Abstract
Measles continues to be a major childhood disease in terms of global morbidity and mortality. In the main areas of its endemicity the only available means of diagnosis are based on clinical criteria: the presence of a maculopapular rash and fever accompanied by cough, coryza, and/or conjunctivitis. We have studied 38 clinically diagnosed cases of measles in Khartoum, Sudan, by means of serology, reverse transcriptase PCR (RT-PCR) on throat swabs and virus isolation from lymphocytes. On the basis of serology, 28 patients were diagnosed as having an acute measles virus (MV) infection, while in 10 cases the clinical symptoms proved to have other causes. It was shown that in cases with low serum immunoglobulin M (IgM) levels, an additional measurement of IgG or virus-neutralizing antibodies was necessary to discriminate between patients with an acute MV infection sampled during an early stage of the disease and patients who had experienced an MV infection in the more distant past. The serological laboratory diagnosis was validated by an MV-specific RT-PCR: for all confirmed measles cases tested a fragment of the correct size which hybridized with a third MV-specific primer could be amplified, while all serologically negative cases were also RT-PCR negative. MV could be isolated from 17 out of 23 of the serologically confirmed cases, demonstrating that virus isolation is less reliable as a diagnostic tool than serology or RT-PCR. This study stresses the urgent need for a rapid diagnostic field test for measles.
Measles is a highly infectious respiratory virus infection, with typical clinical symptoms including maculopapular rash, fever, cough, coryza, and conjunctivitis. The causative agent of the disease, measles virus (MV), is a negative-strand RNA virus of the genus Morbillivirus, family Paramyxoviridae (11). Measles continues to be a major cause of childhood morbidity and mortality worldwide, with an estimated one million fatal cases each year (2). Although the introduction of live attenuated MV vaccines has largely abrogated the endemic circulation of wild-type MV in the industrialized world, vaccination has been less successful in large areas of Africa and Asia (4). This is thought to be the combined result of insufficient vaccination coverage due to limited infrastructure and/or political instability and inherent disadvantages of the live attenuated vaccine such as the need for cold chain maintenance and the interference by maternal antibodies (5, 17).
Considering the World Health Organization (WHO) aim to eradicate measles in the beginning of the next century, more insight is required into the epidemiology and immunopathogenesis of measles in areas where the virus remains endemic. During the course of an eradication campaign, the identification of clinical cases becomes increasingly important. At present, measles diagnosis in third-world countries is almost exclusively based on the evaluation of clinical symptoms. However, due to the immunopathological nature of at least part of the typical clinical symptoms of measles, not all patients infected with MV present typical symptoms (11, 12). Furthermore, not every disease which fulfills the clinical criteria for measles is necessarily caused by an infection with MV (8, 15).
The “gold standard” for laboratory diagnosis of an MV infection is the demonstration of specific immunoglobulin M (IgM) (10), either by a capture enzyme-linked immunosorbent assay (ELISA) (7) or by immunofluorescence (6). A rapid, cheap, and accurate test to detect MV-specific IgM antibodies in a field setting is urgently needed but, at present, is not available. Diagnostic measures based on demonstration of the presence of the virus (either by reverse transcriptase PCR [RT-PCR] or by virus isolation) are equally valid, but generally less practicable in a routine setting. However, the widespread application of lymphoblastoid cell lines instead of the traditional Vero cell cultures for the isolation of wild-type MV strains (13) has greatly facilitated MV isolation procedures. As a spin-off, sequence analysis of the increasing pool of MV strains isolated in different parts of the world has proven to be a powerful tool for molecular epidemiological studies, showing the global distribution of different MV genotypes (1, 3). These studies will be of crucial importance during the final stages of the MV eradication program.
Here, we present the serological and virological characterization of a group of 38 clinical measles cases collected in Khartoum, Sudan, by demonstration of MV-protein-specific serum IgM, IgG, and virus-neutralizing (VN) antibody levels, RT-PCR signals in throat swabs, and MV isolation from peripheral blood mononuclear cells (PBMC).
MATERIALS AND METHODS
Patients.
Clinical materials were collected from infants who met the WHO clinical case definition for measles: “any person with a generalized maculopapular rash (i.e., nonvesicular), and a history of fever of 38°C or more, and at least one of the following: cough, coryza (i.e., runny nose), or conjunctivitis (i.e., red eyes); or: any person in whom a health professional suspects measles” (9). The clinical symptoms were always present at the moment of sampling. Samples were collected after having obtained informed consent from the parents. The collection of clinical specimens was an integral part of an ongoing prospective measles study in Khartoum (started in April 1997), which was approved by the medical ethical committee of the University of Khartoum. The samples collected during the first 6 months of the integral study period are presented here.
Study area.
Most of the patients (n = 30) were sampled through a network which was set up in the residential area Haj Yousif for finding cases of measles. This area of Khartoum has an estimated 500,000 inhabitants, mainly comprised of displaced people from the south and west of Sudan. Health care in the area is provided through volunteer health centers, often by staff members with limited clinical backgrounds. Measles vaccination coverage is low, and endemic MV transmission occurs throughout the year. The cases included in this study did not present as an outbreak, but were spread over the 6-month period. The number of cases observed during this period was not substantially different from that observed in any other period of the year or from any other year between 1995 and the present. The number of reported patients that could not be sampled was less than half of the number of patients included in the study. Eight additional patients were sampled in pediatric hospitals in Khartoum.
Samples.
Clinical specimens collected consisted of a throat swab and a heparinized blood sample (approximately 3 ml). PBMC were isolated by density gradient centrifugation in Khartoum and were frozen in RPMI medium supplemented with 40% fetal bovine serum (FBS) and 10% dimethyl sulfoxide in liquid nitrogen. Plasma and throat swabs were frozen at −70°C.
Serology.
Plasma levels of IgM or IgG specific for the two MV transmembrane glycoproteins, the fusion protein (F) and hemagglutinin (H), were determined by an immunofluorescence assay by using transfected human melanoma cell lines as targets, as previously described (6). Briefly, melanoma cells expressing either the F protein (Mel-JuSo/MV-F) or the H protein (Mel-JuSo/MV-H) or the untransfected parental cell line (Mel-JuSo/wt) were incubated with diluted plasma samples. The samples were prediluted 1:10 in phosphate-buffered saline supplemented with 2% FBS (for measurement of MV-specific IgG) or in GullSorb reagent (Gull Laboratories, Salt Lake City, Utah) to precipitate all plasma IgG for measurement of MV-specific IgM. Subsequently, the samples were diluted 1:10 in phosphate-buffered saline supplemented with 2% FBS to reach a final dilution of 1:100. After 1 h on ice, the cells were washed and stained with fluorescein isothiocyanate-labeled rabbit anti-human IgM or IgG [F(ab′)2 fragments; DAKO, Glostrup, Denmark]. Results are expressed as the fluorescence signal (histogram peak channel) measured on a FACScan (Becton-Dickinson, Mountainview, Calif.) in arbitrary fluorescence units (AFU). Fluorescence signals measured on the untransfected cell line were always below 10 AFU (data not shown).
Plasma levels of IgM specific to the nucleoprotein (N) were measured in a capture ELISA by using peroxidase-labeled purified baculovirus-expressed N (N-PO). Plasma samples were diluted 1:100 in ELISA buffer (Meddens Diagnostics, Brummen, The Netherlands) and were incubated on ELISA plates (Greiner, Alphen a/d Rijn, The Netherlands) coated with rabbit anti-human IgM (Meddens Diagnostics). After 1 h at 37°C, plates were washed in water containing 0.05% Tween-80 and were subsequently incubated with N-PO. Following an additional 1 h incubation at 37°C, the plates were washed again and were subsequently colored using tetramethylbenzidine as a substrate. Extinctions were read in an ELISA reader at 450 nm.
Plasma levels of VN antibodies were measured as previously described (14) with minor modifications. Briefly, serial twofold dilutions of the plasma samples were tested for their ability to neutralize 60 50% tissue culture infective doses of the MV Edmonston strain. Plasma dilutions were prepared in Dulbecco's modified Eagle medium (BioWhittaker, Verviers, Belgium) supplemented with 2% FBS, of which 50 μl was incubated with 50 μl of the virus working dilution in 96-well flat bottom plates (Greiner). After 1 h at 37°C (neutralization phase), Vero cells were added (104 cells in 50 μl per well). Cells were microscopically monitored for cytopathic effects (CPE) during the following week. For each plasma sample, eight dilutions (1:32 to 1:4,096, dilution during neutralization phase) were tested in triplicate. The results are shown as the dilution at which 50% of the cultures was neutralized, calculated as previously described (18), standardized to the WHO international standard (0.2 IU/ml), which was found to have a 50% VN titer of 54.
Virus isolation.
MV was isolated from PBMC by an infectious center assay as previously described (20). PBMC (3.2 × 105) were divided over 8 wells of a 96-well round-bottom plate (Greiner) and stimulated with phytohemagglutinin (Boehringer GmbH, Mannheim, Germany) for 1 h at 37°C, after which twofold serial dilutions were prepared in RPMI medium supplemented with 10% FBS (each dilution range was prepared eight times). Subsequently, a standard amount (5 × 103 per well) of a human Epstein-Barr virus-transformed B-lymphoblastic cell line previously established from a healthy volunteer (GR) was added to each well. In the case of positive virus isolations, CPE were usually observed 2 to 4 days after culture at 37°C. The level of viremia (i.e., the number of MV-infected cells per 106 PBMC) was determined by calculating the number of PBMC per well resulting in 50% of the cultures showing CPE (18) and is presented as the number of MV-infected cells per 106 PBMC.
In the case of a positive MV isolation, as determined by the observation of typical MV-related CPE, the supernatant of one to three wells showing CPE at the upper range of the serial dilutions was harvested and cocultivated with approximately 5 × 106 cells of B-lymphoblastic cell line GR in a 25-cm2-volume culture flask. When these cells showed CPE 2 to 3 days later, cell-free supernatant was harvested and aliquots were frozen at −70°C.
RT-PCR.
The presence of MV genomic RNA in throat swab samples was determined by RT-PCR by using a forward primer in the N gene and a reverse primer in the region between the N gene and the P-C-V gene. Briefly, RNA was isolated from 200-μl of throat swab material by using the High Pure Viral RNA kit (Roche Diagnostics, Almere, The Netherlands) and was analyzed by RT-PCR by using random hexanucleotides for first-strand synthesis. Primers used for amplification were as follows: forward 5′-TTAGGGCAAGAGATGGTAAGG-3′ (MV-N1, position 1090-1110) (19) and reverse, 5′-TTATAACAATGATGGAGGG-3′ (MV-N2, position 1615-1633). PCR products were separated on a 2% agarose gel and blotted onto Hybond N+ membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). Hybridization was performed by using a 32P-labeled oligo probe (5′-GCCATGGCAGGAATCTCGGAA-3′ [MV-prN2, position 1498-1518]).
RESULTS
Laboratory diagnosis by serology.
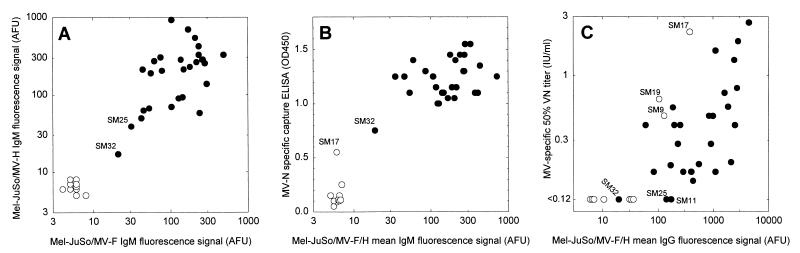
Blood samples were collected from 38 clinically diagnosed measles patients (age range, 5 months to 14 years) within six days after onset of the rash (Table 1). Immunofluorescence with MV-F and MV-H transfected cell lines demonstrated the absence of MV glycoprotein-specific IgM in the plasma of 10 of these patients (Fig. 1A). In one patient, SM32, the result of the assay was indeterminate based on previously established cutoffs (signal, <30 AFU), while the other 27 patients all demonstrated the presence of both MV-F- and MV-H-specific plasma IgM (Fig. 1A). The MV-F- and MV-H-specific immunofluorescence signals showed a good correlation (linear regression analysis, r2 = 0.82). We subsequently compared the mean MV glycoprotein-specific IgM response with the MV N-specific IgM response (Fig. 1B). Of the 10 patients who were IgM negative in the glycoprotein-specific assays, one patient (SM17) gave a low positive result in the N-specific-IgM capture ELISA, while the other nine were negative in this assay. The 27 patients who were glycoprotein-specific-IgM positive and the indeterminate patient (SM32) were also N-specific-IgM positive, although SM32 showed an N-specific signal which was intermediate between SM17 and the 27 high positives.
TABLE 1.
Summary of patient baseline data and results of laboratory assays
| Case designationa | Genderb | Age (mo) | Rash (days after onset) | IgMc (plasma) | RT-PCRd (throat) | MV isolation (PBMC) | No. of infected cells (per 106 PBMC)e |
|---|---|---|---|---|---|---|---|
| SM4 | F | 16 | 1 | + | NDf | − | <3 |
| SM5 | M | 12 | 1 | + | + | ND | ND |
| SM8 | F | 42 | 3 | + | + | ND | ND |
| SM11 | M | 8 | 2 | + | ND | + | 6 |
| SM14 | F | 24 | 2 | + | ND | − | <3 |
| SM16 | M | 18 | 2 | + | ND | − | <3 |
| SM23 | F | 48 | 5 | + | + | + | 6 |
| SM24 | F | 14 | 5 | + | + | + | 10 |
| SM25 | F | 48 | 3 | + | + | + | 925 |
| SM26 | F | 20 | 3 | + | + | + | 50 |
| SM27 | M | 23 | 3 | + | + | + | 3 |
| SM28 | F | 15 | 2 | + | + | + | 350 |
| SM29 | M | 168 | 4 | + | ND | + | 150 |
| SM30 | M | 48 | 3 | + | + | ND | ND |
| SM31 | F | 120 | 4 | + | + | + | 3 |
| SM32 | M | 14 | 2 | +/− | + | + | 9,700 |
| SM33 | F | 96 | 2 | + | + | ND | ND |
| SM34 | M | 132 | 4 | + | + | + | 80 |
| SM37 | M | 7 | 4 | + | + | + | 925 |
| SM38 | M | 11 | 4 | + | ND | − | <3 |
| SM39 | M | 10 | 6 | + | + | − | <3 |
| SM40 | F | 24 | 2 | + | + | + | 200 |
| SM41 | M | 36 | 2 | + | + | ND | ND |
| SM42 | F | 12 | 4 | + | + | + | 525 |
| SM43 | F | 5 | 3 | + | + | − | <3 |
| SM44 | F | 8 | 3 | + | ND | + | 100 |
| SM45 | F | 10 | 3 | + | ND | + | 300 |
| SM46 | F | 23 | 3 | + | + | + | 3 |
| SM1 | F | 48 | 3 | − | − | ND | ND |
| SM2 | F | 48 | 2 | − | − | ND | ND |
| SM3 | M | 54 | 3 | − | − | ND | ND |
| SM9 | M | 15 | 1 | − | − | − | <3 |
| SM17 | M | 42 | 3 | +/− | − | − | <3 |
| SM18 | M | 18 | 4 | − | − | − | <3 |
| SM19 | M | 36 | 2 | − | − | − | <3 |
| SM20 | M | 11 | 3 | − | − | − | <3 |
| SM21 | M | 20 | 4 | − | − | − | <3 |
| SM22 | M | 33 | 4 | − | − | ND | ND |
Laboratory-confirmed MV cases (see results section) are listed in the upper part of the table, and serologically confirmed MV-negative cases are listed in the lower part.
F, female; M, male.
As shown in Fig. 1.
Positive if fragment of correct length which hybridized with the specific probe was amplified.
Determined by infectious center test as described in Materials and Methods.
ND, not done.
FIG. 1.
Relationships between MV-F- and MV-H-specific IgM levels (A), MV-glycoprotein-specific IgM and MV-N-specific IgM levels (B), and MV-glycoprotein-specific IgG and VN antibody levels (C). Laboratory-confirmed MV cases (see Results section) are shown as black symbols, while nonmeasles rash disease cases are shown as open symbols.
On the basis of these MV IgM assays, 36 out of 38 cases could be diagnosed as being either acute MV infections (n = 27) or nonmeasles rash diseases (n = 9). In order to serologically diagnose the two low-positive IgM patients, additional IgG and VN assays were carried out. As shown in Fig. 1C, SM17 had high serum levels of glycoprotein-specific IgG and VN antibodies while SM32 was glycoprotein-specific IgG and VN negative, suggesting that the first was a nonmeasles rash disease patient while the second was sampled at an early stage of an MV infection. Of the other nine MV-IgM-negative cases, two (SM9 and SM19) contained high levels of MV-specific IgG, three contained low levels of MV-specific IgG, and four were completely IgG negative (Fig. 1C).
Laboratory diagnosis by RT-PCR and virus isolation.
In addition to serology, MV-specific RT-PCR on throat swab material and MV isolation from PBMC were carried out with the clinical specimens available. As shown in Table 1, 20 out of 20 serologically confirmed MV cases tested (including SM32) were RT-PCR positive, while all 10 serologically confirmed MV-negative cases (including SM17) were RT-PCR negative. MV could be isolated from PBMC of 17 out of 23 serologically confirmed MV cases (including SM32) but from none of six serologically confirmed MV-negative cases (including SM17, see Table 1). Levels of infected cells were as high as about 10,000 infected cells per 106 PBMC (see Table 1), with the highest level in patient SM32. MV isolation from swab material was far less successful than from PBMC: MV could be isolated from only three of the 20 serologically confirmed cases tested.
DISCUSSION
In the present paper, we have serologically and virologically analyzed 38 clinically diagnosed measles cases in Khartoum, Sudan. Measurement of MV-specific IgM, IgG, and VN antibodies, as well as RT-PCR on throat swab samples and MV isolation from PBMC, demonstrated that in 28 of the 38 cases (74%) the disease was indeed caused by an acute MV infection, but in 10 patients (26%) the disease had another cause.
Misdiagnosis of measles on clinical grounds has often been reported (8, 15, 16). The diagnosis of measles can be difficult, even for experienced practitioners, especially in individuals with a pigmented skin. A number of different infectious agents, including Parvovirus B19, human herpesvirus type 6, Dengue virus, Epstein-Barr virus, Mycoplasma pneumoniae, and Rickettsia conorii, are known to cause symptoms that can easily be confused with measles and are also known to be endemic in this part of Africa (15). In the framework of the envisaged MV eradication program, rapid and reliable assays to diagnose these infections will be crucial.
Measurement of MV-specific IgM antibodies proved to be sufficient to diagnose 36 of the 38 clinically diagnosed measles cases. We measured the IgM antibody response to the three major immunogenic MV proteins: the transmembrane glycoproteins F and H and the internal protein N. For routine diagnostic purposes, a capture ELISA such as the one presented here based on peroxidase-labeled N would be the first choice. In case of low positive specific IgM signals, there are three theoretical possibilities: the patient is in an early stage of an MV infection (with a nascent IgM response), the patient had an MV infection some months ago and is now suffering from a nonmeasles rash disease, or the patient was previously vaccinated and is undergoing a secondary immune response associated with a transient low-level IgM response. The latter category should be discriminated from the first two on the basis of the clinical signs: if the patient has a normal measles rash accompanied with conjunctivitis, the patient is most probably undergoing a primary immune response. Since this was the case for SM17 and SM32, we tried to discriminate between the first two possibilities by measuring MV-specific IgG and VN antibody levels. During the early phase of an MV-specific immune response, specific IgM antibodies appear before or at the same time as specific IgG antibodies. When the levels of IgM antibodies start to decrease a few weeks later, the levels of specific IgG and VN antibodies have reached a plateau value (6, 11). SM17 could be identified as a case of nonmeasles rash disease, since the low MV-specific IgM antibody levels were accompanied by high IgG and VN antibody levels. In contrast, SM32 proved to be a laboratory-confirmed MV infection, since the low levels of MV-specific IgM antibodies were accompanied by undetectable IgG and VN levels. This serological diagnosis was confirmed by RT-PCR and MV isolation: SM17 was negative while SM32 was positive in both assays. Furthermore, assessment of the level of viremia further confirmed that patient SM32 was in an early stage of infection, as this patient had the highest level of infected cells.
To our knowledge, the use of an RT-PCR for the diagnosis of normal measles cases has not been described previously. In our study, an RT-PCR using throat swab materials proved to completely correlate with the serological diagnosis of MV. The RT-PCR described may be used in serologically doubtful cases. However, it will especially be of value in cases of suspected MV infections in immunocompromised individuals in which clinical and serological diagnosis is often impossible. MV isolation from PBMC proved less reliable as a diagnostic tool, since MV could not be isolated from six out of 23 (26%) serologically confirmed MV cases. We hypothesize that in most of these cases this was due to practical problems related to suboptimal sampling, handling, and/or freeze-thawing procedures. Isolation and storage of PBMC in an African setting is often difficult to organize, as the required freezing facilities (−135°C or lower) are rarely available. The success rate of virus isolation from PBMC was significantly higher than the success rate of virus isolation from swab material: in 85% of the serologically confirmed MV cases no MV could be isolated from swabs. This included some of the cases in which the presence of MV genomic RNA was demonstrated by RT-PCR. Apparently, the capacity of viable PBMC to produce new virus particles, especially following phytohemagglutinin stimulation, strongly favors this method of MV isolation. An alternative and generally easy to obtain clinical source for the isolation of MV would be urine. However, this was not evaluated in the present study.
In conclusion, our study has shown that a substantial percentage (26%) of the measles cases identified on the basis of the symptoms specified in the WHO clinical case definition (9) were misdiagnosed. Serological methods were sufficient for a laboratory diagnosis of MV infection: measurement of MV-specific IgM alone could diagnose 95% of the patients, while the remainder could be diagnosed with an additional IgG or VN assay. RT-PCR on throat swab material proved to be an equally valid diagnostic assay, which is, however, less practicable in a routine setting. In combination with virus isolation it does, however, provide the tools for phylogenetic analyses, allowing molecular epidemiological studies which will be of crucial importance in the end stages of the envisaged MV eradication program.
ACKNOWLEDGMENTS
This work was supported by INCO-DC grant IC18CT96-0116 from the European Commission.
We thank all the children who participated in the study and their parents.
REFERENCES
- 1.Anonymous. Expanded programme on immunization (EPI). Standardization of the nomenclature for describing the genetic characteristics of wild-type measles viruses. Wkly Epidemiol Rec. 1998;73:265–272. [PubMed] [Google Scholar]
- 2.Anonymous. Measles: progress towards global control and regional elimination, 1990–1998. Wkly Epidemiol Rec. 1998;73:389–394. [PubMed] [Google Scholar]
- 3.Bellini W J, Rota P A. Genetic diversity of wild-type measles viruses: implications for global measles elimination programs. Emerg Infect Dis. 1998;4:29–35. doi: 10.3201/eid0401.980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements C J, Cutts F T. The epidemiology of measles: thirty years of vaccination. Curr Top Microbiol Immunol. 1995;191:13–33. doi: 10.1007/978-3-642-78621-1_2. [DOI] [PubMed] [Google Scholar]
- 5.Cutts F T, Markowitz L E. Successes and failures in measles control. J Infect Dis. 1994;170(Suppl. 1):S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 6.De Swart R L, Vos H W, UytdeHaag F G C M, Osterhaus A D M E, Van Binnendijk R S. Measles virus fusion protein- and hemagglutinin-transfected cell lines are a sensitive tool for the detection of specific antibodies in a FACS-measured immunofluorescence assay. J Virol Methods. 1998;71:35–44. doi: 10.1016/s0166-0934(97)00188-2. [DOI] [PubMed] [Google Scholar]
- 7.Erdman D D, Heath J L, Watson J C, Markowitz L E, Bellini W J. Immunoglobulin M antibody response to measles virus following primary and secondary vaccination and natural virus infection. J Med Virol. 1993;41:44–48. doi: 10.1002/jmv.1890410110. [DOI] [PubMed] [Google Scholar]
- 8.Ferson M J, Young L C, Robertson R W, Whybin L R. Difficulties in clinical diagnosis of measles: proposal for modified clinical case definition. Med J Aust. 1995;163:364–366. doi: 10.5694/j.1326-5377.1995.tb124630.x. [DOI] [PubMed] [Google Scholar]
- 9.Global Programme for Vaccines and immunization/Expanded Programme on Immunization. Using surveillance data and outbreak investigations to strengthen measles immunization programmes (WHO/EPI/GEN/96.02). Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 10.Grandien M, Osterhaus A D M E, Rota P A, Smaron M F, Wild T F. Laboratory diagnosis of measles infection and monitoring of measles immunization: memorandum from a WHO meeting. Bull W H O. 1994;72:207–211. [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1267–1312. [Google Scholar]
- 12.Helfand R F, Kim D K, Gary H E J, Edwards G L, Bisson G P, Papania M J, Heath J L, Schaff D L, Bellini W J, Redd S C, Anderson L J. Nonclassic measles infections in an immune population exposed to measles during a college bus trip. J Med Virol. 1998;56:337–341. doi: 10.1002/(sici)1096-9071(199812)56:4<337::aid-jmv9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norrby E, Gollmar Y. Appearance and persistence of antibodies against different virus components after regular measles infections. Infect Immun. 1972;6:240–247. doi: 10.1128/iai.6.3.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nur Y A, Groen J, Yusuf M A, Osterhaus A D M E. IgM antibodies in hospitalized children with febrile illness during an inter-epidemic period of measles, in Somalia. J Clin Virol. 1999;12:21–25. doi: 10.1016/s1386-6532(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 16.Oshitani H, Suzuki H, Mpabalwani M, Mizuta K, Kasolo F C, Luo N P, Numazaki Y. Laboratory diagnosis of acute measles infections in hospitalized children in Zambia. Trop Med Int Health. 1997;2:612–616. doi: 10.1046/j.1365-3156.1997.d01-346.x. [DOI] [PubMed] [Google Scholar]
- 17.Osterhaus A D M E, De Vries P, Van Binnendijk R S. Measles vaccines: novel generations and new strategies. J Infect Dis. 1994;170(Suppl. 1):S42–S55. doi: 10.1093/infdis/170.supplement_1.s42. [DOI] [PubMed] [Google Scholar]
- 18.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hygiene. 1938;27:493–497. [Google Scholar]
- 19.Taylor M J, Godfrey E, Baczko K, Ter Meulen V, Wild T F, Rima B K. Identification of several different lineages of measles virus. J Gen Virol. 1991;72:83–88. doi: 10.1099/0022-1317-72-1-83. [DOI] [PubMed] [Google Scholar]
- 20.Van Binnendijk R S, van der Heijden R W J, Van Amerongen G, UytdeHaag F G C M, Osterhaus A D M E. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J Infect Dis. 1994;170:443–448. doi: 10.1093/infdis/170.2.443. [DOI] [PubMed] [Google Scholar]