Abstract
Objective:
To evaluate the effect of a light-emitting diode (LED) and/or low-level laser (LLL) with or without the use of anterior bite jumping appliances (also known as functional appliances [FAs]) on mandibular growth in rats.
Materials and Methods:
Thirty-six 8-week-old male Sprague-Dawley rats weighing 200 g were obtained from Charles River Canada (St. Constant, QC, Canada) and were divided into six groups of six animals each. Groups were as follows: group 1: LLL; group 2: LLL + FA; group 3: LED; group 4: LED + FA; group 5: FA; and group 6: control (no treatment). Mandibular growth was evaluated by histomorphometric and micro computed tomographic (microCT) analyses.
Results:
The LED and LED + FA groups showed an increase in all condylar tissue parameters compared with other groups.
Conclusion:
The LED-treated groups showed more mandibular growth stimulation compared with the laser groups.
Keywords: LED, Laser, Functional appliance, Mandibular growth
INTRODUCTION
Patients with craniofacial underdeveloped lower jaws can have severe psychological and social impacts, especially in growing children, and it can lead to severe airway constriction with associated life-threatening complications such as nonpositional obstructive sleep apnea.1,2 Previous studies have shown that the use of mandibular advancement devices, also known as functional appliances (FAs), can enhance mandibular forward position/projection and may stimulate mandibular forward growth.3,4
On the other hand, other studies have recently shown a device success rate of only 54.8% (failures being attributed mainly to patient noncompliance).5,6 Nonetheless, advancing the mandible with oral appliances was reported to be effective in the short term.5 The long-term efficacy of all of the above-mentioned treatment modalities is unknown. There is increasing evidence that compensatory growth occurs at the temporomandibular joint, and especially the mandibular condyle, in response to altered occlusal function in young, growing animals4,7 and can be stimulated by ultrasound.8–11 It has been also shown that FAs can provide a synergetic effect to the stimulatory effect of low-intensity pulsed ultrasound on mandibular growth.9 However, clinical application of ultrasound in patients required a year on average to obtain a clinically noticeable effect.10 Therefore, there is a need for an alternative noninvasive approach to stimulate mandibular growth with little or no potential side effects in a shorter period of time. Photobiomodulation is a new approach that has been shown to have therapeutic effects on stimulating tissue regeneration and growth. Photobiomodulation uses low-level laser (LLL) or light-emitting diode (LED) light, which have been shown to produce stimulatory effects on fibroblastic and chondral proliferation.12,13 LED and LLL have also been used to accelerate tooth movement14,15 and to minimize orthodontically induced root resorption,15,16 as well as to promote fracture repair.17
The aims of this study were to evaluate any stimulatory effect of LLL or LED on mandibular growth and to determine if there is any synergetic effect between LLL or LED and FAs on mandibular growth stimulation. We hypothesized that LLL or LED can stimulate mandibular growth and that this stimulation can be further augmented with the use of functional appliances.
MATERIALS AND METHODS
This experiment was approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee. Thirty-six 8-week-old male Sprague-Dawley rats weighing 200 g were obtained from Charles River Canada (St. Constant, QC, Canada) and were divided into six groups of six animals each. Groups are presented in Table 1, and they are as follows: group 1: LLL; group 2: LLL + FA; group 3 LED; group 4: LED + FA; group 5: FA; and group 6: control (no treatment). Both LLL and LED (Biolux Inc, Vancouver, BC, Canada) devices produced the same average intensity (10 mW/cm2, which is equal to 6 J/cm2). The wavelength for both is 655 nm (infrared range). These parameters/conditions were selected based on previous studies showing that these LED/LLL parameters have a stimulatory effect on different tissues.12,13
Table 1.
Experimental Groups Used in the Studya
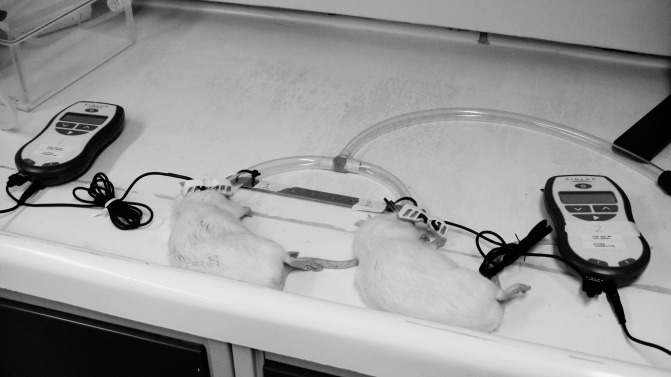
Experimental animals that received LLL or LED were treated on one side (right side), while the left side was used as a self-control, for 10 minutes per day for 28 days while they were under a short period of gas anesthesia (2.5% isoflurane; Figure 1). The LED/LLL applicator size (therapeutic areas) for each applicator was 15.5 mm in length and 8.5 mm in height, and there was a total surface area of 1.3175 cm2. The light was accurately and consistently delivered to the therapeutic point using a custom-made strap that ensured consistent application of the light devices to the condyles. The devices were calibrated for their output before and after finishing the treatment, using a 10D Pin (OSI Optoelectronics, Camarillo, Calif), and the output was consistent. Also, FAs were fitted and cemented to the animals' lower jaw while the animals were under gas anesthesia (2.5% isoflurane). The thickness of the FAs was 5 mm, which allowed the animals to keep their mouths open and repositioned the mandibular condyles downward and forward. To eliminate any effect of using a different diet on animals' weight in groups with an FA and groups with no FA, all animals were fed a soft diet. After 28 days, all animals were euthanized, and dissected mandibles were fixed in 10% formalin and scanned by x-ray microtomography (microCT) then were processed for histological/histomorphometric analysis.
Figure 1.
LED and LLL applied to the animals while they are under gas anesthesia.
MicroCT Imaging of the Dissected Mandibles
Mandibles were scanned using a microCT imager (Skyscan-1076, Skyscan NV, Belgium) at 18-µm resolution, using a tube voltage of 100 kVp, a current of 100 µA, and a power of 10 W. Scan projections were averaged per step, through the 180° of rotation at 0.5°-step increments with 1180 milliseconds exposure time.
The raw image data were reconstructed at a cross-sectional threshold of 0.0–0.046 using NRecon reconstruction software (version 1.4.4, Skyscan NV). Reconstructed images were loaded on the histomorphometric image analysis software (CT-An, Skyscan NV) for the whole hemimandibular bone volume and bone mineral density. Using CT-Vol software, reconstructed images were rendered into three-dimensional (3D) representations for viewing. Regions of interest were manually selected on the right and left sides of the whole mandibular condyles. cTAN software was also used to obtain the 3D analysis from the reconstructed 2D images. In each group, the mandibular condylar bone volume/tissue volume ratio (BV/TV%) was measured and compared between groups.
Histology and Histomorphometric Analysis
The mandibles were decalcified using Cal-EX II (Fisher Scientific, Ottawa, Canada), which was composed of 1.03 M/L formaldehyde and 2.56 M/L formic acid, for about 2 weeks. The samples were processed into paraffin blocks. The condyles were sectioned at a 6-µm thickness and were stained with hematoxylin and eosin. Six samples were taken from each mandibular condyle; the slides were scanned and photographs were taken using a Leica fluorescent digital microscope with CCD digital camera (Leica, Wetzlar, Germany). The analysis of the images was performed using RS Image software 1.73 (Photometric, Roper Scientific Inc, Tucson, Ariz). Four adjacent high-power (40×) microscopic fields (100 µm2 each) in each histology section were analyzed. Images were automatically corrected for brightness and contrast and then converted into eight-bit grayscale. The boundary of the cartilage layer was then identified, and the surface area of the total condylar layer was automatically counted in the selected microscopic fields with the use of image analysis software (Metamorph version 6.1r1). Mandibular condylar layers (Figure 2) were identified. Total surface areas of the mandibular condylar layers were measured representing the readings from the six slides of each sample and were then averaged for each group. The measurements were then compared between groups.
Figure 2.
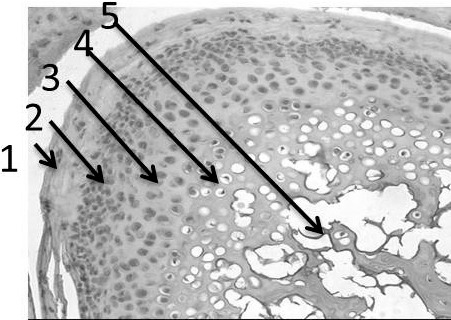
Rat mandibular condyle showing condylar cartilaginous layers for histomorphometric analysis. (1) Fibrocartilage layer. (2) Proliferative layer. (3) Hypertrophic layer. (4) Chondrocyte Layer. (5) Subchondral bone.
Statistical Analysis
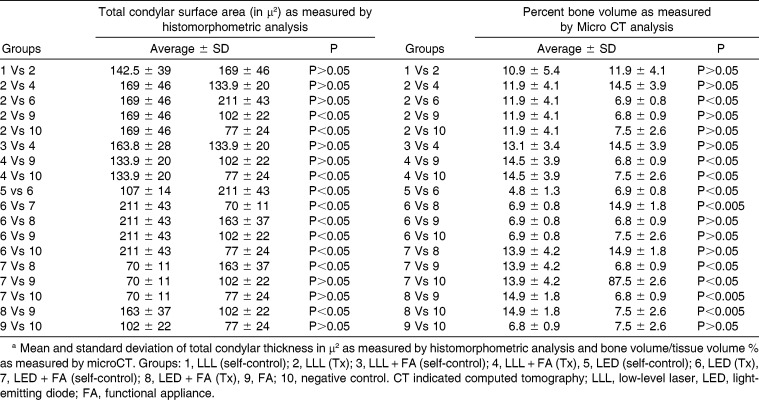
All data are presented as mean ± standard deviation (Table 2) and were analyzed with SPSS version 20.0 software (Chicago, Ill). Independent Student's t-test and one-way analysis of variance with Tukey post hoc test were used for two-group and multiple-group comparisons, respectively. Statistical significance was set at P < .05.
Table 2.
Statistical Comparisons between and among Different Groupsa
RESULTS
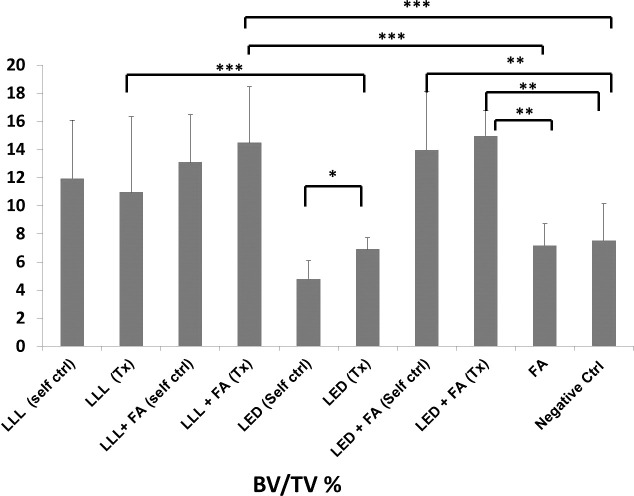
Figure 3 and Table 2 show a comparison of the total surface area of the mandibular cartilage in all groups as measured by histomorphometric analysis in µm2. It can be seen that the LED group showed a significant increase in total condylar cartilage layer compared with all groups. The LLL group also showed a significant increase in condylar cartilage surface area compared with the control or FA groups. The FA did not provide any synergetic effect to either LED or LLL. There were significant differences between treated sides (right side) and self-control sides (left sides) in both the LED and LED + FA treated groups. There was no significant difference between LLL or LLL + FA treatment or self-control groups. Figure 4 and Table 2 show a comparison of microCT analyses between groups. A similar pattern exists as for the significant increase in BV/TV as evaluated by microCT analysis in the LED group compared with the other groups. Also, microCT analyses did not show any synergetic effect between FA or either LED or LLL treatment.
Figure 3.
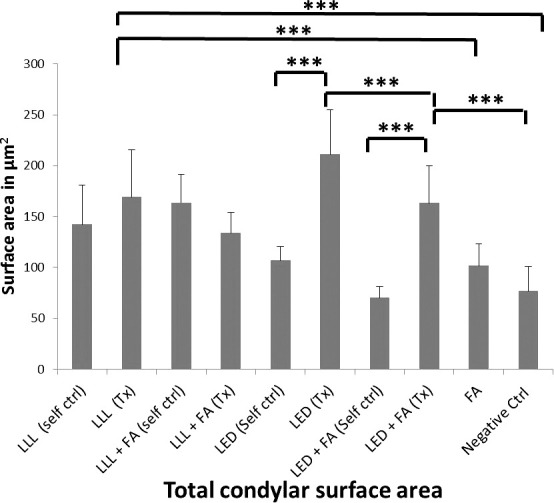
Comparison of the total surface area of the layers in the mandibular condyles in all groups as measured by histomorphometric analysis in µm2. * P < .05; ** P < .01; *** P < .001. It can be seen that LED shows a significant increase in the surface area of the condylar layers.
Figure 4.
Comparison of microCT analyses (BV/TV) between groups. * P < .05; ** P < .01); *** P < .001. It can be seen that the LLL/LED-treated groups when combined with FA showed a statistically significant increase compared with FA or control groups.
DISCUSSION
This study evaluated the possible stimulatory effect of either LED or LLL on mandibular condylar growth with or without FA in rats. Although both LLL and LED have the same wavelength and power output, LED seems to have a greater stimulatory effect on the mandibular condyle compared with LLL or a combination of FA with either LED or LLL. The difference between LLL or LED in stimulatory effect on mandibular growth could be due to the intensity attenuation of LLL while it passes through tissues overlying the mandibular condyle, while LED might have maintained its original power until it reached the mandibular condyles. It has been reported previously that a laser beam scatters through the skin/mucosa, which reduces its energy level to 3% to 6% of its original intensity.18 In comparison, it has been reported that LED irradiation has a low absorption coefficient in hemoglobin and water and, consequently, a high penetration depth in the irradiated tissue.19
Light-mediated photobiomodulation therapy using LLL and/or LED has been shown to stimulate the intracellular production of adenosine triphosphate (ATP).20 The absorption of laser and LED photons by the respiratory chain enzyme cytochrome c oxidase is a response from increasing ATP production.19,20 It has been reported that the difference between LED radiation and LLL radiation is that the latter is a laser with the characteristic of coherency, whereas LED light is not coherent.20 Regardless of the coherent characteristics of LLL compared with LED, LED showed a better stimulatory effect on mandibular growth compared with LLL. The stimulatory effect of LLL or LED on mandibular growth could also be mediated by type I collagen stimulation. A previous study showed that LLL can stimulate type I collagen during tooth movement.21 Although previous studies showed that FAs stimulate mandibular growth through stimulation of different extracellular matrix proteins, including type II collagen and SOX9,22 it seems that FAs and LED or LLL do not have a synergetic effect on similar growth factor or extracellular matrix protein expression. To be confirmed, this assumption requires future studies. The significant differences between treated sides (right side) and self-control sides (left side) in LED- or LLL-treated groups suggest that LED and/or LLL intensities attenuate to a substimulatory level once these irradiations pass through the treated condyles (right condyles), and when they have reached the left side, this is no clinical or possibly cellular stimulation effect. We have confirmed this by measuring light penetration through tissues in our lab, and it has been shown to decline up to 40% from the instant intensity output at 2-mm depth through the tissue from the application surface (data not shown). The possible effects of light on mandibular growth could be due to cellular and subcellular stimulation. It has been previously reported that light stimulates mitochondrial chromophores, photons, proton pumping, and ATP production.23,24 In addition, nitrous oxide (NO) production has been reported to be induced by photon absorption.25–27 Future studies may be needed to investigate such mechanisms in mandibular condylar cells.
CONCLUSIONS
The current study suggests that LED or LLL, when used with presented parameters, have a stimulatory effect on the mandibular surface area, as evaluated by histomorphometric analysis compared with no treatment or FAs.
The current study did not support the hypothesis that a combination of more than one treatment modality (LED, LLL, or FAs) can stimulate mandibular growth more than each treatment modality by itself when evaluated by histomorphomteric analysis. However, microCT evaluation showed an increase in bone volume with LED + FA treatment compared with each treatment modality alone or control groups.
Further studies are needed at the cellular and subcellular level to explore possible different effects of either LED or LLL ± FA on cellular or intracellular signaling that might have led to different histomorphometric and microCT output.
REFERENCES
- 1.Chang ET, Shiao GM. Craniofacial abnormalities in Chinese patients with obstructive and positional sleep apnea. Sleep Med. 2008;9:403–410. doi: 10.1016/j.sleep.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Sunitha C, Kumar SA. Obstructive sleep apnea and its management. Ind J Dent Res. 2010;21:119–124. doi: 10.4103/0970-9290.62806. [DOI] [PubMed] [Google Scholar]
- 3.Rakosi T, Graber TM, Petrovic AC. Dentofacial Orthopedics With Functional Appliances. 2nd ed. St Louis, Mo: Mosby-Year Book; 1997. [Google Scholar]
- 4.Rabie ABM, She TT, Hägg U. Functional appliance therapy accelerates and enhances condylar growth. Am J Orthod Dentofacial Orthop. 2003;123:40–48. doi: 10.1067/mod.2003.45. [DOI] [PubMed] [Google Scholar]
- 5.Lam B, Sam K, Lam JC, Lai AY, Lam CL, Ip MS. The efficacy of oral appliances in the treatment of severe obstructive sleep apnea. Sleep Breath. 2011;15:195–201. doi: 10.1007/s11325-011-0496-y. [DOI] [PubMed] [Google Scholar]
- 6.Tison C, Sébille-Elhage S, Ferri J. Mandibular advancement device: a 5-year long experience in obstructive sleep apnea/hypopnea syndrome. Revue de stomatologie et de chirurgie maxillo-faciale. 2011;112:80–86. doi: 10.1016/j.stomax.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Petrovic A, Stutzmann J, Gasson N. The final length of the mandible: is it genetically predetermined. In: Carlson DS, editor. Craniofacial Biology. Monograph No. 10, Craniofacial Growth Series, Center for Human Growth and Development. Ann Arbor, Mich: University of Michigan; 1981. pp. 105–126. [Google Scholar]
- 8.El-Bialy T, El-Shamy I, Graber TM. Growth modification of the rabbit mandible using therapeutic ultrasound: is it possible to enhance functional appliance results. Angle Orthod. 2003;73:631–639. doi: 10.1043/0003-3219(2003)073<0631:GMOTRM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.El-Bialy T, Hassan A, Albaghdadi T, Fouad HA, Maimani AR. Growth modification of the mandible with ultrasound in baboons: a preliminary report. Am J Orthod Dentofacial Orthop. 2006;130:435.e437–414. doi: 10.1016/j.ajodo.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 10.El-Bialy T, Hassan AH, Alyamani A, Albaghdadi T. Treatment of hemifacial microsomia by therapeutic ultrasound and hybrid functional appliance: a non-surgical approach. Open Access Journal of Clinical Trials. 2010;130:435.e437–414. [Google Scholar]
- 11.Oyonarte R, Zárate M, Rodriguez F. Low-intensity pulsed ultrasound stimulation of condylar growth in rats. Angle Orthod. 2009;79:964–970. doi: 10.2319/080708-414.1. [DOI] [PubMed] [Google Scholar]
- 12.Almeida-Lopes L, Rigau J, Zngaro R, Guidugli-Neto J, Jaeger MM. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med. 2001;29:179–184. doi: 10.1002/lsm.1107. [DOI] [PubMed] [Google Scholar]
- 13.Schultz RJ, Krishnamurthy S, Thelmo W, Rodriguez J, Harvey G. Effects of varying intensities of laser energy on articular cartilage. Lasers Surg Med. 1985;5:577–588. doi: 10.1002/lsm.1900050606. [DOI] [PubMed] [Google Scholar]
- 14.Duan J, Na Y, Liu Y, Zhang Y. Effects of the pulse frequency of low-level laser therapy on the tooth movement speed of rat molars. Photomed Laser Surg. 2012;30:663–667. doi: 10.1089/pho.2012.3220. [DOI] [PubMed] [Google Scholar]
- 15.Ekizer A, Uysal T, Güray E, Akkuş D. Effect of LED-mediated-photobiomodulation therapy on orthodontic tooth movement and root resorption in rats. Lasers Med Sci. In press doi: 10.1007/s10103-013-1405-3. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca PD, de Lima FM, Higashi DT, et al. Effects of light emitting diode (LED) therapy at 940 nm on inflammatory root resorption in rats. Lasers Med Sci. 2013;28:49–55. doi: 10.1007/s10103-012-1061-z. [DOI] [PubMed] [Google Scholar]
- 17.Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int. 1995;4:297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- 18.Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med. 1998;22:97–102. doi: 10.1002/(sici)1096-9101(1998)22:2<97::aid-lsm5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Eells JT, Wong-Riley MT, VerHoeve J, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81:98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Kim YD, Kim SS, Kim SJ, Kwon DW, Jeon ES, Son WS. Low-level laser irradiation facilitates fibronectin and collagen type I turnover during tooth movement in rats. Lasers Med Sci. 2010;25:25–31. doi: 10.1007/s10103-008-0585-8. [DOI] [PubMed] [Google Scholar]
- 22.Rabie AB, She TT, Harley VR. Forward mandibular positioning up-regulates SOX9 and type II collagen expression in the glenoid fossa. J Dent Res. 2003;82:725–730. doi: 10.1177/154405910308200913. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto Y, Arai T, Kikuchi M, Nakajima S, Nakamura H. Effect of low-intensity argon laser irradiation on mitochondrial respiration. Lasers Surg Med. 1992;15:191–199. doi: 10.1002/lsm.1900150207. [DOI] [PubMed] [Google Scholar]
- 24.Eells JT, Wong-Riley MT, VerHoeve J, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 26.Karu TI. Primary secondary mechanisms of action of visible to near–IR radiation on cells. J Photochem Photobiol B. 1998;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 27.Vladimiorv IA, Klebanov GI, Borisenko GG, Osipov AN. Molecular and cellular mechanisms of the low intensity laser radiation effect. Biofizika. 2004;49:339–350. [PubMed] [Google Scholar]