Abstract
Objective:
To determine the total oxidant status (TOS), total antioxidant status (TAS), and the 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels and their interrelationship in the saliva of children undergoing fixed orthodontic therapy.
Materials and Methods:
Thirty children were randomly divided into three groups. The attachments were bonded to all of the teeth using three different orthodontic composites: Transbond XT, Kurasper F, and GrenGloo. The salivary levels of TOS, TAS, and 8-OHdG were determined three times, as follows: before treatment (T1) and at 1 month (T2) and 3 months (T3) following appliance placement. All data were statistically analyzed.
Results:
There were no significant differences in TOS, TAS, and 8-OHdG within the same time periods among the three different orthodontic composites (P > .05). TAS in all composite groups decreased over time. These decreases were found to be significant for Kurasper F and GrenGloo at the T1–T3 and T2–T3 time periods (P < .05). In all composite groups 8-OHdG decreased between T1 and T2 (P < .05). However, 8-OHdG in all composite groups increased from T2 to T3. These differences in 8-OHdG were significant in Kurasper F and GrenGloo (P < .05).
Conclusions:
Fixed orthodontic appliances bonded with the tested composites did not increase the cytotoxicity markers in saliva.
Keywords: Total oxidant status, Total antioxidant status, 8-Hydroxy-2′-deoxyguanosine
INTRODUCTION
Orthodontic composites are often used by pediatric dentists and orthodontists for the bracket-banding process in children with both primary and permanent dentition. However, some components of the orthodontic composites may be released into the oral environment and saliva during fixed-appliance treatment, and even following polymerization.1,2 These components released from orthodontic composites are triethylene-glycoldimethacrylate (TEGDMA), urethane dimethacrylate (UDMA), 2-hydroxyethylmethacrylate (HEMA), bisphenol A, bisphenol A–diglycidyl dimethacrylate (Bis-GMA), and methyl methacrylate. The release of these components and their diffusion may cause various adverse effects in the organism, such as allergic reactions, systemic toxicity, cytotoxicity, mutagenicity, and carcinogenicity.3–5
Although there has been satisfactory development of orthodontic composite materials, the biocompatibility of these materials is usually disregarded in dental practice. Evaluating the cytotoxicity, genotoxicity, and biocompatibility of orthodontic composites and luting cements is as important as considering the physiological or mechanical properties of these materials. However, there are limited studies relating to the cytotoxic effects of orthodontic adhesives.6–9
Antioxidants protect against the potentially harmful effects of processes or reactions that cause excessive oxidation. The total oxidant status (TOS) reflects the present oxidative status. The total antioxidant status (TAS) provides information about the antioxidant capacity of the organism. There are few studies on the antioxidant defense systems of saliva and their relationship to oral disease in children.10,11
Oxidative damage to DNA can be detected by chemical, physical, and enzymatic methods. The levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG) have been used to evaluate DNA damage. It is possible to prove cytotoxicity via oxidative stress by detecting 8-OHdG. Therefore, it has often been used as a biomarker for oxidative damage.12
In the literature, there are some reports6–9,13 on the genotoxic and cytotoxic effects of different orthodontic materials. However, the TOS, TAS, and 8-OHdG levels in the saliva of children undergoing fixed orthodontic therapy have not yet been studied. The aim of the present study was to determine the relationship between the salivary levels of TOS, TAS, and 8-OHdG in children undergoing fixed orthodontic therapy with three different orthodontic composites over a 3-month evaluation period.
MATERIALS AND METHODS
The healthy children, aged between 11 and 17 years (mean age, 13.72 ± 1.7 years) were selected from consecutive patients referring for orthodontic treatment to the Department of Orthodontics at Inonu University, who fulfilled the following inclusion criteria: they were nonsmokers, nondrinkers, were not taking medications, and were not exposed to systemic infections; they had good oral hygiene; they had no decay, fillings, or gingival inflammation; and they had not been using oral-antiseptic solutions or topical fluoride during tooth development.
Their informed consent was obtained, and the study was approved by the Faculty of Medicine Ethic Committee, Inonu University (2011–169). All of the patients underwent lateral and frontal cephalometric radiography and panoramic dental radiography. Three months after the X-ray exposure, orthodontic treatment was initiated.
A total of 30 children who fulfilled inclusion criteria were selected for this study. A power analysis conducted during the planning of the study showed that a sample size of 10 participants would provide sufficient statistical power to discriminate changes in the salivary variables under study. Power analysis was performed with MINITAB version 14.1 software (Minitab Inc, State College, Pa). The selected patients were randomly divided into three equal groups according to the orthodontic composite used in this study (n = 10, alpha level of 0.05, and Power of 0.80). The orthodontic composites used in this study are shown in Table 1. The three orthodontic composites were randomly assigned in a consecutive manner. Transbond XT Light Cure (3M Unitek Ortho Prod, Monrovia, Calif) was applied to the first child who participated in the study. Kurasper F Light Cure (Kuraray Europe GmbH, Frankfurt, Germany) was applied to the second child who participated in the study, and GrenGloo Light Cure (Ormco Corporation, Glendora, Calif) was applied to the third child who participated in the study. This consecutive assignment of orthodontic composite was then repeated for all subsequent participating children. For standardization purposes, Equilibrium 2 brackets and double-direct molar tubes (Dentaurum, Ispringen, Germany) were bonded with three different orthodontic, light-cure composites (according to the manufacturer's instructions) to all teeth in both arches, including the second molars if they had erupted. In each group, 0.012-, 0.014-, and 0.016-inch Rematitan ideal, round, nickel-titanium archwires (Dentaurum) were applied with 0.010 inch Remanium preformed, stainless-steel ligature wires (Dentaurum) at the 2-month interval.
Table 1.
Orthodontic Composite Used in This Studya
Saliva samples were collected three times from each patient. The first samples were collected before the appliance placement and were considered as the controls (T1). One month after the appliance placement, the same investigator took the second set of samples (T2). The final records (T3) were obtained 3 months after appliance placement.
Saliva Collection
Unstimulated saliva samples were collected between 9 AM and noon. Children were instructed not to eat or drink anything for at least 1 hour before collecting the saliva sample and to brush their teeth once in the morning on the day of salivary collection. Children sat in a dental chair at leisure, slightly bent forward, and were instructed to collect the unstimulated saliva into a sterile plastic cup over the course of a 5-minute period. The collected saliva samples were stored at −80°C until biochemical analysis was carried out. The assays for TOS, TAS, and 8-OHdG in saliva were performed in triplicate, and the arithmetic means were calculated.
Assays for TOS and TAS
The TOS in the saliva samples was measured using a novel colorimetric and automated-measurement method for TOS developed by Erel.14 The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Equiv/L).
The TAS in the saliva samples was measured using a novel colorimetric and automated direct-measurement method for TAS developed by Erel.15 In this assay, 2.2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) is incubated with metmyoglobin and hydrogen peroxide to produce ABTS+. Antioxidants present in the saliva cause a reduction in the absorption that is proportional to their concentration. The total antioxidant capacity value of the tested saliva is expressed as an equivalent of the millimolar concentration of Trolox solution (µmol Trolox Equiv/L). The inter- and intra-assay coefficients of variation were 14.62 and 4.85 for TOS and 14.15 and 3.36 for TAS, respectively.
Assay for 8-OHdG Levels
The values of 8-OHdG were determined with test kits involving the enzyme-linked immunosorbent assay (ELISA) method (CAYMAN ELISA Kit 8-hydroxy-2′-deoxyguanosine, Ann Arbor, MI, USA). The sensitivity of Assay Designs' DNA Damage ELISA kit was determined to be 0.59 ng/mL. The inter- and intra-assay coefficients of variation were 4.1 and 5.2, respectively.
Statistical Analysis
The data were analyzed using SPSS for Windows, version 16.0 (SPSS Inc, Chicago, Ill). Descriptive statistics (mean, median, standard deviation, and minimum and maximum values) were calculated. The nonparametric Wilcoxon matched-pairs test was used to analyze the differences in TOS, TAS, and 8-OHdG levels in the saliva at the different time periods within the same orthodontic composite group. The statistical significance of differences in TOS, TAS, and 8-OHdG levels in the saliva at the different time periods within the same orthodontic composite group was accepted at the α < .05 level.
The Kruskal–Wallis test was used to analyze the differences in the TOS, TAS, and 8-OHdG salivary levels for the same time period among the three different orthodontic composite groups. The statistical significance of differences in TOS, TAS, and 8-OHdG salivary levels for the same time period among the three different orthodontic composite groups was accepted at the α < .05 level.
RESULTS
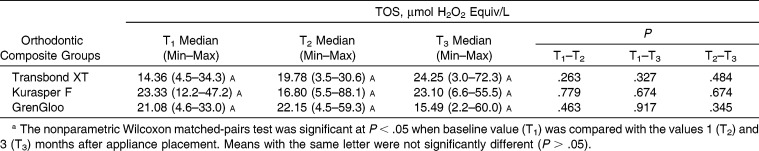
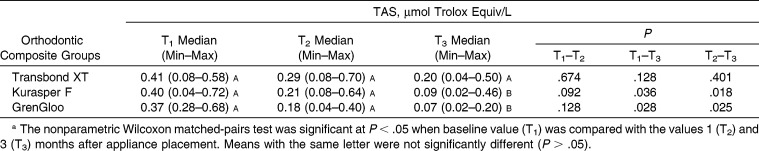
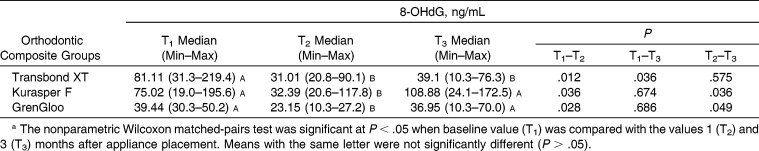
Statistically significant differences were not found in the TOS, TAS, and 8-OHdG levels in the saliva for the same time periods from among the three different orthodontic composites (P > .05) (Tables 2–4). The levels of TAS in all composite groups decreased over time (Table 3). These decreases were found to be statistically significant for Kurasper F and GrenGloo at the T1–T3 and T2–T3 time points (P < .05). The levels of 8-OHdG in all composite groups decreased between T1 and T2 (P < .05) (Table 4). However, the levels of 8-OHdG in all composite groups increased from T2 to T3. These differences in the levels of 8-OHdG in the saliva were statistically significant in the Kurasper F– and GrenGloo-applied groups (P < .05).
Table 2.
Distribution of Total Oxidant Status (TOS) According to Orthodontic Composite Resina
Table 3.
Distribution of Total Antioxidant Status (TAS) According to Orthodontic Composite Resina
Table 4.
Distribution of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) According to Orthodontic Composite Resina
The effect on TOS, TAS, and 8-OHdG levels in the saliva of the three different orthodontic composites used in this study was found to be similar during the 3-month evaluation period.
DISCUSSION
The appliances in fixed orthodontic treatments are fabricated from different alloys. These alloys include nickel, cobalt, and chromium. These metallic ions and monomers released from orthodontic composites have harmful effects on the adjacent oral tissues. In addition, it has been reported16,17 that the mucosa of the mouth may absorb residual monomers and that these monomers may be taken into the digestive system by means of the saliva. Therefore, in the present study we aimed to evaluate the long-term effects of metallic ions and monomers released from orthodontic composites on TOS, TAS, and 8-OHdG levels in the saliva.
Antioxidants are an important part of our diet, and these systems, together with intracellular antioxidants and enzymatic systems, impede inflammation, infections, and tumor formation.18 Some types of inflammation, especially periodontal disease, have been associated with reduced salivary antioxidant status and increased oxidative damage within the oral cavity.19
Oxidative stress has been defined as a disturbance in the oxidant-antioxidant balance, resulting in potential cell damage. It is known that oxidative stress plays a role in the pathogenesis of cancer and many inflammatory diseases, such as diabetes mellitus, atherosclerosis, hypertension, and obesity.20 Antioxidant defense systems have been found to prevent the formation of free radicals and to reduce cell damage. Sculley and Langley-Evans21 stated that women had significantly lower TAS levels than men, regardless of their periodontal health. In addition, the authors reported that salivary TAS increased with increasing caries activity.11,22–26 Tóthová et al.26 found that salivary antioxidant status in children was related to oral hygiene and periodontal status. To assess the results with accuracy, all of the patients who were selected for this evaluation had no fillings, no caries, and no periodontal disease. We found that the levels of TAS in all composite groups decreased over time. These decreases were statistically significant for Kurasper F and GrenGloo at the T1–T3 and T2–T3 time points (P < .05). These decreases may be related to a lack of fillings, lack of caries, good oral hygiene, and fluoride release from the orthodontic composites.
8-OHdG is a biomarker that indicates oxidative damage in a number of disorders, including chronic inflammatory diseases.12 Salivary 8-OHdG levels have been intensively studied in children, including in patients with periodontitis.27 Takane et al.27 reported that salivary 8-OHdG levels appeared to reflect the status of periodontal health. In addition, the latest data showed that the antioxidant capacity decreased and that certain oxidative stress biomarkers increased in periodontitis patients.28–30 We found that the levels of 8-OHdG in all composite groups decreased between T1 and T2 (P < .05). However, the levels of 8-OHdG in all composite groups increased from T2 to T3. These differences in the levels of 8-OHdG were statistically significant in the Kurasper F- and GrenGloo-applied groups (P < .05). These differences may be related to composite content, the release of monomers, and the release of fluoride.
In this study, we found that orthodontic fixed attachments that were bonded with three different orthodontic composites showed no cytotoxic effects in children during the 3-month evaluation period. This finding may result from the influence of the adhesive content and the properties of metallic appliances, either separately or jointly. Transbond XT and Kurasper F contain Bis-GMA and TEGDMA as a main component. GrenGloo contains uncured methacrylate ester monomers. In the literature, Bis-GMA, TEGDMA, UDMA, and HEMA were tested as resin components. Kleinsasser et al.31 stated that TEGDMA, UDMA, and HEMA induced a significant elevation in DNA migration in the Comet assay and that this was a possible sign of genotoxic effects in human salivary glands and lymphocytes. In our in vivo study we applied the same metallic devices and three different commercial compound–type bonding adhesives. This could be a factor leading to the decrease in the release of monomers.
A combination of monomers is generally used in orthodontic composites. Tested orthodontic composites include mostly similar resin matrices. However, Transbond XT also contains Bis-EMA. In addition, a Bis-EMA monomer showed a cytotoxic effect analogous to that of TEGDMA.32 Lee et al.6 stated that all experimental monomers exhibited a dose-dependent cytotoxic effect, and the ranking of the cytotoxicity was GMA > TEGDMA > HEMA. The increased DNA damage in cementation with Kurasper F Light Cure has been mainly attributed to the release of the monomers TEGDMA and Bis-GMA, which are added to this chemical composition.
This study does have some limitations. One limitation is that all subjects were recruited from among children at the University Hospital. This may limit the ability to extrapolate the present findings to the general population. The second limitation is that only patients with good oral hygiene were included in this study. Caries and gingival inflammation may result in a change in terms of the salivary variables.
CONCLUSIONS
The levels of TAS in all composite groups decreased over time.
Fixed orthodontic appliances applied using three tested orthodontic composites have no cytotoxic effects on children. Therefore, tested composites can be used safely by pediatric dentists or orthodontists.
REFERENCES
- 1.Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687–695. doi: 10.1046/j.0909-8836.1998.eos10602ii04.x. [DOI] [PubMed] [Google Scholar]
- 2.Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. Oral Rehabil. 2001;12:1106–1115. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 3.Hensten-Pettersen A. Skin and mucosal reactions associated with dental materials. Eur J Oral Sci. 1998;106:707–712. [PubMed] [Google Scholar]
- 4.Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333–355. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 5.Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Lim BS, Lee YK, Ahn SJ, Yang HC. Involvement of oxidative stress in mutagenicity and apoptosis caused by dental resin monomers in cell cultures. Dent Mater. 2006;22:1086–1092. doi: 10.1016/j.dental.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Hanks CT, Anderson M, Craig RG. Cytotoxic effects of dental cements on two cell culture systems. J Oral Pathol. 1981;10:101–112. doi: 10.1111/j.1600-0714.1981.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 8.Angelieri F, Carlin V, Martins RA, Ribeiro DA. Biomonitoring of mutagenicity and cytotoxicity in patients undergoing fixed orthodontic therapy. Am J Orthod Dentofacial Orthop. 2011;139:e399–e404. doi: 10.1016/j.ajodo.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Vande Vannet BM, Hanssens JL. Cytotoxicity of two bonding adhesives assessed by three-dimensional cell culture. Angle Orthod. 2007;77:716–722. doi: 10.2319/052706-212.1. [DOI] [PubMed] [Google Scholar]
- 10.Pereslegina IA. The activity of antioxidant enzymes in the saliva of normal children. Lab Delo. 1989;11:20–23. [PubMed] [Google Scholar]
- 11.Tulunoglu O, Demirtas S, Tulunoglu I. Total antioxidant levels of saliva in children related to caries, age, and gender. Int J Paediatr Dent. 2006;16:186–191. doi: 10.1111/j.1365-263X.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 12.Shigenaga M, Gimeno C, Ames B. Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci USA. 1989;86:9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Öztürk F, Malkoc S, Ersöz M, Hakki SS, Bozkurt BS. Real-time cell analysis of the cytotoxicity of the components of orthodontic acrylic materials on gingival fibroblasts. Am J Orthod Dentofacial Orthop. 2011;140:e243–e249. doi: 10.1016/j.ajodo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Agaoglu G, Arun T, Izgi B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001;71:375–379. doi: 10.1043/0003-3219(2001)071<0375:NACLIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Kocadereli L, Atac PA, Kale PS, Ozer D. Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle Orthod. 2000;70:431–434. doi: 10.1043/0003-3219(2000)070<0431:SNACIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Goldie MP. Antioxidants in oral health care: making the connection. Int J Dent Hyg. 2005;3:93–95. doi: 10.1111/j.1601-5037.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 19.Brock GR, Butterworth CJ, Mathews JB, Chapple ILC. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 20.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Rad Med Biol. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 21.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105:167–172. doi: 10.1042/CS20030031. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Pandey RK, Agrawal D, Agrawal D. An estimation and evaluation of total antioxidant capacity of saliva in children with severe early childhood caries. Int J Paediatr Dent. 2011;21:459–464. doi: 10.1111/j.1365-263X.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 23.Hegde AM, Rai K, Padmanabhan V. Total antioxidant capacity of saliva and its relation with early childhood caries and rampant caries. J Clin Pediatr Dent. 2009;33:231–234. doi: 10.17796/jcpd.33.3.c730518021m56077. [DOI] [PubMed] [Google Scholar]
- 24.Preethi BP, Reshma D, Anand P. Evaluation of flow rate, pH, buffering capacity, calcium, total proteins and total antioxidant capacity levels of saliva in caries free and caries active children: an in vivo study. Indian J Clin Biochem. 2010;25:425–428. doi: 10.1007/s12291-010-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodwad R, Betigeri AV, Preeti BP. Estimation of total antioxidant capacity levels in saliva of caries-free and caries-active children. Contemp Clin Dent. 2011;2:17–20. doi: 10.4103/0976-237X.79296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tóthová L, Celecová V, Celec P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis Markers. 2013;34:9–15. doi: 10.3233/DMA-2012-00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K. New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol. 2002;73:551–554. doi: 10.1902/jop.2002.73.5.551. [DOI] [PubMed] [Google Scholar]
- 28.Akalin FA, Baltacioğlu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34:558–565. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 29.Canakçi CF, Canakçi V, Tatar A, et al. Increased salivary level of 8-hydroxydeoxyguanosine is a marker of premature oxidative mitochondrial DNA damage in gingival tissue of patients with periodontitis. Arch Immunol Ther Exp (Warsz) 2009;57:205–211. doi: 10.1007/s00005-009-0026-9. [DOI] [PubMed] [Google Scholar]
- 30.Sezer U, Ciçek Y, Canakçi CF. Increased salivary levels of 8-hydroxydeoxyguanosine may be a marker for disease activity for periodontitis. Dis Markers. 2012;32:165–172. doi: 10.3233/DMA-2011-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinsasser NH, Schmid K, Sassen AW, et al. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762–1770. doi: 10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–480. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]