Abstract
Objective:
To test the hypothesis that treatment time, debris/biofilm, and oral pH have an influence on the physical-chemical properties of orthodontic brackets and arch wires.
Materials and Methods:
One hundred twenty metal brackets were evaluated. They were divided into four groups (n = 30) according to treatment time: group C (control) and groups T12, T24, and T36 (brackets recovered after 12, 24, and 36 months of treatment, respectively). Rectangular stainless-steel arch wires that remained in the oral cavity for 12 to 24 months were also analyzed. Dimensional stability, surface morphology, composition of brackets, resistance to sliding of the bracket-wire set, surface roughness of wires, and oral pH were analyzed. One-way analysis of variance, followed by a Tukey multiple comparisons test, was used for statistical analysis (P < .05).
Results:
Carbon and oxygen were shown to be elements that increased expressively and in direct proportion to time, and there was a progressive increase in the coefficient of friction and roughness of wires as a function of time of clinical use after 36 months. Oral pH showed a significant difference between group T36 and its control (P = .014).
Conclusions:
The hypothesis was partially accepted: treatment time and biofilm and debris accumulation in bracket slots were shown to have more influence on the degradation process and frictional force of these devices than did oral pH.
Keywords: Brackets, Orthodontic wires, Properties
INTRODUCTION
The oral environment favors the biodegradation of metal alloys1–3 due to its ionic, thermal, microbiological, and enzymatic conditions. In addition to this, the variations of temperature and pH caused by diet, decomposition of foods, cell debris, oral micro flora, and their by-products are also important factors to be considered when evaluating the clinical behavior of orthodontic components that remain in the oral cavity for months or years.2,3
It is considered3 that the oral medium and time can influence the physical-chemical properties of stainless steel brackets and arch wires, which may act on the clinical performance of these materials and mean an increase in treatment time, as well as alter the color of tooth enamel and lead to caries.4 Therefore, the focus of this in situ study was to test the hypothesis that treatment time, debris/biofilm, and oral pH had an influence on the physical-chemical properties of orthodontic brackets and arch wires.
MATERIALS AND METHODS
The sample was composed of 120 stainless-steel brackets with a slot size of 0.559 × 0.711 mm (0.022 × 0.030-inch; Dental Morelli, São Paulo, Brazil) for premolars and canines in both arches. The brackets were randomly recovered and selected from 45 patients (18–32 years old) with a mean age of 24 years, who concluded orthodontic treatment in time intervals of 12, 24, and 36 months, and 30 pairs of stainless-steel arch wires, distributed as follows: five pairs of stainless-steel arch wires for each cross-section of 0.017 × 0.025-inch, 0.018 × 0.025-inch, and 0.019 × 0.025-inch, (Dental Morelli), which had remained in the oral cavity for 12 and 24 months, respectively.
The inclusion criteria of this study were that the devices were obtained from a private clinic, wires and brackets were of the same brand, and the treatments were performed by a single orthodontist who used the edgewise technique. The exclusion criteria were patients who did not undergo pH measurements in all initial and final times of orthodontic treatment, patients who missed the monthly consultation of maintenance, patients with a diet rich in carbohydrate or sodium, and/or poor oral hygiene.
The brackets were carefully removed with a “pistol” type pair of pliers for the purpose of removing brackets and kept in receptacles with Milli-q deionized water (Millipore, Mass). After this, they were brushed with an electric brush for 10 seconds and rinsed with deionized water to remove any loosely connected integument. After being removed, the wires were cleaned with gauze and 70% alcohol. Afterward, the wires and brackets were kept in sterilized self-sealing packages, until the time of analysis. This study was approved by the Ethics Committee on Human Research, CAAE: 10933512.5.0000.5188.
The brackets were divided into four groups (n = 30 per group): group C (control, without treatment) and groups T12, T24, and T36 (brackets after treatment time of 12, 24, and 36 months, respectively) that were recovered from 15 patients each. Brackets with evident distortions that prevented engagement of the arch wire (0.021 × 0.025-inch) between the wings were discarded. In addition to the brackets recovered, the last stainless-steel retraction wire used in each patient in the last 12 and 24 months was submitted to frictional force and surface roughness tests.
Morphology and Dimensional Stability
Brackets with evident plastic deformations at the base and/or wing were discarded as a result of images in optical microscope (BX60, Olympus Óptica, Tokyo, Japan), at different magnifications (50–200×). After this, 40 brackets (n = 10 per group) were used to evaluate dimensional stability and to take measurements of depth, occlusal, and cervical height of the brackets, by means of optical microscopy (BX60, Olympus). The measurements were taken by a calibrated operator (A.A.; Kappa = 0.88).
Then, orthodontic brackets were randomly selected for analysis by scanning electron microscopy (SEM; JXA 733 Superprobe, Jeol, Tokyo, Japan). A total of 20 randomly selected brackets, with five brackets of each group, were examined. The images obtained by SEM were acquired at various magnifications (20–2000×).
Composition
For this purpose, specimens were vacuum coated with a thin layer of conductive carbon and examined in SEM of energy dispersive x-ray under an electron probe microanalyzer (JXA 733 Superprobe). Secondary electron images and backscattered electron images (BEI) for topography and composition were recorded at 20-kV accelerating voltage.5 The microanalysis was performed in a total of 20 randomly selected brackets, with five brackets of each group evaluating the total area of the brackets (front view image) and three-point surface composition of dark and bright areas, to quantify the elemental composition of the brackets, in the control group (group C) and afterward at the different time intervals of orthodontic treatment (T12, T24, T36).
Frictional Force
To analyze the frictional force between the brackets and stainless-steel arch wires in the control group (group C) and for brackets (n = 10 per group) and arch wires postorthodontic treatment (Groups T12, T24, and T36), a universal test machine (Shimadzu, São Paulo, Brazil) was used with a special device designed for this test, obtained by bonding the brackets with a cyanoacrylate adhesive that WAS standardized 2 to 2 and aligned with a rectangular wire segment of cross section 0.021 × 0.025-inch, with an interbracket distance of 7 mm in each acrylic plate measuring 4 × 15 × 50 mm, with standardization of the run. Thus, the grooves of the brackets remained parallel to the test of the vertical axis of the machine.
Rectangular segments of stainless-steel wires with a length of 11 cm (n = 5 per group) were used. The middle portion of these were joined to the brackets by means of 3-mm elastomeric ligatures of gray color (TP Orthodontics, LaPorte, Ind), immediately before the test, to standardize the joining force, and they were associated with a metal ligature (0.25 mm) loosely inserted into the mesial wing for antirotational effect.
The wire was displaced along the bracket at a rate of 5 mm/min for 1 minute. The levels of force were recorded by a 10-N load cell. The frictional force was calculated from the mean value of forces recorded in millimeters between the first and fifth millimeter of displacement, disregarding the initial static friction, measured in Newton (N).
Surface Roughness
For each group, five wire segments without deformation of 10 mm each were cut and randomly selected from the control and recovered arches, and they were observed by atomic force microscopy (AFM; SPM-9600, Shimadzu, Kyoto, Japan). The samples were fixed to a metal support using adhesive tape. After this, for each wire segment, three surface areas (15 × 15 mm) were randomly selected and analyzed. AFM probes (radius of curvature <10 nm) mounted in consoles (250 µm), operating in contact mode with a silicon nitride tip, were used in a constant of 0.1 N/m. Three-dimensional images (30 × 30 µm) were processed using the software program Gwyddion 3.1 (http://www.gwyddion.net), and the mean arithmetic roughness of the absolute values (Ra) was recorded.
Evaluation of Oral pH
The patients whose oral pH was evaluated were in good periodontal health and did not use medications. The pH was measured at the beginning and end of each orthodontic treatment time: T12, T24, and T36. The patients were instructed to fast for a minimum of 2 hours before saliva collection and to perform tooth brushing in this same period. The saliva was collected for 5 minutes, and then a pH indicator strip of pH 1–14 (J. Prolab, Paraná, Brazil) was put into the collection receptacle. Salivary pH determination with the use of pH indicator strips is made by the colorimetric method, which uses a suitable scale for readout.6 Approximately 10 minutes afterward, the pH at rest was identified by coloring the pH indicator strip. A single evaluator performed the colorimetric method readout (Kappa = 0.8). The pH readouts were always performed under the same conditions of ambient lighting.
Statistical Analysis
The statistical method was chosen based on adherence to the model of normal distribution and equality of variance evaluated by the Kolmogorov-Smirnov and Levene Tests, respectively. The one-way analysis of variance, followed by the Tukey multiple comparisons tests, was used (program SPSS 13.0, Chicago, Ill). For all of the tests, the value of P < .05 was considered statistically significant.
RESULTS
Dimensional Stability
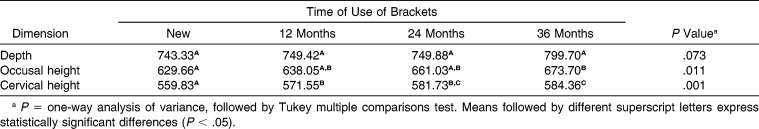
The cervical and occlusal height of the brackets used for 36 months differed significantly from the mean heights of the new devices, and the cervical height also showed significant difference between 12 and 36 months of clinical use (P = .001; Table 1).
Table 1.
Evaluation of Influence of Time on Dimensional Stability (µm) of Brackets
Morphology
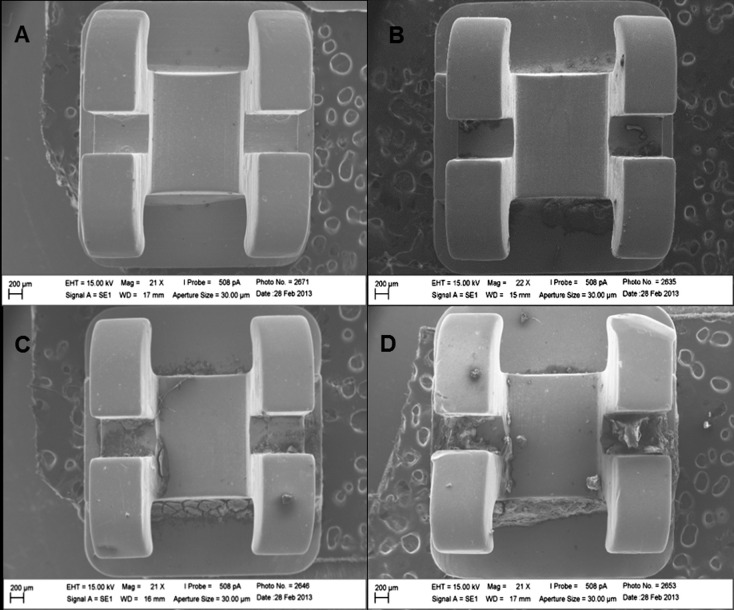
Analysis of the new (Figure 1A) and recovered brackets at T12, T24, and T36 (Figures 1B, 1C, and 1D, respectively) demonstrated that time had a gradual influence on the accumulation of biofilm, debris, and food remainders on the surface and slots of brackets. The presence of grooves and deformations of varying extension could also be observed on the wing surfaces of brackets used for 36 months, even in those recovered from the same patient.
Figure 1.
Images by scanning electron microscopy. (A) Group C (control, new bracket). (B–D) Groups T12, T24, and T36 (brackets recovered after 12, 24, and 36 months of treatment, respectively).
Composition
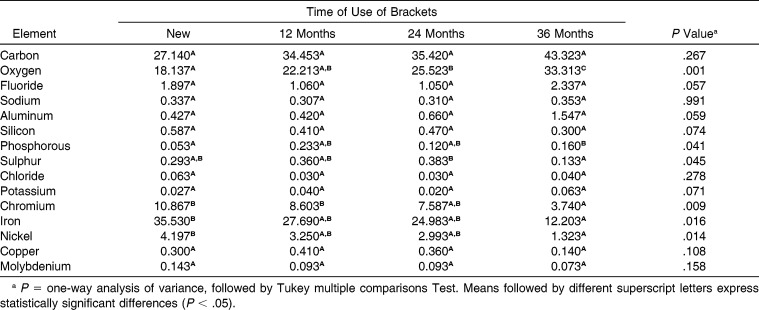
The elemental composition analysis obtained by BEI (Table 2) demonstrated a significance for oxygen between the time of 36 months and all of the other time intervals and between the time interval of 24 months with new brackets (P = .001). The contrary was observed with the elements chromium, iron, and nickel, in which the presence of these was shown to be indirectly proportional to the time.
Table 2.
Influence of Time of Use of Brackets on Their Elemental Composition (Atomic %)
Frictional Force
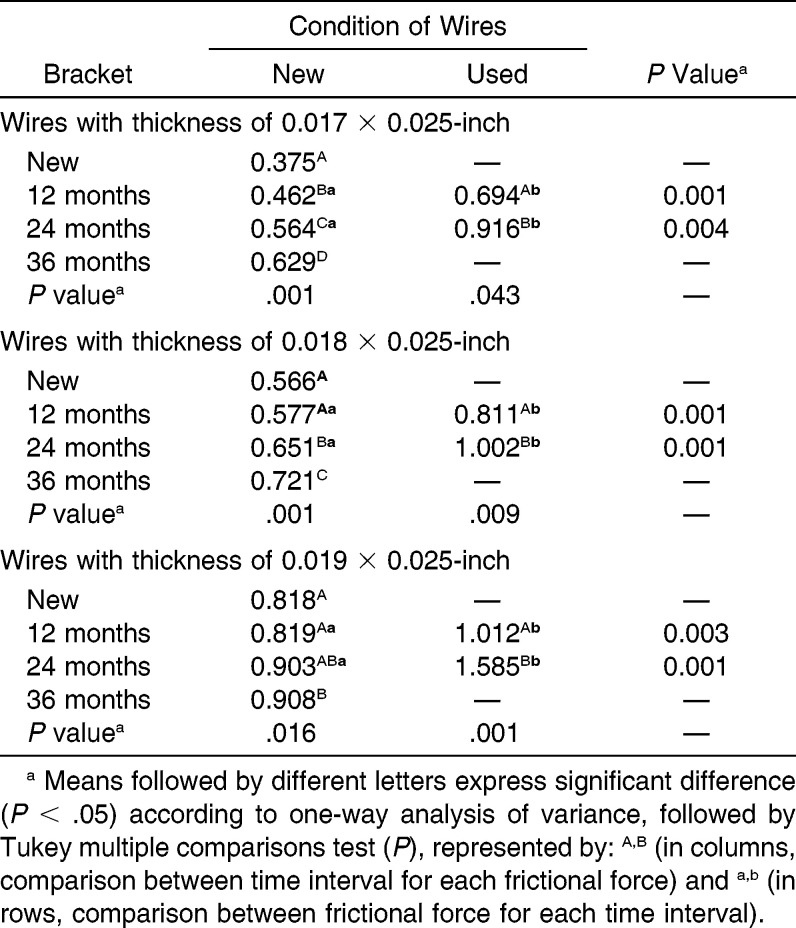
There was a significant difference between the new brackets and those recovered at the time intervals of 12, 24, and 36 months with the new 0.017 × 0.025-inch, and 0.018 × 0.025-inch (P = .001) wires, except between the new brackets, those recovered at a time interval of 12 months associated with new 0.018 × 0.025-inch wires (Table 3). When the new 0.019 × 0.025-inch wire was used, there was a significant difference only between the brackets recovered in the time interval of 36 months and those recovered at 12 months and the new brackets (P = .016).
Table 3.
Influence of Time of Use of Brackets on the Frictional Force (N) That These Exert in Relation to the Orthodontic Wires
Surface Roughness
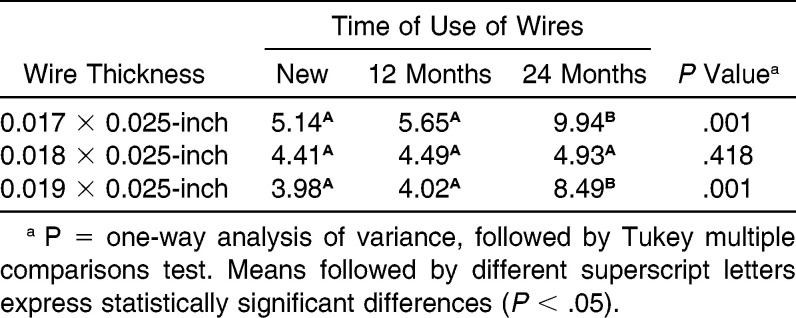
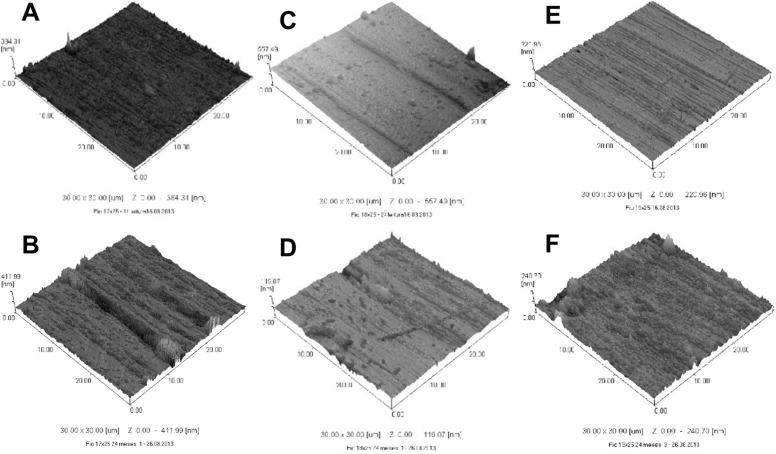
The surface roughness (Table 4) of wires after 24 months of use differed statistically between wires with a thickness of 0.017 × 0.025-inch (Figure 2A,B) and 0.019 × 0.025-inch (Figure 2E,F) for the respective new orthodontic wires (P = .001). The wire thickness of 0.018 × 0.025-inch (Figure 2C,D) demonstrated no significant difference for roughness in the periods evaluated (P = .418).
Table 4.
Influence of Time of Use on Roughness (nm) of Orthodontic Wires
Figure 2.
Images by atomic force microscopy: wire 0.017 × 0.025-inch new (A) and after 24 months (B); wire 0.018 × 0.025-inch new (C) and after 24 months (D); wire 0.019 × 0.025-inch new (E) and after 24 months (F).
Evaluation of Oral pH
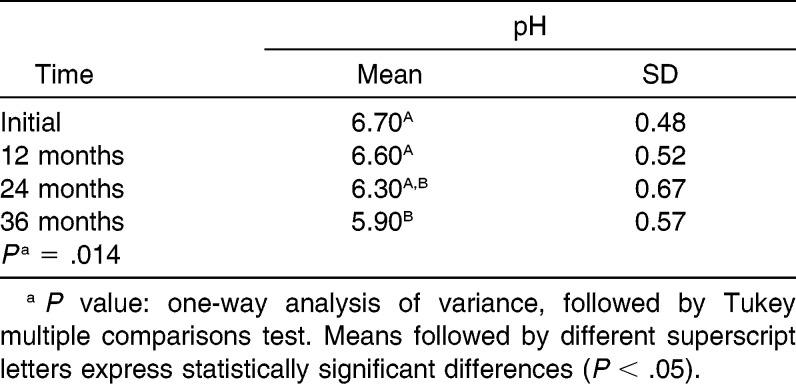
The level of oral pH of patients diminished with the time of use of the orthodontic appliance. There was a statistical difference between the pH measured at 36 months with the pH before treatment and 12 months (P = .014; Table 5).
Table 5.
Evaluation of the Influence of Time on Oral pH
DISCUSSION
In this study, analysis by optical microscopy revealed no significant difference between the depths of brackets, irrespective of their time of use, but the occlusal and cervical heights of brackets used for 36 months differed significantly from the mean heights of new devices. This finding is related to the intraslot wear and/or deformation7,8 due to the dental sliding mechanics, torque, and progressive increase in the thickness of the wires used in the brackets, which is in agreement with the findings of other studies.9,10
The brackets exposed to the oral environment for a longer time of use presented greater signs of pitting corrosion, plastic deformation, gaps, scratches, and deposition of debris.7 The presence of precipitated biofilm found on the recovered brackets is in agreement with the findings of other studies of esthetic8 and metal brackets3 and orthodontic wires.2,11 SEM analysis showed areas of dark surface, which indicated the presence of precipitated biofilm associated with other bright surface areas. Dark areas suggest the presence of elements with low atomic numbers, such as oxygen, consistent with the formation of crystalline particles from biofilm and food debris, and bright surface areas that may be related to silicon, barium, aluminum, iron, and silver.3,12,13 There was also an increased amount of carbon element with time, but this was not significant, as seen in the oxygen element. The presence of silicon and barium may be attributed to contamination by adhesives or orthodontic composites, and inclusions of aluminum are strongly related to corrosion.3,12 The silver cannot be attributed to any clinical discovery, although the transfer of this element may occur through friction between metal surfaces.3
A progressive increase in the element oxygen observed in BEI was found due to the deposition of debris on the surface of brackets, which explains the significant drop in the levels of chromium, iron, and nickel, the main elements responsible for the composition of these devices, corroborating the findings of previous studies.3,14 However, there was a variation in the levels of debris between brackets with the same time of use, which emphasizes the influence of individual variation and personal hygiene methods.7 Although the level of oral pH of patients diminished with the time of use of the orthodontic appliance, it was only after 36 months that there was significant difference. The pH value varied from 6.7 in the beginning to 5.9 at the end of treatment, a reduction below the variation in normality of oral pH in adults (6.2–7.4).15
Variations in pH due to dietary products or the conversion of sugar into acid by dental biofilm determine the limit of saliva capacity to protect teeth, with pH 5.5 being the critical level,6,16 which could also influence orthodontic devices such as corrosion.1,2 In this study, it could be seen that even after 36 months, patients presented a pH above the level considered critical. This suggests that the oral pH was not a determinant factor in the corrosion points of orthodontic brackets and arch wires but can be a potentiating factor in the long term.
When considering the frictional force, the comparison before and after clinical use demonstrated that the frictional force increase was directly proportional to the time of use of brackets as well as orthodontic wire thickness.13 Despite the frictional force of orthodontic appliances in the dry state not corresponding to the actual condition of the oral environment,2 studies3,14 have adopted this methodology as reference. Moreover, the presence of saliva appears to have no significant influence on the reduction of frictional force.17 The samples were analyzed after removal from the oral environment, which may have caused drying of the residues on the orthodontic wire surfaces. However, this drying may also occur during clinical use, when they remain in the oral cavity for months.2
In this study, the increase in frictional force of 0.017 × 0.025-inch wires as a function of time may also be related to some amount of deformation by masticatory forces on this wire, when compared with 0.018 × 0.025-inch and 0.019 × 0.025-inch wires of greater thickness.
In the association between brackets and wires used, there was a significant difference between the brackets recovered after 12 and 14 months, irrespective of the wire thickness used. In addition to wires, the increase in frictional force may also have been generated by the presence of biofilm and dietary debris on the surface and mainly on the bracket slots.3,8 This appears to be an important factor to consider when the force to be applied is calculated.18
The surface roughness of wires is also significantly related to the increase in the levels of frictional force.2,14 The increase in the time of use of wires caused an increase in the levels of surface roughness, irrespective of the orthodontic wire thicknesses. The surface roughness of wires after 24 months of use differed statistically for the wire thicknesses of 0.017 × 0.025-inch and 0.019 × 0.025-inch of the respective new wires. Although the 0.017 × 0.025-inch wire was thinner, it was more predisposed to deformations because of the masticatory forces that could influence the frictional force,14 whereas the 0.019 × 0.025-inch wire presented lower risk of undergoing these deformations, but its thickness allowed little clearance in the bracket-wire relationship, which increases the frictional force. This may explain the fact that the 0.018 × 0.025-inch wire presented more favorable results than the 0.017 × 0.025-inch wire.
CONCLUSIONS
The hypothesis was partially accepted: treatment time and biofilm/debris accumulation in bracket slots were shown to have more influence on the degradation process and frictional force of these devices than oral pH.
The periodic cleaning of the slots of brackets and wire surfaces, added to the reduction in time of clinical use of rectangular wires, may minimize the impact of these alterations on orthodontic treatment.
REFERENCES
- 1.Oh KT, Choo SU, Kim KM, Kim KN. A stainless steel bracket for orthodontic application. Eur J Orthod. 2005;27:237–244. doi: 10.1093/ejo/cji005. [DOI] [PubMed] [Google Scholar]
- 2.Marques ISV, Araújo AM, Gurgel JA, Normando D. Debris, roughness and friction of stainless steel archwires following clinical use. Angle Orthod. 2010;80:521–527. doi: 10.2319/081109-457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regis SJ, Soares P, Camargo ES, Filho OG, Tanaka O, Maruo H. Biodegradation of orthodontic metallic brackets and associated implications for friction. Am J Orthod Dentofacial Orthop. 2011;140:501–509. doi: 10.1016/j.ajodo.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Maia LH, Filho HL, Araújo MV, Ruellas AC, Araújo MT. Incorporation of metal and color alteration of enamel in the presence of orthodontic appliances. Angle Orthod. 2012;82:889–893. doi: 10.2319/092111.599.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliades T, Eliades G, Watts DC. Intraoral aging of the inner headgear component: a potential biocompatibility concern. Am J Orthod Dentofacial Orthop. 2001;119:300–306. doi: 10.1067/mod.2001.111402. [DOI] [PubMed] [Google Scholar]
- 6.Serratine ACP, Silva MRM. Validation of a simplified method for evaluation of salivary pH in children. Pesq Bras Odontoped Clin Integr. 2009;9:217–221. [Google Scholar]
- 7.Eliades T, Bourauel C. Intraoral aging of orthodontic materials: the picture we miss and its clinical relevance. Am J Orthod Dentofacial Orthop. 2005;127:403–412. doi: 10.1016/j.ajodo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Phiton MM, Figueiredo DSF, Oliveira DD, Santos RL. Evaluation of physical properties of esthetic brackets after clinical use: study in situ. J World Fed Orthod. 2013;2:127–132. [Google Scholar]
- 9.Gioka C, Eliades T. Materials-induced variation in the torque expression of preadjusted appliances. Am J Orthod Dentofacial Orthop. 2004;125:323–328. doi: 10.1016/j.ajodo.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama K, Hamada K, Moriyama K, Asaoka K. Degradation and fracture of Ni-Ti superelastic wire in an oral cavity. Biomaterials. 2001;22:257–262. doi: 10.1016/s0142-9612(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 11.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22:317–326. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 12.Gkantidis N, Zinelis S, Karamolegkou M, Eliades T, Topouzelis N. Comparative assessment of clinical performance of esthetic bracket materials. Angle Orthod. 2012;82:691–697. doi: 10.2319/092511-605.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliades T, Gioka C, Zinelis S, Eliades G, Makou M. Plastic brackets: hardness and associated clinical implications. World J Orthod. 2004;5:62–66. [PubMed] [Google Scholar]
- 14.Mendes BAB. Changes in Surface Chemical Composition, Roughness and Friction in Metal Brackets at 12 and 24 Months of Treatment [dissertation] Belo Horizonte, Brazil: Pontifícia Universidade Católica de Minas Gerais; 2012. [Google Scholar]
- 15.Aframian DJ, Davidowitz T, Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12:420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 16.Dawes C. What is the critical pH and why does a tooth dissolve in acid. J Can Dent Assoc. 2003;69:722–724. [PubMed] [Google Scholar]
- 17.Kusy RP, Whitley JQ. Resistance to sliding of orthodontic appliances in the dry and wet states: influence of archwire alloy, interbracket distance, and bracket engagement. J Biomed Mater Res. 2000;52:797–811. doi: 10.1002/1097-4636(20001215)52:4<797::aid-jbm25>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Normando D, Araújo AM, Marques IS, Dias CG, Miguel JA. Archwire cleaning after intraoral ageing the effects on debris, roughness and friction. Eur J Orthod. 2013;35:223–229. doi: 10.1093/ejo/cjr104. [DOI] [PubMed] [Google Scholar]