Abstract
Background and objectives
Type 2 diabetes mellitus (T2DM) patients with Coronavirus Disease 2019 (COVID-19) have poorer prognosis. Inconclusive evidence suggested dipeptidyl peptidase-4 inhibitors (DPP4i) might reduce inflammation and prevent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) entry, hence further evaluation on DPP4i is needed.
Methods
1214 Patients with T2DM were admitted with COVID-19 between 21st January 2020 and 31st January 2021 in Hong Kong. Exposure was DPP4i use within the 90 days prior to admission for COVID-19. Assessed outcomes included clinical deterioration, clinical improvement, low viral load, positive Immunoglobulin G (IgG) antibody, hyperinflammatory syndrome, proportion of IgG antibody, clinical status and length of hospitalization. Multivariable logistic and linear regression models were performed to estimate odds ratios (OR) and their 95% confidence intervals (CI) of event outcomes and continuous outcomes, respectively.
Results
DPP4i users (N = 107) was associated with lower odds of clinical deterioration (OR=0.71, 95%CI 0.54 to 0.93, P = 0.013), hyperinflammatory syndrome (OR=0.56, 95%CI 0.45 to 0.69, P < 0.001), invasive mechanical ventilation (OR=0.30, 95%CI 0.21 to 0.42, P < 0.001), reduced length of hospitalization (-4.82 days, 95%CI –6.80 to –2.84, P < 0.001), proportion of positive IgG antibody on day-3 (13% vs 8%, p = 0.007) and day-7 (41% vs 26%, P < 0.001), despite lack of association between DPP4i use and in-hospital mortality.
Conclusion
DPP4i use was associated with reduced odds of clinical deterioration and hyperinflammatory syndrome. Prospective studies are warranted to elucidate the role of DPP4i in T2DM and COVID-19.
Keywords: COVID-19, DPP4i, Hyperinflammatory syndrome, In-hospital death, Viral clearance
Introduction
As of 29th July 2021, cumulative number of Coronavirus Disease 2019 (COVID-19) cases reported globally was over 195 million, with death toll exceeding 4.1 million [1]. A key feature of COVID-19′s pathophysiology is the presence of hyperinflammatory syndrome, also known as a cytokine storm, proven to be associated with acute respiratory distress syndrome (ARDS) and multiple organ damage in patients [2].
Type 2 diabetes mellitus (T2DM) has been associated with higher mortality and increased risks of intensive care unit (ICU) admission, ARDS and invasive ventilatory requirement among COVID-19 patients [3]. T2DM, characterized by hyperglycemia, represents an impaired immune response with a chronic inflammatory state of increased proinflammatory cytokine production, phagocytic cell dysfunction and defective neutrophil chemotaxis [4]; hence suggesting that T2DM patients with COVID-19 may be especially at risk of hyperinflammatory syndrome. Obesity has also been identified as a significant comorbidity associated with T2DM [5] and hypothesized to worsen prognosis due to hypoventilation, coupled with preadmission low-grade inflammation [6]. Moreover, studies have proposed that chronic inflammation characterizing obesity and T2DM may be traced to a common phenomenon: the upregulation of dipeptidyl peptidase 4 (DPP4), or cluster of differentiation 26 (CD26) in immune cell populations among adipocytes [7, 8].
The most well-known function of DPP4 is the cleavage and inactivation of incretins (e.g., glucagon like peptide-1 (GLP-1)), resulting in reduced insulin secretion [9]. Accordingly, DPP4 inhibitors (DPP4i) can facilitate glycemic control by increasing levels of activated incretins, lowering glucagon secretion and promoting greater glucose uptake into cells [10]. Those leads to its good safety profile for frailty population with lower risks of hypoglycemia and cardiovascular when compared with sulfonylurea (SU) and gastrointestinal adverse events than GLP-1 receptor agonists (GLP-1 RAs) other glucose-lowering medications. Therefore, it is often used as second-line treatment for T2DM after the failure of metformin monotherapy [11, 12]. Preponderance of studies demonstrating pleiotropy of DPP4 activity endows it to be pro-inflammatory [13] which might exacerbate the hyperinflammation of COVID-19. DPP4 itself is also proposed to be a potential receptor for SARS-CoV-2 entry [14]; yet claims regarding its role in viral entry and DPP4i's efficacy in viral entry inhibition remain disputed. Recent studies have also demonstrated lower mortality and anti-inflammatory effects with DPP4i administration in diabetic animals and humans [8, 15]. A recent review by Scheen proposed that DPP4i exhibits anti-inflammatory effects primarily by three routes, namely 1) cytokine control; 2) glycaemic control; and 3) viral entry inhibition [16]. However, due to the recency of such topic on the potential anti-inflammatory effects of glucose-lowering medications, limited studies have investigated the effect of DPP4i in the association between COVID-19 associated hyperinflammatory syndrome and T2DM. None of those studies considered viral clearance dynamics while little was addressed regarding the mechanistic explanation for improved outcomes measured. Therefore, this territory-wide retrospective study aims to bridge the knowledge gap between DPP4i use and the odds of clinical deterioration, clinical improvement, hyperinflammatory syndrome, and viral clearance in T2DM patients hospitalized with COVID-19.
Methods
Data source and study population
Data were extracted from a territory-wide anonymized electronic health records of the Hong Kong Hospital Authority (HA). As the HA is the only public-funded healthcare provider providing management to all COVID-19 cases in Hong Kong, all COVID-19 patients in the territory were captured.
In this study, we analyzed T2DM patients with confirmed COVID-19 infection admitted to public hospitals between 21st January 2020 and 31st January 2021 in Hong Kong SAR, China. COVID-19 was confirmed by positive SARS-CoV-2 viral nucleic acid detected using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay, performed by the Department of Health Public Health Laboratory. Patients with T2DM were identified by International Classification of Primary Care, Version 2 (ICPC2) code of T90 or International Statistical Classification of Diseases and Related Health Problems, 9th Revision, Clinical Modification (ICD-9-CM) codes of 250.x0 or 250.x2.
Date of hospital admission was defined as the index date of patients. Each patient was observed from the index date (baseline, day-0) until the date of in-hospital death, hospital discharge or data cut-off date (30th April 2021), whichever came first.
Definition of DPP4i exposure
Patients were divided into exposure and non-exposure groups. Patients who had received DPP4i from 90 days prior admission to the day before admission for COVID-19 were defined as exposure to use of DPP4i, while others were categorized into DPP4i non-users.
Definition of covariates
Demographics including age and sex, pre-existing comorbidities including hypertension, chronic lung, heart, and kidney diseases, malignancy, and obesity were captured. Use of medications including angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), anticoagulants, antiplatelets, lipid-lowering agents, non-steroidal anti-inflammatory drugs (NSAID) were recorded. Concomitant use of other anti-diabetic agents were also captured (namely GLP1RA, insulin, metformin, SU, thiazolidinedione (TZD), acarbose, and sodium-glucose cotransporter-2 inhibitor (SGLT2i)). DPP4i drugs (subdivided as alogliptin, linagliptin, sitagliptin and vildagliptin) were also captured.
Baseline COVID-19 severity was classified by WHO clinical progression scale. All patients in our cohort obtained a minimum score of 4 (Hospitalized; no oxygen therapy). They were then further classified into moderate (score 4–5) and severe (score 6–9) [17]. Pharmacological treatments for COVID-19 were recorded (namely remdesivir, interferon-β−1b, dexamethasone, tocilizumab). Clinical progress of each patient was recorded, including ICU admission, extracorporeal membrane oxygenation (ECMO), dialysis, occurrence of ARDS and criteria for hyperinflammatory syndrome as defined by Webb et al. (consisting of macrophage activation, hematological dysfunction, coagulopathy and hepatic inflammation) [18]. A comprehensive panel of hematological and biochemical laboratory parameters was obtained, with regular assessments during the course of COVID-19. These included white blood cell count, neutrophil, lymphocyte, platelet, lactate dehydrogenase, creatine kinase, total bilirubin, C-reactive protein, estimated glomerular filtration rate (eGFR), alkaline phosphatase (ALP), alanine transaminase (ALT), hemoglobin, random glucose, hemoglobin A1c (HbA1c)). Ct values refer to the number of cycles of RT-PCR required to detect the gene target, hence a negative relationship between SARS-CoV-2 PCR Ct value and viral load [19].
Definition of outcomes
The primary outcome was clinical deterioration, a composite outcome consisting of in-hospital death or requirement of invasive mechanical ventilation.
A number of secondary outcomes were analyzed: (i) in-hospital death; (ii) requirement of invasive mechanical ventilation (iii) clinical improvement by at least one score reduction on the WHO clinical progression scale ranging from 0 (uninfected with no viral RNA detected) to 10 (dead) [17]; (iv) low viral load (cycle threshold (Ct) value ≥ 30 cycles); (v) positive Immunoglobulin G (IgG) antibody; (vi) incidence of hyperinflammatory syndrome (as defined by Webb et al. [18]); (vii) proportion of IgG antibody; (viii) clinical status as defined by the WHO clinical progression scale; and (ix) length of hospitalization.
Data analysis
Descriptive statistics of baseline characteristics between DPP4i users and non-users were presented as mean and standard deviation for continuous variables, while count and proportion were used for categorical variables. Multiple imputation by chained equations (MICE) was employed to address the missing baseline covariates on admission (Table S1; see supplementary materials associated with this article online) in the exposure and control groups. Each missing value of laboratory data was imputed 20 times using other variables that may impact the outcome.
A logistic regression model was first applied to calculate the propensity scores of each patient in the cohort with the aforementioned covariates. Inverse probability of treatment weighting (IPTW) utilizing the propensity score was then implemented to balance the covariates between groups, in order to reduce the outcome bias caused by differences in baseline characteristics. After the propensity-score weighting, the balance of baseline covariates between treatment groups were further evaluated using the standardized mean difference (SMD). SMDs of ≤ 0.2 indicated sufficient balance between groups.
Multivariable logistic regression model weighed by IPTW was applied to estimate the effects of exposure on binary outcomes, in term of odds ratios (OR) and their 95% confidence interval (CI). Effects of exposure on Ct values, length of hospitalization among survivors and healthcare costs were estimated using multivariable linear regression model weighted by IPTW.
Sensitivity analyses were performed by removing hospital discharge as censoring criterion; limiting follow up period to be at most 90 days. In addition, subgroup analyses were performed on the following: age (≤ 65 and > 65 years), sex, use of invasive mechanical ventilation or ECMO, and ICU admission. All statistical analyses were performed using STATA Version 16 (StataCorp LP, College Station, TX). P-value < 0.05 indicated statistical significance.
Results
Patient cohort
A total of 1214 patients with T2DM who were admitted with COVID-19 between 21st January 2020 and 31st January in Hong Kong were included in this study (Figure S1; see supplementary materials associated with this article online). Among the 107 patients with DPP4i use, alogliptin (n = 22, 20.6%), linagliptin (n = 34, 31.8%), sitagliptin (n = 29, 27.1%) and vildagliptin (n = 22, 20.6%) were prescribed (Table 1 ). DPP4i therapy was continued during hospitalization, while some of them were co-administered with insulin (n = 66, 61.7%), metformin (n = 61, 57.0%), or SU (n = 50, 46.7%). Majority of patients (74.3%) were admitted with non-severe clinical condition without use of oxygen therapy. The most common comorbidity was hypertension (n = 928; 76.4%) in this cohort of COVID-19 patients with diabetes, while 11.6% (n = 141) of patients were obese. Mean random glucose on admission was 10.4 mmol/L for DPP4i group and 9.4 mmol/L for control group, whereas mean HbA1c level was 7.8% for DPP4i group and 7.4% for control group. After multiple imputations and propensity score weighting, all clinical characteristics except anticoagulant use and concomitant use of SU were balanced, with SMDs ≤ 0.2 (Figure S2 and Table S2; see supplementary materials associated with this article online).
Table 1.
Baseline characteristics of hospitalized patients with COVID-19 in patients who used DPP4i and those who did not after multiple imputation and propensity score weighting.
| Before weighting |
After weighting |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | DPP4i (n = 107) |
Control (n = 1107) |
SMD | DPP4i (n = 107) |
Control (n = 1107) |
SMD | ||||
| N / Mean | % / SD | N / Mean | % / SD | % / Mean | SD | % / Mean | SD | |||
| Age, years† | 66.3 | 11.7 | 65.1 | 13.0 | 0.09 | 65.5 | 11.9 | 65.2 | 12.9 | 0.03 |
| ≤ 65 | 49 | (45.8%) | 552 | (49.9%) | 0.08 | (52.4%) | (49.7%) | 0.05 | ||
| > 65 | 58 | (54.2%) | 555 | (50.1%) | (47.6%) | (50.3%) | ||||
| Sex | ||||||||||
| Male | 65 | (60.7%) | 595 | (53.7%) | 0.14 | (58.6%) | (54.4%) | 0.09 | ||
| Female | 42 | (39.3%) | 512 | (46.3%) | (41.4%) | (45.6%) | ||||
| Pre-existing comorbidities | ||||||||||
| Charlson's Index †,‡ | 5.6 | 2.4 | 5.3 | 1.8 | 0.15 | 5.0 | 2.2 | 5.3 | 1.8 | 0.17 |
| Hypertension | 95 | (88.8%) | 833 | (75.2%) | 0.36 | (83.7%) | (76.6%) | 0.18 | ||
| Chronic lung disease | 11 | (10.3%) | 116 | (10.5%) | 0.01 | (8.2%) | (10.4%) | 0.08 | ||
| Chronic heart disease | 24 | (22.4%) | 161 | (14.5%) | 0.20 | (19.7%) | (15.3%) | 0.12 | ||
| Chronic kidney disease | 33 | (30.8%) | 125 | (11.3%) | 0.49 | (14.6%) | (12.5%) | 0.06 | ||
| Liver disease | 14 | (13.1%) | 139 | (12.6%) | 0.02 | (11.1%) | (12.6%) | 0.04 | ||
| Malignancy | 3 | (2.8%) | 38 | (3.4%) | 0.04 | (6.1%) | (3.3%) | 0.13 | ||
| Obesity | 16 | (15.0%) | 125 | (11.3%) | 0.11 | (8.9%) | (11.7%) | 0.09 | ||
| Long-term medications | ||||||||||
| ACEI/ARB | 69 | (64.5%) | 478 | (43.2%) | 0.44 | (50.2%) | (45.1%) | 0.10 | ||
| Anticoagulant | 41 | (38.3%) | 393 | (35.5%) | 0.06 | (26.0%) | (35.6%) | 0.21 | ||
| Antiplatelet | 35 | (32.7%) | 238 | (21.5%) | 0.25 | (27.4%) | (22.7%) | 0.11 | ||
| Lipid-lowering agent | 81 | (75.7%) | 592 | (53.5%) | 0.48 | (58.5%) | (55.6%) | 0.06 | ||
| NSAID | 37 | (34.6%) | 262 | (23.7%) | 0.24 | (30.7%) | (24.8%) | 0.13 | ||
| GLP1RA | 1 | (0.9%) | 8 | (0.7%) | 0.02 | (0.2%) | (1.1%) | 0.12 | ||
| Insulin | 66 | (61.7%) | 351 | (31.7%) | 0.63 | (36.8%) | (34.3%) | 0.05 | ||
| Oral anti-diabetic drugs | ||||||||||
| Metformin | 61 | (57.0%) | 543 | (49.1%) | 0.16 | (59.1%) | (50.1%) | 0.18 | ||
| SU | 50 | (46.7%) | 268 | (24.2%) | 0.48 | (38.5%) | (26.6%) | 0.26 | ||
| TZD | 16 | (15.0%) | 61 | (5.5%) | 0.32 | (10.0%) | (6.5%) | 0.13 | ||
| Acarbose | 0 | (0.0%) | 3 | (0.3%) | NA | (0.0%) | (0.3%) | NA | ||
| SGLT2i | 14 | (13.1%) | 15 | (1.4%) | 0.47 | (3.2%) | (2.6%) | 0.04 | ||
| DPP4i drug | 107 | (100.0%) | 0 | (0.0%) | NA | (100.0%) | (0.0%) | NA | ||
| Alogliptin | 22 | (20.6%) | NA | NA | NA | (25.1%) | NA | NA | ||
| Linagliptin | 34 | (31.8%) | NA | NA | NA | (21.0%) | NA | NA | ||
| Sitagliptin | 29 | (27.1%) | NA | NA | NA | (33.0%) | NA | NA | ||
| Vildagliptin | 22 | (20.6%) | NA | NA | NA | (20.8%) | NA | NA | ||
| Concomitant drug use during hospitalization | ||||||||||
| Remdesivir | 14 | (13.1%) | 179 | (16.2%) | 0.09 | (12.5%) | (16.4%) | 0.11 | ||
| Interferon-β−1b | 68 | (63.6%) | 647 | (58.4%) | 0.10 | (53.0%) | (59.0%) | 0.12 | ||
| Dexamethasone | 37 | (34.6%) | 297 | (26.8%) | 0.17 | (23.6%) | (27.0%) | 0.08 | ||
| Tocilizumab | 4 | (3.7%) | 36 | (3.3%) | 0.03 | (1.8%) | (3.2%) | 0.09 | ||
| ECMO | 1 | (0.9%) | 11 | (1.0%) | 0.01 | (0.4%) | (1.0%) | 0.07 | ||
| Dialysis | 11 | (10.3%) | 51 | (4.6%) | 0.22 | (3.4%) | (5.0%) | 0.08 | ||
| ICU admission on admission | 25 | (23.4%) | 229 | (20.7%) | 0.06 | (13.2%) | (20.7%) | 0.20 | ||
| Admission via emergency department | 58 | (54.2%) | 546 | (49.3%) | 0.10 | (44.8%) | (49.4%) | 0.09 | ||
| Clinical severity on admission by WHO Clinical Progression Scale | ||||||||||
| Score (range 0–10) † | 4.6 | 1.0 | 4.5 | 0.9 | 0.13 | 4.4 | 0.8 | 4.5 | 0.9 | 0.10 |
| No oxygen therapy (Score 4) | 73 | (68.2%) | 829 | (74.9%) | 0.14 | (78.2%) | (74.8%) | 0.08 | ||
| Supplemental oxygen without ventilation (Score 5–6) | 32 | (29.9%) | 265 | (23.9%) | (20.9%) | (24.0%) | ||||
| Mechanical ventilation (Score 7–9) | 2 | (1.9%) | 13 | (1.2%) | (0.9%) | (1.2%) | ||||
| ARDS | 4 | (3.7%) | 25 | (2.3%) | 0.09 | (3.6%) | (2.3%) | 0.07 | ||
| macrophage activation | 0 | (0.0%) | 0 | (0.0%) | na | (0.0%) | (0.0%) | na | ||
| hematological dysfunction | 10 | (9.3%) | 71 | (6.4%) | 0.11 | (4.9%) | (6.5%) | 0.07 | ||
| Coagulopathy | 0 | (0.0%) | 6 | (0.5%) | NA | (0.0%) | (0.6%) | NA | ||
| Hepatic inflammation | 12 | (11.2%) | 81 | (7.3%) | 0.13 | (6.2%) | (7.5%) | 0.05 | ||
| Hyperinflammatory syndrome | 19 | (17.8%) | 137 | (12.4%) | 0.15 | (11.8%) | (12.7%) | 0.03 | ||
| Laboratory parameters [normal range]† | ||||||||||
| White blood cell, × 109/L [3.7–9.2 × 109/L] | 6.6 | 2.8 | 6.0 | 2.4 | 0.21 | 6.2 | 2.4 | 6.1 | 2.5 | 0.03 |
| Neutrophil, × 109/L [1.7–5.8 × 109/L] | 4.7 | 2.4 | 4.2 | 2.2 | 0.22 | 4.2 | 2.2 | 4.2 | 2.3 | 0.02 |
| Lymphocyte, × 109/L [1.0–3.1 × 109/L] | 1.1 | 0.5 | 1.2 | 0.8 | 0.12 | 1.3 | 0.6 | 1.2 | 0.8 | 0.13 |
| Platelet, × 109/L [145–370 × 109/L] | 210.1 | 86.0 | 204.9 | 75.7 | 0.07 | 208.4 | 74.3 | 205.4 | 76.0 | 0.04 |
| Lactate dehydrogenase, U/L [110–210 U/L] | 279.1 | 142.0 | 267.9 | 132.8 | 0.08 | 257.3 | 125.8 | 268.4 | 131.9 | 0.08 |
| Creatine kinase, U/L [26–192 U/L] | 240.8 | 442.7 | 206.0 | 381.5 | 0.09 | 205.5 | 387.9 | 207.2 | 374.7 | 0.00 |
| Total bilirubin, μmol/L [5–27 μmol/L] | 9.0 | 6.0 | 9.2 | 7.0 | 0.04 | 9.3 | 5.4 | 9.2 | 6.8 | 0.01 |
| C-reactive protein, mg/L [<5 mg/L] | 49.6 | 62.4 | 41.1 | 56.1 | 0.15 | 38.4 | 57.5 | 41.5 | 56.4 | 0.05 |
| Cycle threshold value, cycle | 22.5 | 6.5 | 22.5 | 6.4 | 0.00 | 23.3 | 6.3 | 22.5 | 6.4 | 0.13 |
| eGFR, ml/min/1.73m2 [>90 ml/min/1.73m2] | 79.6 | 41.8 | 106.2 | 97.1 | 0.28 | 101.6 | 40.1 | 103.9 | 93.6 | 0.03 |
| ALP, U/L [30–120 U/L] | 82.6 | 35.1 | 74.7 | 31.9 | 0.25 | 80.4 | 30.5 | 74.6 | 31.7 | 0.18 |
| ALT, U/L [<46.5 U/L] | 38.9 | 52.4 | 36.9 | 35.8 | 0.05 | 36.0 | 32.4 | 36.9 | 36.9 | 0.02 |
| Hemoglobin, g/dL [13.4–17.1 g/dL] | 12.5 | 2.0 | 13.2 | 1.7 | 0.41 | 13.3 | 1.9 | 13.1 | 1.7 | 0.12 |
| Random glucose, mmol/L [3–11 mmol/L] | 10.4 | 5.5 | 9.4 | 4.8 | 0.21 | 9.1 | 4.2 | 9.5 | 5.0 | 0.10 |
| Hemoglobin A1c,% [4.8–6.0%] | 7.8 | 2.3 | 7.4 | 2.5 | 0.15 | 7.8 | 2.2 | 7.5 | 2.5 | 0.15 |
Note: ACEI = Angiotensin converting enzyme inhibitor; ALP = Alkaline phosphatase; ALT = Alanine transaminase; ARB = Angiotensin receptor blockers; ARDS = Acute respiratory distress syndrome; AST = Aspartate aminotransferase; ECMO = Extracorporeal membrane oxygenation; eGFR= Estimated glomerular filtration rate; ICU = Intensive Care Unit; NSAID = Nonsteroidal anti-inflammatory drugs; NA = not applicable; SD = Standard deviation; SMD = Standardized mean difference.
Age, Charlson Index, clinical severity, and laboratory parameters on admission are presented in mean ± SD.
The calculation of Charlson Index does not include Acquired Immune Deficiency Syndrome (AIDS).
¶SMD of < 0.2 indicates covariate balance between preadmission use of DPP4i and control groups.
Primary outcome
DPP4i use was associated with lower odds of clinical deterioration (OR=0.71, 95%CI 0.54 to 0.93, P = 0.013). Sensitivity (Table S3; see supplementary materials associated with this article online) and subgroup (Table S4; see supplementary materials associated with this article online) analyses were generally in line with the main results.
Secondary outcomes
DPP4i use was associated with lower odds of hyperinflammatory syndrome (OR=0.52, 95%CI 0.42 to 0.65, P < 0.001) and invasive mechanical ventilation (OR=0.30, 95%CI 0.21 to 0.42, P < 0.001) than those of control (Table 2 ).
Table 2.
Comparison of clinical deterioration, clinical improvement, low viral load, Immunoglobulin G antibody, hyperinflammatory syndrome between the DPP4i and control groups.
| Before weighting |
After weighting |
||||||
|---|---|---|---|---|---|---|---|
| DPP4i |
Control |
DPP4i |
Control |
DPP4i vs Control |
|||
| Outcomes | % (N) | % (N) | % | % | OR† | 95% CI | P-value |
| Primary outcome | |||||||
| Clinical deterioration (composite outcome of in-hospital death or invasive mechanical ventilation) | 21.9% (105) | 19.7% (1094) | 14.9% | 19.9% | 0.71 | (0.54, 0.93) | 0.013 |
| Secondary outcome | |||||||
| Clinical improvement on WHO clinical progression scale by ≥ 1 score | 88.8% (107) | 91.4% (1107) | 91.8% | 91.3% | 1.08 | (0.72, 1.61) | 0.706 |
| Low viral load (Ct value ≥ 30) | 83.0% (94) | 82.8% (1003) | 82.9% | 82.8% | 1.01 | (0.73, 1.38) | 0.969 |
| IgG antibody | 81.3% (107) | 86.5% (1107) | 85.9% | 86.4% | 0.95 | (0.71, 1.29) | 0.761 |
| In-hospital death | 14.0% (107) | 9.0% (1107) | 11.4% | 9.2% | 1.28 | (0.91, 1.79) | 0.151 |
| Invasive mechanical ventilation | 11.4% (105) | 13.7% (1094) | 4.5% | 13.8% | 0.30 | (0.21, 0.42) | <0.001 |
| Hyperinflammatory syndrome | 48.9% (88) | 54.4% (970) | 38.4% | 54.4% | 0.52 | (0.42, 0.65) | <0.001 |
Note: CI = confidence interval; Ct = cycle threshold; IgG = Immunoglobulin G; OR = odds ratio;.
OR > 1 (or < 1) indicates DPP4i was associated with greater (lower) odds of clinical deterioration (composite outcome of in-hospital death or invasive mechanical ventilation), clinical improvement, low viral load, IgG antibody, hyperinflammatory syndrome compared to the control group;.
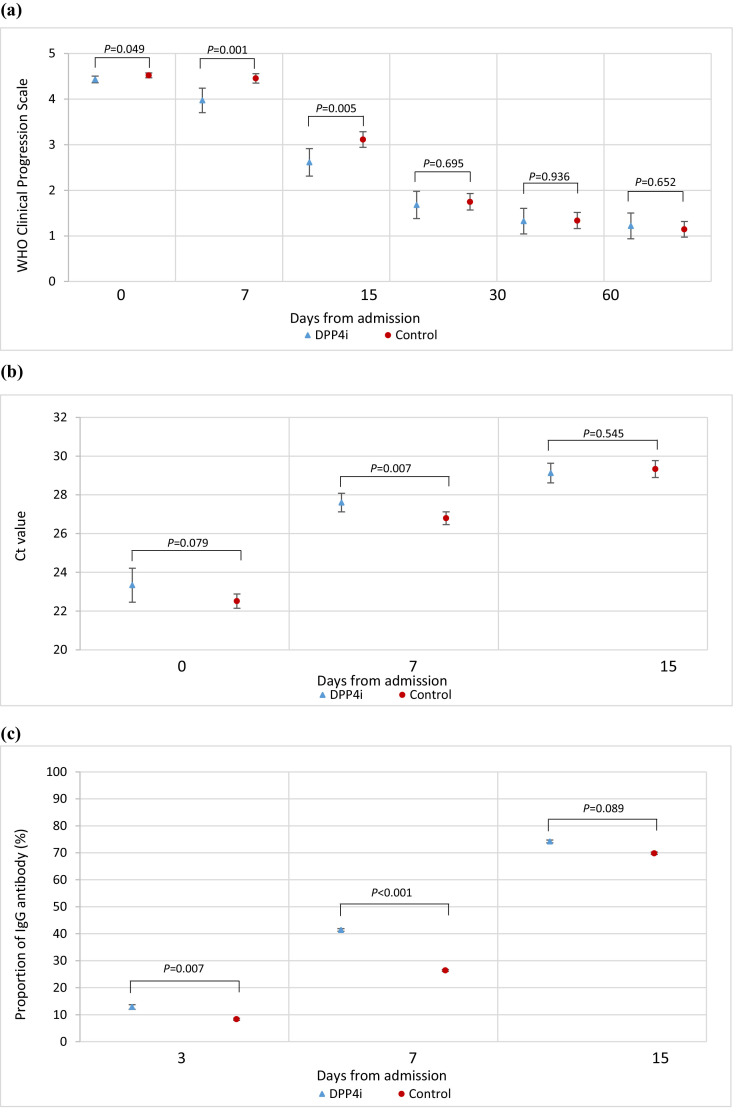
Low viral load was defined as Ct value ≥ 30 cycles. The odds of achieving low viral load (OR=1.01, 95%CI 0.73 to 1.38, P = 0.969) and positive IgG antibody (OR=1.06, 95%CI 0.75 to 1.49, P = 0.752) were comparable between groups while the proportion of positive IgG antibody was significantly higher in DPP4i users on day-3 (13% vs 8%, P = 0.007) and day-7 (41% vs 26%, P < 0.001) (Fig. 1 ). However, DPP4i use was not associated with lower odds in-hospital mortality (OR=1.28, 95% CI 0.91–1.79, P = 0.151).
Fig. 1.
Comparisons of (a) WHO Clinical Progression Scale score, (b) Ct value (c) proportion of IgG antibody among patients between DPP4i and control group.
For survivors, use of DPP4i was associated with a significantly shorter length of hospitalization (−4.82 days, 95%CI –6.80 to –2.84, P < 0.001) compared to control. Fig. 2 illustrated the clinical severity status of the DPP4i users and controls on admission (baseline, day 0) and over the follow-up period (days 7, 15, 30, 60, and 90).
Fig. 2.
Comparisons of clinical status measured by WHO Clinical Progression Scale score from baseline to 90-day among patients between DPP4i and control group.
Discussion
In this retrospective, territory-wide cohort of T2DM patients hospitalized with COVID-19, DPP4i use was associated with lower odds of clinical deterioration and hyperinflammatory syndrome, significantly higher mean Ct values and proportion of positive IgG antibody on day-7, and decreased length of hospital stay when compared to control.
The view for DPP4i to be a repurposed agent as COVID-19 treatment owing to its potential role in inhibiting viral entry and its anti-inflammatory, antifibrotic and immunomodulatory properties was contested in absence of conclusive studies [20]. According to a critical appraisal of literatures (n = 12) by Singh et al., studies reporting positive clinical outcome are generally more than that of negative clinical outcome (5 vs 1, respectively) [21]. Both Fadini et al. and Kim et al. reported insignificant results for mortality [22, 23] while Mirani et al., Solerte et al. and Montastruc et al. measured an independently significant reduction in mortality (HR: 0.13, 95%CI 0.02–0.92; HR: 0.44 95%CI 0.29–0.66) and lower rate of intubation (43% vs 81%) [24], [25], [26]. Several other studies reported quicker hospital discharge and lower risk of progression to severe COVID-19 [27, 28]. Hariyanto, in their systematic review, has reported no significant association between DPP4i use and mortality (OR: 1.14, 95% CI 0.87–1.51), while another systematic review, meta-analysis and meta-regression by Rakhmat et al. has reported a risk ratio of 0.76 (0.60–0.97, P = 0.030) for mortality in COVID-19 patients with T2DM using DPP4i, despite weakened association in patients on concomitant metformin or ACEi [29, 30]. Our study is in line with studies mentioned above as we have proven lower odds of clinical deterioration and length of hospitalization. However, the studies mentioned contain several limitations. Findings by Montastruc et al. may be susceptible to indication bias and shortcomings owing to data collection method [26], and Solerte et al. was challenged on the grounds of imbalanced baseline characteristics [31]. None reported reduction of viral load, detection of IgG antibody nor hyperinflammatory syndrome as an outcome, which our study has succeeded in proving mean Ct value was higher than control, the proportion of positive IgG antibody was significantly higher in DPP4i users on day-3 and day-7 and a significant reduction in the risk of hyperinflammatory syndrome. Severity appears to be associated with time to detection of IgG in both MERS-CoV and SARS-CoV-2 infection (2–3 days longer for more severe cases) [32], hence higher proportion of IgG antibody may indicate better prognosis in DPP4i users. Mechanistic explanations as well as associated findings in our study shall be illustrated below.
Despite contested view, there are evidence and mechanistic explanations for DPP4i's anti-inflammatory potential. Marques et al. has demonstrated reduction in proinflammatory cytokine levels as well as inflammatory gene expression in diabetic mice [8], an observation echoed by several other studies involving COVID-19 patients [21, 29]. Another study by Satoh-Asahara et al. has also found increased serum interleukin-10 (IL-10), an anti-inflammatory cytokine, in diabetic patients treated with sitagliptin (a member of DPP4i) [15]. With reference to studies consulted, DPP4i is proposed to exert anti-inflammatory effects by two distinct pathways mediated by DPP4 pleiotropy and GLP-1 receptors (GLP-1R), eventually uniting at the same transcription factor NF-κB [33, 34].
DPP4 actions are pleiotropic as it is widely distributed on adipocytes, immune cells and various tissues [4]. It has been learnt that when using DPP4i, T-cell proliferation and restoration of immune homeostasis may be facilitated by decreasing DPP4-Adenosine deaminase (ADA) interaction, which has been dysregulated by upregulation of DPP4 expression in obesity and T2DM [7, 35]. Another interacting compound is caveolin-1 (Cav-1). Binding of Cav-1 and DPP4 at the cellular membrane would result in the phosphorylation of Cav-1 thus lowering Nitric Oxide (NO) levels for the activation of NF-κB [33]. On the other hand, DPP4i use is known to raise GLP-1R stimulation known to activate the protein kinase B (Akt) pathway which would raise endothelial Nitric Oxide Synthase derived NO production [34], hence may reduce gene expression and production of proinflammatory cytokines via NF-κB. This hypothesis is supported by studies observing reduced number of inflammatory cells and macrophages within visceral adipose depots, vascular endothelium and pancreatic islets [22, 36]. In our study, such results are replicated by a significant reduction in composite outcomes for hyperinflammatory syndrome associated with DPP4i use.
Another topic worth investigating is the potential for DPP4i to exhibit anti-fibrotic effects. DPP4 was found to potentially promote cytokines and chemokines production as well as smooth muscle cell proliferation by fibroblasts [37]. Hence, DPP4 inhibition may effectively hinder progression of lung fibrosis and potentially reducing requirement for ventilation treatment in COVID-19 patients, a trend supported by significantly reduced risks of clinical deterioration (in-hospital death or invasive mechanical ventilation) and need for invasive mechanical ventilation in our study as well as various in vivo studies investigating lung injury and repair [33].
The second proposed pathway to reduce inflammation was reducing SARS-CoV-2 entry for infection [16]. This hypothesis was based on the findings that there was strong interaction between DPP4 and the S1 region of the spike protein of SARS-CoV-2 through van der Waals’ force and hydrogen bonds [14]. It has become an increasing concern for patients with diabetes and obesity as they were both found to have increased DPP4 expression hence more susceptible to SARS-CoV-2 infection. Accordingly, DPP4i was investigated regarding its effectiveness in preventing the entry of SARS-CoV-2 through membrane-bounded DPP4. Firstly, DPP4i may not act as a competitive inhibitor for SARS-CoV-2 due to difference in binding sites on DPP4. DPP4i binds to the catalytic site of DPP4 while SARS-CoV-2 interacts with DPP4’s residual amino acids in the glycosylation-rich region [38]. Thus, such difference in the binding regions between DPP4i and SARS-CoV-2 with DPP4 suggests that competitive inhibition may not be feasible. Secondly, assuming that DPP4 was indeed a viral receptor for SARS-CoV-2, [31, 38] soluble DPP4 (sDPP4) would have the same affinity for SARS-CoV-2 and increased serum concentration of sDPP4 would reduce SARS-CoV-2 binding with membrane-bounded DPP4, hence lower the rate of viral entry into cells and less severe COVID-19 [16, 38]. By successful prevention of entry, viral proliferation will be halted, hence the severity of infection and inflammation may ultimately be reduced. Currently there were no papers that have researched the efficacy of DPP4i on reducing viral load for COVID-19 patients. Despite the lack of significant results in lowering viral load in DPP4i users when compared to control, in our study, it was found that DPP4i users has higher mean Ct value at day-7 of the observation when compared to the control by which may shed light on the possibility of such mechanism. However, the potential mechanism for viral load reduction remains to be elucidated by further in-vitro, in-vivo and prospective studies as the pathway's feasibility remains doubted.
Final pathway which DPP4i reduces inflammation is by good glycemic control which may reduce inflammation caused by diabetes and COVID-19, as patients who have hyperglycemia and poor glycemic control were found to suffer from low-grade inflammation, supported by elevated cytokine levels when compared to patients who have well-controlled blood glucose levels [39]. DPP4i competitively inhibits the binding of incretins to DPP4, preserving insulinotropic activities of incretins hence increasing insulin secretion and restoring euglycemia as well as immune homeostasis. This hypothesis was also supported by a significant reduction in composite outcomes for hyperinflammatory syndrome associated with the use of DPP4i.
It should be noted that odds of in-hospital mortality, as a classical outcome in most studies investigating the influence of glucose-lowering agents on patient prognosis admitted to the hospital for COVID-19 [40], were not negatively associated with DPP4i use in our study, and instead showed insignificantly a slight increase in OR. In literature, the relationship between DPP4i use and in-hospital mortality is widely disputed [41]. Data from the COVID-PREDICT cohort study in The Netherlands has reported no association between DPP4i treatment and mortality nor a more severe clinical course [42] while a nationwide cohort study published has established a positive relationship between mortality and DPP4i use in their unadjusted univariate model, unlike its adjusted counterpart [43]. The results of our study have added to the inconclusive evidence for DPP4i use and in-hospital mortality and it is hoped that further studies can elucidate the aforementioned relationship.
Despite encouraging results can be seen regarding use of DPP4i with regards to anti-inflammation, there were still several limitations. Firstly, our study strength might be limited by the small sample size regarding to our number of DPP4i users (n = 107). Secondly, residual confounding cannot be fully accounted for due to the observational nature of the study. Nevertheless, propensity score weighting has been performed between DPP4i users and non-users across demographics, comorbidities, and a comprehensive panel of hematological and biochemical laboratory parameters. Thirdly, SARS-CoV-2 viral loads were represented by Ct values in this study. Despite a good correlation, direct quantitative measurements of viral loads would have been preferred if available. Lastly, all data was collected from territory-wide population-based cohorts of T2DM patients with confirmed COVID-19 admitted to public hospitals in Hong Kong. Hence, there may be variations regarding our findings when applied to out-patient setting or other populations with different ethnicity in different regions.
Conclusion
Preadmission use of DPP4i for T2DM patients with COVID-19 was associated with reduced odds of clinical deterioration, hyperinflammatory syndrome, need for invasive mechanical ventilation, and reduced length of hospitalization despite lack of association with in-hospital mortality. Our study may have shed light regarding hyperinflammatory dynamics and viral clearance in mild-to-moderate COVID-19 patients with T2DM, which may be informative to these patient cohorts and associated clinicians. However, prospective studies are necessary to further elucidate the potential role of DPP4i in the pathophysiology of T2DM and COVID-19.
Ethical approval and informed consent
The study protocol was approved by the Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (Reference No. UW 20-493). Given the extraordinary nature of the COVID-19 pandemic, individual patient informed consent was not required for this retrospective cohort study using anonymised data.
Sources of funding
We received financial support from the Health and Medical Research Fund, Food and Health Bureau, Government of the Hong Kong Special Administrative Region, China (grant no. COVID190210). The funders did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing statement
The data that support the findings of this study were provided by the Hong Kong Hospital Authority. Restrictions apply to the availability of these data, which were used under license for this study. Deidentified individual participant data will not be made available.
Acknowledgements
We thank the Hong Kong Hospital Authority for the data provision, and the support from Research Internship Scheme, Li Ka Shing Faculty of Medicine, University of Hong Kong.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.diabet.2021.101307.
Appendix. Supplementary materials
References
- 1.(WHO) WHO. WHO Coronavirus (COVID-19) Dashboard. Updated 29/07/2021. Accessed 30/07, 2021. https://covid19.who.int/
- 2.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalan R., Ang L.W., Tan W.Y., Fong S.W., Tay W.C., Chan Y.H., et al. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother. 2021;23:e48–e51. doi: 10.1093/ehjcvp/pvaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landstra C.P., De Koning E.J. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol. 2021;12:5. doi: 10.3389/fendo.2021.649525. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwok S., Adam S., Ho J.H., Iqbal Z., Turkington P., Razvi S., Le Roux C.W., et al. Obesity: a critical risk factor in the COVID-19 pandemic. Clin Obes. 2020;10:e12403. doi: 10.1111/cob.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong J., Rao X., Deiuliis J., Braunstein Z., Narula V., Hazey J., et al. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques C., Mega C., Gonçalves A., Rodrigues-Santos P., Teixeira-Lemos E., Teixeira F., et al. Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/538737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deacon C., Lebovitz H.E. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18:333–347. doi: 10.1111/dom.12610. [DOI] [PubMed] [Google Scholar]
- 10.Scheen A.J. The safety of gliptins : updated data in 2018. Expert Opin Drug Saf. 2018;17:387–405. doi: 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- 11.Powell W.R., Christiansen C.L., Miller D.R. Meta-Analysis of sulfonylurea therapy on long-term risk of mortality and cardiovascular events compared to other oral glucose-lowering treatments. Diabetes Ther. 2018;9:1431–1440. doi: 10.1007/s13300-018-0443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S., Chai S., Yang J., Cai T., Xu Y., Yang Z., Zhang Y., et al. Gastrointestinal adverse events of dipeptidyl peptidase 4 inhibitors in type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2017;39 doi: 10.1016/j.clinthera.2017.07.036. 1780–9.e33. [DOI] [PubMed] [Google Scholar]
- 13.Klemann C., Wagner L., Stephan M., von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4′s (DPP4) entanglement in the immune system. Clin Experim Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vankadari N., Wilce J.A. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh-Asahara N., Sasaki Y., Wada H., Tochiya M., Iguchi A., Nakagawachi R., et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2013;62:347–351. doi: 10.1016/j.metabol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Scheen A.J. DPP-4 inhibition and COVID-19: from initial concerns to recent expectations. Diabetes Metab. 2021;47 doi: 10.1016/j.diabet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Working Group on the Clinical Characterisation and Management of Covid-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/s1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb B.J., Peltan I.D., Jensen P., Hoda D., Hunter B., Silver A., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tom M.R., Mina M.J. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71:2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drucker D.J. Coronavirus infections and type 2 diabetes—Shared pathways with therapeutic implications. Endocrine Rev. 2020;41:457–470. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A.K., Singh R., Saboo B., Misra A. Non-insulin anti-diabetic agents in patients with type 2 diabetes and COVID-19: a critical appraisal of literature. Diabetes Metab Syndr. 2021;15:159–167. doi: 10.1016/j.dsx.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadini G.P., Morieri M.L., Longato E., Bonora B.M., Pinelle S., Selmin E., et al. Exposure to dipeptidyl-peptidase-4 inhibitors and COVID-19 among people with type 2 diabetes: a case-control study. Diabetes Obes Metab. 2020;22:1946–1950. doi: 10.1111/dom.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M.K., Jeon J.-.H., Kim S.-.W., Moon J.S., Cho N.H., Han E., et al. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44:602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirani M., Favacchio G., Carrone F., Betella N., Biamonte E., Morenghi E., et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. 2020;43:3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 25.Solerte S.B., D'Addio F., Trevisan R., Lovati E., Rossi A., Pastore I., et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43:2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montastruc F., Romano C., Montastruc J.-.L., Silva S., Seguin T., Minville V., et al. Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to intensive care unit in South of France. Therapie. 2020;75:381. doi: 10.1016/j.therap.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wargny M., Potier L., Gourdy P., Pichelin M., Amadou C., Benhamou P.Y., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64:778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee S.Y., Lee J., Nam H., Kyoung Dd-S, Shin D.W., Kim D.J. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab J. 2021;45:251–259. doi: 10.4093/dmj.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hariyanto T.I., Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20:543–550. doi: 10.1007/s40200-021-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakhmat I.I., Kusmala Y.Y., Handayani D.R., Juliastuti H., Nawangsih E.N., Wibowo A., et al. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19)–a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2021;15:777–782. doi: 10.1016/j.dsx.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauck M.A., Meier J.J. Reduced COVID-19 mortality with sitagliptin treatment? weighing the dissemination of potentially lifesaving findings against the assurance of high scientific standards. Diabetes Care. 2020;43:2906–2909. doi: 10.2337/dci20-0062. [DOI] [PubMed] [Google Scholar]
- 32.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrean S., Gao X.-.P., Brovkovych V., Shimizu J., Zhao Y.Y., Vogel S.M., et al. Caveolin-1 regulates NF-κB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 34.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterol. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Bassendine M.F., Bridge S.H., McCaughan G.W., Gorrell M.D. COVID-19 and comorbidities: a role for dipeptidyl peptidase 4 (DPP4) in disease severity? J Diabetes. 2020;12:649–658. doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- 36.Dobrian A.D., Ma Q., Lindsay J.W., Leone K.A., Ma K., cohen J., et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300:e410–e421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantanetti P., Cangelosi G., Ambrosio G. Potential role of incretins in diabetes and COVID-19 infection: a hypothesis worth exploring. Intern Emerg Med. 2020;15:779–782. doi: 10.1007/s11739-020-02389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krejner-Bienias A., Grzela K., Grzela T. DPP4 inhibitors and COVID-19–holy grail or another dead end? Arch Immunol Ther Exp. 2021;69:1–8. doi: 10.1007/s00005-020-00602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L., She Z.-.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikhail N., Wali S. Safety of dipeptidyl peptidase-4 inhibitors in Covid-19: an update. J Infectious Dis Case Rep SRC/JIDSCR-147 DOI. 2021;132:3. https://doi org/1047363/JIDSCR/2021(2) [Google Scholar]
- 42.Meijer R.I., Hoekstra T., van den Oever N.C.G., Simsek S., van den Bergh J., Douma R.A., et al. Treatment with a DPP-4 inhibitor at time of hospital admission for COVID-19 is not associated with improved clinical outcomes: data from the COVID-PREDICT cohort study in the Netherlands. J Diabetes Metab Dis. 2021:1–6. doi: 10.1007/s40200-021-00833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., Ayala-Gutierrez M.M., Fuentes-Jimenez F., Huerta L.J., et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18:359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.