Abstract
Women are disproportionately at risk of acquiring HIV in East and Southern Africa, despite global declines in incidence. Female-initiated HIV prevention methods, like the dapivirine vaginal ring, are needed to end the HIV epidemic. In-depth interviews and focus groups retrospectively explored peer influence on acceptability of and adherence to the ring during the ASPIRE trial, a phase III placebo-controlled trial. Results were analyzed using an inductive analytic approach. Study participants (peers) of all ages and adherence groups developed important interpersonal connections and reported being more open and honest with each other than with external peers or study staff. Study peers who knew each other prior to joining appeared to have a stronger influence on each other’s adherence than peers who met in the study. External peers provided primarily negative input about the ring and study, which sometimes led to ring removals. Peers’ influence on each other’s behavior in both prosocial and detrimental manners could have repercussions on adherence to a biomedical intervention, and consequently, individual disease risk and clinical trial outcomes. Future ring demonstration and implementation studies could use peer networks to intentionally influence uptake and adherence to the ring.
Keywords: Peer influence, HIV prevention, microbicides, sub-Saharan Africa, dapivirine ring
Introduction
Women, particularly young women, continue to be disproportionately at risk of acquiring HIV in East and Southern Africa, despite global declines in incidence1. Current proven methods of HIV prevention such as male and female condoms, while effective, have made a limited impact on the prevention of HIV amongst women in the region for multiple reasons including access and stigma. Male condoms require that men are willing and able to use them, which can pose challenges to some relationships, including the threat of social harms or violence2. Oral pre-exposure prophylaxis (PrEP) can be highly effective amongst women3, though as shown in two large clinical trials from Eastern and Southern- Africa and in PrEP demonstration projects, uptake and adherence has been challenging4–6. Some reasons for low adherence of oral PrEP include male partner disapproval, HIV-related stigma, and the need for daily dosing2,7–11. To successfully end the HIV epidemic, the field must offer HIV prevention method options, particularly those that are female-initiated, and longer-acting3,12–16. The dapivirine vaginal ring is one such new prevention technology that is currently under regulatory review17. The dapivirine vaginal ring offers women a product that can be used discreetly, requires only a monthly change, and has been shown to reduce the risk of HIV acquisition in clinical trials18–20.
To optimize the public health impact of novel prevention methods like the dapivirine vaginal ring, it is imperative to understand influences that may deter and/or encourage their acceptability and use. Findings from the qualitative component of one of the Phase III clinical trials for the dapivirine vaginal ring, Microbicide Trials Network (MTN)-020/ASPIRE, found that despite initial fears about the ring’s appearance and side effects, women ultimately liked it and overcame challenges after experience using it, and with staff and peer support21. Broadly, ASPIRE participants reported barriers to use including social influences (e.g. male partners, community rumors and misconceptions), and attitudes and practices (e.g. hygiene and menstrual related worries, adverse health concerns). The most consistently described influence on acceptability of the ring was male partner relationship dynamics21. Reasons for non-adherence to the ring during ASPIRE were further explored in the primary analysis of a qualitative sub-study, MTN-032/Assessment of ASPIRE and HOPE Adherence (AHA) study, which took place with a subset of former ASPIRE participants after they exited from the ASPIRE trial22. AHA participants generally described their ring use during ASPIRE as consistent, and simultaneously described challenges and reasons for nonuse. The most salient reasons for ring removals and nonadherence to the ring were nondisclosure and disapproval of ring use by male partners, hygiene-related worries (particularly regarding menses), and adverse health concerns22.
Peers are known to influence a wide range of health behavior and health related decision-making, especially during adolescence23–25. For HIV prevention, peers have been shown to have influence over practices such as HIV testing26 and condom use27. Qualitative research with former participants from MTN-003/VOICE, a safety and effectiveness trial of oral tenofovir and Truvada™ tablets and vaginal tenofovir gel, reported that peers and community members exerted negative influence on trial participants regarding research and investigational product use9,10. As noted above, both the primary analyses of the ASPIRE qualitative component and AHA, noted briefly that participants described reliance on information and support from peers as an important influence on their own attitudes and behavior toward the dapivirine vaginal ring21,22. Additionally, a secondary analysis from the ASPIRE qualitative component found that negative rumors about the ring (e.g. that it causes severe health outcomes, or is an object of witchcraft or Satanism) came from women who tried to enroll in ASPIRE but were screened out, enrolled study participants, and the community at large (including peers of study participants). These rumors had a negative effect on women’s decisions to join the trial, women’s adherence to the ring, and sometimes resulted in a lack of support from male partners, family and the community28. During ASPIRE, a range of group activities were implemented to help mitigate the negative social influence of these influencers on ring adherence21,22,28. Building upon these findings, the aim of this secondary analysis using AHA data was to further explore the nuances of peer influences on acceptability of and adherence to the ring during the ASPIRE trial, with the goal of informing future interventions or ring demonstration projects. To note, given that male partners’ and community rumors in particular have been explored in depth in previous publications about the dapivirine ring, they will not be explored in this analysis22, 28–32.
Methods
ASPIRE, the parent study, was a phase III, randomized, double-blind, placebo control trial that enrolled 2629 women at 15 sites in Malawi, South Africa, Uganda and Zimbabwe to either active 25mg dapivirine or placebo vaginal ring (1:1 ratio)18,21,33. ASPIRE took place between August 2012 and June 2015. Starting in March 2013, participant engagement activities (e.g. adherence support meetings, tea parties, movie days or other social events) and group adherence feedback sessions about aggregate adherence at each site compared to other research sites were implemented across all sites. The purpose of the activities was to encourage product adherence and study retention through inspiring a sense of team responsibility and camaraderie amongst participants, and to facilitate social interactions and build rapport between staff and participants21,34. Key inclusion criteria for ASPIRE included being 18–45 years old at time of screening, HIV-negative, sexually active, and using an effective method of contraception33.
AHA was an exploratory sub-study of the ASPIRE trial in which qualitative in-depth interviews (IDI) and focus group discussions (FGD) were conducted with a sub-set of exited ASPIRE participants, primarily to explore factors that affected participants’ adherence to the dapivirine vaginal ring. AHA took place at 7 of the 15 ASPIRE sites, selected by MTN management for ability and prior experience conducting qualitative studies and to represent all four ASPIRE study countries (Malawi, South Africa, Uganda and Zimbabwe). This analysis includes data that was collected from June 2016 through October 2016, 12 to 17 months after ASPIRE trial exit and prior to enrolment into the open-label extension study, HOPE. All AHA participants from all AHA sites were included in the sample for this analysis. Younger participants (those aged 18–21 at the time of ASPIRE enrollment) were intentionally over-sampled in AHA to explore the finding from the ASPIRE trial that the ring provided less protection among the younger women, likely as a result of lower adherence18. Focus group enrollment was stratified by age, where each site held one FGD with younger women and one with older women (age 22–45 at ASPIRE enrollment).
Participants were eligible for recruitment if they had provided written permission to be contacted for future research when they exited ASPIRE and had been on product for a minimum of three consecutive months, or one month if seroconverted. Additionally, since participants discussed their objective biomarker data during interviews, eligibility criteria included only those who had been randomized to the active dapivirine ring arm during ASPIRE, and who had available drug detection data through plasma and/or returned rings. Participants meeting these criteria were recruited in sequential order by each site from a randomly selected list of potential participants generated by the ASPIRE Data Coordinating Center until target numbers were met.
Semi-structured interview guides were developed in English and translated into the local African languages (isiXhosa, isiZulu, Luganda, Shona, and Chichewa). The guides were developed by the qualitative management team with input from site team members. Revisions were made based on site feedback, and role plays were conducted to pilot the guide and assess language and flow. To verify accuracy of the translations, the guides were back-translated into English by a second translator, reviewed by the qualitative data center team at RTI International, and by local institutional review boards (IRB) at each site. The interviewers were hired with discretion from the qualitative teams at each respective site, ensuring they had proper experience in conducting qualitative interviews, were comfortable in the languages spoken by study participants, and with efforts made to ensure they had not served on any role during ASPIRE (e.g. counseling) that would compromise their ability to be impartial. All interviewers attended study specific training by the qualitative management team, which included training on the study interview guides. All interviewers were also required to conduct mock IDIs and FGDs prior to study activation. Follow-up training was conducted by qualitative management or site leadership as needed. Interviews lasted approximately 1 hour 30 minutes, and focus groups lasted approximately 2 hours 30 minutes. Participants were given the option to be interviewed away from the study clinic at a location of their choosing, but all interviews were ultimately conducted in a private location at the study site due to participant preference.
Procedures
Written informed consent for AHA was collected from all participants followed by administration of a questionnaire that captured demographic and behavioral data. A biomarker level results tool was designed for AHA (Figure 1a), which depicted an individual’s levels of dapivirine in their plasma and in the returned rings from ASPIRE quarterly study visits for which data was available. All participants were presented with their personalized biomarker results tool prior to the FGD (for privacy) or during the IDI. Images on the tool were color-coded in green to indicate if the “ring appeared used” (> 95 pg/ml dapivirine in plasma; <22mg dapivirine remaining in returned ring, indicating ~3mg release of dapivirine as would be estimated from one month of continuous use), otherwise they were white. During the interview, participants were also presented with a “trajectory tool” (Figure 1b) and were asked to pick a line to depict their pattern of use of the dapivirine vaginal ring (IDI participants), or what line they think represented ASPIRE participants’ pattern of use in general (FGD participants) throughout the ASPIRE trial. Additional details on study measures and procedures can be found elsewhere22.
Figure 1.
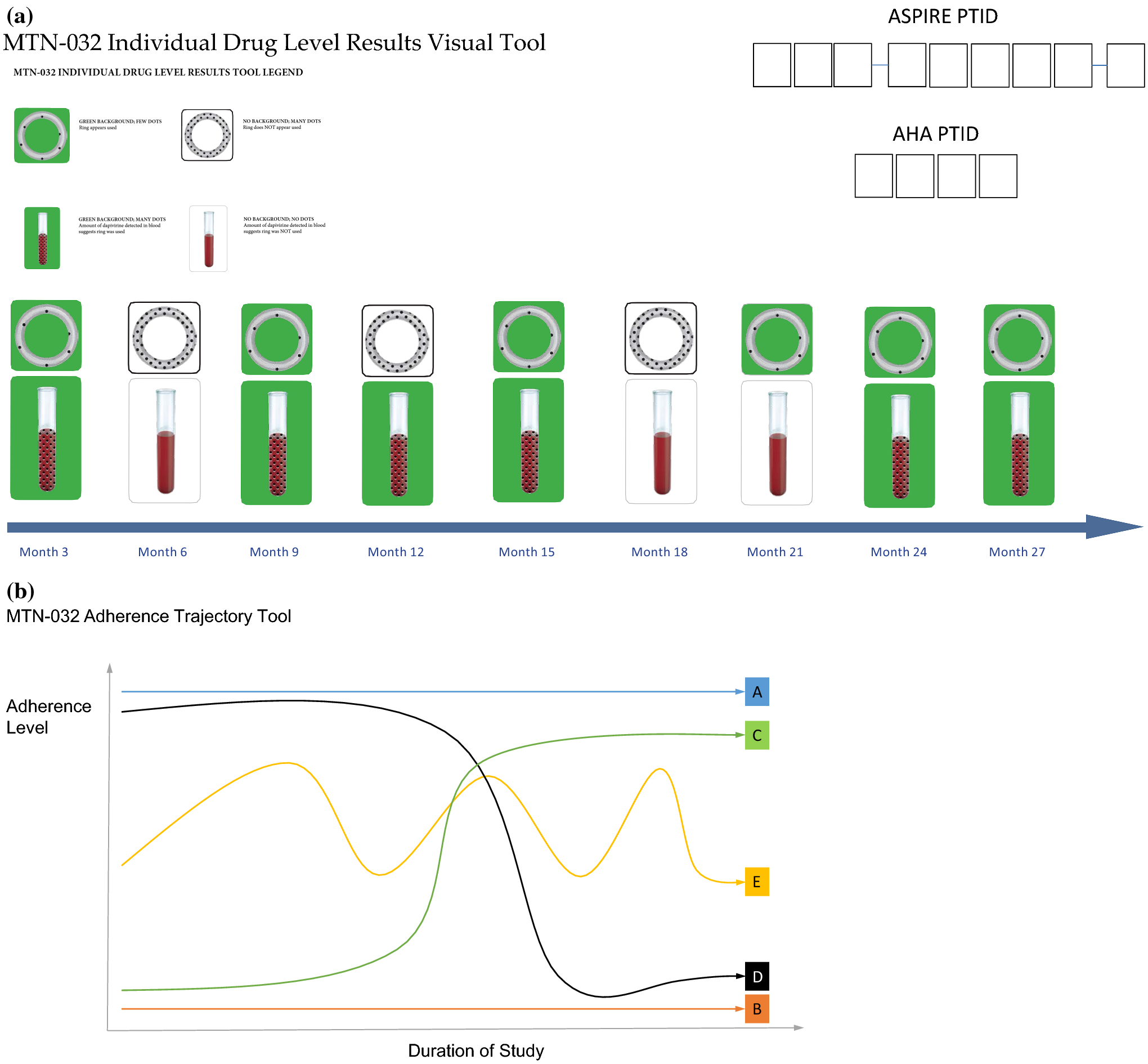
Data collection tools used to discuss adherence
IDIs were used in AHA to explore individual experiences with using the dapivirine ring and with receiving adherence results and FGDs were used to explore group norms and understanding about the ring, the ASPIRE study results and participants’ adherence results. More specifically, IDI guides explored individual experiences in ASPIRE including HIV and general health risks and worries, ring adherence influences, understanding of the ASPIRE study results, and future interest in the ring and the ring’s open-label extension trial, HOPE. FGD guides explored community perceptions about HIV and the ASPIRE study, general participant experiences in ASPIRE and opinions about the ring, perceptions about participants’ adherence to the ring, male partner attitudes, experience with study-led participant engagement activities, understanding of the ASPIRE results, and participants’ future interest in the ring and HOPE. The IDIs and FGDs, conducted in English or local African languages, were audio-recorded and ultimately translated and transcribed into English.
During both the IDIs and FGDs, exploration of peer influences on ring acceptability and adherence occurred through probing topics nested within the semi-structured interview guide. Probes about “other participants” and “other people” occurred in the context of: the participant’s experiences in ASPIRE and changes in attitudes about the study, community perceptions about ASPIRE, feelings about the ring, ring removals, social activities, and ASPIRE results.
Analysis
For the general AHA analysis, the interview guides, research objectives, and themes tracked throughout data collection were used to develop a codebook, which was iteratively updated throughout the coding process. A team of six analysts in the United States, trained in qualitative methods and analysis, with a range of technical backgrounds in the fields of anthropology, global health, and health behavior, were assigned transcripts and applied codes to data sections using Dedoose software (version 7.6.15, Los Angeles, CA). Analysts were not directly involved in data collection for ASPIRE or this sub-study, though four of the six were involved in the ASPIRE qualitative component analysis process. The analysis team, along with the study protocol chair, met weekly to discuss emerging themes, address coding questions and challenges, reach consensus on findings, and re-define codes as necessary. Intercoder reliability was also determined for 10% of transcripts with a mean kappa score of 0.77. Questionnaire-based data were summarized in SAS (version 9.0, Cary, North Carolina, USA). All AHA participants were stratified into four adherence groups based on the distribution of their cumulative plasma and/or residual ring measurements (> 95 pg/ml dapivirine in plasma and <22mg dapivirine remaining in returned ring were classified as “adherent”) as previously described in the primary AHA publication22. Individuals were classified into one of the following groups based on the proportion of data for which they had evidence of ring use: low (0–60% adherent), middle-low (61–80% adherent), middle-high (81–99% adherent) and high (100% adherent).
For this specific analysis on peer influence, we aimed to understand the role of peers on participant ring use. To note, while what constitutes as a ‘peer’ is often not clearly defined in the literature, in a systematic review from sub-Saharan Africa on young people and peer influence on sexual behavior, participants’ peers and/or friends were included, including those who shared demographic characteristics with participants (i.e. other young people, including family members, whom participants might know and interact with and who therefore could be a plausible source of influence)35. For this analysis, we therefore used “peer” if a participant used the term “friend,” if they were talking about a fellow participant(s) in the study, or if they were talking about a family member who was likely to be of similar status or in the same age group, such as a sibling or cousin.
Two members of the analysis team, including the lead author, both of whom were familiar with the data -- from quality control throughout data collection to coding -- extracted data coded in the dataset as “Peer/Family”, “Participants”, or “Activities”. Using an inductive analytic approach36–38, the analysts read the code reports, categorized the data and identified emergent themes and patterns, and created thematic summaries. Summaries were stratified by age (18–21 and 22–45 at entry into ASPIRE) and adherence groups (low, middle-low, middle-high, and high) to contextualize the results. Following this first stage of analysis, similarities and differences across subgroups were discussed, and findings clarified and confirmed. From this, two different types of peers emerged: “study peers” (i.e. those who were participants in the study), and “external peers”, (i.e. those who were not participants in the study). Additionally, within the study peers, a third subgroup emerged, study peers with pre-existing relationships or “pre-existing study peers” (i.e. those who were both participants in the study and who knew each other prior to the study). This “pre-existing study peers” group was created due to the fact that they share both qualities of external peers and study peers, and it became clear from early analysis that having a relationship outside the study as well as sharing the experience of being a study participant and using the ring was an important factor to consider. Furthermore, review of previously created summary reports on the “Adherence” codes and subcodes (i.e. “removal”) was done to examine the role of peers within these subgroups. Interpretation and consensus of study findings were discussed via phone and written documentation with all analysts and co-authors, consisting of the protocol chair and a representative from each site. Site co-author representatives had also been involved in data collection or site data management and analysis for the primary AHA paper and the ASPIRE qualitative component.
The study was approved by the Institutional Review Boards at RTI International, all study sites, and was overseen by the regulatory infrastructure of the US National Institutes of Health and the Microbicide Trials Network.
Results
A total of 187 were enrolled in MTN-032/AHA; 89 participated in a FGD (12 FGDs total, 2 FGDs at each site), and 98 participated in a single IDI22. Basic demographic characteristics of the study sample across all sites are presented in Table 1. Demographics of this sample by cumulative adherence and by ASPIRE age group can be found in a previous publication22. The median age of participants at the time of the AHA interview was 27 years, ranging from 19 to 48 years old, 48% completed secondary school or more and 58% earned their own income. Of the younger participant group, 74% were unmarried and 33% lived with a partner compared to 56% and 64% respectively of the older participant group.
Table 1.
Participant characteristics at time of MTN-032/AHA interview*
| All sites (n=187) | |
|---|---|
| Country of enrollment | |
| South Africa | 93 (50%) |
| Zimbabwe | 31 (17%) |
| Uganda | 29 (16%) |
| Malawi | 34 (18%) |
| Age at time of AHA interview, years a | 27 (28,19–48) |
| Age at time of ASPIRE baseline a | 24 (25,18–44) |
| 18–21 | 69 (37%) |
| 22–45 | 118 (63%) |
| Completed secondary school or more | 48% |
| Earns own income | 109 (58%) |
| Marital Status | |
| Legally | 9 (5%) |
| Traditionally | 56 (33%) |
| Unmarried | 107 (62%) |
| Same partner as when exited ASPIRE | 135 (78%) |
| Currently living with partner | 91 (53%) |
| Primary sex partner has other sex partners | |
| Yes | 32 (19%) |
| No | 41 (24%) |
| Don’t know | 99 (58%) |
unless otherwise specified
median (mean, min-max)
The ways in which peers influenced each other differed somewhat according to whether peers were fellow study participants (study peers), fellow study participants who were previously known (preexisting study peers), or whether peers were external to the study (non-participant peers). As presented below, the data revealed several ways in which peer influence occurred within these subgroups.
Study peers
Participants at all sites, and across all age and adherence groups, reported enjoying the social benefit of being in the study with their fellow study peers. Participants discussed making friends in the study and enjoying the comradery and interactions they had with other participants. A bond formed between fellow participants, as one participant described when talking about meeting friends in the study. While she had not known them prior to the study, when together, she said, “we were like one” (Lilongwe, Malawi; IDI; 22–45; middle-low (61–80%) adherence). Another participant likened her relationship with other participants to that of siblings, “we interacted well as children born by one person and would regret why the day had ended” (Harare, Zimbabwe; FGD; 18–21; middle-high (81–99%) adherence). This connection between participants held emotional importance, like for this participant who explains:
Because it [site events] helped in making new friends and create bonds with other participants so that you won’t feel like it’s a burden to be part of the study, but rather enjoy being in the study. You had people whom you could talk to about other women issues. It was good to know that there were always people whom you can talk to about the ring.
(Durban, South Africa; IDI; 22–45; low (0–60%) adherence)
However, there were some women who specifically noted that they did not make friends, interact with, or share much with other participants. This participant notes:
…I can’t say much because I didn’t have friends who were participants in the study, the only thing I would hear was the mumbling about the time wasted, but about the ring, I didn’t hear much about it.
(Johannesburg, South Africa; IDI; 22–45; middle-low adherence)
Additionally, while not commonly reported, one participant in Zimbabwe talked about times when fellow participants did not get along and conflict ensued.
Generally, however, participants reported an appreciation of the opportunity to meet new people and share about their lives, particularly as part of a group during the activities organized by the study sites (e.g. adherence meetings or social events like tea parties or movie days), or in the study site waiting room. Throughout their time in ASPIRE the participants shared stories and listened to one another about a wide range of ring use and study-related experiences – they “compared notes.” This peer communication allowed participants to share advice with one another when they were experiencing challenges with ring use (e.g. sharing fears and worries) or with their relationships due to using the ring (e.g. explaining how to avoid their partners feeling the ring, like changing sex positions). These discussions also led to both relaying negative rumors about the ring and study and discouraging use to sharing stories of success and ease of use and encouraging use. Although only mentioned by a few women, study peers could also be a source of information on methods they speculated could circumvent the study’s ability to detect non-use. Participants advised each other to dip their rings in tea or boil them in order to make the rings appear used. Some participants would also warn one another that the blood draws would show the study staff if a participant was using the ring or not.
For some, the shared experience of being fellow participants in the study created a feeling of safety to be more open and honest with one another about their ring use and experience in the study than they could with study staff or external peers. This participant explains:
It [other participants’ attitudes] didn’t affect my ring use at all because I think we were just telling each other honestly, you know we were honest to each other hence we couldn’t tell everything to the people that we came to see but amongst ourselves, it was easy for us to talk about how was it how does it feel exactly.
(Johannesburg, South Africa; IDI; 22–45; low adherence)
Because the participants were the ones using the ring, and study staff were not, participants became each other’s confidants, despite having just met one another. Additionally, the power dynamic and fear of judgment from staff made talking to fellow participants easier, as the same participant goes on to explain:
[…] Okay cool we didn’t know each other that much but it was easy because we came to do the same thing you know. So it was easy for us to talk about it rather than talking to a person that we came to see, you know because that person probably, you think she might judge you or say anything that you don’t like but amongst ourselves it was okay, you know it felt good actually more than talking to the nurses.
(Johannesburg, South Africa; IDI; 22–45; low adherence)
Being a part of this group of experienced ring users created a sense of comradery, which also helped them ignore the negative community rumors about the study and ring, as in the case of this woman from Uganda:
I experienced a similar feeling of demoralization [from hearing community rumors about the ring] but I was strengthened to try [to use the ring] because I knew that there were others who are part of the study. If I were to die, I wouldn’t die alone.
(Kampala, Uganda; FGD; 18–21; low adherence)
One unique aspect of this shared experience was that participants were particularly observant of one another. One participant in Durban, South Africa, for example, speculated with her peers in the waiting room about other participants’ health status, observing things like: “If you were called by [clinic staff] to take bloods for the second time then you are fine…But if your file is passed or you are called by the doctor, then nxargha (swearing) it is over for you.” Seeing other participants return for study visits, on the other hand, provided a clue that the ring was working (despite the fact that this was a placebo-controlled trial). She explains:
Yes, that is how we judged people that such a person is still in the study. Maybe when your dates are on the same day then you would see a person whom you last saw six months ago and then you see them. In your mind you thought that they had exited the study. So when you see them your faith gets renewed that the ring does work and it is alright.
(Durban, South Africa; IDI; 22–45; high (100%) adherence)
Study peers’ influence on attitudes and behavior
Beyond simply sharing experiences and advice, participants reported specific instances where their study peers directly influenced their attitudes, such as worries about the ring, and behaviors, such as partner disclosure and support and adherence to the ring, as described below.
Reducing worries about study procedures and the ring
Study peers often helped one another reduce fears related to the ring or study procedures. As one participant explains, she was initially concerned that they were being tested on, “making us monkeys by using the ring.” After staff members and a fellow participant explained how the study worked, “it made sense after hearing people talking about it” (Johannesburg, South Africa; IDI; 22–45; low adherence). Similarly, some participants were fearful of the blood draws, or in the case of this participant, getting a pap smear:
…I would sometimes chat with them about that [fear] and their boldness was encouraging me to the fact that… I’m the only one who was scared of this, so I need to… [Laughs] Because they were not scared of the injection or anything like that.
(Johannesburg, South Africa; IDI; 22–45; middle low adherence)
Another participant discussed the tension between the positive social reinforcement of one’s fears and the need for an internal locus of control to ignore the pressure of group conformity and behave based on one’s own best interest:
…it was nice to see that there were other people who thought the same thing as you. I would be scared to say that I was scared of using the ring at first. It was comforting to find that there were other people who were also scared and I would not have known that if I had not come to the workshops. Another thing about the workshop is that they would stress the importance of focusing on your own life. You must not care too much about other people and what they say because your life is yours alone to live.
[laughs] (Durban, South Africa; IDI; 22–45; middle-low adherence)
Partner disclosure and support
Hearing about other participants’ experience with disclosing or not disclosing their ring use to their partners affected some women’s decision to disclose to their own partners. For example, one younger participant in Durban explains her decision not to disclose to her partner:
Yes, I did hear them saying that the partners would feel the ring but that was because they had told the partners about the ring and they would want them to take the ring out. So having heard those stories, that is when I decided not to tell him because he was going to say that I take the ring out
(Durban, South Africa; IDI; 18–21; low adherence)
While an older participant in Durban expresses her motivation to disclose to her partner:
Okay, there was a lady whom we were within the study and she was married. This lady, the way that she explained the ring to her husband. She was the one person whom she would talk and you would think that if this lady’s husband […] accepted and agreed to her using the ring, then for us as unmarried women… he could just say no because some of them were not even allowed. So yah, that is how it helped me because there was no problem. […] That motivated me.
(Durban, South Africa; IDI; 22–45; high adherence)
Adherence
Participants looked to their fellow study participants for cues, or descriptive norms, on acceptable ring use behavior. Seeing that their peers were using the ring helped them overcome their fears and led them to change their behavior. For example:
It was through these meetings we had where women would share…even the women would say; “I use it and have no problem” and after those months I used it and was strong and during the following months I had gained courage and had no problem with the ring.
(Kampala, Uganda; IDI; 22–45; middle-low adherence)
Women seemed to trust the experience of their study peers and expected to have similar experiences (i.e. no problems with the ring). Furthermore, hearing that women were using the ring without their partners’ knowledge also gave a handful of participants courage to use the ring. A few participants reported that hearing their study peers talk about the positive aspects of the ring (e.g. that the ring protects from HIV; that the ring improves sex) motivated them to use or keep using the ring. One participant specifically noted that she was motivated to use the ring because she thought her friends were using it:
Most of the times when you come here you would find a lot of participants coming here, so you would say a lot of my friends are using the ring, and you would feel that there are a lot of who are using the ring.
(Lilongwe, Malawi; IDI; 22–45; middle-low adherence)
Study peers discussed the site-level adherence feedback as another motivating factor to maintain or improve ring use. Participants indicated a desire to see their site compare favorably with the other sites in the study. Whether their site was falling behind or was leading in adherence, there was a clear sense among participants that they wanted their site to be at the top and they used this information to encourage one another to use the ring.
Alternatively, looking to study peers for social cues regarding ring behavior (e.g. whether others were using the ring or not, if they had side effects, if they had disclosed to their partners, etc.) sometimes led to ring removals. A small subset of mostly older women (and one younger) said that hearing about the negative experiences from fellow participants discouraged them and led to non-use or ring removals. One Ugandan woman describes how she changed her behavior during menses:
That never treated me badly but I used to think about what other women had told me, like for example telling me that it widens you when you are in your periods! Then I also said that may be that [is] why it moves out, but the truth is that it really widens you. […] Then at that point I removed it, so that my periods would end and then I put it back.
(Kampala, Uganda; IDI; 22–45; low adherence)
A participant in Zimbabwe did not use the ring in the beginning of the study due to what others were saying, such as, “This is just a plastic, what could be in there. It’s worthless.” She stopped using the ring at another point in the study because a fellow participant told her she was using a placebo ring (Harare, Zimbabwe; IDI; 22–45; middle-low adherence). As another participant, from Johannesburg, South Africa, plainly put it, “I did not use the ring for the first few months because of peer pressure and being skeptical, not knowing if it’s working or not working” (Johannesburg, South Africa; IDI; 22–45; middle-high adherence).
At one site, when the staff noticed that peers were negatively influencing each other and encouraging one another to not use the ring, they adjusted visit schedules to separate groups of friends.
Because when we were many some would not encourage ring use but some would encourage each other. So the staff realized that at last and they separated us, participants would be shuffled [so that they will not always have the same visit date]. So we were now meeting as women who encouraged each other to use the ring use.
(Harare, Zimbabwe; IDI; 22–45; middle-high adherence)
Some women, however, noted the negative things fellow participants would share with them (e.g. side effects; disliking the ring) and specifically stated that it did not affect their ring use. Other women said they themselves did not experience the problems or side effects they heard others were having and therefore their adherence was not influenced. This participant explains how she chose not to listen to the negative talk:
…even when you go to school and a teacher tells you that this is going to be done like this, a stupid person will always stay stupid and the one who understands it will keep doing what she had been instructed. Whenever I would come here and a health worker says “This is done like this and we are going to do it like this…” I would do it the way I am instructed and that was me.
(Kampala, Uganda; IDI; 18–21; middle-high adherence)
Pre-existing study peers
In addition to making new friends in the study, a subset (~15%; n=30) of the women in this study sample reported knowing a fellow participant prior to joining the study. This included joining the study with their peers, joining because they had peers already in the study, or actively encouraging their peers to join. The types of pre-existing relationships fell into three categories: friends, family (i.e. sisters or other family members such as sister-in-law or cousin), and neighbors or community members. As the participant quoted below insinuates, the implication for these prior relationships is that the ties may have been stronger than other relationships and thus more influential toward adherence behaviors. When asked about what could have been done differently in ASPIRE to improve adherence, and in particular regarding how she feels about support groups for women who use the ring, she explained:
They can group women who are friends so that they can encourage each other and also be open to each other. Yes, let’s say if I have my friend, if she rebukes me it will not hurt me, but if someone else who is not my friend comes and rebukes me - and even if it’s the truth - I will be disappointed… If it’s my friend I will be encouraged…I will say, “Please help me what am I supposed to do here.”
(Harare, Zimbabwe; IDI; 22–45; middle-low adherence)
Of those who talked about pre-existing study peers, many specifically described the influence their previously established relationships had on ring adherence. Different examples of pre-existing peer relationships (stratified by age group), and how they influenced adherence are presented as case studies in Table 2 to illustrate the scope of peer relationships reported.
Table 2.
Case studies of participants who had previously established peer relationships
| Index participant |
Description of prior relationship with study peer(s) | Participant’s narrative of adherence, including peer influence | |
|---|---|---|---|
| OLDER (22–45) | Durban, South Africa; IDI; low (0–60%) adherence | Joined with friends from the neighborhood. Other friends who tried to join and could not -- due to testing positive for HIV or other illnesses -- said researchers gave them HIV. | Hearing about her friends’ experiences scared her, she thought the ring would cause her problems if she used it. She was also scared hearing from other participants about the ring making them sick. She would leave her ring out for a month and then insert it a week before her visit. She also removed the ring when she wasn’t sexually active. One external peer, who is a nurse, also discouraged her from using the ring. |
| Kampala, Uganda; IDI; middle-low (61–80%) adherence | Friend told her about the study and encouraged her to join; her friend ended up moving out of the country and quitting the study at some point. | Both she and her friend removed the ring in the beginning for fear of getting sick but “became strong after.” She changed her behavior after a few months when she gained courage through hearing other study peers talk about their ring use. | |
| Johannesburg, South Africa; IDI; high (100%) adherence | She had three sisters in the study, one of whom convinced her to join. | One sister influenced her to tell her partner about the ring; another sister (the one who encouraged her to join) started having bladder issues and thought it was ring related after consulting a government hospital doctor. This participant told her sister, “no it cannot be the ring, how many people in the study are using it, maybe you have your own problem, but she continued with the study and the other one was the same like me until the study stopped.” She attributes her adherence to just following directions - she describes no influence from other peers or boyfriends. | |
| YOUNGER (18–21) | Harare, Zimbabwe; FGD; low adherence | Two of her friends were already in the study when she joined. She says that many people in the study knew each other already and were from the same area. | She said “me and my friend” would talk about being in pain while on menses and remove the ring. |
| Harare, Zimbabwe FGD; middle low adherence | Joined the study with her friend/relative (same person). | Her friend/relative said the ring was big and would hurt her so they both removed their rings and would only put them back in on the day they were returning to the clinic. Then her friend/relative acquired HIV and told her she should start committing to wear it, so she started using the ring more seriously. | |
| Johannesburg; IDI; high adherence | Had friends who were also in the study. | She did not seem to be influenced by her friends’ concerns about feeling the ring. She told them she didn’t feel it and didn’t have a problem. She would give other participants advice when they had challenges as she had no challenges using the ring and usually forgot she was wearing it. |
Non-participant peers
In addition to the study peers, a number of women mentioned receiving input from external peers -- friends or family who did not participate in the study. In most cases, input from external peers (and other community members) was negative and often related to rumors, such as fears that using the ring would cause health damage to the participant or the study being associated with Satanism. To avoid the negative peer input, and because they feared judgment, some participants chose not to talk to their external peers about their ring use at all. For example:
…I didn’t feel very comfortable talking about the ring with my friends because my friends are those kinds of people that are too judging. So when I was here I got to sit with somebody that was using the same thing that I was using so we share ideas and what not…
(Johannesburg, South Africa; IDI; 22–45; low adherence)
For those who did talk to their peers about the ring, these external peers sometimes negatively influenced the participants’ adherence, as this participant from Malawi explains:
Yea true the main issue was fear, fear overwhelmed us and most of the fear came from our friends in the communities. Some of the things they were saying were the ones that made us to be lazy; maybe we took the issue so serious but when you reach home they could shout at you and then you just decide to take it off and throw it aside [she laughed].
(Lilongwe, Malawi; FGD; 18–21; low adherence)
In contrast, a few participants had external peers (and other family members) who encouraged them to continue with their participation and ignore the negative rumors, though this was not the norm.
They [sisters and grandmother] just said that you were the ones who were explained to. Everything that’s done did not start today so if you have committed yourself…Don’t listen much to what people say about Satanists or what. You are the ones who know very well what was explained about the study.
(Harare, Zimbabwe; IDI; 22–45; low adherence)
Discussion
The purpose of this analysis from the MTN-032/AHA study was to explore the nuances of peer influences on acceptability of and adherence to the dapivirine vaginal ring during the MTN-020/ASPIRE Phase III trial, expanding on findings from the ASPIRE qualitative component21 and primary AHA analysis22. These findings are important as they can inform future interventions or ring demonstration projects. Our three major findings from this analysis were as follows. First, study peers of all ages and adherence groups developed important connections with one another, enabling them to share their nuanced experiences with and opinions about the ring more openly and honestly with each other than with external peers or study staff. At times, these study peer connections mitigated some of the effects from external negative comments. Second, pre-existing study peers appeared to have a stronger influence on each other’s adherence than new study peers. Third, external peers provided primarily negative input to participants about the ring and study, which sometimes led to ring removals.
Previous HIV prevention trials have noted both external and internal peer influence -- from community members and participants -- negatively affecting product adherence and trial participation9,11,39. In fact, community outreach and participant activities in ASPIRE were designed in response to lessons learned from these previous trials to help mitigate such influence34. Our findings also corroborate the ASPIRE trial qualitative component analyses, which found that while participants were initially afraid of the ring’s appearance and possible side effects -- partly as a result of rumors and misconceptions spread by community members and peers -- they gradually became more familiar with ring use through trial progression, in part due to peer support and the study-led activities21,28,40. The notion that peers are an important influence is consistent with the development of peer support and peer education interventions being implemented for a variety of health and wellness domains, including HIV41–43. However, more research is needed about the mechanisms through which peer influence operates within trial settings that are attempting to address sensitive or stigmatized topics, such as HIV. Study participation can include organic or cultivated group experiences (i.e. waiting rooms and/or organized activities) and it would be beneficial if researchers were able to leverage those interactions to guide social influences in positive, prosocial directions (i.e. behaviors that are intended to benefit other people).
In these data, many participants described the important connections that they developed with their study peers throughout the trial. They became one another’s confidants (and occasional conspirators), who mostly supported and encouraged ring use. This relationship-building through shared experience substantiates social psychology principles that humans are motivated to be accepted as part of a group and thus will follow group norms in order to fit in44. Building off these principles as well as findings from previous publications with this population21,22,28, we theorize that at the beginning of ASPIRE, study participants looked more to external sources – family, friends, neighbors and community – for social cues about how to think about the study and the ring because the participants identified more with them than study staff and internal peers, whom they did not yet know or trust. Participants did not yet have sufficient information or experience to trust the study staff and the ring, a novel product of unknown efficacy. Yet, as the study progressed and the study participants became more familiar with the ring, and spent more time with study peers, they likely identified more as members of a new social group, one comprised of ring “experts”. It is feasible that they began looking to their study peers for social cues about the ring and study instead of their external peers due to the perceived similarity with and increased credibility of the former45. Trust within the participants’ social networks was not a topic directly explored in this study, though this analysis suggests that many participants trusted one another as a social reference about the ring, as has also been reported in decision making among adolescents regarding long-acting reversible contraceptives46–48. Future studies on the vaginal ring for HIV prevention, or about other new and potentially stigmatized or sensitive subjects, would benefit from incorporating questions about participants’ trusted sources to either enhance or mitigate their influence in a direction that is beneficial and productive to the research. Additionally, it would be important to explore research trust or mistrust and how that affects participants’ acceptability of and adherence to novel products. Having a lack of trust in research professionals may lead participants to look to their trusted sources (e.g. peers) instead, who may not have the correct information about the product or subject in question, particularly for new or foreign-invented interventions28.
Among the subset of participants who knew other participants prior to joining the study, many talked about how their pre-existing relationships influenced their adherence negatively or how they positively influenced their pre-existing peers’ adherence as sources of support and motivation. The data suggest that for these women, pre-existing connections may have been more influential than new connections. This finding is consistent with the notion that influence is most strongly rooted when it comes from someone who is perceived as credible, and that longer lasting relationships would be more credible than newer established relationships49. The idea that previously established peers are more influential than newer peers, which requires further testing to confirm, could be a key into the success of future ring studies or implementation projects. Social networking analyses (mapping people within a network and linking their relationships and interactions) could be useful to gain more insight into differences between pre-existing and newer peer relationship’s and determine how best to intervene50.
Peer influence is often discussed in the health literature as an adolescent phenomenon23,25,35,48,51–54, however in this analysis, we found peer influence among both younger (18–21) and older (22–45) age groups. Interestingly, it was a small subset of mostly older women who discussed removing the ring or stopping ring use after their fellow participants spoke negatively about it. While it is possible that younger participants were less forthcoming about their negative experiences, the key point is that in this cohort, older participants were also susceptible to peer influence. This range of influence across ages may be explained by social norms theory -- that individual’s behaviors are influenced by perceptions of peer attitudes and behaviors – which is not limited to adolescent health51,55–58. This may have been particularly relevant as the study progressed and the group of ring users became a more solidified social group. It is also important to note that not all participants described themselves as being influenced by other participants, and this is likely because some people were less susceptible to group pressure due to higher self-esteem or self-efficacy to use the ring. Not all interventions focused on encouraging positive peer influence will benefit all participants.
This analysis has several limitations. Exploring the influence of peer relationships was not one of the objectives of the AHA study, although it was tied into the primary objective of exploring socio-contextual issues which affected participants’ adherence to the ring. Therefore, the depth and breadth of questioning around peer relationships varied depending on the participant and the interviewer’s discretion to further explore the topic. This also meant that the topic of trust amongst participants was not directly explored, which would provide valuable detail as to the direction and depth of peer influence. Nevertheless, the fact that these interactions were discussed in such depth by so many participants highlights the fact that the topic is noteworthy and important. Furthermore, the AHA study took place retrospectively 12–17 months after participants exited from ASPIRE, thus there is a potential for recall bias as well as reconstruction of memories being influenced by release of ASPIRE study results (positive mood congruency). For example, if the trial had not been successful, participants may have looked back and thought of fellow peers in a more negative light, blaming others for not using the ring and contributing to the study failure (as occurred in VOICE-D)9. Finally, we used participants’ age at time of ASPIRE to explore age-related differences to help explain ASPIRE results, however both cohorts had aged at the time of this study and thus their retrospective views may be harder to differentiate.
Conclusion
Peers influencing each other’s behaviors in both prosocial and detrimental manners could have repercussions on adherence to a biomedical intervention, and consequently, individual disease risk and clinical trial outcomes. Future trials, particularly regarding new and potentially stigmatized or sensitive subjects, should pay attention to established and newly formed social networks throughout the study in order to properly intervene and either enhance or mitigate peer influence in a direction that is beneficial and productive. One suggestion could be to “infiltrate” established peer groups early on in a study, allowing study counselors or nurses to provide education and mitigate negative rumors from spreading within and beyond these groups. Additionally, peers could be used as peer educators and even product ambassadors, which may lead to greater acceptability, uptake and use of future biomedical products. With proper forethought, peer networks can be harnessed to intentionally influence uptake and adherence to the ring in future ring demonstration and implementation studies.
Acknowledgements
We would like to thank AHA participants for their contribution to this research. The Microbicide Trials Network (MTN) leadership and operating center, FHI 360, and MTN-032 Protocol study team members are acknowledged as critical in the development, implementation, and/or analysis of this study. The full MTN-032 study team can be viewed at https://mtnstopshiv.org/research/studies/mtn-032. This paper benefited greatly from the contributions of Ariane van der Straten for review of and contributions to earlier versions of this manuscript. The study was designed and implemented by the MTN funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.UNAIDS. Global HIV & AIDS Statistics - 2018 fact sheet: UNAIDS;2018. [Google Scholar]
- 2.O’Leary A. Women and HIV in the Twenty-First Century: How Can We Reach the UN 2030 Goal? AIDS Education and Prevention. 2018;30(3):213–224. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure Prophylaxis for HIV Prevention: Where Have We Been and Where Are We Going? JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013/07/01 2013;63:S122–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. February 05 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. August 02 2012;367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celum CL, Delany-Moretlwe S, Baeten JM, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc July 2019;22 Suppl 4:e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS and behavior. 2013;17(6):2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corneli AL, McKenna K, Headley J, et al. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. Journal of the International AIDS Society. 2014;17(3S2):19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. October 23 2015;29(16):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery ET, Mensch B, Musara P, et al. Misreporting of Product Adherence in the MTN-003/VOICE Trial for HIV Prevention in Africa: Participants’ Explanations for Dishonesty. AIDS and behavior. February 2017;21(2):481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PloS one. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid A. More investment needed in developing female-controlled HIV prevention options: UNAIDS; 2016. [Google Scholar]
- 13.van de Wijgert J. Working towards HIV prevention choices for women. The lancet. HIV February 2018;5(2):e60–e61. [DOI] [PubMed] [Google Scholar]
- 14.AVAC. Tracking the Fast-Changing Status of PrEP Around the World. 2018; https://www.avac.org/blog/tracking-prep-status. Accessed November 12, 2018.
- 15.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. August 2 2012;367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 16.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis. February 2012;25(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seltzer H, Solai L. IPM’s Application for dapivirine vaginal ring for reducing HIV risk in women now under review by european medicines agency: international partnership for microbicides; 2017. [Google Scholar]
- 18.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. The New England journal of medicine. February 22 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel A, Bekker L-G, Bukusi E, et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PloS one. 2016;11(3):e0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown E, Palanee-Philips T, Marzinke M, et al. Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection. Paper presented at: JOURNAL OF THE INTERNATIONAL AIDS SOCIETY 2016. [Google Scholar]
- 21.Montgomery ET, van der Straten A, Chitukuta M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. Aids. May 15 2017;31(8):1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery ET, Stadler J, Naidoo S, et al. Reasons for nonadherence to the dapivirine vaginal ring. Aids. 2018;32(11):1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert D, Chein J, Steinberg L. Peer influences on adolescent decision making. Curr Directions Psychol Sci. 2013;22(2):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okonkwo PI, Fatusi AO, Ilika AL. Perception of peers’ behaviour regarding sexual health decision making among female undergraduates in Anambra State. Nigeria. Afr Health Sci. 2005;5(2):107–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Tome G, Matos M, Simoes C, Diniz JA, Camacho I. How can peer group influence the behavior of adolescents: explanatory model. Glob J Health Sci. 2012;4(2):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neary J, Wagner AD, Mugo C, et al. Influence and involvement of support people in adolescent and young adult HIV testing. AIDS Care. 2019;31(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda L, Mash R. Reasons for inconsistent condom use by young adults in Mahalapye, Botswana. Afr J Primary Health Care Fam Med. 2018;10(1):e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitukuta M, Duby Z, Katz A, et al. Negative rumours about a vaginal ring for HIV-1 prevention in sub-Saharan Africa. Cult Health Sex. 2019;21(11):1209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duby Z, Katz A, Musara P, et al. “The state of mind tells me it’s dirty”: menstrual shame amongst women using a vaginal ring in Sub Saharan Africa. Women Health. 2019;60(1):72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duby Z, Katz AWK, Browne EN, et al. Hygiene, blood flow, and vaginal overload: why women removed an HIV prevention vaginal ring during menstruation in Malawi, South Africa, Uganda and Zimbabwe. AIDS Behav; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pleasants E, Tauya T, Reddy K, et al. Relationship type and use of the vaginal ring for HIV-1 prevention in the MTN 020/ASPIRE trial. AIDS Behav; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laborde ND, Pleasants E, Reddy K, et al. Impact of the dapivirine vaginal ring on sexual experiences and intimate partnerships of women in an HIV prevention clinical trial: managing ring detection and hot sex. AIDS Behav. 2018;22(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palanee-Phillips T, Schwartz K, Brown ER, et al. Characteristics of women enrolled into a randomized clinical trial of dapivirine vaginal ring for HIV-1 prevention. PLoS ONE. 2015;10(6):e0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz K, Ndase P, Torjesen K, et al. Supporting participant adherence through structured engagement activities in the MTN-020 (ASPIRE) trial. AIDS Res Hum Retroviruses. 2014;30(S1):A80–A8080. [Google Scholar]
- 35.Fearon E, Wiggins RD, Pettifor AE, Hargreaves JR. Is the sexual behaviour of young people in sub-Saharan Africa influenced by their peers? A systematic review. Soc Sci Med. 2015;146:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–46. [Google Scholar]
- 37.Katz J. Analytic Induction. In: Smelser NJ, Baltes PB, editors. International encyclopedia of the social & behavioral sciences. Oxford: Pergamon; 2001. p. 480–484. [Google Scholar]
- 38.Bhattacharya K. Fundamentals of qualitative research: a practical guide. London: Routledge; 2017. [Google Scholar]
- 39.Magazi B, Stadler J, Delany-Moretlwe S, et al. Influences on visit retention in clinical trials: insights from qualitative research during the VOICE trial in Johannesburg South Africa. BMC Women’s Health. 2014;14:88–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Straten A, Browne EN, Shapley-Quinn MK, et al. First impressions matter: how initial worries influence adherence to the dapivirine vaginal ring. J Acquir Immune Defic Syndr. 2019;81(3):304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramchand R, Ahluwalia SC, Xenakis L, Apaydin E, Raaen L, Grimm G. A systematic review of peer-supported interventions for health promotion and disease prevention. Prev Med. 2017;101:156–70. [DOI] [PubMed] [Google Scholar]
- 42.Morar NS, Naidoo S, Goolam A, Ramjee G. Research participants’ skills development as HIV prevention peer educators in their communities. J Health Psychol. 2018;23(10):1343–9. [DOI] [PubMed] [Google Scholar]
- 43.Naidoo S, Morar NS, Ramjee G. Participants as community-based peer educators: impact on a clinical trial site in KwaZulu-Natal. S Afr J Sci. 2013;109(7–8):01–5. [Google Scholar]
- 44.Aronson E. The social animal. New York: Worth Publishers; 2004. [Google Scholar]
- 45.Hocevar KP, Metzger M, Flanagin AJ. Source credibility, expertise, and trust in health and risk messaging. Oxford: Oxford University Press; 2017. [Google Scholar]
- 46.Anderson N, Steinauer J, Valente T, Koblentz J, Dehlendorf C. Women’s social communication about IUDs: a qualitative analysis. Perspect Sex Reprod Health. 2014;46(3):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoopes AJ, Gilmore K, Cady J, Akers AY, Ahrens KR. A qualitative study of factors that influence contraceptive choice among adolescent school-based health center patients. J Pediatr Adolesc Gynecol. 2016;29(3):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yee L, Simon M. The role of the social network in contraceptive decision-making among young, African American and Latina women. J Adolesc health. 2010;47(4):374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oster E, Thornton R. Determinants of technology adoption: peer effects in menstrual cup take-up. J Eur Econ Assoc. 2012;10(6):1263–93. [Google Scholar]
- 50.Kincaid DL. Social networks, ideation, and contraceptive behavior in Bangladesh: a longitudinal analysis. Soc Sci Med. 2000;50(2):215–31. [DOI] [PubMed] [Google Scholar]
- 51.Beniamino C, Holly S. Social norms and adolescents’ sexual health: an introduction for practitioners working in low and mid-income African countries. Afr J Reprod Health. 2018;22(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Closson K, Dietrich JJ, Lachowsky NJ, et al. Sexual self-efficacy and gender: a review of condom use and sexual negotiation among young men and women in sub-saharan Africa. J Sex Res. 2018;55(4–5):522–39. [DOI] [PubMed] [Google Scholar]
- 53.Romer D, Black M, Ricardo I, et al. Social influences on the sexual behavior of youth at risk for HIV exposure. Am J Public Health. 1994;84(6):977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J. The effect of peers on HIV infection expectations among Malawian adolescents: using an instrumental variables/school fixed effect approach. Soc Sci Med. 1982;2016(152):61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball K, Jeffery RW, Abbott G, McNaughton SA, Crawford D. Is healthy behavior contagious: associations of social norms with physical activity and healthy eating. Int J Behav Nutr Phys activity. 2010;7:86–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheehan DM, Miller RP, Trepka MJ, Smith LR, Latkin C. Role of social network sexual norms and behaviors on the HIV sexual risk behaviors of people who inject drugs in HPTN 037. AIDS Behav. 2019;23(6):1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17(Suppl 1):S102–113. [DOI] [PubMed] [Google Scholar]
- 58.Berkowitz AD. The social norms approach: theory, research, and annotated bibliography. 2004.


