Key Points
SARS-CoV-2 mRNA-1273 vaccine induces an impaired humoral response in patients under anti-CD20 therapy, but cellular response is preserved.
Immunosuppressive therapy for GVHD reduces cellular response with no impact in humoral immunogenicity.
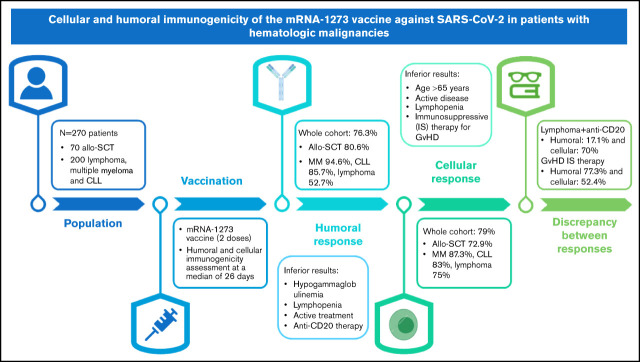
Visual Abstract
Abstract
Recent studies have shown a suboptimal humoral response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines in patients diagnosed with hematologic malignancies; however, data about cellular immunogenicity are scarce. The aim of this study was to evaluate both the humoral and cellular immunogenicity 1 month after the second dose of the mRNA-1273 vaccine. Antibody titers were measured by using the Elecsys and LIAISON anti–SARS-CoV-2 S assays, and T-cell response was assessed by using interferon-γ release immunoassay technology. Overall, 76.3% (184 of 241) of patients developed humoral immunity, and the cellular response rate was 79% (184 of 233). Hypogammaglobulinemia, lymphopenia, active hematologic treatment, and anti-CD20 therapy during the previous 6 months were associated with an inferior humoral response. Conversely, age >65 years, active disease, lymphopenia, and immunosuppressive treatment of graft-versus-host disease (GVHD) were associated with an impaired cellular response. A significant dissociation between the humoral and cellular responses was observed in patients treated with anti-CD20 therapy (the humoral response was 17.5%, whereas the cellular response was 71.1%). In these patients, B-cell aplasia was confirmed while T-cell counts were preserved. In contrast, humoral response was observed in 77.3% of patients undergoing immunosuppressive treatment of GVHD, whereas only 52.4% had a cellular response. The cellular and humoral responses to the SARS-CoV-2 mRNA-1273 vaccine in patients with hematologic malignancies are highly influenced by the presence of treatments such as anti-CD20 therapy and immunosuppressive agents. This observation has implications for the further management of these patients.
Introduction
Patients diagnosed with hematologic malignancies are a particularly vulnerable population that has been severely affected by the coronavirus disease 2019 (COVID-19) pandemic, with a mortality rate as high as 34% to 62% in hospitalized patients1-4 due to variable degrees of immunosuppression caused by disease or treatment. The fact that hematologic patients who survived the illness present a lower rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) seroconversion and a longer persistence of SARS-CoV-2 reverse transcription-polymerase chain reaction testing positivity, particularly those who had received anti-CD20 therapy and stem cell transplant (SCT),5,6 is of particular relevance; this finding calls into question their immune protection after infection or vaccination. Also, previous studies have reported an attenuated humoral response to the recombinant zoster virus vaccine or the hepatitis B virus vaccine in patients diagnosed with B-cell malignancies and multiple myeloma (MM).7-9
Therefore, patients diagnosed with hematologic malignancies have been considered a high-risk population. Their vaccination against SARS-CoV-2 has thus been prioritized despite the exclusion of immunocompromised populations from the pivotal clinical trials that approved the two mRNA-based vaccines (BNT162b2 and mRNA-1273)10,11 and the expectation of a suboptimal immune response.
Recently, the first real-life studies have shown a diminished humoral response to BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in patients diagnosed with chronic lymphocytic leukemia (CLL), MM, and other hematologic malignancies. The rates of seroconversion were between 39% and 88%, with patients diagnosed with B-cell malignancies exhibiting the worst response.12-18 Humoral immunogenicity seems mainly impaired by B cell–directed therapies such as Bruton’s tyrosine kinase (BTK) inhibitors or anti-CD20 monoclonal antibodies, with rates of seroconversion ranging from 0% to 21% within 2 to 8 weeks after 2 doses of the vaccine.12,13,16,18,19
Although SARS-CoV2 mRNA vaccines also induce a cellular response, and studies highlight the important role of T cells in mitigating severe COVID-19,20 data reporting this response in immunocompromised patients are still scarce. Recent studies have revealed a response rate of ∼45% to 50% in patients diagnosed with different hematologic malignancies,21,22 and even a lower rate of 19% in patients after allogeneic SCT (allo-SCT).23 Determination of T-cell response is particularly important in those patients with low or absent humoral response to ensure appropriate strategies to prevent infection in this susceptible population.
The objective of the current study, therefore, was to evaluate immunogenicity (including both a cellular and humoral response) 1 month after a second dose of the mRNA-1273 SARS-CoV-2 vaccine in patients diagnosed with various hematologic malignancies. We also analyzed how this immunogenicity is influenced by specific treatments and the immunologic status of the patients at the time of vaccination.
Methods
This prospective, observational, real-world study was conducted by Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona, Spain. Patients diagnosed with hematologic malignancies were vaccinated through the Spanish vaccination program with the mRNA-1273 SARS-CoV-2 vaccine following the recommendations of the Hematology and Preventive Medicine department of our center. The complete vaccination scheme included 2 vaccine doses administered 28 days apart during the months of March and April 2021.
Blood samples were collected between March and May 2021 at 2 different times: immediately before the first dose and at least 21 days after the second dose, at a median time of 26 days (interquartile range [IQR], 22-28 days).
To establish the basal immunologic condition of the patients, a complete blood count test and total serum immunoglobulin levels were determined in the first blood extraction. Moreover, to identify individuals with previous SARS-CoV-2 asymptomatic infection, a basal serologic analysis was conducted at this point by measuring specific antibodies against both the nucleocapsid (N) and spike (S) antigens of SARS-CoV-2 and also by determining the specific cellular immune response. In the second blood extraction, humoral and cellular immunogenicity of the mRNA-1273 vaccine was assessed by determining the positivity rate of IgG antibodies against spike antigen and the interferon-γ–producing SARS-CoV-2 T cells, respectively.
This study was approved by the Institutional Clinical Research Ethics Committee of the Vall d’Hebron Institute (study number 5818), and all patients provided written informed consent in accordance with the Declaration of Helsinki.
Study population
The study cohort was the entire pool of patients diagnosed with lymphoid neoplasia and monoclonal gammopathies and patients who underwent allo-SCT from 2015 to 2021 at the Vall d’Hebron Hematology department. The median time from transplant to vaccination was 29.6 months (range, 3.7-70.3 months). Patients voluntarily agreed to participate in the study and to receive the complete scheme of vaccination (n = 270).
Assessment of humoral immune response
To assess the humoral immune response to the vaccine, 2 commercial chemiluminescence immunoassays were used: (1) Elecsys Anti-SARS-CoV-2 (Roche Diagnostics, Mannheim, Germany) performed on the Cobas 8800 system (Roche Diagnostics, Basel, Switzerland) for the determination of total antibodies (including IgG, IgM, and IgA) against nucleocapsid SARS-CoV-2 proteins (cutoff, 1.0 index): and (2) LIAISON SARS-CoV-2 TrimericS IgG (DiaSorin, Stillwater, MN) performed on the LIAISON XL Analyzer (DiaSorin, Saluggia, Italy) for the determination of IgG antibodies against the spike glycoprotein of SARS-CoV-2 (cutoff, 13.0 AU/mL).
Assessment of cellular immune response
SARS-CoV-2–specific T-cell response was assessed by the whole blood interferon-γ release immunoassay technology using QuantiFERON SARS-CoV-2 RUO tubes from Qiagen (Hilden, Germany). This technology consists of 2 tubes coated with a combination of SARS-CoV-2 spike antigens (S1, S2, and RBD), a mitogen tube that serves as a positive control, and a Nil tube that serves as a negative control. This test was conducted following the manufacturer’s instructions. Briefly, venous blood samples were collected directly into the four QuantiFERON SARS-CoV-2 RUO tubes, incubated at 37°C for 16 to 24 hours and centrifuged to separate plasma. IFN-γ was measured by chemiluminescence immunoassays using LIAISON QuantiFERON-TB Gold Plus assay (DiaSorin, Italy) with the LIAISON XL Analyzer (DiaSorin, Italy). We used experimentally established cutoff values (Ag1 = 0.051 and Ag2 = 0.442) for the qualitative interpretation of the results.24
Statistical analysis
A descriptive analysis of all included variables in the study was performed. Continuous variables are expressed as median and IQR, and categorical variables are expressed as absolute values and percentages. Univariate logistic regression models were conducted to estimate the association between baseline factors and the following: (1) the cellular immunization rate; and (2) the humoral immunization rate of the mRNA-1273 vaccine. Odds ratios with 95% confidence intervals were reported. The proportion of concordance and discordance between cellular and humoral responses was descriptively calculated in each subgroup of patients. No data imputation was performed, and the data analyses were conducted by using R statistical software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 270 patients were included in this study. Patients’ baseline demographic and clinical characteristics are summarized in Table 1. The median age of the patients was 63 years (IQR, 53-71 years), and 137 patients (51.5%) were female.
Table 1.
Patients’ baseline demographic and disease characteristics (N = 270)
| Parameter | Value |
|---|---|
| Age, median (IQR), y | 63 (53-71) |
| Female sex, N (%) | 137 (51.5) |
| Previous symptomatic COVID-19 infection, N (%) | 27 (10) |
| Previous antibody response to SARS-CoV2, N (%) | 24 (8.9) |
| Previous interferon-γ–producing SARS-CoV-2–reactive T cells, N (%) | 23 (8.5) |
| Underlying disease, N (%) | |
| Lymphoid malignancies and MM cohort | 200 (74.1) |
| CLL | 52 (26) |
| Lymphoma | 85 (42.5) |
| MM | 63 (31.5) |
| allo-SCT | 70 (25.9) |
| Treatment status, N (%) | |
| Lymphoid malignancies and MM cohort | |
| Treatment-naive | 32 (16) |
| On current therapy/<6 mo | 130 (65) |
| Off therapy ≥6 mo | 38 (19) |
| allo-SCT | |
| On current therapy/<6 mo | 4 (5.7) |
| Prophylactic/therapeutic GVHD treatment | 23 (32.9) |
| Type of treatment (current and <6 mo), N (%) | |
| Lymphoid malignancies and MM cohort | |
| Chemotherapy | 8 (6.1) |
| Anti-CD20 monoclonal antibodies ± chemoimmunotherapy | 47 (36.2) |
| BTK inhibitor | 31 (23.8) |
| IMIDs | 30 (23.1) |
| Other target therapies | 14 (10.8) |
| allo-SCT | |
| Target therapies | 4 (5.7) |
| Immunosuppressive agents for GVHD | 23 (32.9) |
| Previous treatment, N (%) | |
| 1 line | 118 (59.6) |
| 2 lines | 57 (28.8) |
| ≥3 lines | 23 (11.6) |
| Disease status of patients, N (%) | |
| Complete response/partial response | 206 (86.6) |
| Stable disease/progressive disease | 32 (13.4) |
| Laboratory parameters, median (IQR) | |
| Absolute neutrophil count, 109/L | 3.8 (2.5-4.8) |
| Absolute lymphocyte count, 109/L | 1.8 (1.2-2.8) |
| IgG, mg/dL | 833 (585-1081.8) |
| IgM, mg/dL | 45 (27-85.75) |
| IgA, mg/dL | 134.5 (60-201.8) |
| Basal immunologic condition, N (%) | |
| Leukopenia (WBC <4 × 109/L) | 38 (14.1) |
| Lymphopenia (ALC <1.0 × 109/L) | 39 (14.4) |
| Neutropenia (ANC <2.0 × 109/L) | 37 (13.7) |
| Low IgG (<700 mg/dL) | 92 (34.1) |
| Low IgM (<40 mg/dL) | 120 (44.4) |
| Low IgA (< 0 mg/dL) | 78 (29.3) |
| Days between second dose of vaccine and antibody test, median (IQR) | 26 (22-28) |
ALC, absolute lymphocyte count;ANC, absolute neutrophil count; WBC, white blood cell count.
In subsequent analysis, patients were divided into 2 different subgroups. First, in the cohort of lymphoid malignancies and MM (n = 200), the underlying malignancy was lymphoma in 42.5% (n = 85) of the patients, followed by MM in 31.5% (n = 63) and CLL in 26% (n = 52). Second, patients who had undergone an allo-SCT (n = 70) were considered a different subgroup regardless of malignancy due to their specific immunologic situation and the immunosuppressive therapy used to prevent or treat graft-versus-host disease (GVHD).
In the cohort of lymphoid malignancies and MM, 65% of the patients were on active therapy or had been previously treated within the prior 6 months. Of those, 47 (36.2%) patients were exposed to anti-CD20 therapy in monotherapy or associated with chemotherapy, 31 (23.8%) received treatment with BTK inhibitors, 30 (23.1%) with immunomodulatory drugs (IMIDs), 8 (6.1%) with chemotherapy alone, and 14 (10.8%) with other target therapies such as daratumumab, brentuximab, proteasome inhibitors, or venetoclax. Thirty-two patients (16%) were treatment naive, and 38 (19%) had finished therapy >6 months ago.
In the allo-SCT cohort (n = 70), 4 patients (5.7%) were on active treatment of their hematologic disease with target therapies, and 23 patients (32.9%) were receiving immunosuppressive agents (steroids, ruxolitinib, sirolimus, or tacrolimus) for prophylaxis or treatment of GVHD. The remaining patients (61.4%) were off therapy for >6 months.
Considering the overall cohort, most of the patients under current or previous treatment had good control of their hematologic disease at the time of vaccination, with 86.6% of them in complete or partial response (206 of 238). At vaccination, 38 (14.1%) patients had a low total leukocyte count (white blood cell count <4 × 109/L), 39 (14.4%) had lymphopenia (absolute lymphocyte count [ACL] <1.0 × 109/L), and 37 (13.7%) had neutropenia (absolute neutrophil count <2.0 × 109/L), but only in 4 patients (1.5%) was this clinically relevant (absolute neutrophil count <1.0 × 109/L). In addition, low basal levels of IgM (<40 mg/dL) were detected in 44.4% of the patients (120 of 270) and low levels of IgG (<700 mg/dL) in 34.1% (92 of 270).
Humoral response to the mRNA-1273 vaccine
Twenty-seven (10%) patients had previous symptomatic COVID-19 in the last year confirmed by nasopharyngeal reverse transcription-polymerase chain reaction swab test, and 89% of them developed positive anti-N antibodies. In addition, 2 patients were considered as previously asymptomatic SARS-CoV-2 infected due to basal positive anti-N antibodies. These 29 patients (11 lymphoma, 3 CLL, 7 MM, and 8 allo-SCT patients) with a history of COVID-19 and basal positive serology were excluded from the analysis examining the humoral immunogenicity of the vaccine.
In the whole cohort (N = 241), the proportion of patients in whom the vaccine elicited a humoral response was 76.3% (Figure 1). The basal immunologic status had a significant influence on the response to the vaccine. Patients with lymphopenia (ACL <1.0 × 109/L) at time of vaccination presented a worse humoral response: 57.1% (20 of 35) vs 79.6% (164 of 206) in patients with a normal lymphocyte count (P = .005). In addition, hypogammaglobulinemia (abnormal low levels of IgG, IgA, and IgM) had a significant negative impact in the humoral response (P ≤ .01 in all comparisons).
Figure 1.
Univariate analysis for humoral immunization rate in patients diagnosed with hematologic malignancies. Analysis of the baseline characteristics that could confer a risk to a reduced humoral immunization rate to the mRNA-1273 vaccine according to overall response rate in patients with basal negative serology immunization and no previously known SARS-CoV-2 infection. CI, confidence interval; CR, complete response; PR, partial response; SD/PD, stable disease/progressive disease.
In the subgroup of lymphoid malignancies and MM (n = 179), 67% elicited a humoral response. Patients diagnosed with MM and CLL presented a high humoral immunization, with a seroconversion of 94.6% (53 of 56) and 85.7% (42 of 49), respectively; in lymphoma patients, only 52.7% (39 of 74) had measurable anti-S antibodies (supplemental Table 1). In these patients, lymphoma as an underlying disease, current or treatment during the last 6 months, and therapy with anti-CD20 monoclonal antibodies during the last 6 months negatively influenced the humoral response. Specifically, treatment-naive patients and those who completed therapy at least 6 months earlier presented a humoral immunization rate of 96.7% (29 of 30) and 91.7% (33 of 36) vs a 63.7% rate (72 of 113) in patients currently under treatment or end of therapy within the previous 6 months before vaccination (P = .004).
Additional observations were made. For example, when humoral response was evaluated according to treatment, a significantly impaired humoral response was detected in patients receiving anti-CD20 therapy during the last 6 months with a humoral response rate of only 17.5% (7 of 40); all 6 patients who received anti-CD20 therapy during the period 6 to 12 months before vaccination developed a humoral response and were cataloged as “off therapy.” Patients treated with IMIDs exhibited the highest humoral response rate of all active therapies, with 100% (26 of 26) seroconversion. In patients with CLL treated with BTK inhibitors (n = 29), the humoral response was observed in 86.2% (n = 25). Given the specific B-cell targeting of BTK inhibition, we quantitatively assessed the humoral response in these patients compared with treatment-naive CLL patients and observed that, despite the positivity rate being similar, patients under BTK inhibition treatment had lower antibody titers (median, 608 vs 95 AU/mL; P < .01) (supplemental Figure 1).
In the allo-SCT population, the humoral response rate was 80.6% (50 of 62). In these patients, only ex vivo CD34+ selection exhibited an association with a lower humoral immunogenicity (P = .03).
Cellular response to the mRNA-1273 vaccine
Thirteen (48%) of the 27 patients with a history of COVID-19 and 10 asymptomatic patients had interferon-γ–producing SARS-CoV-2–reactive T cells before vaccination. In 87 patients, basal-specific T-cell response was not available.
Patients with a history of COVID-19 and basal T-cell immunization (n = 37 [13 lymphoma, 5 CLL, 8 MM, and 11 allo-SCT patients]) were excluded from the analysis to examine the cellular immunogenicity of the vaccine.
In the whole cohort, 184 (79%) of 233 patients developed cellular immunity after the second dose of the mRNA-1273 vaccine (Figure 2). Age in the whole cohort was associated with a diminished cellular response; patients aged >65 years presented a cellular immunity in 73% (73 of 100) of the cases vs 83.5% (111 of 133) in younger patients (P = .05). Patients in response to therapy, either complete or partial, developed higher rates of T-cell response compared with those in stable or progressive disease (80.1% vs 63%, respectively; P = .03). The basal level of immunoglobulins did not have an impact on the cellular response. However, as in the case of humoral response, lymphopenia (ACL <1.0 × 109/L) at time of vaccination was significantly associated with a lower cellular response of 60% (21 of 35) vs 82.3% (163 of 198) in patients with normal lymphocyte count (P = .004).
Figure 2.
Univariate analysis for cellular immunization rate in patients diagnosed with hematologic malignancies. Analysis of the baseline characteristics that could confer a risk to a reduced cellular immunization rate to the mRNA-1273 vaccine according to overall response rate in patients with basal negative serology and T-cell immunization and no previously known SARS-CoV-2 infection. CI, confidence interval; CR, complete response; PR, partial response; SD/PD, stable disease/progressive disease.
In the lymphoid malignancies and MM cohort, patients with MM and CLL presented a high cellular immunization of 87.3% (48 of 55) and 83% (39 of 47), respectively (supplemental Table 2). In the case of lymphoma patients, the cellular response was preserved; 75% (54 of 72) of patients developed SARS-CoV-2–reactive T cells, and this fact was directly related to the unaffected cellular response observed in patients under treatment with anti-CD20 therapy, with 71.1% (27 of 38) of patients exhibiting a positive T-cell response. However, when chemotherapy was associated with anti-CD20 therapy, this response diminished to 50% (9 of 18).
Finally, in the allo-SCT population (n = 59), the cellular response was 72.9% (43 of 59). Presence of active GVHD was related to a lower cellular response: 55.6% (10 of 18) response rate vs 81.8% (27 of 33) in patients without GVHD (P = .05). This situation was directly related to the diminished cellular response induced by treatment with immunosuppressive agents: 52.4% (11 of 21) with treatment vs 85.3% (27 of 33) in patients without any treatment (P = .02).
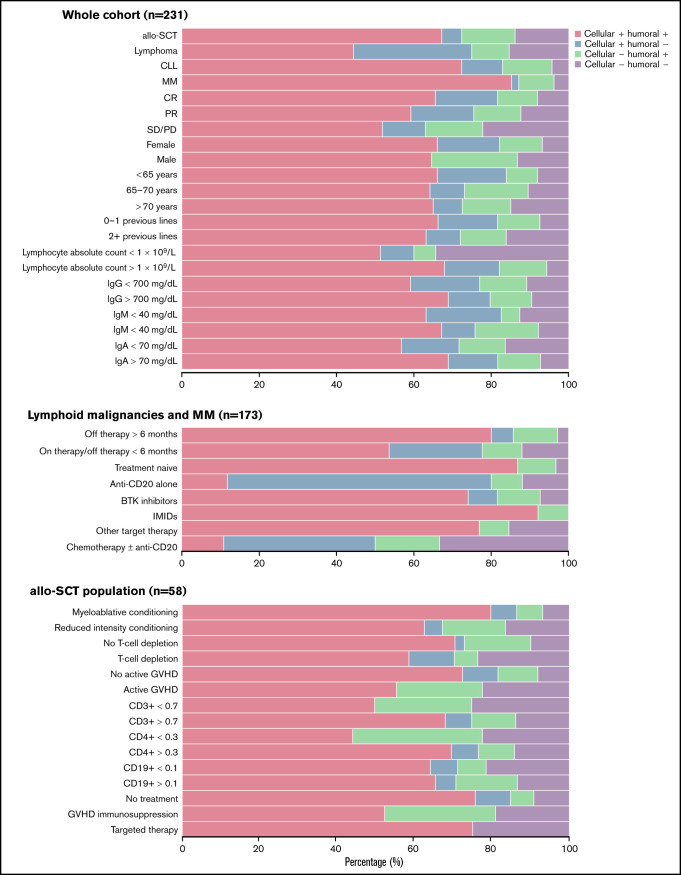
Analysis of coordination between cellular and humoral immune response and the influence of lymphocyte subpopulations
In our series, significant dissociation between the humoral and cellular responses was mainly observed in 2 scenarios: in lymphoma patients treated with anti-CD20 therapy and in the context of immunosuppressive therapy for GVHD (Figure 3).
Figure 3.
Proportion of concordance of humoral and cellular immune response to the mRNA-1273 vaccine according to baseline characteristics in patients diagnosed with hematologic malignancies. CR, complete response; PR, partial response; SD/PD, stable disease/progressive disease.
In the case of lymphoma patients (n = 40) treated with anti-CD20 (humoral response of 17.1% and cellular response of 70%), the discordance rate between responses was 67.5%. Sixty percent (24 of 40) of patients obtained a cellular but not a humoral response, and only 7.5% (3 of 40) obtained a humoral but not a cellular response. Only 10% (4/40) of the patients presented both a cellular and humoral response, and 22.5% (9 of 40) did not develop any response to the vaccine.
In allo-SCT patients with immunosuppressive therapy for GVHD (n = 21), the discordance rate was 28.6% because 6 patients developed only a humoral response. We observed that 52.4% (11 of 21) of patients presented both a humoral and cellular response, and 19% (4 of 21) did not develop any response to the vaccine.
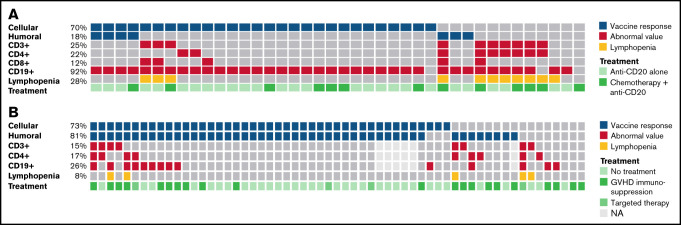
In view of these results, we hypothesized that the main cause of impaired serologic response in patients treated with anti-CD20 was the presence of B-cell aplasia before vaccination. Subsequently, and to confirm this hypothesis, we studied the lymphoid subpopulations by immunophenotyping of cryopreserved peripheral blood mononuclear cells obtained before vaccination in 40 patients with lymphoma under treatment in the last 6 months with anti-CD20 therapy. We observed that 92% (37 of 40) of these patients presented a low count of CD19+ lymphocytes, with just 18% of them (7 of 40) developing specific anti-spike antibodies.
Conversely, 75% of these patients (30 of 40) had preserved CD3+ counts; if we selected only the patients who had received anti-CD20 therapy alone without associated chemotherapy, the value was higher (85% [23 of 27]). In accordance with these data, a cellular response of 83% was observed in patients with normal CD3+ counts, whereas only 30% of patients (3 of 10) with diminished CD3+ counts obtained a positive cellular response (Figure 4).
Figure 4.
Humoral and cellular immune response rate according to lymphocyte subpopulations and administered therapy. (A) In patients undergoing treatment with anti-CD20 therapy (n = 40), the influence of this monoclonal antibody's association with chemotherapy in the lymphocyte subpopulations and in the humoral or cellular response to the vaccine was determined. Abnormal values were considered as follows: CD19, >0.10 × 109/L; CD3, >0.7 × 109/L; CD4, 0.3 × 109/L; CD8, 0.2 × 109/L; and lymphopenia, 1.0 × 109/L. (B) In allo-SCT patients (n = 59), the influence of treatment with immunosuppressive agents or target therapy in the lymphocyte subpopulations and in the humoral or cellular response to the vaccine was determined.
In the allo-SCT cohort, a normal CD19+ count was found in 79.2% (38 of 48) of the patients who achieved a humoral response. Conversely, 4 (36.4%) of 11 patients with abnormal CD19+ counts did not develop a humoral response after vaccination.
It seems that the cellular response in allo-SCT may be influenced by the number of CD4+. We found that those patients who had low levels of CD4+ presented a lower response to the vaccine (44.4% vs 77.3%) (Figure 1).
Finally, we observed that 10 (62.5%) of 16 cellular nonresponders were on treatment with immunosuppressive drugs for GVHD.
Discussion
Clinical trials have proven the humoral and cellular immunogenicity, as well as 94.1% efficacy, with the mRNA-1273 vaccine in preventing COVID-19.11,25,26 However, the lack of representation of immunocompromised patients in these studies hinders the prediction of the immunogenicity of the SARS-CoV-2 vaccine in hematologic patients, particularly in the context of specific antineoplastic treatments or SCT.
Our results confirm inferior humoral and cellular responses in hematologic malignancies of 76.3% and 79%, respectively, compared with a 100% rate of seroconversion and T-cell response previously reported in healthy volunteers.26 However, despite this diminished immunogenicity, these results are encouraging and show similar or even better results than other viral vaccines such as influenza A (H1N1) (seroconversion, 54.3%; cellular response, 35.8%)27 or the recombinant zoster vaccine (Shingrix, GlaxoSmithKline, Philadelphia, PA) (seroconversion, 80.4%; cellular response, 83.7%) in hematologic patients.9
Impaired humoral response, predominantly with the BNT162b2 mRNA vaccine, has also been described in patients with hematologic malignancies,12-20,28,29 particularly in B-cell neoplasms, and especially in the context of different immunosuppressive treatments. In our cohort, patients with lymphoma, current treatment or treatment during the last 6 months, treatment with anti-CD20 monoclonal antibodies, lymphopenia, and low levels of immunoglobulins at the time of vaccination were significantly associated with an inferior humoral response. In agreement with our finding of a 53.4% humoral response in lymphoma patients, Ghione et al19 and Lim et al18 have also reported a low serologic response rate in this population. In our series, the main factor associated with the inferior humoral response was treatment with anti-CD20 therapy. In those patients, we observed the lowest rate of seroconversion, with only 17.1% developing antibodies; this response was expected by the B-cell depletion caused by anti-CD20 and its adverse impact on the production of antibodies.18,19
In CLL, Herishanu et al12 reported a humoral response rate of 39.5%; Roeker et al13 reported a rate up to 52% and Parry et al16 a 75% rate. In these series, active treatment with BTK inhibitors and anti-CD20 immunotherapy were correlated with a considerably lower rate of antibody production, whereas younger age, treatment-naive disease, and absence of active treatment were factors associated with a better humoral response to the vaccine.12,13,16 In our cohort, patients with CLL did not exhibit an inferior response compared with patients with other underlying malignancies.
It should also be noted that in our series, 35% of patients with CLL were treatment naive, and all of them developed antibodies, whereas 82% of CLL patients with active treatment were receiving BTK inhibitors for a long period of time (median of 18 months). In addition, 89.2% of them had a complete/partial response to treatment of their disease, and 57.1% of them had normal levels of immunoglobulins. We observed a high humoral response in patients treated with BTK inhibitors of 86.7% (data apparently contradictory to those described by Herishanu et al12); however, we must emphasize that the methodology used to perform the antibody testing differs between the studies, and recently published data suggest a higher humoral immunogenicity of the mRNA-1273 vaccine compared with the BNT162b2 vaccine in healthy populations,30,31 which could explain the higher seroconversion rate in our cohort. We also measured serum anti–SARS-CoV-2 IgG antibodies and verified that this response was quantitatively weaker, with lower levels of anti-S antibodies compared with those treatment-naive CLL patients (supplemental Figure 1).
Conversely, 94.6% of patients diagnosed with MM had a humoral response. A total of 56.4% (n = 31) of patients were on active treatment; it is remarkable that all 25 of the patients treated with IMIDs developed a humoral response. Those results are compatible with the findings described by Pimpinelli et al17 with an immunization rate of 78.6% that improved to 92.9% in patients receiving ongoing treatment with lenalidomide.
Beyond the recent data emerging regarding the humoral response to SARS- CoV-2 vaccines in hematologic patients, immune protection in seronegative patients could be mediated by T cells. Rydyznski et al20 found that acquisition of adaptive immunity of SARS-CoV-2–specific CD4+ T cells was associated with lower severity of COVID-19, and Bange et al32 showed that patients with hematologic cancer with a greater number of CD8+ T cells had an improved survival from the infection despite an impaired humoral immunity.
The majority (79%) of patients in our series developed a cellular response after vaccination regardless of the hematologic disease. Factors associated with lower probability of cellular response included age >65 years, status of the hematologic disease (stable or progressive disease), treatment with chemotherapy ± anti-CD20 monoclonal antibodies, lymphopenia, or active GVHD and immunosuppressive therapy for GVHD.
We highlight the significant difference between a suboptimal humoral response and a well-preserved cellular response to the vaccine in lymphoma patients, even if they were under treatment with B cell–depleting therapy such as anti-CD20 antibodies. Patients with lymphoma achieved a cellular response rate of 75.3% and 70% in patients treated with antiCD-20 antibodies ± chemoimmunotherapy during the last 6 months. In line with these results, previous data have shown discordance between production of neutralizing antibodies and T-cell response in the context of the varicella zoster virus vaccine33 and more recently in a small number of patients with multiple sclerosis on anti-CD20 therapy receiving SARS-CoV-2 mRNA vaccination.34 Through a flow-cytometric evaluation of lymphocyte subpopulations, we confirmed that among the patients who had received anti-CD20 therapy and had B-cell aplasia, 90.1% of the humoral nonresponders presented a low CD19+ count. Meanwhile, a preserved CD3+ cell count was present in 75% of the cellular responders; chemotherapy-induced leukopenia with a low count of CD3+ negatively influenced the cellular response. In this analysis, we also observed that in the subgroup with allo-SCT, the lower cellular response was associated with a lower CD4+ count and with the use of immunosuppressive agents, reflecting both a quantitative and a qualitative T-cell defect in these patients.
The basal immunologic status at the time of vaccination was one of the more relevant factors associated with the response to the vaccine; however, although hypogammaglobulinemia had a significant negative impact on the humoral response, this variable did not influence cellular response. Lymphopenia was correlated, however, both with an impaired humoral and cellular response to the vaccine compared with patients with a normal lymphocyte count.
Despite concerns regarding the capacity of immunization in allo-SCT, this subpopulation of patients presented remarkable humoral and cellular responses of 81% and 72.4%, respectively; these are similar to the results recently described by Ram et al,23 in which 75% of patients after allo-SCT had evidence of a humoral response. In this study, cellular response was evaluated in 37 patients and was remarkably low (19%). In our cohort, cellular response indeed was lower than the humoral response; active GVHD and current treatment with immunosuppressive agents, as well as low CD4+ counts, were associated with this reduction. The lower CD4+ T cells were observed both in patients receiving immunosuppressive agents as well as in patients who received a CD34+ selection. This reflects a delayed immune reconstitution when using CD34+ selection, which represents an important disadvantage of this platform at the expense of a decreased incidence of acute and chronic GVHD.35,36
In summary, our work shows a high rate of both humoral and cellular response in patients with hematologic malignancies. However, low levels of immunoglobulins, lymphopenia, treatment in the last 6 months, and particularly therapy with rituximab were associated with an inferior humoral response, whereas age (>65 years), status of the disease (in response vs active disease), lymphopenia, and GVHD and immunosuppressive agents in the context of allo-SCT were associated with an impaired cellular response. Patients with these risk factors for a suboptimal immune response are likely to require a booster dose of the vaccine. Moreover, these factors can be taken into account to plan vaccination calendars accordingly. Of note, a high percentage of seronegative patients receiving B cell–depleting therapies developed cellular immunity that could offer protection to COVID-19 severe disease. The efficacy of this cellular immunity against SARS-CoV-2 infection should be clarified in additional studies.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the participation of all the departments that have collaborated in the study and especially the patients and their families for their involvement. The authors also thank the Cellex Foundation for providing research facilities and equipment and the CERCA Programme/Generalitat de Catalunya for institutional support.
Authorship
Contribution: Concept and design were undertaken by P.A., D.V., M.C., E.R., J.E., M.H., and F.B.; data analyses were performed by G.V. and M.J.; collection and assembly of data were conducted by E.R., M.J., and I.R.-C.; laboratory analyses were performed by C.F.-N., J.E., M.M.-G., and D.M.-G.; and samples collection was performed by S.P.-G., G.P., C.H., and C.P.; and all authors contributed to manuscript writing and final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: P.A. has received honoraria from Janssen, Roche, BMS, AbbVie, and AstraZeneca. M. Crespo has received research funding from Pharmacyclics, Genentech, and AstraZeneca. G.V. has received honoraria for speaker activities from Merck Sharp & Dohme and an advisory role from AstraZeneca. F.B. has received honoraria from Janssen, Gilead, Roche, BMS, AbbVie, AstraZeneca, Novartis, and Lilly. M. Campins has participated in advisory boards and has received research funding from GSK, Sanofi, Pfizer, Novavax, and Janssen. P.B. declares having received honoraria from Amgen, BMS, Kite-Gilead, Incyte, Miltenyi Biotec, Novartis and Pfizer not related with the present article. The remaining authors declare no competing financial interests.
Correspondence: Pau Abrisqueta, Servei d’Hematologia, Vall d’Hebron Hospital Universitari, Experimental Hematology, Vall d’Hebron Institute of Oncology (VHIO), Vall d’Hebron Barcelona Hospital Campus, Passeig Vall d’Hebron 119-129, 08035 Barcelona, Spain; e-mail: pabrisqueta@vhio.net.
References
- 1.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passamonti F, Cattaneo C, Arcaini L, et al. ; ITA-HEMA-COV Investigators . Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737-e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS-CoV2-IgG in patients with malignant disease and association with anticancer therapy. Nat Can. 2021;2(4):392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda H, Tsukune Y, Watanabe N, et al. Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20(11):774-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zent CS, Brady MT, Delage C, et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia. 2021;35(6):1788-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagnew AF, Ilhan O, Lee WS, et al. ; Zoster-039 Study Group . Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988-1000. [DOI] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2703-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542-e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138(9):811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996-1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA Covid-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021;27(9):788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Gallo M, Esperalba J, Pujol-Borrell R, et al. Commercialized kits to assess T-cell responses against SARS-CoV-2 S peptides. A pilot study in health care workers [published online ahead of print 25 September 2021]. Med Clin (Barc). doi: 10.1016/j.medcli.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson LA, Anderson EJ, Rouphael NG, et al. ; mRNA-1273 Study Group . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu L, McPhee R, Huang W, et al. ; mRNA-1273 Study Group . A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lavallade H, Garland P, Sekine T, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96(2):307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583-e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4(9):e2124331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021; 27(7):1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrino J, McNeil SA, Lawrence SJ, et al. Safety and immunogenicity of inactivated varicella-zoster virus vaccine in adults with hematologic malignancies receiving treatment with anti-CD20 monoclonal antibodies. Vaccine. 2017;35(14):1764-1769. [DOI] [PubMed] [Google Scholar]
- 34.Apostolidis S, Kakara M, Painter M, et al. Altered cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis and anti-CD20 therapy. medRxiv 2021; doi: 10.1101/2021.06.23.21259389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roldan E, Perales MA, Barba P. Allogeneic stem cell transplantation with CD34+ cell selection. Clin Hematol Int. 2019;1(3):154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewin SR, Heller G, Zhang L, et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood. 2002;100(6):2235-2242. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.