Abstract
Ubiquitin-proteasome-mediated destruction of rate-limiting proteins is required for timely progression through the main cell cycle transitions. The anaphase-promoting complex (APC), periodically activated by the Cdh1 subunit, represents one of the major cellular ubiquitin ligases which, in Saccharomyces cerevisiae and Drosophila spp., triggers exit from mitosis and during G1 prevents unscheduled DNA replication. In this study we investigated the importance of periodic oscillation of the APC-Cdh1 activity for the cell cycle progression in human cells. We show that conditional interference with the APC-Cdh1 dissociation at the G1/S transition resulted in an inability to accumulate a surprisingly broad range of critical mitotic regulators including cyclin B1, cyclin A, Plk1, Pds1, mitosin (CENP-F), Aim1, and Cdc20. Unexpectedly, although constitutively assembled APC-Cdh1 also delayed G1/S transition and lowered the rate of DNA synthesis during S phase, some of the activities essential for DNA replication became markedly amplified, mainly due to a progressive increase of E2F-dependent cyclin E transcription and a rapid turnover of the p27Kip1 cyclin-dependent kinase inhibitor. Consequently, failure to inactivate APC-Cdh1 beyond the G1/S transition not only inhibited productive cell division but also supported slow but uninterrupted DNA replication, precluding S-phase exit and causing massive overreplication of the genome. Our data suggest that timely oscillation of the APC-Cdh1 ubiquitin ligase activity represents an essential step in coordinating DNA replication with cell division and that failure of mechanisms regulating association of APC with the Cdh1 activating subunit can undermine genomic stability in mammalian cells.
To support error-free development and ensure tissue homeostasis of multicellular organisms, eukaryotic cells evolved multiple layers of tightly controlled molecular pathways that coordinate the progression through distinct phases of the cell cycle. These mechanisms ultimately converge on regulating the activity of cyclin-dependent kinases (CDKs), which by phosphorylating their critical substrates catalyze progression through the main cell cycle transitions (40, 44, 50). Besides the active role of CDKs, timely and rapid inactivation of those CDKs that fulfilled their functions appears to be equally important in promoting cell cycle progression (21). The ubiquitin-proteasome-mediated destruction of the cyclin subunits represents a key mechanism supporting the timing of CDK inhibition (14, 22). Covalent attachment of polyubiquitin chains priming the mitotic cyclins for degradation by the proteasome is catalyzed by the anaphase-promoting complex (APC) ubiquitin ligase, a large multiprotein particle composed of at least 10 subunits (41, 48, 69). As such, APC possesses little ubiquitin ligase activity unless it is activated by a direct interaction with either of the two additional subunits, Cdc20 (fizzy in Drosophila melanogaster) or Cdh1 (fizzy-related) (10, 24, 57, 60, 66). APC activity during the cell cycle is highly periodic. At the metaphase-anaphase transition, APC associates with Cdc20, an event which strictly requires previous phosphorylation of the APC core by mitotic kinases (25, 59). In budding yeast, the activated APC-Cdc20 complex initiates sister chromatid separation by triggering destruction of the securin Pds1 (7, 8) and facilitates exit from mitosis by initiating degradation of the mitotic cyclin Clb5 (58). APC-Cdh1 assembles later in anaphase. In sharp contrast to Cdc20, Cdh1 activates both interphase and mitotic APC, and its binding to the APC core is negatively regulated by phosphorylation of the Cdh1 subunit itself (19, 24, 25, 32, 70). By targeting mitotic cyclins such as Clb2 for degradation, APC-Cdh1 contributes to abrupt silencing of mitotic CDK activity, a regulatory step essential for reestablishment of preinitiation complexes on origins of DNA replication (43). During the exit from mitosis, Cdc20 is degraded (52, 68), whereas Cdh1 remains bound to APC throughout G1 (10, 19, 24, 70). The resulting postmitotic activity of APC-Cdh1, at least in Saccharomyces cerevisiae, appears to be critically important for establishment and maintenance of the G1 phase (17, 29, 57, 66). Consistently, also during Drosophila development, the Cdh1 homologue fizzy-related is expressed only in those cell cycles that contain a G1 phase (60). The physiological significance of persistent APC activity during G1 could at least partly reflect prevention of precocious accumulation of the mitotic cyclins. In addition, APC-Cdh1 may also control accumulation of other S-phase-promoting factors such as Dbf4 (5, 11, 47, 67), as well as inhibitors of initiation of DNA replication, exemplified by geminin (38). Collectively, all of the above listed evidence points to an important role of APC-Cdh1 in both mitotic exit and regulation of DNA replication. Apart from the crucial importance of APC-dependent proteolysis for cell cycle progression, several reports have suggested a role for APC-Cdh1 activity in quiescent cells (2, 13).
Recently, we have witnessed tremendous progress in understanding the molecular anatomy of the APC in yeast and vertebrate experimental systems. The need to elucidate APC function and identify its natural substrates in human somatic cells has recently become apparent from studies demonstrating a potential link between APC-dependent proteolysis and cancer. Thus, kinetochore-associated APC regulators Mad2, Mad3, and Bub1 were found down-regulated or mutated in subsets of tumors and directly implicated in contributing to genomic instability (3, 16, 30). Molecular cloning of human securin (73) unexpectedly revealed the identity of Pds1 with the PTTG oncogene overexpressed in several types of human cancer (9, 55). Our own results showed that APC-Cdh1 assembly is controlled by the pRb-E2F tumor suppressor pathway which is frequently deregulated during multistep tumorigenesis (32). Here we have generated novel experimental tools allowing positive or negative modulation of the APC ubiquitin ligase activity by conditional manipulation of APC-Cdh1 assembly or ablation of Cdh1 function by neutralizing antibodies, respectively. We present data supporting an essential role of periodic oscillation of the APC-associated ubiquitin ligase activity for proliferation and genome integrity of human cells.
MATERIALS AND METHODS
Plasmids and gene transfer.
Human Cdh1 cDNA was tagged on the amino terminus with myc epitope and subcloned into the pBI tetracycline-responsive plasmid (Clontech). Expression plasmids coding for puromycin resistance, i.e., pBabePuro, for a constitutively active mutant of the retinoblastoma protein, i.e., pRbΔcdk, and for cyclin B1-luciferase were reported previously (32, 36). The cycE-Luc reporter plasmid (pCE −3565/+263) (1) was a gift from P. Jansen-Dürr. Plasmids 6xE2F-Luc containing six E2F-responsive elements in front of the TATA box and Myc-Luc containing four Myc-responsive E boxes cloned into the pG12-promoter vector and pCMV-LacZ reporter plasmids were previously described (56).
Cell culture.
U-2-OS-TA, a U-2-OS human osteosarcoma cell line with stably integrated tetracycline-regulated transcriptional activator and a neomycin resistance gene (37), was transfected with the pBI plasmid containing myc-Cdh1 along with the pBabePuro plasmid in a 10:1 ratio. Puromycin-resistant clones were isolated and cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum (FCS), G418 (400 μg/ml), puromycin (1 μg/ml), and tetracycline (2 μg/ml). Induction of myc-Cdh1 expression by removal of tetracycline was performed according to procedures previously published (37). Synchronization of cells in S phase was achieved by addition of aphidicolin (5 μg/ml) into culture medium for 18 h. Metaphase-arrested cells were obtained by incubating the cells in the presence of nocodazole (40 ng/ml) for 12 h.
Immunochemical techniques.
Antibodies used in this study included rabbit polyclonal antibodies to human Cdc20 (SC 8358; Santa Cruz), p27 (PC52; Calbiochem), mitosin (14C10; GeneTex), and CENP-F (NB500-101; Novus Biologicals). Rabbit sera to human Cdh1 and Cdc27 were previously described (13, 24). Rabbit sera against cyclin A and human Pds1 were obtained from M. Pagano and H. Zou, respectively. Mouse monoclonal antibodies included CC03 to cyclin B1 (Calbiochem); PL-5 to human Plk1 (15); 9E10 to myc epitope (gift from G. Evan); CTR-453 to pericentriolar proteins (donated by M. Bornens); KH-95 to E2F-1 (Santa Cruz), DCS-141 to Mcm7 (C. S. Sørensen, C. Lukas, E. R. Kramer, C. Gieffers, J.-M. Peters, J. Bartek, and J. Lukas, unpublished data); HE-12 and HE-172 to cylin E (34), and A78720 to Aim1 (Transduction Laboratories). Monoclonal antibody to Skp2 was provided by W. Krek. Immunoprecipitation, immunoblotting, and in situ immunocytochemical techniques including detection of bromodeoxyuridine (BrdU) incorporated into newly synthesized DNA were described earlier (33, 35). Cyclin E-associated kinase activity using histone H1 as a substrate was assessed essentially as previously described (56).
Microinjection.
Affinity-purified rabbit antibody to Cdh1 (Sat105 [13]) or purified nonimmune rabbit immunoglobulin G (IgG) (Sigma) was microinjected (2 mg/ml) into R-12 cells (34) grown on glass CELLocate coverslips (Eppendorf) and synchronized in G0 by incubation for 48 h in serum-free medium. Alternatively, expression plasmids for myc-Cdh1, cyclin E, and pRbΔcdk (25-μg/ml needle concentration) were coinjected in combinations specified in the figure legends. The cells were subsequently stimulated by the addition of medium containing 10% FCS supplemented with BrdU (100 μg/ml). All microinjections were performed with a Zeiss-AIS system exactly as previously described (33, 34).
Reporter assays.
U-2-OS Cdh1 cells were electroporated with 1 μg of the reporter plasmids containing Myc, cyclin E, and E2F promoter fragments regulating the expression of the luciferase gene, together with 0.5 μg of the internal control pCMV-LacZ. The cells were harvested after 48 h of culture with or without tetracycline as indicated in the figure legends. The resulting luciferase and β-galactosidase activities were measured with a Lumat LB 9501 luminometer (Berthold) and an UltroSpec 2000 spectrophotometer (Pharmacia Biotech), respectively.
RT-PCR.
U-2-OS Cdh1 cells were induced to express myc-Cdh1 by removal of tetracycline from the culture medium for the time specified in figure legends. Total RNA was isolated using a Triazol reagent kit (Gibco-BRL) according to manufacturer's instructions. Conditions for reverse transcription (RT) and PCR amplification including the primer sequences for cyclin E and the porphobilinogen deaminase (PBGD) housekeeping gene have been described in detail (56).
Flow cytometry.
Cells were trypsinized and fixed in 70% ice-cold methanol for 20 min, washed in phosphate-buffered saline, and incubated for 30 min at 37°C in propidium iodide (PI) buffer (10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 50 μg of PI per ml, and 10 μg of RNase A per ml). The stained cells were acquired by the FACSCalibur flow cytometer (Becton Dickinson), and the DNA content was analyzed using CellQuest software.
In vitro ubiquitination assay.
Cyclin B fragments (amino acids 13 through 110) from sea urchins were iodinated as previously described (24). [35S]methionine- and [35S]cysteine-labeled Xenopus Pds1, Plx, and geminin and human Aim1, cyclin A, and Cdc20 proteins were prepared by coupled transcription-translation reactions in rabbit reticulocyte lysate (Promega). To obtain highly pure Cdh1-activated APC, the inactive APC core was purified from interphase Xenopus egg extracts. Under such conditions, APC is not phosphorylated and thus not bound to Cdc20. Since Xenopus eggs do not contain any endogenous Cdh1, such APC was highly and specifically activated by baculovirus-expressed human Cdh1. Conversely, specific APC-Cdc20 ubiquitin ligase was generated by using APC purified from mitotic Xenopus oocytes, which contain high cyclin B-Cdc2 activity essential to modify the APC core to a status activatable by baculovirus-expressed human Cdc20. The in vitro ubiquitination reaction was performed essentially as previously described (25). Samples were analyzed by sodium dodecyl sulfate-5 to 15% polyacrylamide gel electrophoresis and phosphorimaging.
RESULTS
Generation of human cell lines allowing conditional manipulation of APC-Cdh1 assembly during the cell cycle.
Human U-2-OS cells engineered to express myc-tagged Cdh1 in a tetracycline-dependent manner rapidly accumulated the myc-Cdh1 protein upon removal of tetracycline from culture medium (Fig. 1A). Densitometric measurements revealed that the degree of myc-Cdh1 overexpression varied among different clones, ranging from a five- to eightfold increase compared to the endogenous protein (fivefold in clone A6, shown as an example in Fig. 1A). All phenotypic and biochemical consequences of the myc-Cdh1 induction were identical and essentially reproduced in both higher- and lower-expressing clones, and unless stated otherwise, the data shown below were obtained from experiments performed with clone A6.
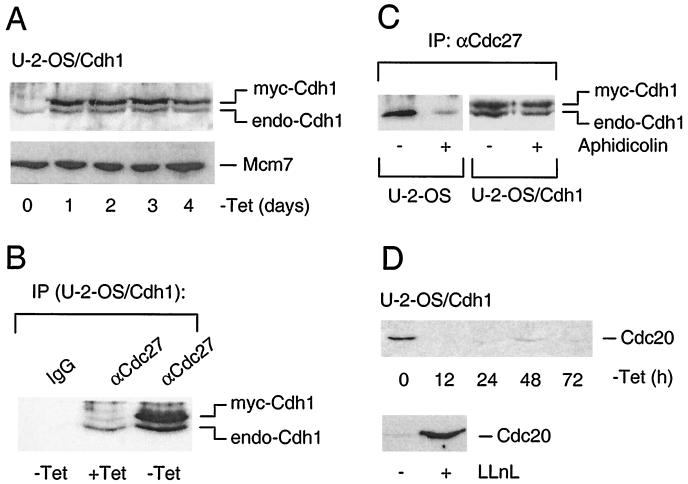
FIG. 1.
Conditionally elevated myc-Cdh1 associates with endogenous APC irrespective of cell cycle position. (A) U-2-OS Cdh1-inducible cells (clone A6) were grown in the absence of tetracycline and at the indicated time points, the abundance of endogenous and myc-tagged Cdh1 was determined by Western blotting using a rabbit polyclonal antibody raised against the full-length Cdh1 protein. Western blotting of stable Mcm7 protein is provided as a loading control. (B) Exponentially grown U-2-OS Cdh1 cells were induced to express ectopic Cdh1 as indicated by removal of tetracycline for 24 h. The cell extracts were immunoprecipitated (IP) either with an unrelated control antibody (IgG) or with antiserum against the structural APC component Cdc27 (αCdc27). The relative amount of Cdh1 bound to the immunopurified APC was determined by Western blotting essentially as described for panel A. (C) Parental U-2-OS and U-2-OS Cdh1 cell lines were synchronized in S phase by aphidicolin or mock treated with the solvent (dimethyl sulfoxide) as indicated. In U-2-OS Cdh1 cells, the myc-Cdh1 transgene was induced by removal of tetracycline for an additional 24 h (in the constant presence of aphidicolin). The ability of Cdh1 to interact with APC was then determined exactly as described for panel B. (D) U-2-OS Cdh1 cells were induced by the removal of tetracycline, and at the indicated time points, the cell extracts were analyzed by Western blotting for the abundance of the Cdc20 protein. In parallel, the cells induced to express myc-Cdh1 for 24 h were incubated either with solvent (dimethyl sulfoxide) or with a proteasome inhibitor LLnL for 12 h before immunoblotting with anti-Cdc20 antibody.
Immunoprecipitation analyses verified that the myc-Cdh1 protein induced in exponentially growing U-2-OS cells was functional, as it readily associated with the cellular APC, immunopurified through its Cdc27 subunit (Fig. 1B). Moreover, induction of myc-Cdh1 resulted in formation of stable APC complexes also in cells synchronized in S phase by aphidicolin (Fig. 1C), which means under conditions when APC and Cdh1 normally do not associate due to CDK-dependent phosphorylation of the Cdh1 subunit (10, 24, 32). We concluded that the degree of elevation in Cdh1 protein conditionally achieved in our cell lines saturates the cellular capacity to modify Cdh1 during S phase and results in stable, nonoscillating APC-Cdh1 association throughout the cell cycle. These results suggest that Cdh1 protein levels relative to other APC subunits and to its upstream regulators such as cyclin A (32) must be tightly controlled and must not exceed a certain threshold to avoid untimely APC activation.
The next question was whether the ectopic Cdh1 also affects recruitment of Cdc20, another activating subunit, into APC complexes. Interestingly, Western blotting analysis revealed that following myc-Cdh1 induction, Cdc20 protein rapidly disappeared and remained virtually undetectable as long as the cells grew without tetracycline (Fig. 1D). Reduction of the Cdc20 protein was caused by acceleration of its turnover since inhibition of the proteasome restored the Cdc20 protein levels to the values observed in exponentially growing cells (Fig. 1D). The rapid disappearance of the Cdc20 protein following myc-Cdh1 induction indicated that mammalian Cdc20 is degraded in an APC-Cdh1-dependent manner in vivo. Collectively, these data demonstrated that the APC activity in our inducible cell lines was largely (if not completely) generated by APC-Cdh1 complexes.
Nonperiodic APC-Cdh1 association prevents G2 and M events and causes endoreplication.
Next we asked how the U-2-OS cells would respond to transient or prolonged loss of periodicity of APC-dependent proteolysis. Following removal of tetracycline from the culture medium, the cells rapidly ceased to multiply (Sørensen et al., unpublished data). Detailed flow cytometry analysis revealed that induction of myc-Cdh1 dramatically deregulated cell cycle progression at multiple transition points. First we noticed that such cells transiently but reproducibly accumulated in G1 (Fig. 2A). Detailed measurements of G1 length in cells released from metaphase arrest confirmed that induction of myc-Cdh1 approximately doubled the time required for progression through G1 and delayed entry into S phase for 6 to 8 h (Fig. 2B). However, cells continuously expressing myc-Cdh1 proved unable to sustain a stable G1 arrest and, between 36 and 48 h after induction, forced progressive accumulation of cells with a 4 N DNA content (Fig. 2A). Multiparameter flow cytometry combining measurements of DNA distribution with BrdU incorporation also revealed that the rate of nucleotide incorporation in myc-Cdh1-expressing cells was 2.1-fold slower compared to that in the S-phase cells from the same clone not expressing the myc-Cdh1 transgene. Consistently, when released from aphidicolin-mediated early S-phase arrest, myc-Cdh1-expressing cells traversed the S phase more slowly compared to the control cells (Sørensen et al., unpublished data). Nevertheless, this slow but uninterrupted DNA synthesis led to a progressive increase in cellular ploidy culminating between days 4 and 5, when the majority of the cells expressing myc-Cdh1 acquired DNA content higher than 4 N (Fig. 2A). The cells at this stage contained enlarged nuclei (Fig. 2C) consistent with the increased DNA content detected by flow cytometry. Immunostaining with antibodies specific for pericentriolar proteins revealed that such cells failed to separate centrosomes and create bipolar microtubule-generating centers (Fig. 2C), an event which otherwise marks the transition between G2 phase and initial stages of mitosis (39). Together with complete lack of any morphological signs of mitosis (Sørensen et al., unpublished data), these data suggest that deregulated activation of APC-Cdh1 ubiquitin ligase in U-2-OS cells precluded productive cell division and promoted slow but continuous DNA replication, uncoupled from mitosis.
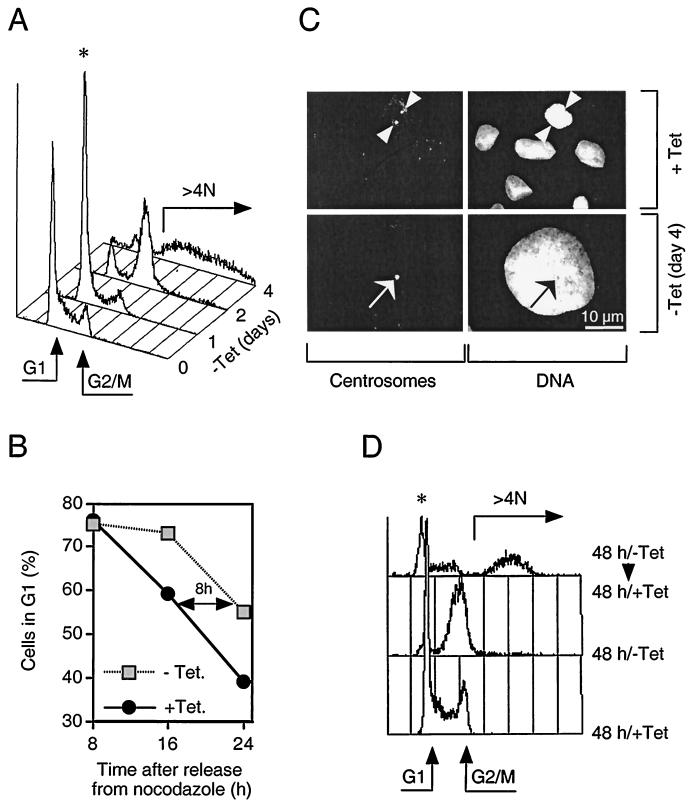
FIG. 2.
Induction of myc-Cdh1 prevents cell division and promotes endoreplication. (A) U-2-OS Cdh1 cells were stimulated to express myc-Cdh1 by removal of tetracycline (−Tet). At the indicated time points after myc-Cdh1 induction, the cells were fixed, stained with propidium iodide, and analyzed for DNA content by single-parameter fluorescence-activated cell sorting. Arrows indicate the position of G1 and G2/M cells as well as the degree of endoreplication (over 4 N DNA content); the asterisk indicates the initial transient accumulation of cells in G1 phase. (B) U-2-OS Cdh1 cells were synchronized in metaphase by incubating in the presence of nocodazole. Rounded mitotic cells were collected and replated into a drug-free medium. At the same time, expression of myc-Cdh1 was induced by removing tetracycline from the medium (−Tet). At indicated time points, the cells were fixed, and their cell cycle distribution was determined by flow cytometry. (C) U-2-OS Cdh1 cells were seeded on glass coverslips and either cultured in the constant presence of tetracycline (+Tet) or induced to express myc-Cdh1 for 4 days (−Tet day 4). The cells were immunostained with an antibody against pericentriolar proteins (left panels). DNA was counterstained with Hoechst 33258 (right panels). The representative images at both time points show identical fields for centrosome and DNA staining, respectively (arrowheads, separated centrosomes; arrows, unseparated centrosomes). (D) U-2-OS Cdh1 cells were either grown in the constant presence of tetracycline (+Tet) or induced to express myc-Cdh1 by removal of tetracycline for 48 h (−Tet). When indicated, the expression of ectopic Cdh1 was silenced by readdition of tetracycline into the culture media, and the cells were incubated for an additional 48 h (−Tet/+Tet). The DNA content was analyzed by FACS.
Ultimately, prolonged induction of ectopic Cdh1 appeared to be lethal, as evidenced by progressive cell detachment detectable after 5 days of myc-Cdh1 induction (Sørensen et al., unpublished data). However, shorter exposure of cells to active APC-Cdh1 was partially reversible, albeit with serious consequences for genomic integrity. Readdition of tetracycline and silencing of the myc-Cdh1 expression after 12 to 48 h of its induction restored cell division in a significant proportion of cells (Fig. 2D) (Sørensen et al., unpublished data). Superimposing of fluorescence-activated cell sorting histograms revealed that, although such cells resumed cell cycle progression, they appeared to either decrease or increase the ploidy compared to cells never exposed to an excess of ectopic Cdh1 (Fig. 2D). Collectively, these results indicate the critical importance of periodic oscillation of the APC-Cdh1 ubiquitin ligase activity for coordinating S phase with cell division and consequently for protecting the stability of the genome in mammalian cells.
APC-Cdh1 induces destruction of mitotic regulators.
Western blotting analysis revealed that a number of known or putative APC substrates regulating entry into, progression through, and exit from mitosis, such as cyclin B1, cyclin A, securin Pds1, the polo-like kinase Plk1, the kinetochore-associated proteins mitosin-CENP-F, the Aurora-like midbody-associated protein Aim1 (Fig. 3A), and Cdc20 (Fig. 1D), were quantitatively degraded following myc-Cdh1 induction. The degradation was proteasome dependent (Fig. 3A, right panels; Fig. 1D) and relatively fast; for instance, cyclin B1 protein began to decline at around 3 h and reached a nadir at 6 h after myc-Cdh1 induction (Fig. 3B) (Sørensen et al., unpublished data). Thus, it appears that in mammalian cells, APC-Cdh1 can induce rapid degradation of many known or putative APC substrates directly involved in regulating cell division. In several independent experiments, we have noticed that myc-Cdh1-induced destruction of cyclin A appeared to be less quantitative and delayed compared to that of cyclin B1 (Fig. 3A) (Sørensen et al., unpublished data). This phenomenon was especially pronounced when myc-Cdh1 was expressed in cells arrested in S phase by aphidicolin (Fig. 3B), suggesting that S-phase cells may contain a subpopulation of cyclin A specifically protected from APC-mediated proteolysis.
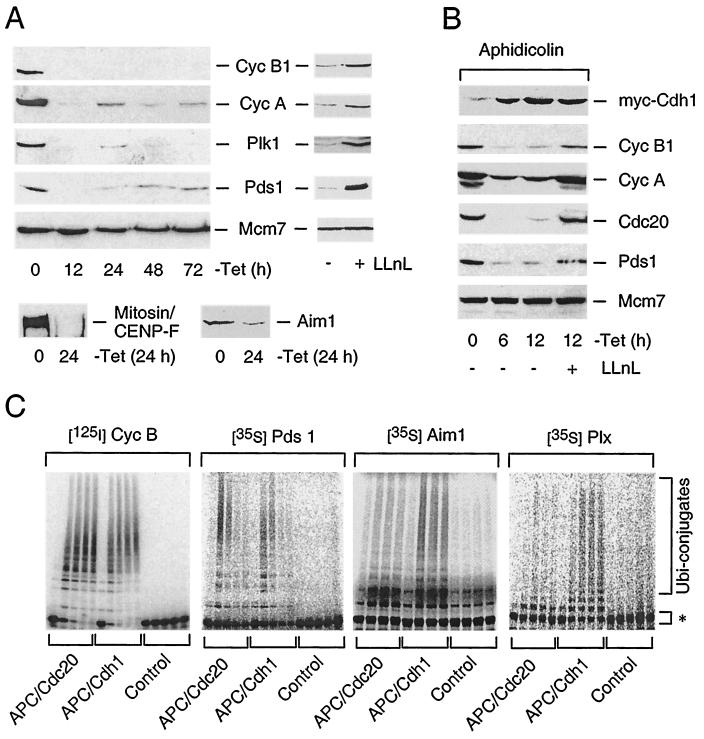
FIG. 3.
APC-Cdh1 ubiquitinates and targets for destruction multiple regulators of cell division. (A) U-2-OS Cdh1 cells were induced to express myc-Cdh1 by the removal of tetracycline (−Tet), and the abundance of the indicated proteins (all APC substrates) was analyzed by immunoblotting with specific antibodies (Mcm7 protein serves as a loading control). When indicated, proteasome function was inhibited by addition of LLnL into the culture medium between 24 and 36 h after removal of tetracycline. (B) U-2-OS Cdh1 cells were presynchronized in S phase by aphidicolin before induction of myc-Cdh1 with or without concomitant inhibition of the proteasome by LLnL. At the indicated time points, the cell lysates were prepared and analyzed by Western blotting with the indicated antibodies. (C) Xenopus APC was activated with baculovirus-expressed human Cdh1 and Cdc20, respectively. Radioactively labeled substrates (marked by asterisks) were added to the ubiquitination reaction and analyzed at 0, 5, 10, 20, and 30 min. In the control reaction Cdh1-activated APC was used without addition of E2 enzymes.
To directly address the issue of the apparently broad substrate recognition by the human APC-Cdh1 ubiquitin ligase, we reconstituted the APC-dependent ubiquitination reaction in vitro. Consistent with the data obtained with the inducible clones, cyclin B, Pds1, Aim1, and Plx (Xenopus homolog of human Plk1 kinase) were efficiently ubiquitinated by APC activated by Cdh1 (Fig. 3C). Also other APC substrates such as geminin, cyclin A, and Cdc20 were efficiently modified by Cdh1-activated APC in an in vitro ubiquitination assay (Sørensen et al., unpublished data). Direct comparison of APC ubiquitin ligase activities catalyzed by Cdh1- and Cdc20-activating subunits, respectively, revealed that, while APC-Cdh1 efficiently ubiquitinated all substrates tested, APC-Cdc20 was less efficient in modification of Plx and Aim1 (Fig. 3C). We concluded that once assembled, APC-Cdh1 is capable of ubiquitinating and priming for rapid destruction a broad range of key regulators of multiple steps of mammalian cell division.
Failure to inactivate APC-Cdh1 at the G1/S transition promotes E2F-dependent up-regulation of cyclin E-Cdk2.
Contrary to the destruction of mitotic regulators, induction of myc-Cdh1 promoted progressive up-regulation of cyclin E-Cdk2 (Fig. 4A), which in mammalian cells represents the major CDK activity responsible for initiation of DNA replication (18, 28, 45, 46). This was achieved by at least two distinct mechanisms. First, starting from 24 h after myc-Cdh1 induction, we observed a rapid decline of the p27 protein, a potent inhibitor of Cdk2 kinase activity (51, 65), accompanied by accumulation of Skp2, the F-box protein recruiting p27 to the SCF ubiquitin ligase (Fig. 4A) (4, 62). We did notice the transient drop of the Skp2 protein shortly after replating the cells into tetracycline-free medium; nevertheless, the progressive increase of the Skp2 levels after 24 h was reproducibly observed in several independent experiments and distinct U-2-OS-Cdh1 clones (Sørensen et al., unpublished data). This was not due to stabilization of the Skp2 protein, whose turnover was actually somewhat accelerated (Sørensen et al., unpublished data), potentially explaining its initial transient decline (Fig. 4A). The gradual accumulation of Skp2 was likely a consequence of stimulation of its transcription, which was detected in myc-Cdh1-expressing cells (Fig. 4B). Second, myc-Cdh1 induction lead to a marked increase of the cyclin E protein itself (Fig. 4A). Pulse-chase assays did not show any significant changes in cyclin E protein stability (Sørensen et al., unpublished data). However, RT-PCR analysis (Fig. 4B) and Northern blot analysis (Sørensen et al., unpublished data) revealed that the transcription of cyclin E mRNA increased approximately fivefold in cells expressing myc-Cdh1. Consistently, reporter assays performed with a cyclin E promoter fragment showed marked superactivation in cells induced to express myc-Cdh1 (Fig. 4C). This could not be explained solely by the myc-Cdh1-induced changes in the cell cycle position since similar results were obtained when the expression of ectopic Cdh1 was induced in cells arrested by aphidicolin in early S phase (Fig. 4D). Cyclin E expression is controlled by a concerted action of the two major G1 transcriptional activities, namely, those associated with E2F and Myc (reference 56 and references therein). Several pieces of evidence suggested that the accelerated transcription of cyclin E reflected the increased activity of endogenous E2F. First, the Cdh1-induced activation of the cyclin E promoter could be fully repressed by coexpression with pRbΔcdk (Sørensen et al., unpublished data), a phosphorylation-deficient retinoblastoma protein which was previously demonstrated to be a potent repressor of all known E2F isoforms (34). Second, parallel luciferase measurements revealed that induction of ectopic Cdh1 led to a severalfold increase of the activity associated with endogenous E2F but not with Myc transcription factors (Fig. 4C). Finally, induction of myc-Cdh1 led to an increased accumulation of at least one member of the E2F transcription factor family, namely, E2F-1 (Fig. 4A). Also in this case, active APC-Cdh1 did not significantly affect E2F-1 protein stability (Sørensen et al., unpublished data) but rather promoted increased transcription of the E2F-1 gene (Fig. 4B). Taken together, these data strongly suggested that nonperiodic activation of APC-Cdh1 created conditions permissive for gradual accumulation and elevated activity of the E2F transcription factors and consequently for increased synthesis of at least some cell cycle regulators capable of initiating and maintaining DNA synthesis, which, in the concomitant absence of cell division, likely generated the major force promoting progressive endoreplication.
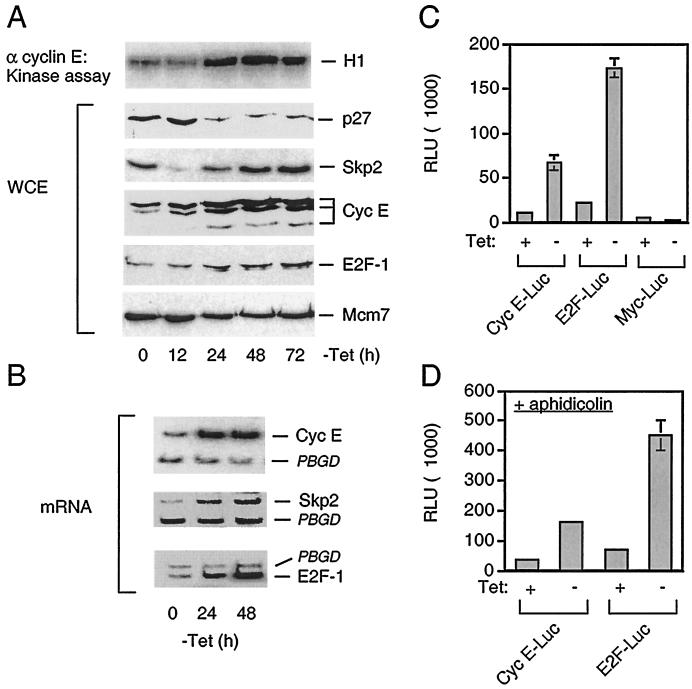
FIG. 4.
Constitutive APC-Cdh1 assembly stimulates E2F-dependent superactivation of cyclin E-Cdk2. (A) U-2-OS Cdh1 cells were induced exactly as described in the legend for Fig. 3A. The kinase activity associated with cyclin E was measured using histone H1 as a substrate (upper panel). The abundance of the p27, Skp2, cyclin E, and E2F-1 proteins was determined in whole cell extracts (WCE) by Western blotting using specific antibodies as indicated. α cyclin E, anti-cyclin E. (B) Total mRNA was isolated from U-2-OS-Cdh1 cells induced to express myc-Cdh1 for the indicated time, and the transcription of endogenous cyclin E, Skp2, E2F-1, and control housekeeping PBGD genes was determined by RT-PCR. (C) U-2-OS Cdh1 cells were transiently transfected with the indicated reporter plasmids and were either kept uninduced (+Tet) or stimulated to express myc-Cdh1 for 24 h (−Tet). The activity associated with each reporter construct was measured by standard luciferase reporter assays and normalized for transfection efficiency by subtracting the values associated with cotransfected β-Gal reporter (RLU, relative light units). (D) U-2-OS Cdh1 cells were presynchronized in early S phase by incubation with aphidicolin. Luciferase activity associated with the indicated reporter constructs was measured essentially as described for panel C, except that the cells remained arrested with aphidicolin throughout the whole assay period.
Functional status of APC-Cdh1 ubiquitin ligase modulates the ability of cyclin E-Cdk2 to initiate DNA replication.
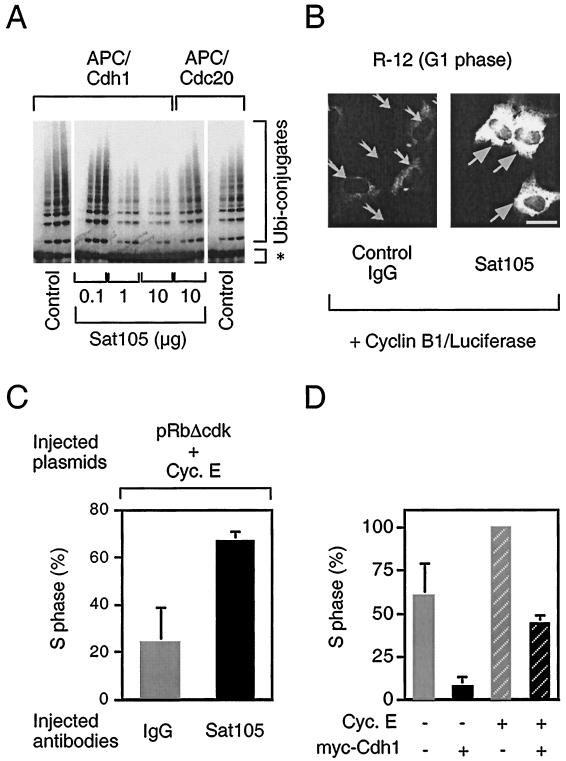
The data obtained with our inducible cell lines showed that, on one hand, persistent APC-Cdh1 interaction delayed both the passage through G1/S transition and the progression through S phase. On the other hand, induction of myc-Cdh1 gradually increased the activity associated with the S-phase-promoting cyclin E-Cdk2 holoenzyme and caused progressive overreplication of the genome. Although counterintuitive at first glance, this complex consequence of nonperiodic APC-Cdh1 activity on DNA synthesis could be reconciled if Cdk2, by itself capable of supporting limited DNA replication, requires an APC-sensitive auxiliary factor to set a correct timing of initiation and subsequent rate of DNA synthesis. To test such predictions, we employed an inverse approach to our previous experiments, and rather than overexpressing ectopic Cdh1, we ablated the function of the endogenous protein by means of a specific anti-Cdh1 neutralizing antibody. We performed these experiments in immortalized rat fibroblasts (R-12 cell line) which could be synchronized by mitogen depletion and thus provide an accurate system to monitor events at the G1/S transition (33, 37). First we tested the capability of Sat105, a highly specific anti-Cdh1 antibody (13; Sørensen et al., unpublished data) to interfere with Cdh1 function. Indeed, when added in the in vitro ubiquitination reaction, Sat105 progressively inhibited Cdh1-dependent polyubiquitination of cyclin B in a dose-dependent manner (Fig. 5A). This effect was specific for APC-Cdh1 because APC-Cdc20 complexes were not inhibited even by high doses of these antibodies (Fig. 5A). Moreover, Sat105 inhibited endogenous APC-Cdh1 activity also in vivo. When microinjected into G1 fibroblasts, which naturally contain highly active APC-Cdh1, Sat105 significantly stabilized a cyclin B1-luciferase reporter protein (Fig. 5B), which serves as a sensitive marker for APC-dependent proteolysis (32).
FIG. 5.
Inactivation of APC-Cdh1 cooperates with cyclin E-Cdk2 to initiate DNA replication. (A) APC-dependent ubiquitination reaction of radiolabeled cyclin B (marked by asterisk) specifically catalyzed by Cdh1 and Cdc20, respectively, was reconstituted in vitro essentially as described in the legend to Fig. 3B. Purified anti-Cdh1 antibody (Sat105) was added into the reaction in increasing amounts as indicated. Control, no antibody added. (B) Serum-starved R-12 cells were microinjected with the control (IgG) or anti-Cdh1 (Sat105) antibodies, respectively (2-mg/ml needle concentration), together with the expression plasmid for cyclin B1-luciferase reporter (5 μg/ml). Subsequently, the cells were stimulated with 10% FCS for 8 h to reenter G1 phase and assayed for accumulation of the reporter by immunostaining with antiluciferase antibody. Arrows point to the productively injected cells in representative fields; both images were taken under identical exposure times. Bar, 10 μm. (C) Serum-starved R12 cells were microinjected with indicated antibodies as in panel B together with expression plasmids for pRbΔcdk and cyclin E (Cyc. E) (both 25 μg/ml). The cells were stimulated with FCS supplemented with BrdU (100 μg/ml) for 20 h, fixed, and assayed for S-phase entry by immunodetection of BrdU. (D) Serum-starved R-12 cells were microinjected with cyclin E and myc-Cdh1 expression plasmids (25 μg/ml) as indicated, stimulated with FCS containing BrdU (100 μg/ml) for 24 h, and evaluated as in panel C.
Having validated the ability of Sat105 to functionally neutralize Cdh1, we next tested whether inactivation of APC-Cdh1 could affect the length of G1. Indeed, microinjection of Sat105 into synchronized rat fibroblasts consistently resulted in a moderate (15 to 20%) increase in cells incorporating BrdU when compared to control-injected cells (Sørensen et al., unpublished data). To specifically address whether the functional status of APC-Cdh1 influences the S-phase-promoting potential of cyclin E-Cdk2, we generated a model system based on two recent findings obtained in our laboratories. First, rat fibroblasts deprived of de novo expression of E2F target genes by means of a constitutively active allele of the retinoblastoma protein (pRbΔcdk) undergo G1 arrest, which could be rescued by coexpression of cyclin E from a heterologous promoter (6, 23, 34). Second, pRbΔcdk greatly stimulates assembly of APC-Cdh1 holoenzyme even in the presence of active cyclin E-Cdk2 (32). Hence, expression of pRbΔcdk together with cyclin E generates a defined system in which S phase is generated largely by the cyclin E-Cdk2 kinase activity in the concomitant presence of functional APC. Importantly, under such conditions, coinjection of Sat105 significantly accelerated S-phase entry compared to control cells injected with nonspecific antibody (Fig. 5C). More detailed analysis at multiple time points revealed that under these experimental conditions, the Sat105-microinjected cells accumulated in S phase approximately 6 h earlier compared to control cells (Sørensen et al., unpublished data). Conversely, both the mitogen-induced S phase and accelerating effect of ectopic cyclin E on G1/S transition were significantly reduced upon comicroinjection with expression plasmid for myc-Cdh1 (Fig. 5D). Taken together, these data indicate that cancellation of the APC ubiquitin ligase activity at the G1/S transition and throughout S phase allows accumulation of at least one unstable protein, which augments cyclin E-Cdk2-mediated initiation of DNA replication.
DISCUSSION
Periodic assembly of APC with Cdh1 represents an essential step in coordinating multiple cell cycle transitions in human cells.
Our data demonstrate that periodic regulation of APC-Cdh1-mediated proteolysis appears crucial for productive cell division of mammalian cells. Failure to inactivate APC-Cdh1 ubiquitin ligase in a timely manner led to unscheduled destruction of key G2/M regulators such as mitotic cyclins, Plk1, and Pds1 and consequently precluded cell cycle progression beyond the G2 phase. Our finding that Cdc20 represents a bona fide in vivo substrate for APC-Cdh1 ubiquitin ligase is fully consistent with the recent report, published while this manuscript was being prepared, that ubiquitination of Cdc20 in an in vitro-reconstituted reaction is specifically catalyzed by APC-Cdh1 (49). The observation that human APC-Cdh1 catalyzes ubiquitination and promotes destruction of the above G2/M regulators with similar rapid kinetics was unexpected, given the evidence from lower eukaryotes arguing for more restricted substrate specificity conferred by Cdc20 or Cdh1, respectively (57, 66). For instance, yeast Cdh1, even when overexpressed, induced complete destruction of Clb2 yet only partial elimination of Pds1. Our results suggest that the situation is different and that the APC-Cdh1 ubiquitin ligase may regulate abundance of a broad range of proteins containing appropriate recognition signals such as the destruction box or the newly identified KEN box (49). This notion is further supported by recent data from budding yeast cells in which Pds1 proteolysis in G1 has been shown to depend on Cdh1, not Cdc20 (54). In contrast, APC-Cdc20 appears to have a more restricted substrate specificity, at least in vitro where it can ubiquitinate Aim1 and Plk1 less well than APC-Cdh1. Cdc20 and Cdh1 may therefore differ mostly in their mode of regulation and the resulting temporal difference in their ability to activate the APC, whereas their substrate specificities may be more similar than previously proposed. In addition, we identified mitosin-CENP-F and Aim1 as two novel KEN box-containing proteins specifically degraded in an APC-Cdh1-dependent manner. It is intriguing that mitosin-CENP-F localizes to kinetochores (53, 72), suggesting that kinetochore-associated proteins may not only help to sequester and inhibit APC upon activation of the spindle checkpoint (69) but could actually be themselves targeted for APC-mediated proteolysis. This observation further underscores the need for a tight control of APC-Cdh1 activity, to ensure timely and spatially correct segregation of sister chromatids in anaphase. The Aurora and Ipl1-like midbody-associated protein Aim1 has been recently found to play an essential role in cytokinesis in mammalian cells (64). Our finding that Aim1 protein is also degraded in an APC-Cdh1-dependent manner helps explain the periodic accumulation of this protein during the cell cycle (64) and suggests that Cdh1, through regulation of Aim1 protein turnover, supports timely exit from mitosis.
Besides the negative effect on cell division, conceptually consistent with findings made in yeast, Drosophila, and Xenopus laevis (69), our results revealed a novel aspect of APC-mediated proteolysis, namely, that dissociation of Cdh1 from the APC core at the G1/S transition appears to play a central role in protecting cells from genomic instability by helping to restrict chromosomal duplication to only one round per cell division cycle. We could demonstrate that untimely activation of APC-Cdh1 during S phase interfered with the natural oscillation of some factors that were rate limiting for G1/S transition, such as cyclin E or E2F-1, and as such likely contributed to uninterrupted DNA synthesis and severe rereplication of the genome. Moreover, de novo refiring of at least some origins without intervening mitosis could be further augmented by APC-Cdh1-mediated destruction of the newly emerging class of APC substrates such as geminin, which at least in Xenopus embryos functions as a potent inhibitor of initiation of DNA replication (38). This, together with high cyclin E-Cdk2 activity could overpower a potential lack of other APC targets, which may normally accumulate at the G1/S transition and cooperate with cyclin E-Cdk2 to efficiently initiate DNA replication in a timely manner. Although the identity of such protein(s) remains elusive, our in vivo APC-Cdh1 neutralization data strongly argue for its existence (Fig. 5C and D). One plausible candidate is Dbf4, which together with its catalytic subunit Cdc7 was demonstrated as an essential factor for S-phase entry in various organisms (5, 11, 47, 67), and yeast Dbf4 was recently shown to be a bona fide substrate for APC-mediated proteolysis (11, 47). Finally, several other observations opened up the possibility that the persistent replication-promoting activity in cells expressing ectopic Cdh1 could be facilitated also by a potential cross talk between APC and SCF ubiquitin ligases. High and nonperiodic APC-Cdh1 activity apparently supported progressive accumulation of Skp2 F-box protein, a crucial SCF component, and thus created conditions permissive for a rapid turnover of p27 CDK inhibitor that would otherwise restrain S-phase-promoting CDK activities. How APC-Cdh1 induces accumulation of Skp2 remains to be established. The reported requirement of Skp2 for S phase (71), the sharp increase of Skp2 accumulation at the G1/S transition (31), and increased transcription of the Skp2 gene in myc-Cdh1-expressing cells (this study) indicate that Skp2 expression could be regulated, at least in part, by E2F.
One plausible explanation for the gradually increasing E2F-mediated transcription could be the degradation of cyclin A observed in myc-Cdh1-expressing cells. In such a scenario, APC-Cdh1 would not directly induce E2F-dependent transcription but rather would prevent its physiological silencing, which normally occurs at the end of S phase following the completion of genome duplication. It has been previously demonstrated that cyclin A-Cdk2 phosphorylates the E2F-heterodimerizing partner DP-1 and thereby cancels the ability of E2F–DP-1 complexes to bind DNA (26). It has also been shown that failure to displace E2F from DNA results in the inability to exit S phase (27), the phenotype very similar to what we describe here as a consequence of the deregulated APC-Cdh1 activity. Hence, one plausible way to gradually amplify cellular E2F activity in our system would be based on autostimulation of E2F-1 synthesis, promoted by reduced levels of cyclin A upon myc-Cdh1 induction.
The involvement of cyclin A as a mediator of some crucial phenotypic consequences of nonperiodic APC-Cdh1 assembly raised an important issue of complexity in terms of the functional interplay between APC-Cdh1 and E2F-cyclin A. We have previously demonstrated that, during S phase, E2F-regulated genes including cyclin A prevent Cdh1 from interacting with the APC core and thus ensure that the APC ubiquitin ligase would not be switched on prematurely (32). The data presented in this study show that approximately fivefold elevation of Cdh1 protein was sufficient to revert this dependency and induce destruction of a major pool of cellular cyclin A. However, our observation that S-phase cells retained detectable amounts of cyclin A at the time when induction of myc-Cdh1 nearly completely eliminated other APC substrates suggests the existence of a fraction of cyclin A specifically protected against APC-dependent proteolysis. Indeed, factors capable of interfering with vertebrate cyclin A but not cyclin B destruction have been recently reported in the literature (12). Availability of such an APC-insensitive pool of cyclin A would be consistent with the reported role of cyclin A-Cdk2 in phosphorylating Cdh1 and thus securing that cells beyond the G1/S transition would not prematurely reactivate destruction of other APC targets (32). In fact, our observation that despite steadily increasing cyclin E-Cdk2 activity in myc-Cdh1-expressing cells, Cdh1 remained bound to functional APC further supports cyclin A but not cyclin E as a direct Cdh1 kinase in mammalian cells. The interspecies differences between mammals and the genus Drosophila, in which cyclin E-Cdk2 has been proposed as a physiological fizzy-related kinase, could be explained by at least several distinctions in cyclin-CDK biochemistry including the dominant catalytic partners of cyclin A and its subcellular localization (61). Finally, very recent advances in the proteolysis field indicate that degradation of mammalian cyclin A might be unexpectedly complex; during this study, Nakayama and colleagues reported that cyclin A but not cyclin B can be efficiently ubiquitinated by SCFSkp2 ubiquitin ligase (42). Based on our finding that elevation of myc-Cdh1 stimulates transcription of the Skp2 F-box protein and given that Skp2 and cyclin A proteins physically interact (71), it is plausible that in our system, as well as during unperturbed cell cycle progression (42), the steady-state levels of cyclin A result from a delicate balance between its E2F-regulated transcription and its protein turnover controlled by both APC and SCF ubiquitin ligases.
Taken together, the cellular mechanisms involved in regulating the periodicity of APC-Cdh1 assembly in mammalian cells appear to play a crucial role in coupling DNA replication with productive cell division. In fact, Cdh1 appears to join the small number of cell cycle genes such as the myc, p53, cyclin E, and p21 genes, the deregulation of which appears capable of inducing genomic instability. It is tempting to speculate that at least some mechanisms involved in control of APC-Cdh1 assembly could be deregulated during multistep tumorigenesis, for instance in those human tumors that aberrantly accumulate mitotic B-type cyclins during G1 (20). Our finding that mitosin-CENP-F is targeted by APC-Cdh1 for destruction opens up a possibility to search for potential defects affecting stability of kinetochore-associated proteins and raise the question whether these could explain at least some aspects of aneuploidy detected in the majority of human tumors. Finally, our observation that APC-Cdh1 directly determines the level of the human Pds1 (PTTG) and Aim1 proto-oncogenes raises the possibility that aberrations in posttranscriptional regulation including proteolysis might account for their elevated expression in human malignancies (9, 55, 63).
ACKNOWLEDGMENTS
We are grateful to C. Giefers, H. Zou, M. Pagano, M. Bornens, W. Krek, and G. Evan for donating diverse antibodies and to H. Zou, T. McGarry, K. Helin, P. Jansen-Dürr, J. Cleveland, and Y. Terada for cDNAs and reporter constructs.
This work was supported by grants from the Danish Cancer Society, the Human Frontier Science Program (RG-299/97), the Danish Medical Research Council (9600821), and the Austrian Industrial Research Promoting Fund (FFF 3/12801).
REFERENCES
- 1.Botz J, Zerfass-Thome K, Spitkovsky D, Delius H, Vogt B, Eilers M, Hatzigeorgiou A, Jansen-Dürr P. Cell cycle regulation of the murine cyclin E gene depends on an E2F binding site in the promoter. Mol Cell Biol. 1996;16:3401–3409. doi: 10.1128/mcb.16.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K, Markowitz S D, Kinzler K W, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 4.Carrano A C, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Collyer T, Hardy C F. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol Cell Biol. 1999;19:4270–4278. doi: 10.1128/mcb.19.6.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew Y P, Ellis M, Wilkie S, Mittnacht S. pRB phosphorylation mutants reveal role of pRB in regulating S phase completion by a mechanism independent of E2F. Oncogene. 1998;17:2177–2186. doi: 10.1038/sj.onc.1202443. [DOI] [PubMed] [Google Scholar]
- 7.Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Fix O, Peters J M, Kirschner M W, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez A, Ramos-Morales F, Romero F, Rios R M, Dreyfus F, Tortolero M, Pintor-Toro J A. hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms: evidence for a transcriptional activation function of hPTTG. Oncogene. 1998;17:2187–2193. doi: 10.1038/sj.onc.1202140. [DOI] [PubMed] [Google Scholar]
- 10.Fang G, Yu H, Kirschner M W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira M F, Santocanale C, Drury L S, Diffley J F. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol Cell Biol. 2000;20:242–248. doi: 10.1128/mcb.20.1.242-248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funakoshi M, Geley S, Hunt T, Nishimoto T, Kobayashi H. Identification of XDRP1: a Xenopus protein related to yeast Dsk2p binds to the N-terminus of cyclin A and inhibits its degradation. EMBO J. 1999;18:5009–5018. doi: 10.1093/emboj/18.18.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gieffers C, Peters B H, Kramer E R, Dotti C G, Peters J M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glotzer M, Murray A W, Kirschner M W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 15.Golsteyn R M, Schultz S J, Bartek J, Ziemiecki A, Ried T, Nigg E A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Shiratori Y, Kato N, Inoue T, Omata M. Mutational inactivation of mitotic checkpoint genes, hsMAD2 and hBUB1, is rare in sporadic digestive tract cancers. Jpn J Cancer Res. 1999;90:837–840. doi: 10.1111/j.1349-7006.1999.tb00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irniger S, Nasmyth K. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- 18.Jackson P K, Chevalier S, Philippe M, Kirschner M W. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaspersen S L, Charles J F, Morgan D O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 20.Keyomarsi K, Pardee A B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King R W, Deshaies R J, Peters J M, Kirschner M W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 22.King R W, Peters J M, Tugendreich S, Rolfe M, Hieter P, Kirschner M W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 23.Knudsen E S, Buckmaster C, Chen T T, Feramisco J R, Wang J Y. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer E R, Gieffers C, Holzl G, Hengstschlager M, Peters J M. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- 25.Kramer E R, Scheuringer N, Podtelejnikov A V, Mann M, Peters J-M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Jr, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 27.Krek W, Xu G, Livingston D M. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 28.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 29.Kumada K, Su S, Yanagida M, Toda T. Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Trainer A H, Friedman L S, Thistlethwaite F C, Evans M J, Ponder B A, Venkitaraman A R. Mitotic checkpoint inactivation fosters transformation in cells lacking the breast cancer susceptibility gene, Brca2. Mol Cell. 1999;4:1–10. doi: 10.1016/s1097-2765(00)80182-3. [DOI] [PubMed] [Google Scholar]
- 31.Lisztwan J, Marti A, Sutterluty H, Gstaiger M, Wirbelauer C, Krek W. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukas C, Sørensen C S, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters J M, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 33.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 35.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 36.Lukas J, Petersen B O, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047–1057. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukas J, Sørensen C S, Lukas C, Santoni-Rugiu E, Bartek J. p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene. 1999;18:3930–3935. doi: 10.1038/sj.onc.1202777. [DOI] [PubMed] [Google Scholar]
- 38.McGarry T J, Kirschner M W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 39.Meraldi P, Lukas J, Fry A M, Bartek J, Nigg E A. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 40.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 41.Morgan D O. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama K, Nagahama H, Minamishima Y A, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noton E, Diffley J F. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol Cell. 2000;5:85–95. doi: 10.1016/s1097-2765(00)80405-0. [DOI] [PubMed] [Google Scholar]
- 44.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 45.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshiro G, Owens J C, Shellman Y, Sclafani R A, Li J J. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol Cell Biol. 1999;19:4888–4896. doi: 10.1128/mcb.19.7.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters J M. SCF and APC: the Yin and Yang of cell cycle regulated proteolysis. Curr Opin Cell Biol. 1998;10:759–768. doi: 10.1016/s0955-0674(98)80119-1. [DOI] [PubMed] [Google Scholar]
- 49.Pfleger C M, Kirschner M W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 50.Pines J. Cyclins, CDKs and cancer. Semin Cancer Biol. 1995;6:63–72. doi: 10.1006/scbi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 51.Polyak K, Lee M H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 52.Prinz S, Hwang E S, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- 53.Rattner J B, Rao A, Fritzler M J, Valencia D W, Yen T J. CENP-F is a ca. 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton. 1993;26:214–226. doi: 10.1002/cm.970260305. [DOI] [PubMed] [Google Scholar]
- 54.Rudner A D, Hardwick K G, Murray A W. Cdc28 activates exit from mitosis in budding yeast. J Cell Biol. 2000;149:1361–1376. doi: 10.1083/jcb.149.7.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saez C, Japon M A, Ramos-Morales F, Romero F, Segura D I, Tortolero M, Pintor-Toro J A. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999;18:5473–5476. doi: 10.1038/sj.onc.1202914. [DOI] [PubMed] [Google Scholar]
- 56.Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–3509. doi: 10.1128/mcb.20.10.3497-3509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwab M, Lutum A S, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 58.Shirayama M, Toth A, Galova M, Nasmyth K. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- 59.Shteinberg M, Protopopov Y, Listovsky T, Brandeis M, Hershko A. Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem Biophys Res Commun. 1999;260:193–198. doi: 10.1006/bbrc.1999.0884. [DOI] [PubMed] [Google Scholar]
- 60.Sigrist S J, Lehner C F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 61.Sprenger F, Yakubovich N, O'Farrell P H. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr Biol. 1997;7:488–499. doi: 10.1016/s0960-9822(06)00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 63.Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. [PubMed] [Google Scholar]
- 64.Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 66.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 67.Weinreich M, Stillman B. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 1999;18:5334–5346. doi: 10.1093/emboj/18.19.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinstein J. Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc: a mammalian homolog of CDC20/Fizzy/slp1. J Biol Chem. 1997;272:28501–28511. doi: 10.1074/jbc.272.45.28501. [DOI] [PubMed] [Google Scholar]
- 69.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 70.Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 72.Zhu X, Mancini M A, Chang K H, Liu C Y, Chen C F, Shan B, Jones D, Yang-Feng T L, Lee W H. Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cell Biol. 1995;15:5017–5029. doi: 10.1128/mcb.15.9.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou H, McGarry T J, Bernal T, Kirschner M W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]