8) | 1805 | ||
| March | 1780 (94.0) | 113 (6.0) | 1893 | ||
| April | 1658 (93.0) | 124 (7.0) | 1782 | ||
| May | 1532 (91.6) | 140 (8.4) | 1672 | ||
| June | 178 (92.7) | 14 (7.3) | 192 | ||
Of the children, 899 (11.4%) had a history of diarrhea with the highest prevalence of ARI (21.9%). About 4662(58.9) were not vaccinated against any type of vaccine-preventable infectious with the highest prevalence of ARI (7.9%). Regarding nutritional status, 959 (12.12%) were wasted and 3129(39.5%) were stunted with the highest prevalence of ARI (9.9%) and (9.7%) respectively. The majority, 6655 (84.1%) of children received no drugs for intestinal parasites in the last 6 months, and 4707(59.5%) of them not received vitamin A recently with the highest proportion on the prevalence of ARI (9.5%) (Table 3).
Table 3.
Nutritional and Comorbidity Characteristics of ARI among Under Five Children in rural Ethiopia from January 18 to June 27, 2016 (n = 7911)
| Variables | Categories | Presence of ARI | Total | P-value | |
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| Duration of breastfeeding | Never breast feeding | 272 (94.4) | 16 (5.6) | 288 | 0.000 |
| Ever breastfeeding, not currently | 3978 (93.8) | 261 (6.2) | 4239 | ||
| Still breast feeding | 3041 (89.9) | 343 (10.1) | 3384 | ||
| Receive vitamin A in last 6 months | No | 4259 (90.5) | 448 (9.5) | 4707 | 0.000 |
| Yes | 3032 (94.6) | 172 (5.4) | 3204 | ||
| Had diarrhea recently | No | 6589 (94) | 423 (6) | 7012 | 0.000 |
| Yes | 702 (78.1) | 197 (21.9) | 899 | ||
| Acute malnutrition of child | No | 6427 (92.4) | 525 (7.6) | 6952 | 0.011 |
| Yes | 864 (90.1) | 95 (9.9) | 959 | ||
| Chronic malnutrition of a child | No | 4465 (93.4) | 317 (6.6) | 4782 | 0.000 |
| Yes | 2826 (90.3) | 303 (9.7) | 3129 | ||
| Drugs for intestinal parasites | No | 6128 (92.1) | 527 (7.9) | 6655 | 0.534 |
| Yes | 1163 (92.6) | 93 (7.4) | 1256 | ||
| Anemia status of the child | Not anemic | 4575 (93.4) | 323 (6.6) | 4898 | 0.000 |
| Anemic | 2716 (90.1) | 297 (9.9) | 3013 | ||
| Vaccination status | No | 4293 (92.1) | 369 (7.9) | 4662 | 0.758 |
| Yes | 2998 (92.3) | 251 (7.7) | 3249 | ||
Factors associated with acute respiratory infection among under-five children
In multivariable logistic regression analysis, age of the child, wealth index of household, mother’s education level, had received vitamin A recently, had diarrhea, stunted, source of drinking water, maternal occupation, the month of data collection, anemia status, and the region were significantly related to the acute respiratory infections of under-five children.
The impact of many factors on childhood ARI status was assessed using a multivariable logistic regression model, and the significant findings are summarized in the following section. Based on a multivariable logistic regression analysis, the odds of ARI decreased as the age of the child increased. The odds of incidence of ARI among under-five children in the age group of less than 6 months,6–11 month, 12–23 month,24–35 month, 36–47 month, were (AOR = 1.936, 95% CI: 1.243–3.014),(AOR = 2.783,95%CI:1.806–4.288),(AOR = 2.47095%CI:1.697–3.597), (AOR = 1.987, 95% CI:1.420–2.779) and (AOR = 1.729, 95% CI:1.238–2.416) times more likely experiencing ARI than those children in the age group 48–59 months respectively (Table 4).
Table 4.
Associations between ARI and predictor variables among children under five in rural Ethiopia from January 18 to June 27, 2016 (n = 7911)
| Variables | Categories | Presence of ARI | COR(95% CI) | AOR (95% C.I) | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Age of the child | 48–59 months (ref) | 1593 | 61 | 1.0 | 1.0 |
| Less than 6 months | 814 | 71 | 2.278 (1.601–3.240) | 1.936 (1.243–3.014)* | |
| 6–11 months | 694 | 91 | 3.424 (2.446–4.793) | 2.783 (1.806–4.288)* | |
| 12–23 months | 1326 | 164 | 3.230 (2.386–4.372) | 2.470 (1.697–3.597)* | |
| 24–35 months | 1421 | 127 | 2.334 (1.706–3.194) | 1.987 (1.420–2.779)* | |
| 36–47 months | 1443 | 106 | 1.918 (1.389–2.650) | 1.729 (1.238–2.416)* | |
| Wealth index | Rich (ref) | 1410 | 70 | 1.0 | 1.0 |
| Poor | 4670 | 458 | 1.975(1.526–2.558) | 2.170(1.631–2.887)* | |
| Middle | 1211 | 92 | 1.530(1.111–2.108) | 1.388(0.992–1.943) | |
| Duration of breastfeeding | Still breastfeeding (ref) | 3041 | 343 | 1.0 | 1.0 |
| Never breast feeding | 272 | 16 | 0.522(0.311–0.874) | 0.884(0.507–1.544) | |
| Ever breastfeeding, not currently | 3978 | 261 | 0.582 (0.492–0.688) | 0.942 (0.724–1.226) | |
| Mothers educational level | Secondary and above (ref) | 308 | 9 | 1.0 | 1.0 |
| No education | 5262 | 451 | 2.933 (1.501–5.731) | 2.050 (1.017–4.133)* | |
| Primary | 1721 | 160 | 3.182 (1.608–6.295) | 2.387 (1.176–4.845)* | |
| Receive vitamin A in last 6 months | Yes (ref) | 3032 | 172 | 1.0 | 1.0 |
| No | 4259 | 448 | 1.854 (1.546–2.224) | 1.926 (1.578–2.351)* | |
| Had diarrhea recently | Yes (ref) | 702 | 197 | 1.0 | 1.0 |
| No | 6589 | 423 | 0.229 (0.190–0.276) | 0.257 (0.210–0.314)* | |
| Mothers currently working | Working (ref) | 1783 | 164 | 1.0 | 1.0 |
| Not working | 5508 | 456 | 0.9 (0.747–1.084) | 0.773 (0.630–0.948)* | |
| Wasted | Yes (ref) | 864 | 95 | 1.0 | 1.0 |
| No | 6427 | 525 | 0.743 (0.590–0.935) | 0.788 (0.614–1.011) | |
| Stunted | Yes (ref) | 2826 | 303 | 1.0 | 1.0 |
| No | 4465 | 317 | 0.662 (0.562–0.780) | 0.663 (0.552–0.796)* | |
| Type of water source | Improved (ref) | 2577 | 144 | 1.0 | 1.0 |
| Not improved | 4714 | 476 | 1.807 (1.491–2.191 | 1.715 (1.395–2.109)* | |
| Region | Rural Dire Dawa (ref) | 243 | 10 | 1.0 | 1.0 |
| Rural Tigray | 716 | 104 | 3.530 (1.815–6.863) | 3.628 (1.817–7.244)* | |
| Rural Afar | 804 | 61 | 1.844 (0.930–3.653) | 1.446 (0.711–2.941) | |
| Rural Amhara | 733 | 79 | 2.619 (1.335–5.137) | 2.410 (1.192–4.872)* | |
| Rural Oromia | 1217 | 179 | 3.574 (1.863–6.857) | 3.516 (1.784–6.928)* | |
| Rural Somali | 1042 | 53 | 1.236 (0.620–2.464) | 1.132 (0.553–2.317) | |
| Rural Benishangul gumuz | 725 | 18 | 0.603 (0.275–1.325) | 0.545 (0.241–1.230) | |
| Rural SNNPR | 987 | 86 | 2.117 (1.084–4.137) | 2.377 (1.181–4.784)* | |
| Rural Gambela | 473 | 18 | 0.925 (0.420–2.034) | 0.832 (0.367–1.886) | |
| Rural Harari | 351 | 12 | 0.831 (0.353–1.953) | 0.805 (0.333–1.944) | |
| Month of data collection | June(ref) | 178 | 14 | 1.0 | 1.0 |
| January | 496 | 71 | 1.820 (1.001–3.310) | 2.125 (1.127–4.010)* | |
| February | 1647 | 158 | 1.220 (0.691–2.152) | 1.535 (0.841–2.801) | |
| March | 1780 | 113 | 0.807 (0.454–1.436) | 0.989 (0.538–1.819) | |
| April | 1658 | 124 | 0.951 (0.536–1.688) | 1.231 (0.670–2.260) | |
| May | 1532 | 140 | 1.162 (0.656–2.056) | 1.319 (0.720–2.414) | |
| Anemia status | Anemia (ref) | 2716 | 297 | 1.0 | 1.0 |
| Not anemic | 4575 | 323 | 0.646 (0.548–0.761) | 0.636 (0.532–0.761* | |
*Significant at . Ref = reference category, Hosmer and Lemeshow's goodness of model test was found to be chi-square of 19.614 with a p-value of 0.12 which implies the goodness of the model to predict the outcome
The odds of under-five children developing ARI among mothers who had no education to have ARI was 2.050 (AOR 2.050; 95% CI 1.017–4.133) times more likely affected by ARIs as compared with children whose mothers’ had secondary and above educational level and children whose mothers had primary education was 2.387 times more likely compared with children whose mothers had secondary and above education level (AOR 2.387; 95% CI 1.176–4.845). Moreover, children within the poorest quintile were 2.170 times more likely to develop cases (AOR = 2.170, 95% CI: 1.631–2.887), compared with children from the richest households.
The odds of ARI among not stunted children were 33.7% less likely to develop ARI compared to stunted children (AOR 0.663; 95% CI 0.552–0.796). Maternal occupation also has a statistically significant association with ARI; accordingly, compared with children of employed mothers, children whose mothers were not worked had 22.7% reduced odds of having ARI (AOR = 0.773; 95% CI 0.630–0.948).
According to this study, Vitamin A consumption of children in the last six months was found that it had a significant effect on the ARIs. The odds of a child who had not received vitamin A recently was 92.6% (AOR = 1.926, 95% CI 1.578–2.351) more likely to suffer from ARI compared to a child who received vitamin A recently. Indeed, the odds of Under-five children who had no Diarrhea recently were 74.3% (AOR = 0.257, CI: 0.210–0.314) times less likely to experience ARI compared with a child who had Diarrhea recently. Anemia has been stated as one of the risk factors of ARI. When compared to a kid who had anemia, the child who did not have anemia was 36.4% (AOR = 0.636 95 percent CI: 0.532–0.761) less likely to develop ARI and the frequency of ARI was strongly linked to the season, the prevalence of ARI in January was 2.125 times (AOR = 2.125 95% CI:1.127–4.010) higher than in the reference group.
The source of drinking water also has a significant effect on ARI. The odds of children using an unprotected source of drinking water were 71.5% (AOR = 1.715, 95% CI: 1.395–2.109) times more likely to have ARI as compared with a child using a protected source of drinking water. Province was also significantly related to ARI symptoms (P < 0.05). The odds of having ARI symptoms for children in rural Afar, rural Somali, rural Benishangul Gumuz, rural Gambela, and rural Harari were not significantly different as compared to rural Dire Dawa. Children in rural Tigray regional state (AOR: 3.628, 95% CI: 1.817–7.244), Children who lived in rural Amhara (AOR: 2.410, 95% CI: 1.192–4.872), rural Oromia (AOR: 3.516, 95% CI: 1.784–6.928), and rural South Nation Nationalities Peoples regional states (AOR: 2.377, 95% CI: 1.181–4.784) were significantly higher risk of ARI symptoms as compared to rural Dire Dawa.
Discussions
The current study aimed at evaluating the prevalence of, and risk factors associated with, AR in rural Ethiopia based on the Ethiopian Demographic and Health Survey data. In the study overall prevalence of ARI was found to be 7.8%. Our findings are comparable to those of a study conducted by [31] in Zambia, where the prevalence of ARI was reported to be 8% and 6.9% in Iraq by [32]. In contrast to our findings, a study conducted in New Delhi slums estimated the overall prevalence of ARI among children under the age of five to be around 4.5% for one month [33]. ARI affected 21.3% of Bangladeshi children under the age of five in the two weeks before the survey [17]. Differences in study populations, study sites, age categories investigated, the method used to assess the outcome variable, comorbidities, and variations in the study period and season could all contribute to the variation in ARI proportions.
The study showed that there is an enormous disparity in the distribution of ARIs in children across the different regional states of rural Ethiopia. According to the descriptive data analysis, the highest prevalence of ARI occurred in rural Oromia and rural Tigray at 12.8% and 12.7% respectively and the lowest, 2.4%, in rural Benishangul Gumuz region. It is in line with [34, 35]. There could be numerous reasons for the regional variations in illness distributions; high-risk areas for ARI were found in the northern and central parts of the country, particularly rural Tigray and rural Oromia. These were highland areas, implying a high prevalence of ARI among children under the age of five. Children are indoors for longer periods when the weather is cold, putting them in close contact with a variety of bacteria, fungi, and viruses. When the temperature drops, flu, viruses, and bacteria are more likely to remain stable in the air, forming respiratory droplets and causing damage to the airways, allowing bacteria to cause infections, most commonly ARI in the lungs. Another reason could be that many of the children in these areas were under the age of one year, and the majority of households in these areas relied on charcoal and cow dung as a source of fuel.
In this study, age of the child, wealth index of household, maternal education, received vitamin A supplement, had diarrhea recently, stunting, maternal occupation, source of drinking water, anemia status, the month of data collection, and region of the residence were found to be significantly associated with ARI symptoms. Children aged 48–59 months have a lower risk of developing ARI symptoms. This finding is consistent with studies undertaken in Ahmadabad city and other low- and middle-income nations [36, 37]. The prevalence of ARI is particularly common among children aged 6–36 months, according to studies conducted in the People's Republic of China and South India [38, 39]. Compared to a child more than 48 months, the chances of the child at the earlier age experiencing acute respiratory infection symptoms are higher. It is obvious that as a child grows older, he or she will have greater resistance to diseases such as cough and diarrhea [14, 25]. Because children's immunity develops as they grow older, they are more prepared to battle infection.
Children from the poorest families are more likely to contract ARI than those from wealthier households. These findings are supported by the study done in Bangladesh, residence in poor households conferred 1.3 times (95% CI 1.09–1.55) higher odds of ARI in young children [17]. Under-five children those whose family were from the low socio-economic class was also significantly associated with ARI [40–43]. This is due to wealthier households tend to afford better nourishment and health care for their children. Wealthier families can also minimize their children’s exposure to risk factors like unsanitary environments and contaminated water [44]. These findings are supported by other studies which found that higher poverty levels increase the risk of ARI and diarrhea [45, 46]
Children whose mothers have no or only a primary education have a much higher risk of ARI than children whose mothers have a secondary or higher degree of education. This statement is consistent with earlier findings [14, 47]. This could be because education has improved mothers' ability to apply basic health knowledge and has facilitated their ability to manipulate their environment, including health care facilities, work more effectively with health professionals, follow treatment recommendations, and keep their environment clean. Furthermore, educated mothers have more power over their children's health decisions.
Children with stunted were shown to have a greater prevalence of ARI. This finding is also in line with studies conducted in India, specifically in Solapur and Gujarat, Ethiopia, and other developing countries [22, 36, 39, 43, 48–50]. This finding indicates that malnourished children have inadequate immunity and are susceptible to a variety of diseases, including ARI.
A kids who had not recently received vitamin A was 92.6% more likely to develop ARI than a child who had recently received vitamin A. Promoting Vitamin A supplementation for all rural children could improve their health. Several studies backed up our conclusions [17].
In this study, under-five children from rural areas with a history of diarrhea were more affected by ARI. This research backed up a study conducted in northeast Ethiopia's Oromia zone, which found that children with a history of diarrhea were three times more likely to get ARI than their peers [51]. Similar studies have been carried out in southwest Ethiopia and Ghana [52, 53]. In addition, a study done in Bangladeshi found that children with a history of diarrhea were more likely to develop ARI [54]. The reason is children who have a concomitant illness like diarrhea may have a lowered immunity, making them more susceptible to a disease like ARI. The reason for this is that children with a comorbid ailment, such as diarrhea, anemia may have decreased immunity, making them more vulnerable to diseases like ARI.
In rural Ethiopia, the source of drinking water is another key environmental element that influences the ARI of children under the age of five. This study found that children who drink water from an unprotected/unimproved source are more likely to have ARI symptoms than children who drink water from a protected/improved source. This result was supported by [21, 55]. This was due to the fact that contaminated water was thought to be the leading cause of acute respiratory infection.
Mother’s occupation was found to be a significant factor of ARIs. Compared to children of mothers currently working those children whose mothers have no work in any sectors have a significantly lower risk of ARI. This means mothers to stays at home may keep their child in a good and clear environment. This result was supported by [14, 34]. The other explanation might be that, mothers who had work could be exposed to certain chemicals, pollutants, or toxic fumes within the working environment, thereby transmitting the infection to their children may be increased. Furthermore, because the majority of mothers in developing countries like Ethiopia work in the informal sector and lower-status occupations, the amount of income for these mothers is low and would be a significant effect on ARIs of under five children.
The odds of the child suffering from ARI were higher in January. Because this study was carried out during the peak of the dry season which is characterized by dry and dusty harmattan winds. It is consistent with the previous study done by [56–58]. Anemia or low hemoglobin status has also been stated as one of the risk factors of ARI. This finding was consistent with the studies conducted by [59]. Similar significance was also shown in other studies like [60, 61]. Because most healthy children can fight infection with their natural defenses, children with impaired immune systems are more susceptible to infection. Anemia amplifies this effect by reducing the body's natural defenses. So, when the current study was compared to previous similar studies, there was a strong link between anemia and ARI. As a result, preventing anemia and detecting anemia early can help to lower the incidence of acute respiratory tract infection.
Finally, ARI among under-five children was a significant association with regions. It revealed that the probability of children living in rural Tigray, rural Amhara, rural Oromia, and rural SNNPR regions have higher ARI symptoms before two weeks preceding the survey than children who living in rural Dire Dawa regions. It is consistent with a previous study done by [34]. While, the odds of children living in rural Somali, rural Benishangul-Gumuz, rural Gambela, and rural Afar, being have ARI symptoms before two weeks are significantly not different from those living in rural Dire Dawa.
Strengths and weaknesses of the study
A strength of this study was that it assessed many important determinant factors including socioeconomic status, maternal and child nutrition, environmental, health, and other factors contributing to ARI in a cross-sectional study design. This study's findings should help policymakers better grasp this rapidly evolving health hazard and adopt appropriate legislative steps. Aside from our significant contributions, our research contains a number of flaws. First, the symptoms of ARIs were reported by mothers and were not the result of clinical examinations. Because the variables were self-reported, they are prone to reporting bias. Second, no data on household hygiene practices, which is a critical predictor of infectious diseases in people of all ages, was available. However, this gap is expected to be overcome, to a great extent, by the inclusion of sanitation variables. Finally, because the data was secondary, no causal inferences regarding the correlations could be drawn. Therefore, the reader of this article should take into account the above barriers.
Conclusions
In this study from a rural setting in Ethiopia, significant factors for ARI in under-five children were child age, maternal/caregiver occupation, economic status of the family, received vitamin A recently, had diarrhea recently, anemia status, the month of data collection, region, mother’s education level, source of drinking water and nutritional status of the child. The occurrence of ARI could be reduced by implementing interventions to improve economic status, nutrition status, mother’s educational level and by increasing community awareness regarding protected water, diarrhea, anemia, morbidity, and vitamin A supplementation for the prevention of ARI amongst under-five children.
Acknowledgements
The author wishes to express his gratitude to the Central Statistical Agency for allowing us to use the data in this study.
Abbreviations
- ALRI
Acute Lower Respiratory Infections
- AOR
Adjusted Odds Ratio
- ARI
Acute Respiratory Infections
- AURI
Acute Upper Respiratory Infections
- CI
Confidence Interval
- CSA
Central Statistical Agency
- EA
Enumeration Areas
- EDHS
Ethiopia Demographic Health Survey
- FMoH
Federal Ministry of Health
- SNNPR
South Nations Nationalities Peoples Region
- WHO
World Health Organizations
Authors' contributions
AMM developed the proposal, designed, analyzed the data, writes up the paper and developed the manuscript. The author read and approved the final manuscript.
Funding
No funding was received for this work.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In this study, we used population based secondary datasets available online with all identifier information removed. MEASURE DHS gave the author permission to use the data. DHS Program is consistent with the standards for ensuring the protection of respondents’ privacy. ICF International assures that the survey complies with the Human Subjects Protection Act of the U.S. Department of Health and Human Services. The description of consent process is available from: http://goo.gl/ny8T6X.
Consent for publication
Not applicable.
Competing interests
The author has no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Broor S, Parveen S, Bharaj P, Prasad VS, Srinivasulu KN, Sumanth KM, Kapoor SK, Fowler K, Sullender W. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE. 2007;2(6):e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simoes EAF, Cherian T, Chow J, Shahid-Salles SA, Laxminarayan R, Jacob John T. Acute respiratory infections in children. In: Disease control priorities in developing countries. 2nd edition. 2006.
- 3.Khalek EA, Abdel-Salam DM. Acute respiratory tract infections in children under 5 years of age in Upper Egypt. Int J Community Med Public Health. 2016;3(5):1161–1166. [Google Scholar]
- 4.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassebaum N, Kyu HH, Zoeckler L, Olsen HE, Thomas K, Pinho C, Bhutta ZA, Dandona L, Ferrari A, Ghiwot TT, Hay SI. Child and adolescent health from 1990 to 2015: findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA pediatr. 2017;171(6):573–592. doi: 10.1001/jamapediatrics.2017.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, Fullman N, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. IJMS. 2016;17(12):2120. doi: 10.3390/ijms17122120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO:Children reducing mortality 2018," https://www.who.int/news-room/fact-sheets/detail/, Accessed 4 Feb 2019.
- 9.Anjum MU, Hashim R, HafizMuhammad T. Acute respiratory tract infections (ARIS) TPMJ. 2017;24(02):322–325. [Google Scholar]
- 10.Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA CSA and ICF; 2016.
- 11.Mir AA, Imtiyaz A, Fazili A, Iqbal J, Jabeen R, Salathia A. Prevalence and risk factor analysis of acute respiratory tract infections in rural areas of Kashmir valley under 5 years of age. Int J Med Public Health. 2012 doi: 10.4103/2230-8598.108392. [DOI] [Google Scholar]
- 12.Misganaw A, Haregu TN, Deribe K, Tessema GA, Deribew A, Melaku YA, Amare AT, et al. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990–2015: findings from the Global Burden of Disease Study 2015. Popul Health Metrics. 2017;15(1):1–17. doi: 10.1186/s12963-017-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abejew AA, Tamir AS, Kerie MW. Retrospective analysis of mortalities in a tertiary care hospital in Northeast Ethiopia. BMC Res Notes. 2014;7(1):1–6. doi: 10.1186/1756-0500-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinzón-Rondón ÁM, Aguilera-Otalvaro P, Zárate-Ardila C, Hoyos-Martínez A. Acute respiratory infection in children from developing nations: a multi-level study. Toxicol Lett. 2016 doi: 10.1016/j.toxlet.2016.07.636. [DOI] [PubMed] [Google Scholar]
- 15.Adesanya OA, Chiao C. A multilevel analysis of lifestyle variations in symptoms of acute respiratory infection among young children under five in Nigeria. BMC Public Health. 2016;16(1):1–11. doi: 10.1186/s12889-016-3565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulkadir MB, Abdulkadir ZA, Johnson WB. An analysis of national data on care-seeking behaviour by parents of children with suspected pneumonia in Nigeria. S Afr J Child Health. 2016;10(1):92–95. [Google Scholar]
- 17.Azad KM. Risk factors for acute respiratory infections (ARI) among under-five children in Bangladesh. J Sci Res. 2009;1(1):72–81. [Google Scholar]
- 18.Kanungo S, Bhowmik K, Mahapatra T, Mahapatra S, Bhadra UK, Sarkar K. Perceived morbidity, healthcare-seeking behavior and their determinants in a poor-resource setting: observation from India. PLoS ONE. 2015;10(5):e0125865. doi: 10.1371/journal.pone.0125865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abuka T. Prevalence of pneumonia and factors associated among children 2–59 months old in Wondo Genet district, Sidama zone, SNNPR. Ethiopia. Cur Pediatr Res. 2017;21(1):19–25. [Google Scholar]
- 20.Mekuriaw Alemayehu KA, Hardeep R. Household fuel use and acute respiratory infections in children under five years of age in Gondar city of Ethiopia. J Environ Earth Sci. 2014;4(7):77–85. [Google Scholar]
- 21.Desalegn B, Suleiman H, Asfaw A. Household fuel use and acute respiratory infections among younger children: an exposure assessment in Shebedino Wereda, Southern Ethiopia. Afr J Health Sci. 2011;18(1–2):31–36. [Google Scholar]
- 22.Fekadu GA, Terefe MW, Alemie GA. Prevalence of pneumonia among under-five children in Este Town and the surrounding rural Kebeles, Northwest Ethiopia: a community based cross sectional study. SJPH. 2014;2(3):150–155. [Google Scholar]
- 23.Sanbata H, Asfaw A, Kumie A. Association of biomass fuel use with acute respiratory infections among under-five children in a slum urban of Addis Ababa, Ethiopia. BMC Public Health. 2014;14(1):1–8. doi: 10.1186/1471-2458-14-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidu AA, Ameyaw EK, Ahinkorah BO, Baatiema L, Appiah F. Ecological zone and symptoms of acute respiratory infection among children under five in Ghana: 1993–2014. SSM Population Health. 2019;8:100414. doi: 10.1016/j.ssmph.2019.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harerimana J-M, Nyirazinyoye L, Thomson DR, Ntaganira J. Social, economic and environmental risk factors for acute lower respiratory infections among children under five years of age in Rwanda. Arch Public Health. 2016;74(1):1–7. doi: 10.1186/s13690-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilabuko JH, Nakai S. Effects of cooking fuels on acute respiratory infections in children in Tanzania. Int J Environ Res Public Health. 2007;4(4):283–288. doi: 10.3390/ijerph200704040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishwajit G. Household wealth status and overweight and obesity among adult women in B angladesh and Nepal. Obes Sci Pract. 2017;3(2):185–192. doi: 10.1002/osp4.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvaraj K, Chinnakali P, Majumdar A, Krishnan IS. Acute respiratory infections among under-5 children in India: a situational analysis. J Nat Sc Biol Med. 2014;5(1):15. doi: 10.4103/0976-9668.127275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinyemi JO, Morakinyo OM. Household environment and symptoms of childhood acute respiratory tract infections in Nigeria, 2003–2013: a decade of progress and stagnation. BMC Infect Dis. 2018;18(1):1–12. doi: 10.1186/s12879-018-3207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senaviratna NA, Cooray TM. Diagnosing multicollinearity of logistic regression model. AJPAS. 2019 doi: 10.9734/ajpas/2019/v5i230132. [DOI] [Google Scholar]
- 31.Mulambya NL, Nanzaluka FH, Sinyangwe NN, Makasa M. Trends and factors associated with acute respiratory infection among under five children in Zambia: evidence from Zambia’s demographic and health surveys (1996–2014) Pan Afr Med. 2020 doi: 10.11604/pamj.2020.36.197.18799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siziya S, Muula AS, Rudatsikira E. Diarrhoea and acute respiratory infections prevalence and risk factors among under-five children in Iraq in 2000. Ital J Pediatr. 2009;35(1):1–9. doi: 10.1186/1824-7288-35-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta RK, Kumar A, Singh P. Factor analysis of acute respiratory infections among under-fives in Delhi slums. Indian Pediatr. 1999;36(5):1144–1149. [PubMed] [Google Scholar]
- 34.Jabessa S. Multilevel analysis of acute respiratory infection symptoms among under five children in Ethiopia. J Biom Biostat. 2015;6(4):1. [Google Scholar]
- 35.Amsalu ET, Akalu TY, Gelaye KA. Spatial distribution and determinants of acute respiratory infection among under-five children in Ethiopia: Ethiopian Demographic Health Survey 2016. PLoS ONE. 2019;14(4):e0215572. doi: 10.1371/journal.pone.0215572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prajapati B, Talsania N, Sonaliya KN. A study on prevalence of acute respiratory tract infections (ARI) in under five children in urban and rural communities of Ahmedabad district, Gujarat. Natl J Commun Med. 2011;2(2):255–259. [Google Scholar]
- 37.Savitha MR, Nandeeshwara SB, Kumar MP, Raju CK. Modifiable risk factors for acute lower respiratory tract infections. Indian J Pediatr. 2007;74(5):477–482. doi: 10.1007/s12098-007-0081-3. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Wang S, Zhang L, Xu C, Bian C, Wang Z, Ma Y, et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infections in Jinan, China. Clin Dev Immunol. 2013 doi: 10.1155/2013/210490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma D, Kuppusamy K, Bhoorasamy A. Prevalence of Acute Respiratory Infections (ARI) and their determinants in under five children in urban and rural areas of Kancheepuram District, South India. Ann Trop Med Public Health. 2013 doi: 10.4103/1755-6783.133700. [DOI] [Google Scholar]
- 40.Prajapati B, Talsania N, Sonaliya KN. Study on prevalence of acute respiratory tract infections (ARI) in under five children in Lucknow district. Natl J Med Res. 2014;4(4):298–302. [Google Scholar]
- 41.Goel K, Ahmad S, Agarwal G, Goel P, Kumar V. A cross sectional study on prevalence of acute respiratory infections (ARI) in under-five children study on prevalence of acute respiratory infections (ARI) in under-five children. Commun Med Health Educ. 2012;2(9):2–5. [Google Scholar]
- 42.Banda B, Mazaba M, Mulenga D, Siziya S. Risk factors associated with acute respiratory infections among under-five children admitted to Arthur's Children Hospital, Ndola, Zambia. J Health Sci. 2016;3:153–159. [Google Scholar]
- 43.Cardoso AM, Coimbra CE, Jr, Werneck GL. Risk factors for hospital admission due to acute lower respiratory tract infection in guarani indigenous children in southern Brazil: a population-based case-control study. Trop Med Int Health. 2013 doi: 10.1111/tmi.12081. [DOI] [PubMed] [Google Scholar]
- 44.Siziya S, Muula AS, Rudatsikira E. Correlates of diarrhoea among children below the age of 5 years in Sudan. Afr Health Sci. 2013;13(2):376–383. doi: 10.4314/ahs.v13i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adesanya OA, Chiao C. Environmental risks associated with symptoms of acute respiratory infection among preschool children in North-Western and South-Southern Nigeria Communities. Int J Environ Res Public Health. 2017;14(11):1396. doi: 10.3390/ijerph14111396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budhathoki SS, Bhattachan M, Yadav AK, Upadhyaya P, Pokharel PK. Eco-social and behavioural determinants of diarrhoea in under-five children of Nepal: a framework analysis of the existing literature. Trop Med Health. 2016 doi: 10.1186/s41182-016-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geberetsadik A, Worku A, Berhane Y. Factors associated with acute respiratory infection in children under the age of 5 years: evidence from the 2011 Ethiopia Demographic and Health Survey. Pediatric Health Med Ther. 2015;6:9. doi: 10.2147/PHMT.S77915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirji G, Shashank KJ, Shrikant SW, Reddy D, Naik H. Socio-demographic profile of children under the age of five admitted for acute lower respiratory tract infections in a tertiary care hospital. Int J Contemp Pediatr. 2014;1:106–109. [Google Scholar]
- 49.Jackson S, Mathews KH, Pulanić D, Falconer R, Rudan I, Campbell H, Nair H. Risk factors for severe acute lower respiratory infections in children–a systematic review and meta-analysis. Croat Med J. 2013;54(2):110–121. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prajapati B, Talsania NJ, Lala MK, Sonalia KN. Epidemiological profile of acute respiratory infections (ARI) in under five age group of children in urban and rural communities of Ahmedabad district, Gujarat. Int J Med Sci Public Health. 2012;1(2):52–58. [Google Scholar]
- 51.Dadi AF, Kebede Y, Birhanu Z. Determinants of pneumonia in children aged two months to five years in urban areas of Oromia Zone, Amhara Region, Ethiopia.". OALib. 2014 doi: 10.4236/oalib.1101044. [DOI] [Google Scholar]
- 52.Deribew A, Tessema F, Girma B. Determinants of under-five mortality in Gilgel gibe field research center, Southwest Ethiopia. Ethiop J Health Dev. 2007;21(2):117–124. [Google Scholar]
- 53.Schmidt W-P, Cairncross S, Barreto ML, Clasen T, Genser B. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol. 2009;38(3):766–772. doi: 10.1093/ije/dyp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahman SS, Khatun A, Azhar BS, Rahman H, Hossain S. A study on the relationship between nutritional status and prevalence of pneumonia and diarrhoea among preschool children in Kushtia. PRIJ. 2014 doi: 10.5171/2014.805309. [DOI] [Google Scholar]
- 55.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity, Hoboken: John Wiley & Sons; 2005. [Google Scholar]
- 56.Sikolia DN, Mwololo K, Cherop H, et al. The Prevalence of acute respiratory infections and the associated risk factors: a study of children under five years of age in Kibera Lindi Village, Nairobi, Kenya. J Natl Inst Public Health. 2002;51:67–72. [Google Scholar]
- 57.Rahman MM, Shahidullah M. Risk factors for acute respiratory infections among the slum infants of Dhaka city. Bangladesh Med Res Counc Bull. 2001;27(2):55–62. [PubMed] [Google Scholar]
- 58.Tazinya AA, Halle-Ekane GE, Mbuagbaw LT, Abanda M, Atashili J, Obama MT. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med. 2018;18(1):1–8. doi: 10.1186/s12890-018-0579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malla T, Pathak OK, Malla KK. Is low hemoglobin level a risk factor for acute lower respiratory tract infections? J Nepal Paediatr Soc. 2010;30(1):1–7. [Google Scholar]
- 60.SheikhQuyoomHussain MA, Wani JG, Ahmed J. Low hemoglobin level a risk factor for acute lower respiratory tract infections (ALRTI) in children. JCDR. 2014;8(4):PC01. doi: 10.7860/JCDR/2014/8387.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avhad Y, Wade P, Ghildiyal RG. Anemia as a risk factor for lower respiratory tract infections (LRTI) in children. Int J Contemp Med Res. 2016;3(12):3512–3514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.