Abstract
Heat shock protein 27 (HSP27) confers cellular protection against a variety of cytotoxic stresses and also against physiological stresses associated with growth arrest or receptor-mediated apoptosis. Phosphorylation modulates the activity of HSP27 by causing a major change in the supramolecular organization of the protein, which shifts from oligomers to dimers. Here we show that phosphorylated dimers of HSP27 interact with Daxx, a mediator of Fas-induced apoptosis, preventing the interaction of Daxx with both Ask1 and Fas and blocking Daxx-mediated apoptosis. No such inhibition was observed with an HSP27 phosphorylation mutant that is only expressed as oligomers or when apoptosis was induced by transfection of a Daxx mutant lacking its HSP27 binding domain. HSP27 expression had no effect on Fas-induced FADD- and caspase-dependent apoptosis. However, HSP27 blocked Fas-induced translocation of Daxx from the nucleus to the cytoplasm and Fas-induced Daxx- and Ask1-dependent apoptosis. The observations revealed a new level of regulation of the Fas pathway and suggest a mechanism for the phosphorylation-dependent protective function of HSP27 during stress and differentiation.
The small heat shock protein HSP27 is expressed at various levels in different cell types and tissues. HSP27 is regulated at both the transcriptional and posttranslational levels (2). Like those of other heat shock proteins, its concentration increases severalfold over the basal level under specific stressful environmental conditions that activate the heat shock transcription factor. Such overexpression confers cellular resistance to a variety of stimuli that induce cell death with either necrotic or apoptotic features, including physical and chemical stress, growth factor withdrawal, and activation of death receptors (14, 20, 22, 28, 32, 34, 39–41, 65). HSP27 is also regulated at the posttranslational level by stress and by cytokines and growth factors that activate stress-activated protein kinase 2 (SAPK2) p38, the upstream activator of the HSP27 kinase mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 (12, 15, 18, 21, 29, 31, 37, 40, 50, 57). Phosphorylation causes a major change in the quaternary structure of HSP27, which shifts from large 600- to 800-kDa homotypic multimers down to dimers and monomers (27, 49). A similar shift from high-molecular-weight to dimeric complexes also occurs in the Saccharomyces cerevisiae homologue of HSP27, HSP26, suggesting conservation of an important regulatory mechanism (17).
Two biochemical activities of HSP27 have been well documented. HSP27 phosphorylation modulates actin dynamics. In vitro, unphosphorylated monomers but not phosphorylated monomers or large multimers have been shown to block polymerization of actin (3, 42, 43). In vivo, actin polymerization and reorganization are modulated by HSP27 concentration and phosphorylation in response to growth factors (33, 51). During stress, HSP27 phosphorylation-mediated microfilament reorganization contributes to filament stabilization in some conditions but mediates cell blebbing in others (15, 18, 19, 30, 33, 34). HSP27 also possesses chaperone activities. In vitro, high-molecular-weight complexes of HSP27 bind denatured proteins and prevent their aggregation by keeping them in a renaturation-competent state (11, 24, 35). A recent study with an HSP27 relative from yeast suggested that this function involves the initial binding of the denatured peptides on HSP27 dimers, followed by the reassembly of the HSP27-denatured peptide complexes into high-molecular-weight structures. This suggested that the phosphorylation-induced dissociation of the mammalian HSP27 multimers into dimers may similarly promote the chaperone function (17). An in vivo chaperone function of HSP27, however, remains to be demonstrated.
HSP27 has a strong protective activity against a number of cytotoxic agents, including heat shock, oxidative stress, chemotherapeutic agents, and cytokines (14, 20, 22, 28, 32, 34). While some of these agents may affect protein structure or microfilament integrity, it appears unlikely that the known biochemical activities of HSP27 can explain so wide a spectrum of protective activities. A better explanation could come from its capacity to block apoptotic processes. In neuronal cells, for example, HSP27 overexpression protects against both thermal and ischemic stress and against apoptosis induced by nerve growth factor withdrawal or retinoic acid treatments (36, 65). HSP27 also blocks cytotoxic drug-induced apoptosis in tumor cells (13, 14). The expression and phosphorylation status of HSP27 are also modulated in several mammalian cells during differentiation and entry in the G1 phase of the cell cycle, and this may play an essential role in preventing apoptosis induction linked to cessation of cell growth (4, 14, 37, 40, 54–56). For example, HSP27 was shown to accumulate and to be essential for preventing apoptosis during leukemia-inhibitory factor withdrawal-induced differentiation of embryonic stem cells and dopamine-mediated differentiation of rat olfactory neurons (37, 40). The possibility that HSP27 has a specific target in a key apoptotic signaling or execution pathway is supported by the report that HSP27 can prevent activation of pro-caspase 9 after etoposide treatment and can inhibit apoptosis induced by activation of the Fas receptor (13, 41).
Regulation of cell death through apoptosis is of major importance in the embryonic development, as well as the functioning, of a multicellular organism. A key regulator of apoptosis is Fas (also named CD95 and APO-1), which belongs to the tumor necrosis factor death receptor superfamily and which is expressed constitutively on the surfaces of many cells from many tissues (45). Upon binding its ligand, Fas-L, Fas recruits the adapter Fas-associated death domain (FADD; also called MORT1) through an intracellular 80-amino-acid motif called the death domain. The interaction is mediated by a similar death domain in the C-terminal end of FADD, while the N terminus of FADD mediates interaction with caspase 8 (MACH [FLICE]), an interleukin-1β-converting enzyme family cysteine protease (5, 6, 9, 44). Activated caspase 8 in turn activates downstream caspases, leading to cleavage of essential substrates and cell death. An alternative and independent apoptotic pathway induced by Fas involves Daxx, a protein normally associated with the nuclear substructures named ND-10 or PML oncogenic domain (POD) (7, 8, 61, 66). The mechanism by which Daxx conveys the Fas apoptotic signal is not totally clear. Mouse Daxx has been described as an adapter of Fas that can recruit and activate apoptosis signal-regulated kinase 1 (Ask1), a mitogen-activated protein kinase kinase kinase. Activation of Ask1 can then lead to the activation of stress-activated protein kinase 1–Jun N-terminal kinase (SAPK1/JNK) (7, 8, 66). The role of SAPK1/JNK activation in Daxx-mediated cell death is, however, unclear. It was also proposed that Daxx enhancement of Fas-induced apoptosis results from the PML-mediated association of Daxx with POD (61). In either case, it was concluded that Daxx acts at a proximal postreceptor step in Fas signaling.
In the present study, we found that HSP27 in its phosphorylated dimeric form but not in its unphosphorylated multimeric form interacts both in vitro and in vivo with Daxx, preventing association of Daxx with Fas and Ask1 and induction of apoptosis by Daxx when coexpressed with Ask1. The physiological relevance of this interaction was investigated in 293 cells. We showed that stimulation of Fas results in two temporally distinguishable apoptotic processes: a fast process, which was dependent on FADD and which was inhibited by a dominant-negative form of FADD or the pancaspase inhibitor z-VAD-fmk, and a slow process, which was independent of caspases, dependent on Daxx, and enhanced by Ask1. The Daxx-dependent pathway was inhibited by expression of HSP27 but not by an HSP27 phosphorylation mutant which fails to form dimeric complexes and to interact with Daxx. Taken together, the observations suggest a new mechanism for regulating the Fas pathway and for the phosphorylation-dependent protective function of HSP27 during stress and differentiation. Inhibition of Daxx-dependent apoptosis may be a major mechanism of HSP27-mediated protection, particularly in some differentiated or tumor cells in which the FADD-dependent pathway of Fas-induced apoptosis is blocked.
MATERIALS AND METHODS
Plasmids and reagents.
pSVHa27WT encodes the full-length sequence of Chinese hamster HSP27 (HaHSP27) (27). pSVHa27AA and pSVHa27EE are similar to pSVHa27WT but encode serine-to-alanine and serine-to-glutamate substitutions at positions 15 and 90, respectively (27). pCINHu27WT expresses full-length human HSP27 (HuHSP27). It was constructed by cloning the EcoRI insert of pGAD10-HuHSP27 (27) in pCI-NEO (Promega). GST–Fas-DD, a fusion protein comprising glutathione S-transferase (GST) and the death domain of Fas (Fas-DD), was produced in bacteria from the plasmid pGST-Fas-DD, made by inserting the HincII-HindIII insert from the Fas-encoding clone 927519 (American Type Culture Collection [ATCC]) into pGEX 4T-3 (Amersham Pharmacia Biotech). GST-HuHSP27 and GST–55.1 were produced similarly from plasmids pGST-HuHSP27 and pGST-55.1, made from pCINHu27WT and pGADGH-55.1, respectively. pGADGH-55.1 was obtained in a two-hybrid screen in this study and is described later. It encodes the carboxy-terminal end (residues 472 to 740) of human Daxx. pCINHuDaxx expresses full-length human Daxx protein and was constructed with the insert of a Daxx cDNA (26). pCIN-AAD and pCIN-DaxxC express residues 497 to 625 (apoptosis activation domain [AAD]) and 497 to 740 (DaxxC) of Daxx, respectively. They were constructed from pGADGH-55.1 and clone 184860 from ATCC. Briefly, pCIN55.1 was produced with the EcoRI-XhoI insert of pGADGH-55.1 cloned in pCI-NEO. The EcoRI-BglII insert of pCIN55.1 was replaced by the EcoRI-BglII insert of clone 184860 to produce pCIN-DaxxC. pCIN-AAD was produced by removing the C-terminal part of pCIN-DaxxC by AvaII digestion. pCINmyc-FBD was produced by inserting an AvaII-XhoI fragment of pGADGH-55.1 into pCINmyc. pCINmyc was produced by inserting a DNA cassette coding for an initiating methionine and c-myc epitope (MEQKLISEEDL) into the multicloning site of pCI-NEO. pcDNA3-HA-Ask1 expresses full-length human Ask1 tagged with the influenza virus hemagglutinin (HA) epitope (52). pCINMyc-FADD expresses a full-length FADD tagged in its N terminus with the myc epitope. The FADD cDNA was produced by PCR amplification of a human kidney library (Clontech) and inserted into pCINmyc. pCINMyc-FADD was digested with AccI to produce pCINMyc-FADD-DN, which lacks the first 79 amino acids of FADD. pCMVβ, which expresses β-galactosidase, was from Clontech. All constructs newly developed in this study were confirmed by DNA sequencing.
SB203580 was from Calbiochem, human Fas-activating antibody (clone CH11) was from Upstate Biotechnology, and z-VAD-fmk was from Enzyme Systems Products. 4′,6-Diamidino-2-phenylindole (DAPI) was from Sigma. Antibodies L2R3 against HaHSP27 and anti-Hu against HuHSP27 have been described before (28, 34). The anti-Daxx EL-68 antibody was developed in rabbits after injection of bacterially produced GST–55.1. Anti-HA (12CA5) is a mouse monoclonal antibody recognizing the YPYDVPDYA peptide sequence from human influenza virus HA protein (Roche Molecular Biochemicals Corporation). Antimyc (9E10) is a mouse monoclonal antibody recognizing theEQKLISEEDL peptide sequence from the human c-Myc protein (ATCC).
Two-hybrid screening and analyses.
The two-hybrid screening procedures have been described in detail elsewhere (27). Briefly, the full-length HaHSP27 cDNA contained in pSVHa27WT was cloned in phase with LexA cDNA in the pBTM116 vector to produce the bait fusion protein LexA-HaHSP27. The LexA-HaHSP27 construct was used to screen a HeLa cell cDNA library cloned at the EcoRI-XhoI site of the pGADGH vector (Clontech). Screening was done using Saccharomyces cerevisiae reporter strain L40. Colonies that grew on triple-selective media (lacking histidine, leucine, and tryptophan) were tested for β-galactosidase activity using a colony lift filter assay. Colonies were replica plated, and one set was transferred to a filter disk (Whatman Inc., Clifton, N.J.), lysed by freezing, and incubated at 30°C with Z buffer (0.1 M NaPO4, 10 mM KCl, 1 mM MgSO4, 0.27% β-mercaptoethanol, 0.33 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [Boehringer Mannheim Biochemicals]/ml, pH 7.0) until the blue coloration appeared (4 to 12 h). The plasmids from positive clones were purified and cotransfected with LexA-ras (val 12) and LexA-lamin C (64) to eliminate false-positive clones. Only clone 55.1, which coded for the carboxy-terminal end (residues 472 to 740) of Daxx, was considered in this study (see Fig. 1A).
FIG. 1.
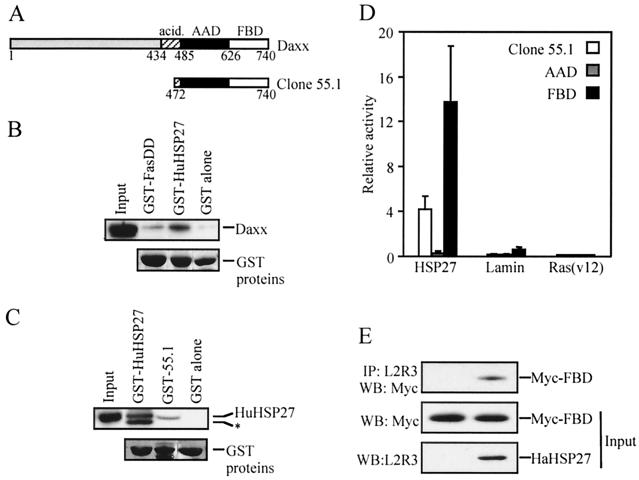
HSP27 interacts with Daxx. A partial cDNA of Daxx (clone 55.1) was obtained in a two-hybrid screen of a HeLa cell cDNA library using HaHSP27 as a bait. (A) Schematic representation of the Daxx sequences contained in clone 55.1. Represented are the acidic domain (acid.), the AAD, and the FBD of human Daxx. Residue numbers at domain boundaries are indicated. (B and C) GST pull-down assays. (B) Daxx is retained on immobilized HuHSP27. Extracts from pCINHuDaxx-transfected 293 cells were incubated with immobilized GST–Fas-DD, GST-HuHSP27, or GST. Retained Daxx protein was detected by Western blotting using antibody EL-68. Also shown are the GST fusion proteins in the same gel stained with Ponceau red. For convenience, bands were aligned at the same level. The input track represents 6.7% of the total extract. (C) HuHSP27 is retained on immobilized Daxx sequences contained in clone 55.1. Extracts from pCINHu27WT-transfected 293 cells were incubated with immobilized GST-HuHSP27, GST–55.1, or GST. Retained HuHSP27 protein was detected by Western blotting using an anti-HuHSP27 antibody. Also shown are the GST fusion proteins in the same gel stained with Ponceau red. For convenience, bands were aligned at the same level. The asterisk indicates a GST-HuHSP27 degradation product recognized by anti-Hu. The input track represents 7.5% of the total extract. (D and E) HSP27 interacts with the Fas binding domain of Daxx. (D) Two-hybrid interaction assay was performed with yeast after transfection of LexA-HuHSP27 or the negative controls LexA-lamin C and LexA-ras (val 12) with pGADGH-55.1, GAL4-AAD, or GAL4-FBD. The interaction was quantified by measuring β-galactosidase activities, which are shown in arbitrary units. (E) 293 cells were transfected with pCINmyc-FBD or with pCINmyc-FBD and pSVHa27WT. Extracts (6.1% of the total) were analyzed by Western blotting (WB) with anti-Myc antibody 9E10 to detect Myc-FBD protein or with anti-HaHSP27 antibody L2R3 to detect HaHSP27 expression (input). The extracts were also immunoprecipitated (IP) with L2R3 and then probed with 9E10 to detect coimmunoprecipitated Myc-FBD.
Further two-hybrid β-galactosidase assays with pairs of GAL4 activation domain and LexA binding domain plasmids were performed. LexA-HuHSP27 was produced by inserting into pBTM116 full-length HuHSP27 cDNA from a pGADGH-HuHSP27 plasmid obtained in the same two-hybrid screening. GAL4-AAD and GAL4-FBD (see Fig. 1A) were produced by inserting AvaII-digested clone 55.1 cDNA encoding the N- and C-terminal parts, respectively, in phase with the coding sequence for the GAL4 activation domain contained in the pGADGH plasmid. All constructs were confirmed by DNA sequencing. Pairs of vectors were cotransfected in yeast strain L40 and grown in selective media. Cells were lysed by chloroform and processed to evaluate β-galactosidase expression by o-nitrophenyl-β-d-galactopyranoside (Sigma) hydrolysis, which was measured at 420 nm.
Cell culture and transfection.
293 cells were maintained at 37°C in a 5% CO2 humidified atmosphere in Dulbecco's modified Eagle medium containing 2.2 g of NaHCO3 and 4.5 g of glucose/liter and supplemented with 10% fetal calf serum. Transfection was done by calcium phosphate precipitation as described before (28) except that 50 μM chloroquine was added for the first 5 h of transfection.
GST pull-down assay and coimmunoprecipitation.
Exponentially growing 293 cells were plated onto 6-cm-diameter dishes at 0.75 × 106 to 1.0 × 106 cells per well and transfected 24 h later with the appropriate plasmid (4 to 10 μg). The cells were lysed 24 to 48 h later in buffer EM2 containing 50 mM HEPES (pH 7.6), 50 mM NaCl, 1% NP-40, 5 mM EDTA, 10% glycerol, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (Sigma) and centrifuged at 17,000 × g for 10 min at 4°C. Supernatants were used either for GST pull-down assays or immunoprecipitation. Cell extracts (150 to 300 μl) were incubated with 1 to 10 μg of GST-fused proteins adsorbed on glutathione-Sepharose (Amersham Pharmacia Biotech) or with 10 μl of the L2R3 anti-HaHSP27 antibody (34) adsorbed on protein A-Sepharose (50% [vol/vol]) (Amersham Pharmacia Biotech) at 4°C for 60 to 90 min. After incubation, beads were washed two times in 300 μl of buffer EM2. Proteins retained on beads and total cell extracts were subjected to electrophoresis on a sodium dodecyl sulfate-polyacrylamide gel and then transferred to a nitrocellulose membrane. HuHSP27, HaHSP27, and Daxx proteins were revealed by immunoblotting using anti-Hu, L2R3, or EL-68 antibodies, respectively, and diluted 1/2,500 in 10 mM Tris-HCl (pH 7.4)–150 mM NaCl–1% (wt/vol) free fatty acid powder milk. Ask1-HA and Myc-FBD were revealed using 1/5,000 dilutions of anti-HA and anti-Myc antibodies, respectively. Primary antibodies were detected using anti-rabbit or anti-mouse immunoglobulin G coupled to horseradish peroxidase (diluted 1/5,000) (Jackson Laboratory). GST-fused proteins were visualized by Ponceau red staining on a nitrocellulose membrane before immunoblotting.
Cell death assay.
Exponentially growing 293 cells were plated at a concentration of 0.5 × 105 to 1.0 × 105 cells per well onto six-well dishes coated with 0.1% gelatin. The next day, the cells were transfected with pCMVβ together with the plasmids to be tested. Concentrations of plasmids between groups within an experiment were varied slightly (0.1 to 0.5 μg/well) to maintain equal expression of the tested proteins. Within all experiments, the level of protein expression was kept constant, as verified by Western blot analysis of total cell lysates as described above. Apoptosis induction was measured by counting under the microscope the number of β-galactosidase-positive cells with apoptotic morphology. pCMVβ-transfected cells were fixed with 0.05% glutaraldehyde in phosphate-buffered saline for 10 min and stained for β-galactosidase expression by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside hydrolysis. Cells were considered apoptotic if they showed intense cell blebbing or were round and condensed. More than 200 blue cells were analyzed per well. The percentage of apoptotic cells was 100 times the number of blue cells with apoptotic morphology divided by the total number of blue cells in each condition. Specific apoptosis is the difference between the percentage of blue cells with apoptotic morphology in cultures transfected with the test plasmid and that in cultures transfected with an empty vector. Basal apoptosis ranged from 3 to 7%. Values presented are averages plus standard deviations of triplicate determinations.
Immunofluorescence microscopy.
Immunolabeling of endogenous or transfected Daxx was performed on 293 cells growing on gelatin-coated six-well dishes. Cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (137 mM NaCl, 5 mM KCl, 10 mM Na2HPO4, 11 mM glucose, pH 7.2) for 20 min and permeabilized with 0.1% saponin for 15 min. EL-68 antibody diluted 1/100 was used to detect Daxx. The Daxx-antigen-antibody complex was revealed with fluorescein isothiocyanate (FITC)-labeled anti-rabbit immunoglobulin G antibodies (Jackson Laboratory) diluted 1/50. Nuclei were revealed by DAPI staining (10 μg/ml). Epifluorescence microscopy was done on a Nikon Eclipse E600 microscope equipped with a B2-A filter for FITC detection and a UV-1A filter for DAPI staining. Pictures were obtained with a Micromax cooled digital camera system (Princeton Instruments).
RESULTS
HSP27 dimers interact with Daxx and block Daxx-Ask1 and Daxx-Fas interaction.
To identify proteins which could mediate the protective function of HSP27, a two-hybrid screening of a library of HeLa cell cDNA fused to the activation domain of Gal4 was done using HaHSP27 fused to the LexA DNA-binding domain as bait. Among the positive clones obtained, four identical clones typified by clone 55.1 specifically interacted in a two-hybrid assay with HaHSP27 and HuHSP27, but not with ras (val 12) and lamin C (data not shown). Sequence analysis revealed that clone 55.1 corresponded to the carboxy terminus of the human Daxx protein from amino acid 472 to 740 (Fig. 1A). Clone 55.1 contained the sequences corresponding to two characterized domains of mouse Daxx: the AAD, which is sufficient for the apoptosis activity of Daxx and which corresponds to human amino acids 485 to 626, and the Fas binding domain (FBD), which corresponds to amino acids 626 to 740 of human Daxx and which mediates interaction of mouse Daxx with the death domain of Fas (Fas-DD) (66).
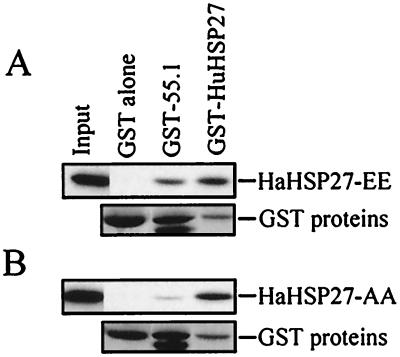
The interaction between Daxx and HSP27 was confirmed in a GST pull-down assay. Some Daxx from extracts of Daxx-transfected 293 cells was retained on immobilized GST- HuHSP27 but not on immobilized GST. Conversely, some HuHSP27 expressed in 293 cells was retained on immobilized GST–55.1 but not on GST alone. As expected for a multimeric protein, HSP27 was retained on GST-HSP27, and, as previously demonstrated, Daxx was retained on immobilized GST–Fas-DD (Fig. 1B and C). Transient expression in yeast and immunoprecipitation indicated that the FBD domain of Daxx was sufficient to mediate the interaction of Daxx with HSP27. In yeast, AAD did not interact with HSP27 whereas FBD alone produced a signal even stronger than that obtained with the protein coded for by clone 55.1 (Fig. 1D). In extracts from mammalian cells transfected with Myc-tagged FBD and HaHSP27, Myc-tagged FBD could be coimmunoprecipitated by HSP27 antibody L2R3 (Fig. 1E). To determine whether the interaction between Daxx and HSP27 could be modulated by phosphorylation, we expressed in 293 cells two previously characterized HSP27 phosphorylation mutants. In HaHSP27-AA and HaHSP27-EE, Ser15 and Ser90 were replaced by nonphosphorylatable and pseudophosphorylated residues, respectively. HaHSP27-AA is expressed in cells mainly as a homotypic multimer, whereas HaHSP27-EE is expressed as a dimer (27). In extracts from transfected 293 cells, HaHSP27-AA and HaHSP27-EE interacted efficiently with immobilized GST- HuHSP27, indicating that the proteins were functional; however, HaHSP27-EE interacted with the Daxx protein encoded by clone 55.1 much more efficiently than HaHSP27-AA (Fig. 2).
FIG. 2.
HSP27 phosphorylation modulates the interaction with Daxx. Extracts from 293 cells transfected with pSVHa27EE (A) or pSVHa27AA (B) were incubated with immobilized GST, GST–55.1, or GST-HuHSP27. Retained HSP27 proteins were detected by Western blotting using antibody L2R3. Also shown are the GST fusion proteins in the same gel stained with Ponceau red. For convenience, bands were aligned at the same level. The input tracks represent 5% of the total extracts.
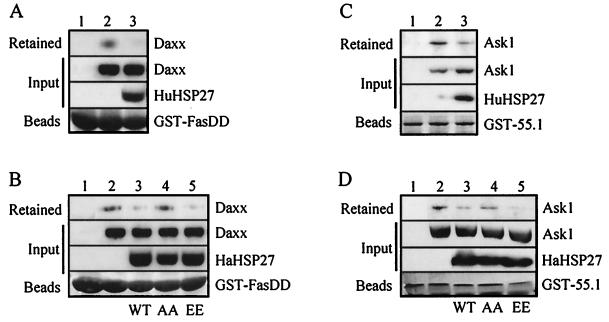
Daxx has been proposed to mediate cell killing by interacting with both the death domain of Fas, Fas-DD, and Ask1. The interaction between Daxx and Ask1 was confirmed by showing that Ask1 from extracts of 293 cells was retained on immobilized GST–55.1 (Fig. 3C, lane 2) or coimmunoprecipitated with antibodies against Daxx (data not shown). Similarly, Daxx was retained on immobilized GST–Fas-DD (Fig. 3A, lane 2). The 293 cells have little constitutive expression of HSP27 (data not shown). We tested the effect of overexpressing HSP27 on Daxx-Ask1 and Daxx-Fas interactions. When extracts from HuHSP27- or HaHSP27-expressing 293 cells were mixed with extracts of cells expressing Daxx or Ask1, the binding of Ask1 to GST–55.1 or of Daxx to Fas-DD was strongly inhibited (Fig. 3A to D, lanes 3). Expression of HaHSP27-AA had, however, little effect on the interaction between Daxx and Ask1 or Fas, while HaHSP27-EE was as effective, if not more effective, in blocking the interaction than wild-type HSP27 (Fig. 3B and D, lanes 4 and 5). The differential effects of HaHSP27-AA and HaHSP27-EE on Daxx-Ask1 and Daxx-Fas interactions were consistent with the failure of the multimeric HaHSP27-AA to interact with Daxx. It was concluded that the binding of HSP27 to Daxx prevents Daxx interaction with both Ask1 and Fas.
FIG. 3.
HSP27 competes with Fas (A and B) and Ask1 (C and D) for interacting with Daxx. 293 cells were transfected with either pCIN-Daxx, pcDNA3-HA-Ask1, pCINHu27WT, pSVHa27WT, pSVHa27AA, or pSVHa27EE. Extracts from Daxx- (A and B) or HA-Ask1 (C and D)-expressing cells were incubated with immobilized GST–Fas-DD or GST–55.1, respectively, either alone (lanes 2) or after being mixed with extracts from cells expressing either species of HSP27 (lanes 3 to 5) as indicated in the input lanes (HuHSP27 or HaHSP27-WT, -AA, or -EE). Retained Daxx and Ask1 were detected by Western blotting using anti-Daxx EL-68 and anti-HA 12CA5 antibodies, respectively. Ponceau red-stained GST fusion proteins are shown on the bottom of each panel. The input lanes in panels A to D represent 0.6, 0.3, 7.5, and 7.5% of the total extracts, respectively.
Daxx-induced apoptosis is inhibited by interaction of phosphorylated HSP27 with Daxx.
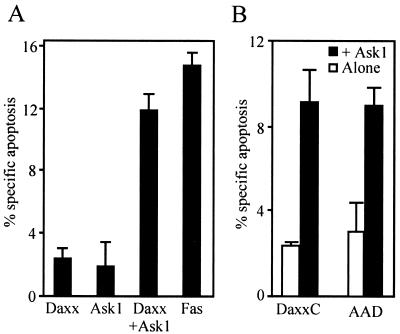
Cells were transfected with Daxx and/or Ask1 together with β-galactosidase. Twenty-four hours later, β-galactosidase-positive cells were monitored for apoptotic morphology. Overexpression of Daxx or Ask1 alone induced little apoptosis. However, cotransfection of Daxx together with Ask1 led to apoptosis in proportions similar to that observed when, as a control, Fas was overexpressed in the cells (Fig. 4A). Daxx-Ask1-transfected cells showed typical apoptosis features such as cytoplasmic shrinking and nuclear condensation at 24 h and nuclear fragmentation at 48 h (DAPI staining [data not shown]). In comparison, apoptosis induced by expression of Fas or FADD appeared faster, with most apoptotic cells showing fragmented nuclei already at 24 h (data not shown). Expression of DaxxC or Daxx-AAD with Ask1 also strongly induced apoptosis, confirming that the AAD domain is sufficient to induce apoptosis (Fig. 4B).
FIG. 4.
Coexpression of Daxx and Ask1 induces apoptosis in 293 cells. 293 cells were cotransfected with various combinations of pCIN-Daxx, pCIN-DaxxC, pCIN-AAD, pcDNA3-HA-Ask1, and pCIN-Fas, as indicated. pCMVβ was also cotransfected as a marker to identify transfected cells. Twenty-four hours later, the percentages of β-galactosidase-positive cells with apoptotic morphology were calculated after correcting for apoptosis in cells transfected with the marker gene only.
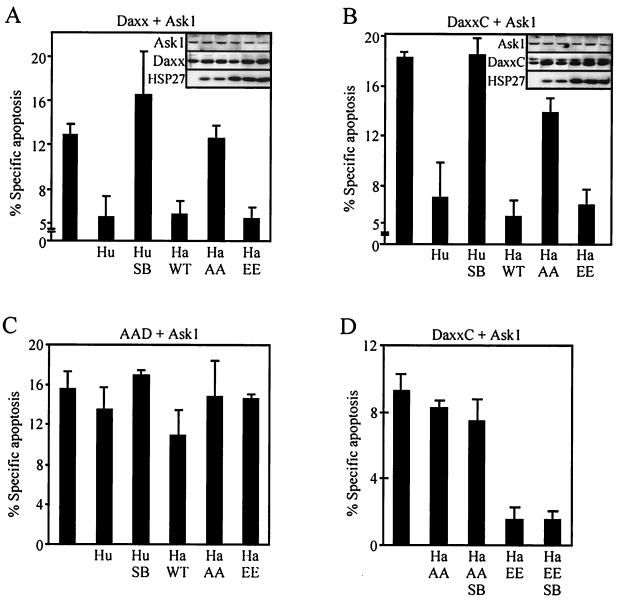
We next determined whether HSP27 could block Daxx-induced apoptosis in vivo. Expression of HuHSP27 or HaHSP27 blocked the apoptosis induced by expression of Ask1 with either Daxx or DaxxC by up to 70% (Fig. 5A and B). Because in preliminary experiments we observed that cotransfection of multiple plasmids often influenced the expression of individual proteins, the expression of the proapoptotic proteins was carefully monitored in the various experimental groups. As shown in the insets of Fig. 5A and B, the inhibitory activity of HSP27 was observed for equal quantities of expressed Ask1 and Daxx or DaxxC. As observed before for the interaction between Daxx and HSP27, the protection was also dependent on the phosphorylation status of HSP27. At equal levels of expression, HaHSP27-EE blocked cell death as efficiently as wild-type HaHSP27, whereas HaHSP27-AA provided very little protection. To confirm that phosphorylation was regulating the protective function of HSP27, we tested the effect of expressing HSP27 in the presence of SB203580. SB203580 is a specific inhibitor of p38 (10). By inhibiting basal p38 activity and thereby lowering MAPKAP kinase 2 activity and HSP27 phosphorylation level, SB203580 lowers the concentration of the active HSP27 dimers (data not shown), forcing wild-type HSP27 into the inactive high-molecular-weight oligomeric state. As expected, SB203580 abrogated the protective effect of HuHSP27 on Daxx-Ask1- or DaxxC-Ask1-induced apoptosis. To rule out the possibility that the observed effects of SB203580 could be the result of blocking other functions of p38, SB203580 was also used in combination with HaHSP27-EE and HaHSP27-AA. SB203580 had no effect on the activity of the phosphorylation mutants, indicating that HSP27 phosphorylation really modulated the protective activity of HSP27 (Fig. 5D). To confirm that HSP27 protection was the result of its interaction with Daxx, we looked at the effect of HSP27 on cell death induced by AAD. As expected, HSP27 could not block AAD-induced apoptosis, which was consistent with the finding that HSP27 does not interact with AAD (Fig. 5C).
FIG. 5.
HSP27 blocks apoptosis induced by coexpression of Daxx and Ask1. Apoptosis was induced by the cotransfection of the appropriate plasmids to yield the coexpression of Ask1 with either Daxx (A), DaxxC (B and D), or AAD (C), with or without HuHSP27 (Hu), HaHSP27 (Ha WT), or the phosphorylation mutants HaHSP27-AA (Ha AA) and HaHSP27-EE (Ha EE). pCMVβ was also transfected in all cases to identify transfected cells. Twenty-four hours after transfection, the cells were processed to determine the number of β-galactosidase-positive cells with apoptotic morphology. When indicated (Hu SB), the cells were incubated for the last 16 h in the presence of SB203580 (5 μM). (A and B insets) Western blots showing the concentrations of Ask1, Daxx or DaxxC, and HSP27 in an aliquot of the cell lysates for each condition corresponding to the histograms. Gel tracks in the insets are in the same order as in the histograms.
Role of HSP27 in Fas-induced apoptosis.
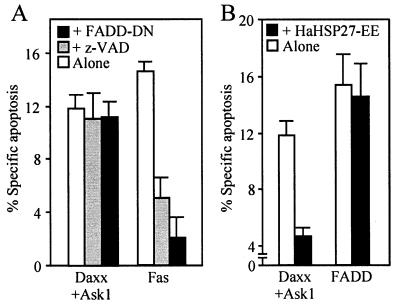
The finding that HSP27 could block the apoptotic activity of Daxx suggested that it could modulate Fas-induced apoptosis. Activation of Fas by incubation of cells with a Fas-activating antibody or by overexpression of Fas induces two independent pathways of cell death, one Daxx dependent and presumably sensitive to HSP27 and the other FADD dependent and leading to the activation of caspase 8. Consistent with the existence of these two distinct pathways, we found that apoptosis induced by overexpression of FADD was insensitive to coexpression of HaHSP27-EE, which in the same experiment blocked Daxx-Ask1-induced cell death (Fig. 6B). Conversely, Daxx-induced cell death was not influenced by coexpression of a dominant-negative form of FADD (FADD-DN) which blocked apoptosis induced by the expression of Fas. Treatments with the pancaspase inhibitor z-VAD-fmk, like treatments with FADD-DN, blocked Fas-induced cell killing but had no effect on Daxx-induced cell death (Fig. 6A). The latter result was highly reproducible (see Fig. 7 and 8) and suggested, contrary to previous findings (66), that Daxx-induced cell killing was caspase independent.
FIG. 6.
Specificity of the Daxx and FADD arms of the Fas pathway. (A) Effect of expressing FADD-DN or inhibiting the caspases with z-VAD-fmk on apoptosis induced by overexpression of Daxx and Ask1 or Fas. (B) Effect of expression of pseudophosphorylated HSP27 (HaHSP27-EE) on apoptosis induced by overexpression of Daxx and Ask1 or FADD. 293 cells were transfected with the appropriate plasmids together with pCMVβ, and apoptosis was measured 24 h later in β-galactosidase-positive cells. When indicated, z-VAD-fmk (50 μM) was added 12 h before staining.
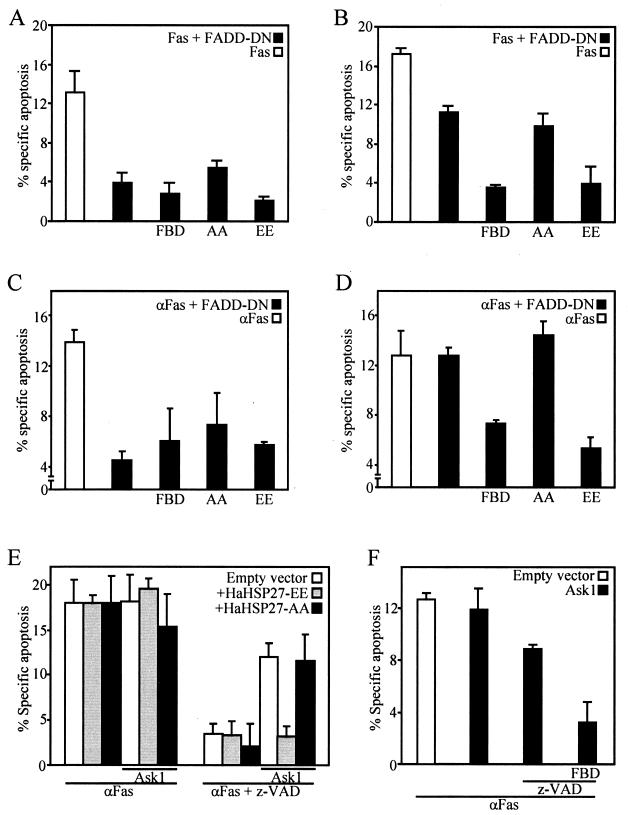
FIG. 7.
HSP27 inhibits the Daxx-sensitive arm of Fas-induced apoptosis. (A to D) Effect of expression of Daxx-FBD, HaHSP27-AA, or HaHSP27-EE, together with FADD-DN, on apoptosis induced 24 h (A and C) or 48 h (B and D) after either overexpressing Fas by transfection (A and B) or stimulating Fas with anti-Fas (C and D). Anti-Fas (100 ng/ml) was added 24 h after transfection of other plasmids. (E and F) Overexpressing Ask1 amplifies the relative importance of the Daxx pathway and of HSP27 inhibition of apoptosis induced 24 h after Fas activation. The cells were transfected with the appropriate plasmids in various combinations as indicated. Anti-Fas (100 ng/ml) was added 12 h after transfection. z-VAD-fmk (50 μM) was added 1 h before Fas activation. pCMVβ was also transfected in all cases, and apoptosis in the β-galactosidase-positive cells was measured.
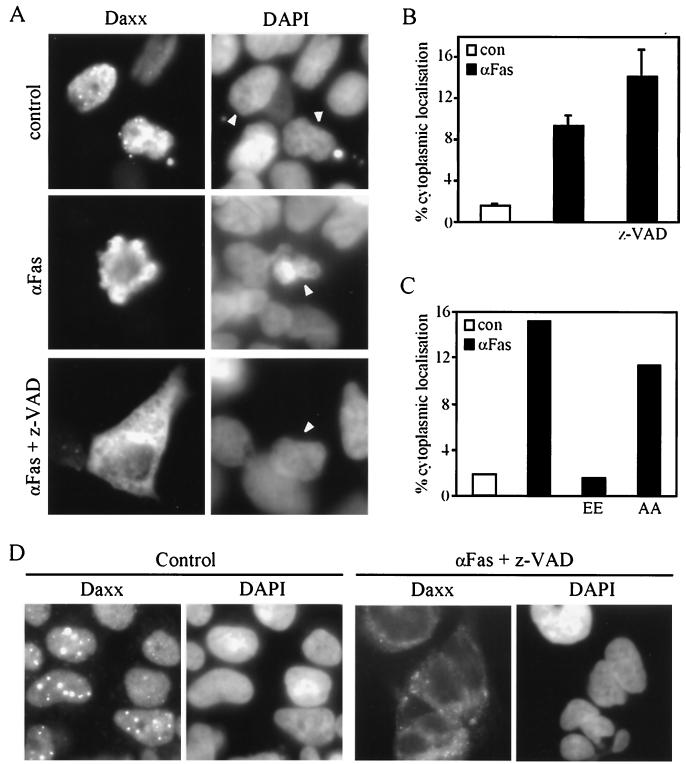
FIG. 8.
Fas-induced translocation of Daxx from the nucleus to the cytoplasm is inhibited by HSP27. (A to C) 293 cells were transfected with plasmid pCINHuDaxx, alone or together with plasmid pSVHa27AA (AA) or pSVHa27EE (EE). Twelve hours after transfection the cells were left untreated (control or con) or treated with 100 ng of anti-Fas activating antibody/ml for 12 h before fixation. Where indicated, z-VAD-fmk (50 μM) was added to the cells 30 min before Fas stimulation. Daxx was localized by immunofluorescence using anti-Daxx EL-68 (A). Nuclei were revealed by DAPI staining. Arrowheads indicate nuclei of transfected cells. The percentages of cells in which Daxx was localized in the cytoplasm (as illustrated in panel A) were determined by direct counting under the microscope. (D) Immunolocalization of endogenous Daxx. The procedure was the same as for panel A except that the cells were treated directly without transfection. Endogenous Daxx was not seen in panel A because of a much shorter exposure time (15-fold) than that used for panel D.
Since HSP27 can block only one arm of the Fas pathway, its effects on Fas-induced apoptosis necessarily depend on the relative contributions of the FADD and Daxx pathways. We found a strong time dependency for the relative contributions of FADD and Daxx to apoptosis induced by either overexpressing Fas (Fig. 7A and B) or activating endogenous Fas (Fig. 7C to F). Expression of FADD-DN, a dominant-negative form of FADD which can bind Fas but which lacks the caspase-8 binding domain, or the presence of z-VAD-fmk had a major inhibitory effect on Fas-induced apoptosis measured at 24 h, suggesting that the FADD pathway played a major role at early times (Fig. 7A, C, and E). Accordingly, inhibiting the Daxx pathway by expressing FBD in cells in which the FADD pathway was also blocked by the presence of FADD-DN had little additional protective effect (Fig. 7A and C). Under these conditions, HSP27 also had no effect. At 48 h poststimulation, however, the contribution of the FADD pathway became less important. Cell death could not be prevented efficiently for such a long period of time with FADD-DN alone and required that Daxx also be inhibited. Under these conditions, HSP27-EE, but not HSP27-AA, was as efficient as FBD in blocking Fas-induced apoptosis (Fig. 7B and D). The contribution of the Daxx pathway could, however, be detected also at 24 h when the Daxx pathway was enhanced by expressing Ask1. In the presence of a higher concentration of Ask1, Fas-induced apoptosis measured at 24 h was only partially blocked by z-VAD-fmk and z-VAD-fmk-resistant apoptosis was efficiently blocked by FBD or HSP27-EE but not by HSP27-AA (Fig. 7C and D). The results indicated that Fas induced a rapid apoptotic process which is mainly dependent on the FADD pathway and the activation of caspases. Blocking the FADD pathway reveals the existence of a longer-term apoptotic process, dependent on the Daxx-Ask1 module and inhibitable by FBD and phosphorylated HSP27. The result that Ask1 expression has no effect on the total amount of cell death when the FADD pathway is functional (Fig. 7E) suggests that the same individual cells are killed by the two pathways; i.e., when both processes are operational, they are overlapping rather than complementary.
HSP27 prevents Fas-induced relocalization of Daxx.
Daxx is a nuclear protein, and therefore the mechanism by which it mediates Fas-induced apoptosis is unclear. It has been demonstrated that Fas, Ask1, and Daxx form a multiprotein complex after stimulation of Fas, implying a change in the intracellular localization of Daxx (7). By immunofluorescence using antibody EL-68, both endogenous Daxx and Daxx expressed at a high concentration after transfection were found localized in the nucleus, in many cases in speckles (Fig. 8A and D) identified previously as the POD (67). In unstimulated cells, only 1 to 2% of the cells show cytoplasmic Daxx. Twelve hours after stimulation of Fas, this proportion typically increased to some 10% and Daxx was often localized in the blebs of cells starting apoptosis. The exclusion of Daxx from the nucleus was not a consequence of apoptosis. Blocking the early phase of apoptosis with z-VAD-fmk either had no effect or caused an even higher proportion of the cells to accumulate Daxx in the cytoplasm, likely due to the accumulation of the preapoptotic cells by z-VAD-fmk (Fig. 8B). Similar results were obtained with the endogenous Daxx in untransfected cells (Fig. 8D and data not shown). To determine the effect of HSP27 on Fas-induced translocation of Daxx, cells were cotransfected with Daxx and either HSP27-AA or HSP27-EE and then exposed to the activating antibody of Fas. Expression of HSP27 efficiently blocked Fas-induced Daxx translocation. As found for the activity of HSP27 on Daxx-mediated apoptosis, HSP27-EE but not HSP27-AA was active in blocking the translocation of Daxx (Fig. 8C).
DISCUSSION
HSP27 was initially characterized as a protein whose phosphorylation and concentration were modulated by heat shock and which had a potent ability to provide thermoresistance (28). Subsequent studies showed that its spectrum of protection extends well beyond elevated temperatures, providing increased survival to cells exposed to a large variety of stimuli, including oxidative stress, tumor necrosis factor and Fas death receptor activation, and several commonly used anticancer drugs (14, 20, 22, 28, 32, 34, 39–41, 65). HSP27 concentration and phosphorylation status were also found to vary among different tissues and after treatments that induce growth or differentiation, and it was shown that the increased HSP27 concentration that accompanies differentiation was essential to prevent cell death associated with growth arrest (4, 14, 37, 40, 54–56). Various mechanisms have been proposed for its protective activities. HSP27 modulates actin polymerization in vitro, and expression of HSP27 stabilizes actin stress fibers in vivo during oxidative stress (3, 15, 18, 19, 30, 33, 34). HSP27 possesses a chaperone activity in vitro. It binds denatured proteins and prevents their aggregation (11, 24). HSP27 also has been proposed to decrease intracellular reactive oxygen species (38). However, conclusive evidence for a role of any of these mechanisms in cell protection is lacking. Because apoptosis represents a common mechanism of cell death in both physiological and toxic conditions, the recent discovery that HSP27 could block apoptosis induced by cytotoxic drugs and by activation of the death receptor Fas (13, 41) provided a putative explanation for the general protective function of HSP27. In the present study, we report for the first time the functional association of HSP27 with a protein known as a mediator of apoptosis. We showed that HSP27 associates both in vitro and in vivo with Daxx, preventing its interaction with Fas and Ask1 and inhibiting its ability to induce apoptosis.
Daxx was first described as an adapter of the Fas receptor, capable of mediating Fas-induced apoptosis along a pathway distinct from that mediated by the other Fas adapter, FADD. It was shown that Daxx binds through its C-terminal domain FBD, an intracellular domain of Fas, in a manner that did not compete with the binding of FADD (7, 8, 66). A Fas mutant impaired in its capacity to bind FADD but still capable of binding Daxx mediated apoptosis, which developed slower than FADD-mediated apoptosis and which was enhanced by the expression of Ask1. Our results are also consistent with the existence of two parallel pathways downstream of Fas. We found that cell death generated by coexpressing Daxx with Ask1, in contrast to that generated by expressing FADD, was not inhibited by z-VAD-fmk but could be totally blocked by HSP27. In 293 cells, which were used as a cell model to study the in vivo significance of Daxx-HSP27 interactions, we found that in the presence of a dominant-negative form of FADD, which can bind Fas but which cannot recruit caspases, activation of Fas triggered a Daxx-dependent death pathway that became evident at 48 h and that could then be totally inhibited by the Fas-binding domain of Daxx or by HSP27. When Ask1 was overexpressed, a FADD-independent, Daxx-dependent, HSP27-sensitive pathway of apoptosis could also be evidenced at 24 h. Thus our results are consistent with the idea that Daxx can mediate cell death downstream of Fas. Whether this pathway plays a major role in a given situation will depend on the concentration of a number of key molecules influencing the relative strengths of the FADD (e.g., FLIP; see below) and Daxx (e.g., Ask1, Daxx, and HSP27) pathways. In particular, the results predict that cell types that express HSP27 constitutively would not possess a very active Daxx pathway and hence that FADD might appear in these cells as the only pathway leading to apoptosis downstream of Fas. Studies with FAD−/− and caspase 8−/− mice suggested that thymocytes and embryonic fibroblasts have a very weak if not totally inefficient Daxx pathway downstream of Fas (63, 67).
Although the notion that Daxx plays a role in apoptosis is well accepted, the exact mechanism by which it can mediate the action of Fas is controversial (7, 8, 61, 66). In particular, a major problem has been the finding that Daxx is a nuclear protein, a result which appeared inconsistent with the demonstration that upon Fas stimulation Daxx mediates the formation of a multiprotein complex made of Fas, Ask1, and Daxx (7, 8, 66). We confirmed that Daxx is mostly nuclear under control conditions but found that upon activation of Fas, endogenous or overexpressed Daxx becomes cytoplasmic in a large percentage of the cells, thus explaining its interaction with Ask1 and Fas. It is unclear why a previous study failed to detect such a translocation of Daxx (61). One difficulty is that activation of Fas is very toxic, and under some conditions the cells may be killed before any cytoplasmic Daxx is detected or Daxx may be observed only in dying cells (Fig. 8A), thereby producing data which are difficult to interpret. Here we found that cytoplasmic Daxx was highly reproducibly detected when Fas was stimulated in the presence of z-VAD-fmk to block FADD-dependent apoptosis. A second possible cause for the variable results obtained with the localization of Daxx may be the variable level of expression of HSP27 found in different cell lines. Daxx translocation did not occur in cells that expressed HSP27. Intriguingly, our finding that a high concentration of HSP27 in a cell extract can block the interaction of Fas or Ask1 with Daxx may also explain some of the controversial results on the capacity of Daxx to interact with these proteins (61).
HSP27 exists in cells as large homotypic multimers in dynamic equilibrium with a smaller proportion of dimers. Dimers are formed through stable intermolecular interactions of the carboxy-terminal α-crystallin-like domain. The dimers further multimerize in some 24-mers through additional intermolecular interactions mediated by the amino-terminal domain. During stress, phosphorylation of HSP27 destabilizes the amino-terminal interactions, resulting in a shift in the equilibrium between dimers and multimers and in a rapid accumulation of dimers as the major species (27). The protective activity of HSP27 against Daxx-mediated apoptosis was totally dependent on the oligomerization and phosphorylation status of HSP27. Wild-type HSP27, which is distributed in dimers and multimers that totally dissociate into dimeric molecules upon phosphorylation, and the pseudophosphorylated HSP27-EE mutant, which forms exclusively dimeric molecules irrespective of phosphorylation (27), could interact with Daxx, block its interaction with Fas and Ask1, and protect from apoptosis. In contrast, the nonphosphorylatable HSP27-AA mutant, which forms stable oligomeric molecules that do not dissociate upon phosphorylation (27), did not interact with Daxx and did not block its interaction with the other proteins or apoptosis. This observation is consistent with several reports showing the phosphorylation-dependent functions of HSP27. A nonphosphorylatable human or Chinese hamster HSP27 mutant could not protect cells against heat shock or actin filaments from the disruptive effect of oxidative stress or activation of the cholecystokinin A receptor (20, 34, 53). Pseudophosphorylated HSP27 was, in contrast, as effective as wild-type HSP27 in protecting against heat shock or cholecystokinin-induced actin filament disruption (unpublished results; 53). Furthermore, dissociation into dimers, which for the yeast homologue HSP26 occurs spontaneously upon raising the temperature in vitro, has also been shown to enhance HSP26 chaperone activity (17). A previous correlation between the phosphorylation state of HSP27 and its capacity to protect cells against stress, together with the present results, suggests the hypothesis that phosphorylation-induced changes in the supramolecular organization of HSP27 expose a protective domain in the N-terminal region of the protein (27).
HSP27 is phosphorylated upon cell stimulation by a number of growth factor agonists, such as epidermal growth factor, vascular endothelial growth factor, and thrombin; cytokines such as lipopolysaccharide, tumor necrosis factor alpha, and interleukin-1β; and stress such as that due to heat shock and oxidants. It is phosphorylated by MAPKAP kinase 2, a serine kinase activated through direct phosphorylation by the MAP kinase p38 (12, 15, 18, 21, 29, 31, 37, 40, 50, 57). In some cell lines, activation of Fas also results in the activation of p38, which can be either FADD and caspase dependent or dependent on the interaction between Daxx and Ask1 (8, 25, 62). This mechanism is, however, not well understood and clearly not universal. In the 293 cell lines used here, neither activation of Fas by overexpression of Fas or exposure to a Fas-activating antibody nor coexpression of Daxx and Ask1 led to a significant activation of JNK or p38 (data not shown). A similar lack of activation of JNK by the Daxx-Ask1 axis in HT1080 cells was previously reported (61). At any rate, it is interesting that, at least in some specific cellular contexts, the interaction between Daxx and HSP27 may define a new mechanism for cross talk between different signaling pathways that activate HSP27 phosphorylation and Fas, and even in some cases an autoregulatory loop within the Fas pathway.
The contribution of the HSP27-Daxx interaction in the several protective activities of HSP27 remains to be determined. Although there is at least one experimental condition in which HSP27 overexpression has been shown to confer significant protection against Fas-induced apoptosis (41), HSP27 is not expected to contribute in a major manner to Fas-induced cell death in most cases. Since HSP27 only blocks the Daxx-dependent arm of the Fas pathway, protection of Fas-induced cell death by HSP27 is expected to be significant only in cellular contexts where FADD-dependent apoptosis is blocked. Intriguingly, there are a number of physiological and pathological situations where this may occur. Upon viral infection, for example, two potent inhibitors of the FADD pathway are produced: CrmA, which inhibits caspase 8, and v-FLIP, which prevents recruitment and activation of caspase 8 (48, 59). In monocytes undergoing differentiation into macrophages, in lymphocytes upon activation, in some tumor cells, and in motoneurons, upregulation of a cellular homologue of v-FLIP, c-FLIP, provides resistance to FAS-mediated apoptosis (1, 16, 23, 46, 47). The phosphorylation and expression levels of HSP27 are also modulated during differentiation of macrophages in different populations of lymphocytes as a function of the cell cycle and are highly variable in different tumors (4, 14, 37, 40, 54–56, 58, 60). There is thus a possibility that in those cases, expression and phosphorylation of HSP27 contribute to the regulation of the Fas pathway. More studies will be required, however, to determine the exact role of Daxx in Fas-mediated apoptosis and possibly other mechanisms of toxic cell death and the contribution of the HSP27-Daxx interaction to the general mechanism of protection of this heat shock protein.
ACKNOWLEDGMENTS
We thank M. Kiriakidou and H. Ichijo for providing us with the Daxx cDNA-containing plasmid and pcDNA3-HA-ASK1, respectively. We also acknowledge the technical assistance of C. Champagne and E. Pellerin.
This work was supported by Medical Research Council of Canada grant MT-7088. S.J.C. received a studentship from the Medical Research Council of Canada.
REFERENCES
- 1.Algeciras-Schimnich A, Griffith T S, Lynch D H, Paya C V. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–5211. [PubMed] [Google Scholar]
- 2.Arrigo A P, Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto R I, Tissières A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 335–373. [Google Scholar]
- 3.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 4.Benndorf R, Kraft R, Otto A, Stahl J, Bohm H, Bielka H. Purification of the growth-related protein p25 of the Ehrlich ascites tumor and analysis of its isoforms. Biochem Int. 1988;17:225–234. [PubMed] [Google Scholar]
- 5.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 7.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 8.Chang H Y, Yang X, Baltimore D. Dissecting Fas signaling with an altered-specificity death-domain mutant: requirement of FADD binding for apoptosis but not Jun N-terminal kinase activation. Proc Natl Acad Sci USA. 1999;96:1252–1256. doi: 10.1073/pnas.96.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 10.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 11.Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaestel M, Schroder W, Benndorf R, Lippmann C, Buchner K, Hucho F, Erdmann V A, Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- 13.Garrido C, Bruey J M, Fromentin A, Hammann A, Arrigo A P, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 14.Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo A P, Chauffert B, Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res. 1997;57:2661–2667. [PubMed] [Google Scholar]
- 15.Guay J, Lambert H, Gingras-Breton G, Lavoie J N, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 16.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 17.Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White H E, Chen S, Saibil H R, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 19.Huot J, Houle F, Rousseau S, Deschesnes R G, Shah G M, Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huot J, Houle F, Spitz D R, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 21.Huot J, Lambert H, Lavoie J N, Guimond A, Houle F, Landry J. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur J Biochem. 1995;227:416–427. doi: 10.1111/j.1432-1033.1995.tb20404.x. [DOI] [PubMed] [Google Scholar]
- 22.Huot J, Roy G, Lambert H, Chrètien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- 23.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 24.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 25.Juo P, Kuo C J, Reynolds S E, Konz R F, Raingeaud J, Davis R J, Biemann H P, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiriakidou M, Driscoll D A, Lopez-Guisa J M, Strauss J F., III Cloning and expression of primate Daxx cDNAs and mapping of the human gene to chromosome 6p21.3 in the MHC region. DNA Cell Biol. 1997;16:1289–1298. doi: 10.1089/dna.1997.16.1289. [DOI] [PubMed] [Google Scholar]
- 27.Lambert H, Charette S J, Bernier A F, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 28.Landry J, Chrètien P, Lambert H, Hickey E, Weber L A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 30.Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat shock protein of 27 kDa (Hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- 31.Landry J, Lambert H, Zhou M, Lavoie J N, Hickey E, Weber L A, Anderson C W. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 32.Lavoie J N, Gingras-Breton G, Tanguay R M, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 33.Lavoie J N, Hickey E, Weber L A, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 34.Lavoie J N, Lambert H, Hickey E, Weber L A, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee G J, Roseman A M, Saibil H R, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis S E, Mannion R J, White F A, Coggeshall R E, Beggs S, Costigan M, Martin J L, Dillmann W H, Woolf C J. A role for HSP27 in sensory neuron survival. J Neurosci. 1999;19:8945–8953. doi: 10.1523/JNEUROSCI.19-20-08945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehlen P, Coronas V, Ljubic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo A P. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- 38.Mehlen P, Kretz-Remy C, Briolay J, Fostan P, Mirault M E, Arrigo A P. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem J. 1995;312:367–375. doi: 10.1042/bj3120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehlen P, Kretz-Remy C, Preville X, Arrigo A P. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 40.Mehlen P, Mehlen A, Godet J, Arrigo A P. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- 41.Mehlen P, Schulze-Osthoff K, Arrigo A P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 42.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miron T, Wilchek M, Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988;178:543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- 44.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 45.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 46.Perlman H, Pagliari L J, Georganas C, Mano T, Walsh K, Pope R M. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raoul C, Henderson C E, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 49.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo A P, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 50.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 51.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 52.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schäfer C, Clapp P, Welsh M J, Benndorf R, Williams J A. HSP27 expression regulates CCK-induced changes of the actin cytoskeleton in CHO-CCK-A cells. Am J Physiol. 1999;277:C1032–C1043. doi: 10.1152/ajpcell.1999.277.6.C1032. [DOI] [PubMed] [Google Scholar]
- 54.Spector N L, Ryan C, Samson W, Levine H, Nadler L M, Arrigo A P. Heat shock protein is a unique marker of growth arrest during macrophage differentiation of HL-60 cells. J Cell Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- 55.Spector N L, Samson W, Ryan C, Gribben J, Urba W, Welch W J, Nadler L M. Growth arrest of human B lymphocytes is accompanied by induction of the low molecular weight mammalian heat shock protein (Hsp28) J Immunol. 1992;148:1668–1673. [PubMed] [Google Scholar]
- 56.Stahl J, Wobus A M, Ihrig S, Lutsch G, Bielka H. The small heat shock protein hsp25 is accumulated in P19 embryonal carcinoma cells and embryonic stem cells of line BLC6 during differentiation. Differentiation. 1992;51:33–37. doi: 10.1111/j.1432-0436.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 57.Stokoe D, Engel K, Campbell D G, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 58.Tetu B, Lacasse B, Bouchard H L, Lagace R, Huot J, Landry J. Prognostic influence of HSP-27 expression in malignant fibrous histiocytoma: a clinicopathological and immunohistochemical study. Cancer Res. 1992;52:2325–2328. [PubMed] [Google Scholar]
- 59.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 60.Thor A, Benz C, Moore D, Goldman E, Edgerton S, Landry J, Schwartz L, Mayall B, Hickey E, Weber L A. Stress response protein (srp-27) determination in primary human breast carcinomas: clinical, histologic, and prognostic correlations. J Natl Cancer Inst. 1991;83:170–178. doi: 10.1093/jnci/83.3.170. [DOI] [PubMed] [Google Scholar]
- 61.Torii S, Egan D A, Evans R A, Reed J C. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs) EMBO J. 1999;18:6037–6049. doi: 10.1093/emboj/18.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toyoshima F, Moriguchi T, Nishida E. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J Cell Biol. 1997;139:1005–1015. doi: 10.1083/jcb.139.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham K B, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 64.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 65.Wagstaff M J, Collaco-Moraes Y, Smith J, de Belleroche J S, Coffin R S, Latchman D S. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Cado D, Chen A, Kabra N H, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]