ABSTRACT
Kinetic characterization of ribosomal translocation is important for understanding the mechanism of elongation in protein synthesis. Here we have optimized a popular fluorescent-mRNA based translocation assay conducted in stopped-flow, by calibrating it with the functional tripeptide formation assay in quench-flow. We found that a fluorescently labelled mRNA, ten bases long from position +1 (mRNA+10), is best suited for both assays as it forms tripeptide at a fast rate equivalent to the longer mRNAs, and yet produces a large fluorescence change upon mRNA movement. Next, we compared the commonly used peptidyl tRNA analog, N-acetyl-Phe-tRNAPhe, with the natural dipeptidyl fMet-Phe-tRNAPhe in the stopped-flow assay. This analog translocates about two times slower than the natural dipeptidyl tRNA and produces biphasic kinetics. The rates reduce further at lower temperatures and with higher Mg2+ concentration, but improve with higher elongation factor G (EF-G) concentration, which increase both rate and amplitude of the fast phase significantly. In summary, we present here an improved real time assay for monitoring mRNA-translocation with the natural- and an N-Ac-analog of dipeptidyl tRNA.
KEYWORDS: Ribosome, protein synthesis, translocation, pyrene mRNA, N-acetyl Phe-tRNA, EF-G, GTP hydrolysis
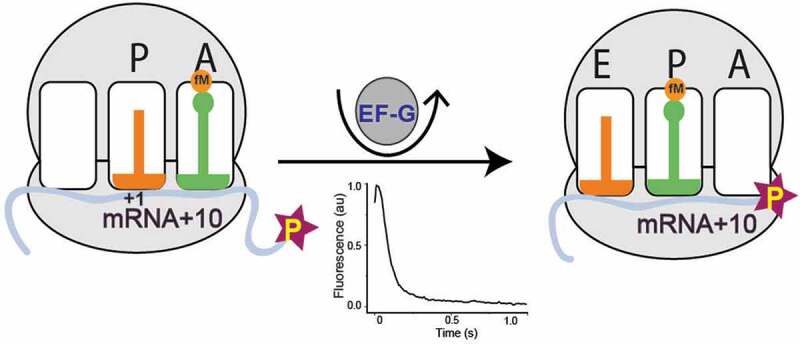
GRAPHICAL ABSTRACT
Introduction
Protein synthesis by the ribosome, during which the genetic information encoded in messenger RNA (mRNA) is translated into proteins, is central to all cellular life. In living cells, ribosomes spend most of their time in peptide chain elongation and the average elongation rate is closely tied to the growth rate of the cell. Therefore, understanding the mechanism of peptide elongation has been a central focus of the ribosome field since the early days of protein synthesis research.
Elongation of the polypeptide chain occurs at a high rate in cells, of about 10–20 amino acids per second per ribosome [1]. The elongation cycle comprises two major events, peptide bond formation and ribosomal translocation. The first step involves elongation factor Tu (EF-Tu), which delivers the aminoacyl tRNAs to the A site and the second step involves elongation factor G (EF-G), which translocates codon-anticodon paired mRNA and tRNAs from A- to P site and P- to E site on the ribosome. In an in vitro translation system, fully reconstituted with purified translation components from Escherichia coli, the mean time for tripeptide formation starting from the 70S initiation complex (70S IC) has been determined almost as short as in vivo, 110 – 150 ms [2,3]. The process includes binding of elongation factor Tu (EF-Tu)•aminoacyl tRNA•GTP ternary complex (TC) to the 70S IC; first peptide bond formation; elongation factor G (EF-G)–mediated ribosomal translocation; binding of the second TC and formation of the second peptide bond. By subtracting the time taken for two peptide bond formations (starting from the binding of TC) from the total time of tripeptide formation, the mean time for EF-G driven ribosomal translocation can been estimated [2,3]. This includes multiple sub-steps of EF-G cycle such as EF-G binding, GTP hydrolysis, mRNA-tRNA movement, ribosomal rearrangements, and EF-G release – occurring in a sequential manner. There are individual assays for monitoring some of the sub-steps listed here, such as fluorescently labelled mRNA or tRNA based assay to follow mRNA-tRNA movement in stopped-flow [4–9] and FRET-based assays for monitoring ribosomal dynamics [10–14] during translocation. However, the components and conditions used in these assays as well as in the functional assay of ribosomal translocation (e.g. tripeptide formation) often differ, which makes it challenging to obtain relatable rates from these assays.
In 2003, Studer et al. first developed a fluorescence based real-time kinetic assay to monitor mRNA movement during ribosomal translocation, by using short, synthetic mRNAs labelled with pyrene at the 3ʹ end [4]. The assay starts by rapid mixing of EF-G to a pre-translocation (pre-T) ribosomal complex programmed with a 3ʹ pyrene labelled mRNA and the peptidyl-tRNA analog N-acetylated Phe-tRNAPhe (NAc-Phe-tRNAPhe) placed in the ribosomal A site. During mRNA translocation, the mRNA moves by one codon and as a result the pyrene residue enters in the mRNA channel or moves closer to the ribosome. This change in the environment of the pyrene dye reduces its fluorescence emission. Thus, mRNA movement can be followed directly in real-time by monitoring the change in pyrene fluorescence using a stopped-flow equipped with a fluorescence detector. Due to simple design and the ease of operation, this assay has been popular in kinetic studies related to ribosomal translocation [4,5,11,15–28]. However, this assay, originally referred to as ‘mRNA translocation’ assay [4,5], cannot reflect on the post-mRNA movement steps including ribosomal rearrangement and EF-G release, which can in fact take longer time than the mRNA movement during one cycle of elongation. Thus, calibration of the fluorescence based assay for mRNA movement with the functional assay for translocation by tripeptide formation is crucial for obtaining relatable rates in these two powerful assays, which together can elucidate the mechanism and the limiting factors of ribosomal translocation.
In the original study, an mRNA labelled with pyrene, nine bases downstream of the start codon (mRNA+9) was proposed as the best mRNA for this assay [4] based on the fact that it produced the largest change in the fluorescence signal upon translocation. However, recent results from our laboratory indicate that mRNA+9 is almost two-fold slower in translocation than longer, unlabelled mRNAs, determined by the tripeptide formation assays using quench-flow [2]. Therefore, our primary aim in this work is to identify an mRNA which produces a high-fluorescence signal in the stopped-flow based mRNA movement assay, while simultaneously undergoing a complete elongation cycle, measured by tripeptide formation in quench-flow, with rates comparable to those of longer unlabelled mRNAs. To this end, we systematically characterized pyrene-labelled mRNAs of different lengths (+9 to +12; Fig. 1) in a tripeptide formation assay in quench-flow in parallel to the fluorescent-mRNA based stopped-flow assay. Our careful comparison identified mRNA+10, one base longer than the commonly used mRNA+9 (Fig. 1), as the most suitable mRNA for obtaining physiologically relevant kinetic rates for translocation. Not only did mRNA+10 produce the second-highest fluorescence change in stopped-flow, it simultaneously showed fast tripeptide-formation kinetics – comparable with longer mRNAs. We have further optimized the conditions using the popular the peptidyl-tRNA analog N-acetylated Phe-tRNAPhe (NAc-Phe-tRNAPhe) in the stopped-flow based assay. This analog translocates significantly slower than the natural peptidyl tRNAs and produces biphasic kinetics [24,25,29], making rate analysis challenging. By varying temperature, and titrating Mg2+, EF-G and NAc-Phe-tRNAPhe in the reaction, we have successfully derived the best conditions for obtaining a predominant fast phase (~90%) in this assay. These results are not only important for identifying the potential reason for the biphasic kinetics, but also for explaining disparities in results obtained from this assay in the literature.
Figure 1.

Sequence of the mRNAs without or with the pyrene dye (the circled P) attached covalently to the 3ʹ end. The mRNAs include SD sequences (grey colour) and code for a tripeptide fMet-Phe-Leu. The A of the start codon AUG is counted as +1. The mRNA names are based on the number of nucleotides starting from the +1 position
Materials and methods
Components and buffer preparation
All his-tagged translation factors (IF1, IF2, IF3, EF-Tu, EF-Ts and EF-G) and Leu and Phe aminoacyl tRNA synthetases (LeuRS and PheRS) (in-house laboratory clones) were over expressed in E. coli BL21(DE3) cells and purified using affinity chromatography on Ni-IMAC column from GE Healthcare [30,31]. 70S ribosomes (E. coli MRE600) and f[3H]Met-tRNAfMet were prepared according to Antoun et. al. [32]. NAc-Phe-tRNAPhe was prepared as described by Haenni et al, 1966 with minor modifications [33].
Six mRNAs were designed similar to those in Studer et al. [4] (Fig. 1) and purchased from IBA GmbH, Germany. All mRNAs used in this study contain a strong Shine-Dalgarno sequence (UAAGGAGG) and a small open reading frame encoding the peptide sequence Met-Phe-Leu. The open reading frame (underlined in Fig. 1) was followed by the 3ʹ end either immediately (mRNA+9 and mRNA+9 nodye) or after one, two or three additional nucleobases (mRNA+10, mRNA+10 nodye, mRNA+11 and mRNA+12). All mRNAs, except those where ‘nodye’ is mentioned in the name, had a pyrene fluorophore covalently attached to the 3ʹ end with a short carbon linker (IBA GmbH, Germany).
All experiments were performed in HEPES-polymix buffer, pH 7.5 (5 mM HEPES (pH 7.5), 100 mM KCl, 5 mM NH4Cl, 0.5 mM CaCl2, 5 mM Mg(CH3COO)2, 8 mM putrescine, 1 mM spermidine and 1 mM dithioerythritol) at 37°C. The reaction buffers contained energy pump components ATP (1 mM), GTP (1 mM), phosphoenolpyruvate (PEP, 10 mM), pyruvate kinase (PK, 50 µg/ml), myokinase (MK, 2 µg/ml). Addition of ATP and GTP led to the free Mg2+ concentration 2 mM, which is close to the physiological range in bacteria E. coli [34]. PK and MK were not added in translocation assay with NAc-Phe-tRNAPhe. ATP and GTP were purchased from GE Healthcare. 3H-Met was from Perkin-Elmer. All other chemicals and reagents were from Sigma-Aldrich.
Di- and tripeptide formation assays using quench-flow
For measuring f[3H]Met-Phe dipeptide formation with time, mix A containing 70S ribosome (1 µM), initiation factors (IF1, IF2, IF3, 2 µM each), f[3H]Met-tRNAfMet (1.5 µM), MFL mRNA(s) (1.2 µM) (Fig. 1), and mix B containing EF-Tu (10 µM), EF-Ts (1 µM), tRNAPhe (4 µM), Phe (200 µM), PheRS (0.5 µM) were incubated separately at 37°C for 15 min. The reaction was started by rapid mixing of equal volume of both mixes in a Quench-flow (RQF 3-KinTek Corporation). The reactions were quenched at different time points using formic acid (17% final). The samples were treated with 0.5 M KOH, to release the peptides from the tRNAs. The relative amounts of f[3H]Met and f[3H]Met-Phe were determined by Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) (C18 column) with on-line radiation detection as described by Holm et al. [3]. The dipeptide formed over time was fitted to a single exponential function (, where k corresponds to the apparent rate constant and A to the total amplitude. The mean time for the first peptide bond formation starting from binding of TC and including entire EF-Tu cycle, (τp1), was determined as 1/k.
In order to follow the f[3H]Met-Phe-Leu tripeptide formation starting from the 70S IC, the reaction mixes were prepared as above with addition of EF-G (10 µM), tRNALeu1 (4 µM), Leu (200 µM) and Leu-synthetase (0.5 µM) to the mix B. For conducting the reaction and to analyze the product, the same procedure as the dipeptide experiment was followed. The fraction of tripeptide formed (f[3H]Met-Phe-Leu) was fitted with a model equation composed of three consecutive irreversible steps as previously described [2,3]. The total mean time for tripeptide formation (τtripeptide) was obtained by calculating the reciprocal of the rate constants.
For determination of the mean time of the second peptide bond formation (τp2) starting from binding of the second TC in a tripeptide reaction, three mixes were prepared. The mixes A and B were prepared with 2X concentration of all components used in the dipeptide reaction. The mix B also contained 2 µM EF-G. The mix C contained EF-Tu (5 µM), EF-Ts (1 µM), EF-G (9 µM), Leu (200 µM), Leu-synthetase (0.5 µM), tRNALeu1 (4 µM). After individual incubation at 37°C for 15 minutes, one volume of each of A and B were manually mixed, which would result in formation of f[3H]Met-Phe-tRNAPhe and translocate it to the P site. To this, two volumes of the mix C were rapidly added in quench-flow. The quenching of the reaction and the separation of the peptides using RP-HPLC was done as described above. The fraction of tripeptide, f[3H]Met-Phe-Leu formed over time was fitted with a single exponential function in order to determine τp2.
The mean time of EF-G cycle referred here as translocation, τtranslocation, was calculated by subtraction of τp1 and τp2 from τtripeptide as shown in Fig. 2A and Table 1 [2]. The final rate constants and the corresponding mean times were obtained by averaging the individual values obtained from at least three independent experiments and expressed with standard deviation.
Figure 2.
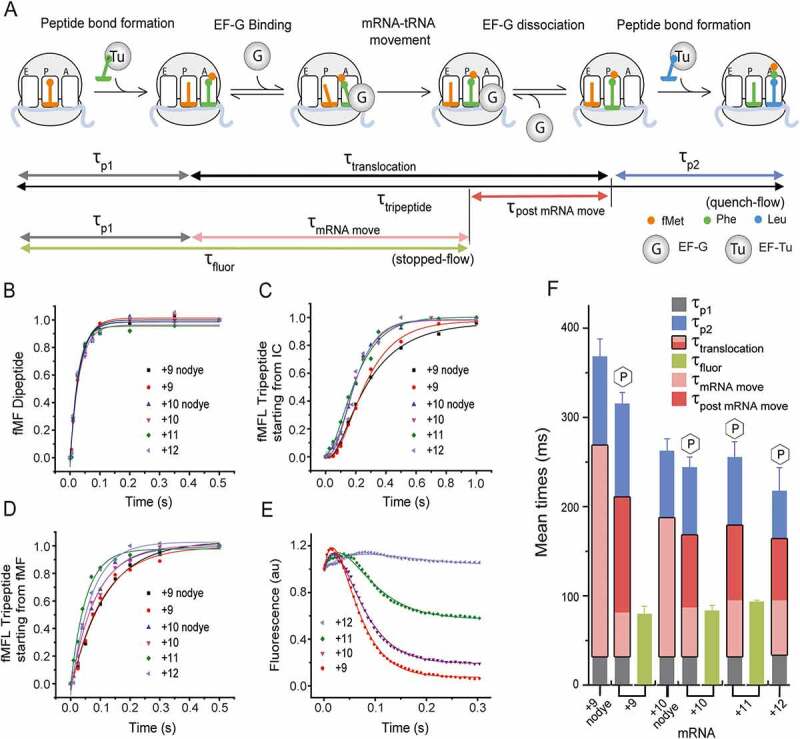
Determination of the optimal length of the mRNA for fast rates and large fluorescence change
(A) Schematic representation of the peptide elongation cycle on the ribosome starting from the 70S IC. The process includes two peptide bond formation steps and one translocation step driven by EF-G. The di- and tripeptide formation experiments were conducted in quench-flow, where an elongation factor mix containing the respective TCs (5 µM) was rapidly mixed with the 70S IC (0.5 µM) containing mRNA and fMet-tRNAfMet in the P site. By fitting the kinetic data as described in the Materials and Methods, the mean times of the first peptide bond formation (τp1), second peptide bond formation (τp2) and tripeptide formation (τtripeptide) were determined. Both τp1 and τp2 include time starting from binding of TC and entire EF-Tu cycle. The mean time of a full translocation reaction (τtranslocation) was calculated as [τtripeptide– (τp1+ τp2)]. In parallel, the kinetics of translocation starting from the 70S IC was followed in stopped-flow, where the fluorescence change of the 3ʹ pyrene labelled mRNAs was monitored in real time. The mean time τfluor, indicated total time of all events starting from the 70S IC up to and including mRNA movement. The mean time for mRNA movement, τmRNA move, was determined by subtracting τp1 from τfluor. (B) Kinetics of the first peptide bond formation and (C) tripeptide formation in quench-flow starting from the 70S IC with the mRNAs as indicated.(D) Kinetics of the second peptide bond formation starting from the post-translocation complex with fMet-Phe-tRNAPhe in P site. (E) Kinetics of EF-G mediated mRNA movement during translocation in a stopped-flow starting from the 70S IC, monitored by the changes in pyrene fluorescence (343 nm excitation, 360 nm long-pass filter) with the mRNAs.(F) Bar diagram for direct comparison of the mRNAs for the mean times of different steps of elongation. The three stacked bars indicate τtripeptide obtained by quench-flow with clear demarcations for τp1 (grey), τtranslocation (pink/red) and τp2 (blue). For fluorescent mRNAs τtranslocation (pink/red) is divided into τmRNA mov (pink) and τpost mRNA move (red). For mRNAs without dye entire τtranslocation is in pink. The stopped-flow based mean times τfluor are presented by the green bars. The error bars represent standard deviation.
Table 1.
The mean time of different steps of elongation starting from either 70S IC or pre-T complex measured by quench-flow and stopped-flow
| A. Reaction starting from 70S IC, the elongation mix contains natural peptidyl tRNA | |||||||
|---|---|---|---|---|---|---|---|
| Quench-flow measurement |
Stopped-flow measurement | Derived mean times following the model shown in Figure 2A |
|||||
| mRNA | τp1 (ms) | τtripeptide (ms) | τp2 (ms) |
τfluor (ms) |
τtranslocation (τtripeptide – (τp1 + τp2)) (ms) | τmRNA mov (τfluor – τp1) (ms) |
τpost mRNA move (τtranslocation – τmRNA mov) (ms) |
| MFL+9 nodye | 32 ± 2 | 368 ± 20 | 100 ± 6 | - | 236 ± 15 | - | - |
| MFL+9 | 30 ± 2 | 315 ± 13 | 119 ± 15 | 79 ± 9 | 180 ± 18 | 49 ± 9 | 131 ± 9 |
| MFL+10 nodye | 32 ± 3 | 261 ± 15 | 75 ± 6 | - | 154 ± 16 | - | - |
| MFL+10 | 31 ± 3 | 244 ± 12 | 76 ± 10 | 83 ± 6 | 137 ± 16 | 52 ± 7 | 85 ± 16 |
| MFL+11 | 31 ± 3 | 255 ± 18 | 66 ± 8 | 93 ± 2 | 158 ± 20 | 62 ± 4 | 96 ± 20 |
| MFL+12 |
33 ± 3 |
217 ± 27 |
56 ± 6 |
- |
128 ± 28 |
- |
- |
| B. Reaction starting from pre-TC with NAc-Phe- tRNAPhe in the A sitef | |||||||
| |
Quench-flow measurement |
Stopped-flow measurement |
|||||
| mRNA |
τtranslocation + τp2 (ms) |
τmRNA mov (ms) |
|
|
|
|
|
| MFL+9 | 2381 ± 227 | 112 ± 6 | |||||
| MFL+10 | 1667 ± 139 | 119 ± 7 | |||||
See Materials and Methods and Figures 2A and 3A for derivation of the mean time parameters. The results are average of minimum three identical replicates with standard deviation.
Ribosomal translocation in stopped-flow with pre-T complex containing natural dipeptidyl tRNA in the A site
Mixes A and B, same as in the quench-flow experiments for tripeptide formation starting from the 70S IC, were formed and incubated at 37°C for 15 min. Equal volume of A and B were mixed in a stopped-flow instrument (Applied Photophysics) and the fluorescence emission was followed with time using a 360 nm long-pass filter (Comar Optics Ltd). The excitation wavelength was 343 nm, typical for pyrene [4,35]. The fluorescence traces showed an initial small increase followed by a predominant monophasic decrease. The traces were fitted to a double exponential function using the equation , where k1 and k2 are the apparent rate constants, and A1and A2 are respective amplitudes of the first (increasing) and second (decreasing) phases respectively. The τfluor, which includes the mean times for first peptide bond formation (τp1) and the movement of the fluorescent mRNA (τmRNA move), was determined as 1/k1 + 1/k2. The τmRNA move value was then estimated by subtracting τp1 obtained from the quench-flow experiments from τfluor (Table 1). It should be noted that τmRNA move is shorter than τtranslocation. This is likely due to the fact that τtranslocation includes also time for ribosomal rearrangement and EF-G release prior to next EF-Tu TC binding and the mRNA-fluorescence based assay cannot detect those. It is unlikely that the time gap indicates any event before mRNA movement as τp1 includes peptide bond formation, which happens after EF-Tu release and immediately prior to EF-G binding. Therefore, we indicate the time gap between τtranslocation and τmRNA move as τpost mRNA move, which is the mean time for the post mRNA movement steps. τpost mRNA move was estimated by subtracting τmRNA move from τtranslocation (Table 1). The experiments were repeated at least three times and the average rate constants with standard deviation were determined.
Ribosomal translocation in stopped-flow and quench-flow with pre-T complex containing NAc-Phe-tRNAPhe
Ribosomal translocation and peptidyl transfer experiment with different length of mRNAs were also carried out starting from pre-T complex with peptidyl tRNA analog NAc-Phe-tRNAPhe in A site and deacylated tRNAfMet in the P site. The pre-T complex was formed by first incubating 1 µM 70S with 1.2 µM MFL coding mRNA and 2 µM tRNAfMet at 37°C for 15 min, followed by 20 min incubation with addition of 10 µM NAc-Phe-tRNAPhe.
The pre-T complex (1 µM) was mixed with EF-G (10 µM) in a stopped-flow and the change in pyrene fluorescence was followed as described above. As the traces were biphasic, they were fitted with the double exponential function, . τmRNA move was derived from the rate of the major phase, k1 in most of the cases.
For estimation of the mean time of the complete elongation cycle (τtranslocation + τp2, see the scheme in Fig. 3A), equal volumes of the pre-T complex (1 µM) and the elongation mix (10 µM EF-G and 2 µM Leu TC comprising [3H]Leu-tRNALeu1) were rapidly mixed in a quench-flow and NAc-Phe-[3H]Leu formation was determined by separating the peptides in RP-HPLC as described above. The data are fitted with a single exponential function.
Figure 3.
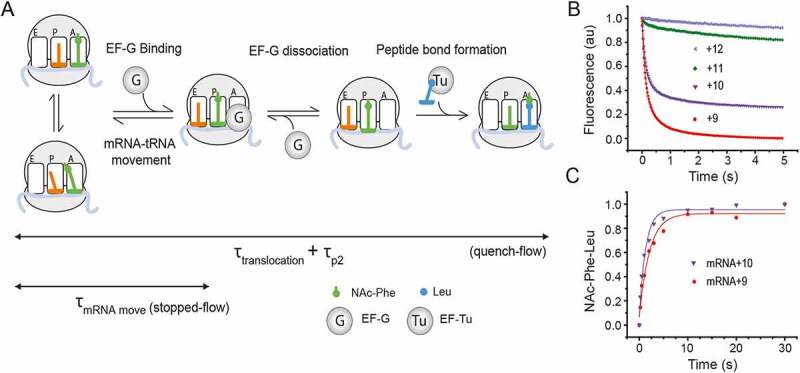
Kinetics of translocation of the pre-T complex containing NAc-Phe-tRNAPhe and pyrene labelled mRNAs
(A) Schematic representation of the peptide elongation cycle on the ribosome starting from the pre-equilibrated pre-T complexes, which are in equilibrium between the classical and the hybrid state. EF-G binding and GTP hydrolysis lead to mRNA movement by one codon. The ribosome complex undergoes certain structural rearrangements and EF-G releases, thereby leaving the ribosome ready for the next peptide bond formation. The meantime of the whole elongation cycle (τtranslocation + τp2) was determined by rapidly mixing NAc-Phe-tRNAPhe containing pre-T complex (0.5 µM) with EF-G (5 µM) and Leu TC (1 µM) in a quench-flow and following the formation of NAc-Phe-Leu against time. In parallel, the kinetics of the mRNA movement was followed in stopped-flow, where EF-G (5 µM) was rapidly mixed to a NAc-Phe-tRNAPhe containing pre-T complex (0.5 µM). As a result of the mRNA movement, the fluorescence of the 3ʹ pyrene labelled mRNAs decreases, which is monitored in stopped-flow against time. The τmRNA move was determined from the reciprocal of the rates obtained from the fluorescence traces.(B) Kinetics of mRNA movement upon EF-G binding to the pre-T complexes containing NAc-Phe-tRNAPhe and pyrene labelled mRNAs: mRNA+9 (red circle), mRNA+10 (violet inverted triangle), mRNA+11 (green diamond) and mRNA+12 (light purple left triangle). The pyrene fluorescence was monitored with 360 nm long-pass filter (343 nm excitation).(C) Kinetics of NAc-Phe-Leu formation in a quench-flow by rapid mixing of a pre-T complex with EF-G and Leu TC.
The translocation assay with pre-T complex was conducted in stopped-flow by varying (i) the mRNAs (Fig. 1); (ii) NAc-Phe-tRNAPhe concentration; (iii) EF–G concentration (0.5–10 µM); (iv) temperature (20, 25, 30 and 37°C), (v) Mg2+ concentration by adding additional 1–10 mM Mg(CH3COO)2. Further, the translocation assays with pre-T complex were also conducted in quench-flow to compare the mRNAs.
Results
The mRNA+10 translocates at the same rate as the longer mRNAs
All six mRNAs listed in Fig. 1 were subjected to dipeptide (fMet-Phe) and tripeptide (fMet-Phe-Leu) formation assay in quench-flow. The tripeptide formation assays were conducted starting from (i) the 70S IC and (ii) 70S post-translocation complex containing dipeptidyl tRNA in the P site. The mean times for formation of the first peptide bond (τp1), determined from the dipeptide assay were identical (~31 ms) for all mRNAs. This indicates that neither mRNA length nor pyrene labelling had any effect on this reaction (Fig. 2B, grey bars in Fig. 2F, Table 1A). The mean time of fMet-Phe-Leu tripeptide formation (τtripeptide), and the mean time of formation of the second peptide-bond (τp2), however, varied for the mRNAs, with unlabelled mRNA+9 (mRNA+9 nodye, Fig. 1) and pyrene-labelled mRNA+9 being the slowest ones (Fig. 2C, Fig. 2D, Fig. 2F, Table 1A). τtripeptide and τp2 for mRNA+9 were 315 ± 13 ms and 119 ± 15 ms, respectively. These mean times were at least 70 and 45 ms longer than those for the longer mRNAs. In contrast, the τtripeptide and τp2 values for mRNA+10 were 244 ± 12 ms and 76 ± 10 ms, respectively, similar to those for the longer mRNAs (Table 1A). When the unlabelled mRNAs were compared, mRNA+9 nodye was significantly slower than mRNA+10 nodye, in both tripeptide and second peptide bond formation (Fig. 2C, Fig. 2D, Fig. 2F, Table 1A).
The mean time of translocation (τtranslocation) was determined by subtracting the sum of τp1 and τp2 from τtripeptide. The τtranslocation for mRNA+9 was 180 ± 18 ms, while τtranslocation for all other mRNAs (including mRNA+10) was ~140 ms, 40 – 50 ms faster than for mRNA+9 (red bars in Fig. 2F, Table 1A). Thus, the mRNA+9 is clearly defective in overall translocation. Comparison of the τp2 values also demonstrate that the mRNA+9 is also defective in the subsequent peptide-bond formation, where it takes about 43 ms longer than mRNA+10 and longer mRNAs. Interestingly, these defects are ameliorated in mRNA+10 by addition of just one additional base at the 3ʹ end of the mRNA+9, which is as fast as the longer mRNAs in these vital steps. For convenience, all rates are summarized in Supplementary Table 1A.
The mean time of mRNA movement in translocation does not depend on the mRNA length but the mean time of post mRNA movement does
Stopped-flow experiments were conducted by addition of EF-Tu in ternary complex with natural aminoacyl tRNAs and EF-G to 70S IC carrying pyrene-labelled mRNAs of different lengths, to obtain information about both the amplitude and the rate of the fluorescence change induced by mRNA movement during translocation (Fig. 2E). The whole experiment involves several steps, namely binding of the ternary complex, peptide bond formation, EF-Tu release, binding of EF-G and subsequent mRNA movement and translocation. The mRNAs, +9, +10 and +11, showed an initial short increase followed by a prominent single-phase decrease in pyrene fluorescence similar to our previous report [36]. While the monophasic fluorescence decay indicates mRNA movement during translocation, as also suggested by previous studies [4,5,11,16,17,19,20,25,37], the initial fluorescence increase probably happens during the prior events listed above. As explained in the Materials & Methods section, experimental data were fitted with a double exponential function to estimate the τfluor, i.e. the sum of τp1 and τmRNA move (Table 1A).
In our experiments the mean time of all steps, up to and including mRNA movement (τfluor), was ~80 ms for the +9, +10 and +11 fluorescent mRNAs (purple bars in Fig. 2F, Table 1). Thus, the mean time of mRNA movement (τmRNA move), estimated by subtracting τp1 from τfluor was ~50 ms for all three mRNAs, thereby suggesting that the mean time of mRNA movement (τmRNA move) is not dependent on the length of the mRNA. The rates are listed in Supplementary Table 1A.
It is clearly noticeable from Table 1 that τmRNA move is much shorter than τtranslocation. This means that post mRNA movement steps of translocation, namely ribosomal rearrangement and EF-G release occupy significant fraction of the total time for translocation. The mean time of this step, presented as τpost mRNA move is estimated by subtraction of τmRNA move from τtranslocation. This phase is longest for mRNA+9, 131 ± 9 ms. In contrast, the mRNA+10 or longer mRNAs spent about 90 ms in this step. Thus, the mRNA+9 is grossly defective in the post-mRNA movement steps of ribosomal translocation, taking about 40 ms longer than the longer mRNAs.
In agreement with the results obtained by Studer et al. [4], the relative amplitude of the fluorescence change decreased with increasing length of the mRNA. mRNA+9 and mRNA+10 showed the largest and the second-largest fluorescence change, respectively (Fig. 2E). In comparison, mRNA+11 had a roughly 50% smaller fluorescence amplitude and almost no change in fluorescence could be detected with mRNA+12. Hence we conclude that the mRNA+12 or longer mRNAs labelled with fluorescent dye at the 3ʹ are not suitable for this stopped-flow based assay.
Since the mRNA with the largest fluorescence change, mRNA+9, translocated significantly slower than the longer mRNAs and lead to much slower formation of the second peptide-bond (Fig. 2F, Table 1A), functional studies with this mRNA are likely to be compromised. However, mRNA+10 restored the rates of these two steps to comparable magnitude as with longer unlabelled mRNAs, while maintaining near-maximum fluorescence change. Thus, mRNA+10 is undoubtedly the best mRNA for this assay.
The dipeptidyl tRNA analog NAc-Phe-tRNAPhe is less efficient in translocation than natural dipeptidyl tRNAs
The pre-T complex containing natural peptidyl tRNAs in the ribosomal A site is inherently unstable on the timescale of typical laboratory work. Thus, tRNAs charged with N-acetylated amino acids are commonly used as A-site peptidyl tRNA analogs to mimic the pre-T complex. The binding of such analogs is a factor-independent equilibrium process and the substrates can therefore be supplied in large excesses [4,5,11,16,17,19,20,25,37]. We designed a set of experiments to compare translocation of a pre-T complex formed by pre-equilibration with a commonly used peptidyl tRNA analog NAc-Phe-tRNAPhe to a pre-T complex containing natural fMet-Phe-tRNAPhe, formed as described above, in continuous progression (without pre-equilibration), starting from the 70S IC (Fig. 3A).
First we conducted the stopped-flow based assay using the pyrene-labelled mRNAs. Irrespective of the length of the mRNAs (+9 to +12), translocation of NAc-Phe-tRNAPhe produced a biphasic fluorescence-decay curve. The fast phase accounted for 80–90% of the amplitude and the slow phase accounted for the remaining 10%– 20% (Fig. 3B, Supplementary Table 2). Contrary to the reactions with natural dipeptidyl tRNAs (Fig. 2E), no initial increase in fluorescence could be seen (Fig. 3B). Our biphasic decay curves were similar to previous reports [5,11,19,20,25]. However, since the fast phase was significantly larger than the slow phase in our assay (Fig. 3B), we estimated τmRNA move as a reciprocal of the rate k1 (Supplementary Table 2). For both mRNA+9 and mRNA+10, the τmRNA move with NAc-Phe-tRNAPhe was ~115 ms, which means that under identical reaction conditions, NAc-Phe-tRNAPhe leads to at least two times slower mRNA movement than the natural dipeptidyl tRNA, which takes about 50 ms (τmRNA move = ~50 ms) (Table 1A). Addition of EF-Tu and EF-Ts in the NAc-Phe-tRNAPhe reaction mix did not change the rate of mRNA movement (Supplementary Figure 1). As shown in Fig. 3B, both mRNA+9 and mRNA+10 produced significant change in pyrene fluorescence upon translocation with NAc-Phe-tRNAPhe. In contrast, the mRNA+11 and mRNA+12 produced very small fluorescence changes and therefore accurate translocation rates could not be determined.
Table 2.
Effect of varying temperature, EF-G and Mg2+ concentration, EF-G mutation and GTP analogs on mRNA movement during ribosomal translocation with NAc-Phe-tRNAPhe.
| k1(s−1) | k2 (s−1) | A1/(A1+ A2) | τmRNA move (ms) | |
|---|---|---|---|---|
| A. EF-G (μM) | ||||
| 0.5 | 2.8 ± 0.5 | 0.6 ± 0.05 | 0.49 ± 0.03 | 357 ± 67 |
| 1.25 | 4.4 ± 0.1 | 0.92 ± 0.01 | 0.76 ± 0.06 | 227 ± 5 |
| 2.5 | 6 ± 0.2 | 0.72 ± 0.1 | 0.84 ± 0.04 | 167 ± 6 |
| 5 | 8.4 ± 0.5 | 0.66 ± 0.2 | 0.88 ± 0.06 | 119 ± 7 |
| 10 | 10.3 ± 0.3 | 0.4 ± 0.07 | 0.91 ± 0.02 | 97 ± 3 |
| B. Temperature (°C) | ||||
| 37 | 8.4 ± 0.5 | 0.66 ± 0.2 | 0.88 ± 0.06 | 119 ± 7 |
| 30 | 3.2 ± 0.1 | 0.56 ± 0.02 | 0.74 ± 0.01 | 313 ± 10 |
| 25 | 1.25 ± 0.06 | 0.30 ± 0.01 | 0.62 ± 0.01 | 800 ± 38 |
| 20 | 0.35 ± 0.07 | 0.09 ± 0.02 | 0.47 ± 0.02 | 2857 ± 571 |
| C. Extra Mg2+ (mM) | ||||
| 1 | 5.8 ± 1.3 | 0.71 ± 0.01 | 0.66 ± 0.01 | 172 ± 39 |
| 2 | 3.2 ± 0.2 | 0.43 ± 0.03 | 0.62 ± 0.03 | 313 ± 20 |
| 3 | 1.9 ± 0.2 | 0.28 ± 0.11 | 0.52 ± 0.02 | 526 ± 55 |
| 5 | 0.8 ± 0.3 | 0.17 ± 0.04 | 0.48 ± 0.05 | 1250 ± 469 |
| 10 | 0.19 ± 0.01 | 0.04 ± 0.01 | 0.49 ± 0.01 | 5263 ± 277 |
The biphasic traces of pyrene fluorescence (Figure 5) are fitted with double exponential function. k1, k2, and A1, A2 are the rate constants and amplitudes of the fast and the slow phases respectively. See Materials and Methods for derivation of τmRNA move. The results are average of minimum three experimental replicates with standard deviation.
We also checked NAc-Phe-tRNAPhe in quench-flow for translocation and NAc-Phe-Leu peptide bond formation (Fig. 3C). The length of one elongation cycle (τtranslocation + τp2) with NAc-Phe-tRNAPhe was significantly longer than with natural dipeptidyl tRNAs. While τtranslocation + τp2 was about 300 ms for the dipeptidyl tRNAs, it was almost 2 sec with NAc-Phe-tRNAPhe (Table 1A, B). Thus, the use of NAc-Phe-tRNAPhe in translocation leads to large functional defects. Interestingly, τtranslocation + τp2 is comparatively shorter with mRNA+10 than with mRNA+9, adding further evidence that mRNA+10 is better suited for functional assays than mRNA+9. The rates are reported in Supplementary Table 1B.
Optimizing the stopped-flow based assay with the peptidyl tRNA analog NAc-Phe-tRNAPhe
To identify the optimal conditions for both translocation rate and fluorescence signal amplitude, we systematically titrated NAc-Phe-tRNAPhe, EF-G, and Mg2+, and varied temperature in the fluorescent-mRNA based translocation assay in stopped-flow.
First, NAc-Phe-tRNAPhe was titrated by supplying it with the EF-G containing elongation mix such that translocation would require first binding of NAc-Phe-tRNAPhe to the ribosomal A site. Increase in NAc-Phe-tRNAPhe concentration increased the rate of the fluorescence decay in a linear fashion (Fig. 4A) from 0.005 s−1 at 0.5 µM to 0.013 s−1at 5 µM. As EF-G mediated translocation is much faster (about 8 s−1), the rate of the fluorescence change here is limited by association of NAc-Phe-tRNAPhe to the 70S IC. It allowed us to estimate the binding parameter of NAc-Phe-tRNAPhe to the 70S IC. By plotting the observed rates vs. NAc-Phe-tRNAPhe concentrations, the dissociation constant KD was determined to be 2.7 ± 0.2 µM (Fig. 4B).
Figure 4.
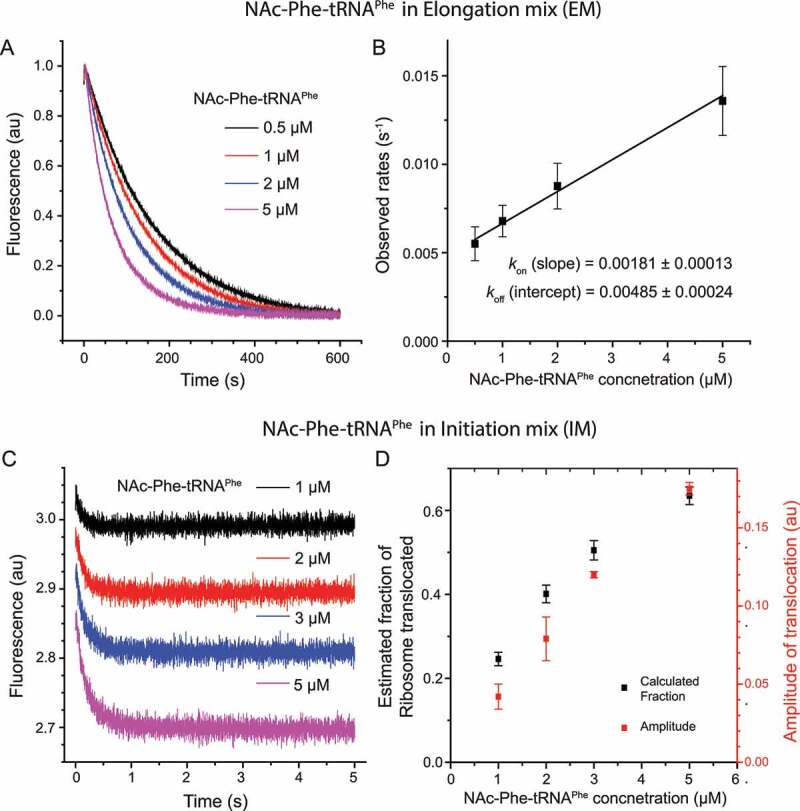
NAc-Phe-tRNAPhe titration in the 3’ pyrene-labelled mRNA+10 based translocation reaction in stopped-flow
(A) Pyrene fluorescence traces obtained by adding NAc-Phe-tRNAPhe (0.5–5 µM) in the elongation mix (EM), which was mixed rapidly with the 70S IC (0.5 µM) carrying mRNA+10 and tRNAfMet. (B) The plot of the observed rates of pyrene fluorescence change in (A) versus NAc-Phe-tRNAPhe concentration. The data are fitted with linear equation to obtain the binding parameters. The KD was determined as koff/kon = 2.7 ± 0.2 µM. (C) Pyrene fluorescence traces obtained by adding NAc-Phe-tRNAPhe (1–5 µM) in the initiation mix (IM) containing 70S IC (0.5 µM), which was mixed rapidly with EM containing EF-G (5 µM). The start point of each fluorescence trace is offset vertically for easy comparison. The data is fitted with double exponential function and presented in Supplementary Table 3. (D) Correlation of the calculated and experimentally determined fractions of the 70S pre-TC, obtained from the KD value (B) and fluorescence amplitudes (C), respectively. Data represent average of three replicates with standard deviation.
Next, NAc-Phe-tRNAPhe was titrated (1–5 µM) into the 70S IC mix (0.5 µM), allowing it to pre-equilibrate with the ribosomes to form a pre-T complex. Here, the rates of fluorescence decay were similar over the entire concentration range, but the fluorescence amplitude changed as a function of NAc-Phe-tRNAPhe concentration (Fig. 4C, Supplementary Table 3). The fractions of pre-T complex were directly estimated from the amplitudes of fluorescence traces in Fig. 4C (Supplementary Table 3). These were compared with the fractions of the pre-T complex calculated from the KD value (Fig. 4B). We observed a substantial correlation between the two, showing that NAc-Phe-tRNAPhe binding to ribosome under this condition is considerably weak (Fig. 4D). On the basis of these results, 2 µM or higher concentration of NAc-Phe-tRNAPhe is required for near-stoichiometric formation of the pre-T complex and un-ambiguous estimation of translocation rates.
Next, EF-G was titrated (0.5 to 10 µM) in the reaction with NAc-Phe-tRNAPhe (Fig. 5A). As expected, translocation was faster at higher EF-G concentrations (Table 2A): τmRNA move decreased to around 100 ms at 10 µM from 357 ± 67 ms at 0.5 µM of EF-G. Interestingly, the biphasic nature of the fluorescence traces also changed. At 0.5 µM EF-G concentration the amplitude of the fast phase was ~50%, which increased to ~90% with EF-G equal to or more than 5 µM (Fig. 5B, Table 2A). From the rates of translocation at varying EF-G concentrations, the Michaelis-Menten parameters for NAc-Phe-tRNAPhe translocation, (kcat = 11.9 ± 0.8 s−1 and kcat/KM = 5.4 ± 1 µM−1 s−1), were determined (Supplementary Figure 2). The same assay with natural dipeptidyl tRNAs starting from the 70S IC, showed kcat = 22.8 ± 2.3 s−1 and kcat/KM = 9.1 ± 0.14 µM−1 s−1 (Supplementary Figure 2). These results clearly demonstrate that NAc-Phe-tRNAPhe is about two times less efficient than the natural dipeptidyl-tRNAs in EF-G mediated translocation.
Figure 5.
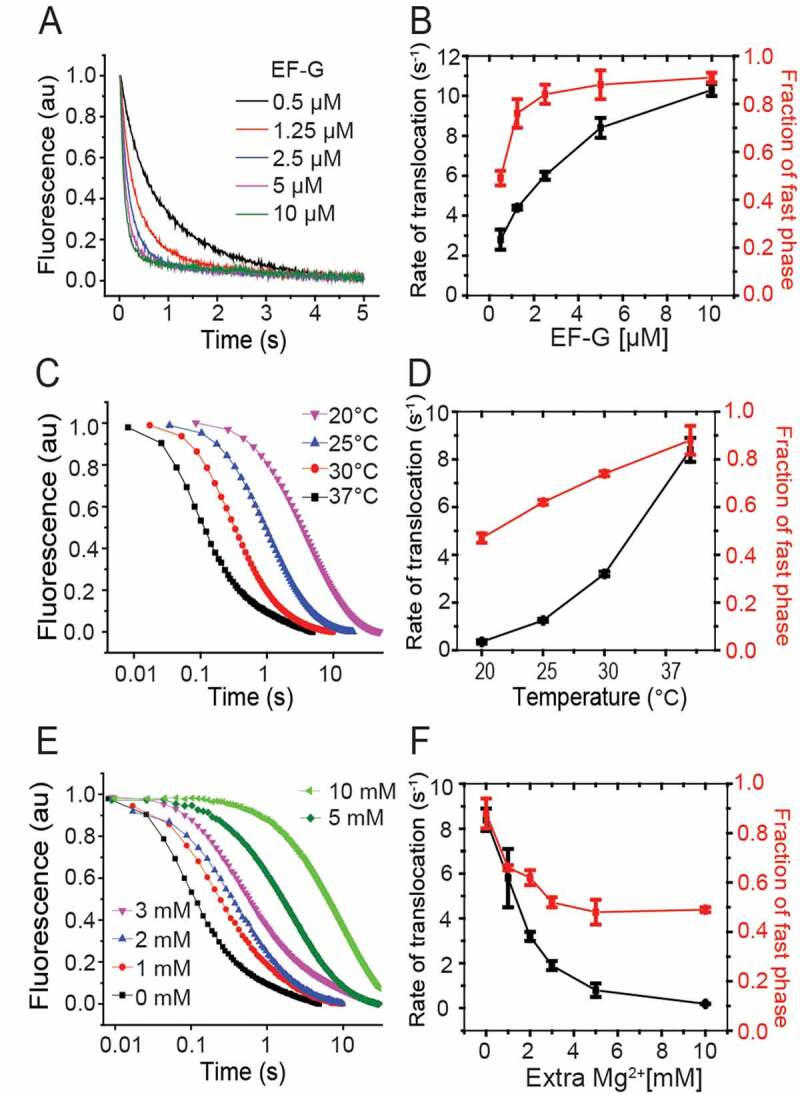
Kinetics of NAc-Phe-tRNAPhe translocation with varying temperature and concentrations of EF-G and Mg2+
The kinetics of the mRNA movement during translocation was followed in stopped-flow, where EF-G was rapidly mixed to a NAc-Phe-tRNAPhe containing pre-T complex (0.5 µM). The left panels show the pyrene fluorescence traces monitored with 360 nm long-pass filter (343 nm excitation). The right panels present the rates and amplitudes of the fast phase of the fluorescence traces (Table 2). The plots represent measurements with varying concentration of EF-G (A, B), different temperature (C, D) and varying Mg2+ concentration (E, F). The rates and the fractional amplitude of the fast phase are summarized in Table 2.
Next we studied the effect of temperature and Mg2+ ion concentration on the rate of translocation using the pyrene–mRNA assay (Fig. 5C–F). These two parameters have varied a lot in the existing literature [3,4,11,16,25,36,38]. Irrespective of the temperature (20, 25, 30 and 37°C) and Mg2+ concentration (1-10 mM Mg2+), we obtained biphasic fluorescence traces with varying amplitudes of the fast and the slow phase (Fig. 5C,E, Table 2). As expected, the rates were lower at lower temperatures and higher Mg2+ concentrations (Table 2B, C), as also seen by Feldman et al [17,36]. The reactions at 20°C were ~25 times slower than at 37°C (Fig. 5C, Table 2B). Similarly, addition of 10 mM extra Mg2+ (at 37°C) slowed the reaction by ~30 times (Fig. 5E, Table 2C). This effect of Mg2+ concentration is comparable to its effect on translocation with native tRNAs , as published previously [2,17]. Interestingly, the fractional amplitude of the fast phase (A1/(A1+ A2)) also changed with decreasing temperature and increasing Mg2+ (Fig. 5D,F, Table 2B,C). Our results closely match the rates reported in the literature under similar conditions [5,11,16,19,20,25].
Discussion
The fluorescent-mRNA based assay, originally developed by Studer et al. [4], is popularly used to study ribosomal translocation in real time using stopped-flow [4,5,11,16,17,19,20,25,37]. Our main objective in this work was to optimize this assay by calibrating it with the quench-flow based translocation measurements using unlabelled native substrates.
First, we aimed to identify the optimal length of the pyrene-labelled mRNA that would not only produce a high fluorescence signal, but would also show kinetics comparable to those obtained with longer, natural mRNAs. For that, we tested pyrene-labelled mRNAs of different length (+9 to +12), in the fluorescent-mRNA based assay in stopped-flow, and in parallel, in tripeptide-formation assay in quench-flow. Our results show that mRNA+9, suggested by the original work [4] and used extensively in the literature [4,11,16,17,19,25], is slow in overall translocation and subsequent peptide bond formation (Fig. 2C,D,F and Table 1).Thus, the kinetic rates obtained with mRNA+9 may not be physiologically relevant. However, extending the mRNA by just one more nucleotide rectifies these rate issues. The mRNA+10 translocates as fast as the longer mRNAs and causes no issues with downstream elongation (Fig. 2C,D,F and Table 1). When compared in the fluorescence based stopped-flow assay, all mRNAs moved at similar rates, suggesting that the speed of mRNA movement during translocation is not dependent on the mRNA length (Fig. 2F, Table 1). However, the amplitude of the fluorescence decay showed strong mRNA-length dependence, mRNA+9 being the best one and mRNA+10 being the second best (Figures 2E, Figures 3B). When mRNAs longer than 10 bases were tested, the fluorescence change was small as reported earlier [4], and therefore not suitable for kinetic analysis. Probably for these longer mRNAs, the dye at the 3ʹ end cannot enter the ribosome milieu by one round of translocation. In conclusion, mRNA+10 is clearly the best mRNA for obtaining relatable rates in the fluorescent-mRNA based stopped-flow assay and the quench-flow based tripeptide assay without compromising the fluorescence signal.
When the mRNAs are tested in tripeptide formation assay for determining the mean time of a complete translocation cycle starting from 70S IC, those mRNAs, truncated immediately after the A-site codon (mRNA+9 and mRNA+9 nodye, Fig. 1) show large defects in the post mRNA-movement steps of translocation. These late steps of translocation have recently been implicated in determining both the overall rate [39,40] and possibly accuracy [41] of the process. Most likely, abrupt truncation of these mRNAs leads to problems with ribosomal rearrangement such as reverse swivel of the small-subunit head domain. It is also possible that these truncated mRNAs are partially destabilized in the A site, making the recruitment of the second ternary complex defective and thereby slowing down the second peptide bond formation. It is interesting that addition of just one extra nucleotide, thereby extending the mRNA into the mRNA channel downstream of the A site [42] alleviates these issues. Addition of the pyrene dye at the 3ʹ end also seems to partially improve the situation as labelled mRNA+9 is faster than the unlabelled mRNA+9 in translocation (Fig. 2F, Table 1). These results imply that base interaction in the mRNA channel downstream to the A-site codon is possibly important to anchor the mRNA during translocation.
Another important aspect of this work is that we carefully compared the peptidyl tRNA analog NAc-Phe-tRNAPhe with the natural dipeptidyl tRNA (fMet-Phe-tRNAPhe) in the fluorescent-mRNA based translocation assay. The rate of translocation with NAc-Phe-tRNAPhe is about two times lower than with the natural dipeptidyl tRNA (Supplementary Figure 2 and Table 1). This result is not too surprising given that the acetyl group is significantly smaller than an amino acid (f-Met in this case). However, NAc-Phe-tRNAPhe sits quite stably in the ribosomal A-site than the natural dipeptidyl tRNAs, which has half-life of only 10 seconds. This advantage cannot be ignored.
Another notable difference was in the nature of the declining phase of the fluorescence traces. It was clearly biphasic with NAc-Phe-tRNAPhe, as also reported by many other studies [5,11,17,19,20,25] (Fig. 4C). In contrast, the natural dipeptidyl tRNAs lead to monophasic fluorescence decay (Fig. 2E) [36]. In NAc-Phe-tRNAPhe translocation assays, increasing temperature led to significantly larger fast phases, while increasing Mg2+ had the opposite effect (Fig. 5D, Fig. 5F, Table 2B, C). Increase in the concentration of EF-G also led to predominant (~90%) fast phase (Fig. 5B, Table 2A). Although we cannot determine the exact cause of the differences between the biphasic vs. monophasic curves, we notice that such biphasic curves are frequently reported in the literature, whenever the pre-T complex has been allowed to pre-equilibrate by incubation. This is irrespective of whether the reaction was done with peptidyl tRNA analogs [5,11,19,25,28] or natural peptidyl tRNAs [10,17,38,43]. In contrast, when the pre-T complex was formed by continuous progression from the 70S IC and translocated immediately, the fluorescence decay was monophasic (Fig. 2E) [36]. We suspect, in line with an earlier report [29] that the two phases of the biphasic curves likely represent different translocation rates from the two conformational states of the pre-T ribosome, which are known to populate at equilibrium [13,14,44]. Without pre-equilibrium, the pre-T ribosomes reach a uniform conformational state and therefore show a monophasic transition, a suggestion tentatively supported by recent single-molecule FRET data [45]. EF-G binding likely facilitates transition of the pre-T complex to a translocation-competent state, thereby resulting in near-monophasic kinetics at high concentration.
We also noticed differences in the fluorescence traces obtained with NAc-Phe-tRNAPhe compared to the natural dipeptidyl tRNA. When translocation was initiated by addition of NAc-Phe-tRNAPhe TC and EF-G (in EM) to the programmed 70S IC, an initial short increase of fluorescence could be seen (Fig. 4A). This was absent when translocation was conducted by addition of EF-G to a pre-T complex containing NAc-Phe-tRNAPhe (Fig. 4C). Since the latter did not involve any natural steps for formation of the pre-T complex, we assume that the initial increase in the former case indicates structural rearrangement in the ribosome or the mRNA related to peptide bond formation and EF-Tu release.
The low affinity of NAc-Phe-tRNAPhe for the ribosomal A site during formation of the pre-T complex should also be noted. Binding affinity measurements obtained by titrating NAc-Phe-tRNAPhe into the EF-G mix indicated a fairly high KD (2.7 ± 0.2 µM) (Fig. 4B). This implies that the low fluorescence amplitude obtained with low concentration of NAc-Phe-tRNAPhe is due to its poor binding to the pre-T complex (Figures 4C, D). However, it is unlikely that the slow phase in translocation with NAc-Phe-tRNAPhe results from this poor affinity, since the rate of binding of NAc-Phe-tRNAPhe was significantly slower (0.013 s−1) than the rate of the slow phase (about 0.6 s−1) under our experimental condition (Fig. 4A,B).
In summary, this study demonstrates pyrene labelled mRNA+10 as the most suitable mRNA for real-time translocation assay as well as the functional tripeptide formation assay. We further report the best reaction conditions for the fluorescent-mRNA based translocation assay with NAc-Phe-tRNAPhe.
Supplementary Material
Acknowledgments
We sincerely thank Xueliang Ge and Narayan Prasad Parajuli for their help in manuscript preparation and making the illustrations.
Funding Statement
This work was supported by Carl Tryggers Stiftelse för Vetenskaplig Forskning [CTS 18:338, CTS 19: 806]; Knut och Alice Wallenbergs Stiftelse [KAW 2017.0055]; Vetenskapsrådet [2016-06264]; Vetenskapsrådet [2018-05498]; Wenner-Gren Foundations [UPD2017-0238, UPD2018-0306] to S.S.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Young R, Bremer H.. Polypeptide-chain-elongation rate in Escherichia coli B/r as a function of growth rate. Biochem J. 1976;160(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Borg A, Ehrenberg M.. Determinants of the rate of mRNA translocation in bacterial protein synthesis. J Mol Biol. 2015;427(9):1835–1847. [DOI] [PubMed] [Google Scholar]
- [3].Holm M, Borg A, Ehrenberg M, et al. Molecular mechanism of viomycin inhibition of peptide elongation in bacteria. Proc Natl Acad Sci U S A. 2016;113(4):978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Studer SM, Feinberg JS, Joseph S. Rapid kinetic analysis of EF-G-dependent mRNA translocation in the ribosome. J Mol Biol. 2003;327(2):369–381. [DOI] [PubMed] [Google Scholar]
- [5].Feinberg JS, Joseph S. Ribose 2ʹ-hydroxyl groups in the 5ʹ strand of the acceptor arm of P-site tRNA are not essential for EF-G catalyzed translocation. RNA. 2006;12(4):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilden B, Savelsbergh A, Rodnina MV, et al. Role and timing of GTP binding and hydrolysis during EF-G-dependent tRNA translocation on the ribosome. Proc Natl Acad Sci U S A. 2006;103(37):13670–13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Betteridge T, Liu H, Gamper H, et al. Fluorescent labeling of tRNAs for dynamics experiments. RNA. 2007;13(9):1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pan D, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25(4):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Svidritskiy E, Ling C, Ermolenko DN, et al. Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome. Proc Natl Acad Sci U S A. 2013;110(30):12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cunha CE, Belardinelli R, Peske F, et al. Dual use of GTP hydrolysis by elongation factor G on the ribosome. Translation (Austin). 2013;1:e24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ermolenko DN, Noller HF. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat Struct Mol Biol. 2011;18(4):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Holtkamp W, Cunha CE, Peske F, et al. GTP hydrolysis by EF-G synchronizes tRNA movement on small and large ribosomal subunits. Embo J. 2014;33(9):1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blanchard SC, Kim HD, Gonzalez RL Jr., et al. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci U S A. 2004;101(35):12893–12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cornish PV, Ermolenko DN, Noller HF, et al. Spontaneous intersubunit rotation in single ribosomes. Mol Cell. 2008;30(5):578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Devaraj A, Shoji S, Holbrook ED, et al. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA. 2009;15(2):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dorner S, Brunelle JL, Sharma D, et al. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat Struct Mol Biol. 2006;13(3):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feldman MB, Terry DS, Altman RB, et al. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nat Chem Biol. 2010;6(3):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Garcia-Ortega L, Alvarez-Garcia E, Gavilanes JG, et al. Cleavage of the sarcin-ricin loop of 23S rRNA differentially affects EF-G and EF-Tu binding. Nucleic Acids Res. 2010;38(12):4108–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo Z, Noller HF. Rotation of the head of the 30S ribosomal subunit during mRNA translocation. Proc Natl Acad Sci U S A. 2012;109(50):20391–20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khade PK, Joseph S. Messenger RNA interactions in the decoding center control the rate of translocation. Nat Struct Mol Biol. 2011;18(11):1300–1302. [DOI] [PubMed] [Google Scholar]
- [21].Liu H, Chen C, Zhang H, et al. The conserved protein EF4 (LepA) modulates the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 2011;108(39):16223–16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu H, Pan D, Pech M, et al. Interrupted catalysis: the EF4 (LepA) effect on back-translocation. J Mol Biol. 2010;396(4):1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Q, Fredrick K. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 2013;41(1):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salsi E, Farah E, Dann J, et al. Following movement of domain IV of elongation factor G during ribosomal translocation. Proc Natl Acad Sci U S A. 2014;111(42):15060–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Salsi E, Farah E, Ermolenko DN. EF-G activation by phosphate analogs. J Mol Biol. 2016;428(10):2248–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi X, Khade PK, Sanbonmatsu KY, et al. Functional role of the sarcin-ricin loop of the 23S rRNA in the elongation cycle of protein synthesis. J Mol Biol. 2012;419(3–4):125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ticu C, Nechifor R, Nguyen B, et al. Conformational changes in switch I of EF-G drive its directional cycling on and off the ribosome. Embo J. 2009;28(14):2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Walker SE, Shoji S, Pan D, et al. Role of hybrid tRNA-binding states in ribosomal translocation. Proc Natl Acad Sci U S A. 2008;105(27):9192–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xie P. An explanation of biphasic characters of mRNA translocation in the ribosome. Biosystems. 2014;118:1–7. [DOI] [PubMed] [Google Scholar]
- [30].Ge X, Mandava CS, Lind C, et al. Complementary charge-based interaction between the ribosomal-stalk protein L7/12 and IF2 is the key to rapid subunit association. Proc Natl Acad Sci U S A. 2018;115(18):4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mandava CS, Peisker K, Ederth J, et al. Bacterial ribosome requires multiple L12 dimers for efficient initiation and elongation of protein synthesis involving IF2 and EF-G. Nucleic Acids Res. 2012;40(5):2054–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Antoun A, Pavlov MY, Tenson T, et al. Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering. Biol Proced Online. 2004;6(1):35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Haenni AL, Chapeville F. Behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966;114(1):135-+. [DOI] [PubMed] [Google Scholar]
- [34].Koripella RK, Holm M, Dourado D, et al. A conserved histidine in switch-II of EF-G moderates release of inorganic phosphate. Sci Rep. 2015;5(1):12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kierzek R, Li Y, Turner DH, et al. 5ʹ-amino pyrene provides a sensitive, nonperturbing fluorescent-probe of Rna secondary and tertiary structure formation. J Am Chem Soc. 1993;115(12):4985–4992. [Google Scholar]
- [36].Borg A, Holm M, Shiroyama I, et al. Fusidic acid targets elongation factor G in several stages of translocation on the bacterial ribosome. J Biol Chem. 2015;290(6):3440–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Juette MF, Terry DS, Wasserman MR, et al. Single-molecule imaging of non-equilibrium molecular ensembles on the millisecond timescale. Nat Methods. 2016;13(4):341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rodnina MV, Savelsbergh A, Katunin VI, et al. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature. 1997;385(6611):37–41. [DOI] [PubMed] [Google Scholar]
- [39].Wasserman MR, Alejo JL, Altman RB, et al. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat Struct Mol Biol. 2016;23(4):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Flis J, Holm M, Rundlet EJ, et al. tRNA translocation by the Eukaryotic 80S Ribosome and the impact of GTP hydrolysis. Cell Rep. 2018;25(10):2676–2688 e2677. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alejo JL, Blanchard SC. Miscoding-induced stalling of substrate translocation on the bacterial ribosome. Proc Natl Acad Sci U S A. 2017;114(41):E8603–E8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gao YG, Selmer M, Dunham CM, et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science. 2009;326(5953):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mohr D, Wintermeyer W, Rodnina MV. Arginines 29 and 59 of elongation factor G are important for GTP hydrolysis or translocation on the ribosome. Embo J. 2000;19(13):3458–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim HD, Puglisi JD, Chu S. Fluctuations of transfer RNAs between classical and hybrid states. Biophys J. 2007;93(10):3575–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jamiolkowski RM, Chen C, Cooperman BS, et al. tRNA fluctuations observed on stalled ribosomes are suppressed during ongoing protein synthesis. Biophys J. 2017;113(11):2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


