ABSTRACT
Interleukin-1 (IL-1) is an inflammatory cytokine associated with tumor invasiveness and metastasis. We recently found that baseline IL-1 in melanomas promoted resistance to immunotherapy by creating an immunosuppressive tumor microenvironment and that IL-1 produced in response to CD40 agonist also induced resistance to therapy. Here, we discuss how naturally occurring and immunotherapy-induced IL-1 in tumors causes immune suppression and resistance to immunotherapy, and we discuss targeting the IL-1 pathway to enhance the efficacy of immunotherapy.
KEYWORDS: Inflammation, IL-1, cancer, immunotherapy
Introduction
Although inflammation helps eliminate pathogens and heals injuries, it can induce long-lasting disorders like autoimmune diseases, asthma, and cancers. Interleukin-1 (IL-1) is a well-known inflammatory cytokine that can cause sterile inflammation and infection-induced inflammation. IL-1 is also associated with tumor invasiveness and metastasis. The two forms of IL-1, IL-1α and IL-1β, are derived from different genes but are functionally similar, and both bind to IL-1 receptor type 1 (IL-1R1).1 However, other receptors, such as IL-1R2, can act as decoy receptors and bind to pro-IL-1, thus preventing it from being cleaved by proteases and released as the active form.2 Furthermore, IL-1 receptor antagonist binds to IL-1R1, thereby inhibiting its activation.3 IL-1 has been associated with decreased survival of patients with melanoma,4 and endogenous IL-1 facilitates the growth of human melanoma.5 However, the role of IL-1 in suppression of anti-tumor immunity is not well studied.
We recently reported that baseline IL-1α in melanomas promoted resistance to immunotherapy by creating an immunosuppressive tumor microenvironment and that IL-1α produced in response to CD40 agonist also promoted resistance to immunotherapy.6 Here, we provide a brief overview of the role of IL-1 in suppression of anti-tumor immunity and the possibility of enhancing the efficacy of cancer immunotherapies by targeting the IL-1 signaling pathway.
Sources of IL-1 in the tumor microenvironment
Baseline IL-1
We recently reported that IL-1α in tumors increased with increasing tumor size, and inflammatory monocytes are the key cells that produce IL-1 in the tumor microenvironment.6 Since larger tumors contain more necrotic cells, we speculate that the DAMP (damage-associated molecular pattern) pathway plays an essential role in IL-1 induction through toll-like receptor–nuclear factor kappa B (TLR–NF-kB) activation in the tumor microenvironment. Indeed, it has been reported that DAMPs and acidic environments can induce the secretion of IL-1β independently of the inflammasome7 or through the inflammasome.8 Microbes and microbial products in the tumor can also induce IL-1 through TLR–NF-kB activation.
Immunotherapy-induced IL-1
We found that CD40 activation triggered IL-1 production in the tumor microenvironment;6 however, we did not study the mechanism by which CD40 activation induces IL-1 production. Other investigators showed that CD40 activation induced IL-1β-converting enzyme (Caspase-1) activity in vascular smooth muscle and endothelial cells, thus inducing IL-1β production.9 It would be interesting to know whether IL-1 can also be induced through CD40 ligand–CD40–NF-kB activation.
IL-1 mediates suppression of anti-tumor immunity
Our findings showed that both baseline IL-1 and CD40 agonist-induced IL-1 mediated increased accumulation of polymorphonuclear myeloid-derived suppressive cells (PMN-MDSCs) in tumors.6 Our unpublished data showed that tumor-associated monocytes and macrophages are the key cells that express IL-1R1 on the surface. Therefore, it may be that the IL-1 signaling cascade in monocytes and macrophages produces PMN-MDSC-recruiting chemokines (CXCL1, CXCL2, and CXCL5), leading to PMN-MDSC trafficking into tumors. Since PMN-MDSCs are the key cells expressing programmed cell death ligand 1 (PD-L1) in the tumor microenvironment, an increase in PMN-MDSCs increased PD-L1 expression in tumors.6 These consequences inhibited tumor-specific CD8+ T-cell immunity (Figure 1). Thus, IL-1 induces an immunosuppressive tumor microenvironment and resistance to immunotherapy through PD-L1-expressing PMN-MDSCs.
Figure 1.
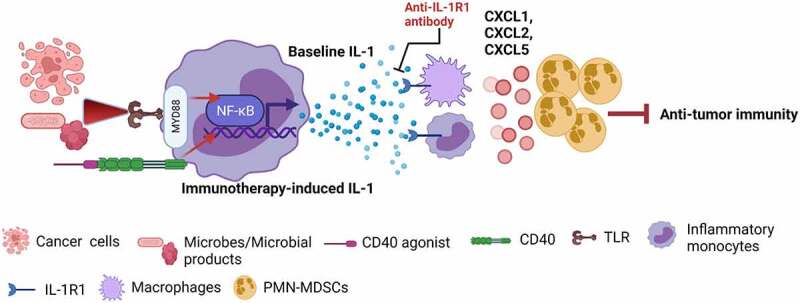
Baseline and immunotherapy-driven IL-1 suppresses anticancer immunity. Activation of the TLR–NF-kB pathway through necrotic cancer cells or microbes/microbial products induces baseline IL-1 production by inflammatory monocytes, and activation of the CD40–NF-kB pathway through CD40 agonist induces immunotherapy-driven IL-1 production by inflammatory monocytes. In response to IL-1, IL-1R1-expressing monocytes and macrophages produce PMN-MDSC-recruiting chemokines (CXCL1, CXCL2, and CXCL5), leading to PMN-MDSC trafficking into tumors and PMN-MDSC-mediated suppression of anti-tumor immunity. These consequences make tumors resistant to immunotherapy. Blocking the IL-1 signaling pathway by anti-IL-1R1 antibody overcome IL-1-mediated resistance to immunotherapies by stopping the PMN-MDSC trafficking into the tumor microenvironment (Created with BioRender.com)
IL-1: interleukin-1; IL-1R1: IL-1 receptor type 1; MyD88: myeloid differentiation primary response protein 88; NF-κB: nuclear factor-kappa B; TLR: toll-like receptor; PMN-MDSCs: polymorphonuclear myeloid-derived suppressive cells
Similarly, using preclinical tumor models and patient samples, Theivanthiran et al.10 found that activation of CD8+ T cells through programmed cell death protein 1 (PD-1) blockade induced a PD-L1/NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome signaling cascade and resulted in the recruitment of PMN-MDSCs into tumors and suppression of anti-tumor immunity.
Targeting the IL-1 pathway in cancer to overcome resistance to immunotherapies
We found that blocking the IL-1 pathway by IL-1R1 antibody overcame IL-1-mediated resistance to immunotherapies (CD40 agonist and anti-PD-1 antibodies) through eliminating the PMN-MDSCs in the tumor microenvironment.6 Our finding that the primary source of IL-1 is the monocytes, not the tumor cells, indicates that IL-1-mediated immune suppression can be found in various cancers. Furthermore, most immunotherapies induce IL-1 in cancer through direct or indirect activation of monocytes/macrophages. Thus, immunotherapy-induced IL-1 production and IL-1-mediated immune suppression in the tumor are generalized phenomena not limited to melanoma.
Kaplanov et al. reported that blocking IL-1β enhanced the efficacy of PD-1 blockade against breast cancer.11 A recent study by Aggen et al. showed that the combination of IL-1β blockade with either anti-PD-1 or a tyrosine kinase inhibitor had greater anti-tumor activity than either monotherapy alone against mouse renal cell carcinoma.12
A human IL-1R1 blockade antibody, anakinra, is already approved by the US Food and Drug Administration (FDA) for treating rheumatoid arthritis. Our findings suggest that anakinra can be repurposed for cancer treatment as a monotherapy or in combination with other immunotherapies.
Future perspective
Because IL-1 induces innate and acquired resistance to immunotherapy, it could serve as a predictive marker for various immunotherapies for melanoma and a wide variety of other cancers. It is essential to learn whether FDA-approved immunotherapies and immunotherapies in clinical trials induce IL-1 and whether blocking the IL-1 pathway increases the efficacy of these immunotherapies. PMN-MDSCs are the critical cells that cause immune suppression in the tumor microenvironment, and there is no effective strategy for deleting them. If IL-1 signaling is indeed a primary mechanism for recruiting PMN-MDSCs in tumors, targeting IL-1 would be a safe and effective strategy to eliminate PMN-MDSCs in the tumor microenvironment, converting immunologically cold tumors to hot tumors. Furthermore, since IL-1 can induce immune-related adverse events in response to immunotherapies through its downstream cytokines IL-6, IL-8, and C-reactive protein, blocking IL-1 signaling may reduce the severity of immune-related adverse events in addition to improving the efficacy of immunotherapy. Thus, targeting the IL-1 pathway can benefit cancer patients in multiple ways.
Funding Statement
This work was supported by the Department of Defense Idea Award CA160521 (to M.S.) and The University of Texas MD Anderson Cancer Center Specialized Program of Research Excellence in Melanoma (P50CA093459 to M.S.)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
- DAMP
damage-associated molecular pattern
- FDA
US Food and Drug Administration
- IL-1
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- IL-1R1
IL-1 receptor type 1
- IL-1R2
IL-1 receptor type 2
- MyD88
myeloid differentiation primary response protein 88
- NF-κB
nuclear factor kappa B
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- PMN-MDSCs
polymorphonuclear myeloid-derived suppressive cells
- TLRs
toll-like receptors
References
- 1.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118(2):503–3. Epub 2000/ 08/11. doi: 10.1378/chest.118.2.503. PubMed PMID: 10936147. [DOI] [PubMed] [Google Scholar]
- 2.Chiu JW, Binte Hanafi Z, Chew LCY, Mei Y, Liu H. IL-1alpha processing, signaling and its role in cancer progression. Cells. 2021;10(1):92. Epub 2021/ 01/13. doi: 10.3390/cells10010092. PubMed PMID: 33430381; PMCID: PMC7827341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. Epub 1998/ 05/23. doi: 10.1146/annurev.immunol.16.1.27. PubMed PMID: 9597123. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136(10):2352–2360. Epub 2014/ 10/30. doi: 10.1002/ijc.29297. PubMed PMID: 25353097. [DOI] [PubMed] [Google Scholar]
- 5.Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizee G, Poindexter N, Grimm EA. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res. 2011;9(11):1537–1550. Epub 2011/ 09/29. doi: 10.1158/1541-7786.MCR-11-0279. PubMed PMID: 21954434; PMCID: PMC3387749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Xiao Z, Bavisi K, Roszik J, Melendez BD, Wang Z, Cantwell MJ, Davis RE, Lizee G, Hwu P, et al. IL-1alpha mediates innate and acquired resistance to immunotherapy in melanoma. J Immunol. 2021;206(8):1966–1975. Epub 2021/ 03/17. doi: 10.4049/jimmunol.2000523. PubMed PMID: 33722878; PMCID: PMC8023145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edye ME, Lopez-Castejon G, Allan SM, Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J Biol Chem. 2013;288(42):30485–30494. Epub 2013/ 09/12. doi: 10.1074/jbc.M113.478941. PubMed PMID: 24022484; PMCID: PMC3798512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajamaki K, Nordstrom T, Nurmi K, Akerman KE, Kovanen PT, Oorni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem. 2013;288(19):13410–13419. Epub 2013/ 03/27. doi: 10.1074/jbc.M112.426254. PubMed PMID: 23530046; PMCID: PMC3650379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schonbeck U, Mach F, Bonnefoy JY, Loppnow H, Flad HD, Libby P. Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes elaboration of active interleukin 1beta. J Biol Chem. 1997;272(31):19569–19574. Epub 1997/ 08/01. doi: 10.1074/jbc.272.31.19569. PubMed PMID: 9235962. [DOI] [PubMed] [Google Scholar]
- 10.Theivanthiran B, Evans KS, DeVito NC, Plebanek M, Sturdivant M, Wachsmuth LP, Salama AK, Kang Y, Hsu D, Balko JM, et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest. 2020;130(5):2570–2586. Epub 2020/ 02/06. doi: 10.1172/JCI133055. PubMed PMID: 32017708; PMCID: PMC7190922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, Dinarello CA, Voronov E, Apte RN. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116(4):1361–1369. Epub 2018/ 12/14. doi: 10.1073/pnas.1812266115. PubMed PMID: 30545915; PMCID: PMC6347724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, Chaimowitz MG, Lopez-Bujanda ZA, Spina CS, Hawley JE, et al. Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: multidimensional analyses. Clin Cancer Res. 2021;27(2):608–621. Epub 2020/ 11/06. doi: 10.1158/1078-0432.CCR-20-1610. PubMed PMID: 33148676; PMCID: PMC7980495. [DOI] [PMC free article] [PubMed] [Google Scholar]


