Key Points
CLL patients who received venetoclax monotherapy achieved a significantly higher response rate to anti–COVID-19 vaccines compared with those who received BTKi.
Seronegative patients after anti–COVID-19 double-dose mRNA vaccination have a global response rate of 35% after dose 3.
Abstract
Immunocompromised individuals such as patients with chronic lymphocytic leukemia (CLL) are at risk of impaired immune responses to vaccination. The objective of our study was to evaluate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibody responses in patients with CLL after the first, second, and third doses of the BNT162b2 or mRNA-1273 vaccines and after a single dose for patients with confirmed previous COVID-19. In all, 530 patients were included in the study. Patients received 2 doses at a 4-week interval and a third dose if they were seronegative after the second dose. Response rate was 27% after dose 1 and 52% after dose 2. Post-dose 2 treatment-naïve patients had the highest response rate (72%) followed by patients previously treated by chemoimmunotherapy (60%). Among patients receiving therapy, those receiving Bruton tyrosine kinase inhibitor alone (22%) or in combination with anti-CD20 monoclonal antibodies or venetoclax (0%) had the poorer response rate whereas patients who received venetoclax monotherapy achieved a significantly higher response rate (52%). A multivariable analysis identified age older than 65 years, ongoing CLL treatment, and gamma globulin ≤6 g/L as independent predictors of the absence of seroconversion. Post-dose 2 seronegative patients had a global response rate of 35% after dose 3. This study provides an argument for the use of a third dose and for prophylactic SARS-CoV-2 neutralizing monoclonal antibodies.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for an ongoing pandemic. Although phase 3 messenger RNA (mRNA) vaccine trials demonstrated excellent efficacies, a number of subpopulations, including immunocompromised individuals, were excluded from these trials.1,2
Chronic lymphocytic leukemia (CLL) is associated with immune dysregulation as a result of hypogammaglobulinemia, impaired cellular immunity, and therapy-related immunosuppression. Impaired immune responses to vaccination have been previously reported in CLL, notably with attenuated vaccine-induced immunity in patients heavily treated or undergoing treatment with Bruton tyrosine kinase inhibitors (BTKi’s).3-5 Furthermore, in a recent Israeli study, a suboptimal response was reported after 2 doses of COVID-19 mRNA vaccine.6 In view of our preliminary results, the French National Authority for Health recently recommended the use of a third dose of vaccine in patients with CLL. The objective of our study was to evaluate SARS-CoV-2–specific antibody responses after the first, second, and third doses of the BNT162b2 and mRNA-1273 vaccines and after a single dose for patients with confirmed previous COVID-19.
Methods
Data were collected retrospectively from 17 French Innovative Leukemia Organization (FILO) centers and the Association de Soutien et d’Information à la Leucémie Lymphoïde Chronique et la Maladie de Waldenström (SILLC [the French CLL patients’ association]). The study was approved by the Health Data Hub. All patients provided informed consent. Patients received 2 vaccine doses at a 4-week interval and a third dose if they were seronegative. The third dose was administered at 6 to 8 weeks after dose 2. SARS-CoV-2–immunoglobulin G (IgG) anti-spike levels were measured at ∼4 weeks after each dose. Anti-spike antibodies were tested with available assays approved by the French Ministry of Health. The analysis of IgG anti-spike titers was performed only when tested with Abbott SARS-CoV-2 IgG II Quant assay (detailed statistical methods are provided in supplemental Methods).
Results and discussion
From March to July 2021, a total of 530 patients were included in this study (patients’ clinical data are summarized in supplemental Table 1). Vaccine response was evaluated for 158 patients with CLL after dose 1, for 506 patients after dose 2, and for 95 patients after dose 3. BNT162b2 vaccine was administered to 377 patients (71%) and mRNA-1273 vaccine was administered to 76 patients (14%). For the remaining 77 (15%) of 530 patients, the type of mRNA vaccine was not specified by investigators. Median age of patients was 71 years (range, 37-93 years). A total of 218 patients with CLL (40%) were treatment naïve (TN), 136 (26%) had received previous treatment for CLL and were off therapy, and 176 (34%) were receiving therapy. In the previously treated patients, the median time from administration of the last treatment and the first dose of vaccine was 47 months (range, 2-232 months).
After dose 1, the global response rate was 27% (43 of 158), lower than the reported 34% in the British cohort.7 TN and previously treated patients had similar response rates of 34% (23 of 67) and 33% (12 of 36), respectively, and their response rate was higher than that of patients receiving therapy (15%; 8 of 55; P = .02). Among the 55 patients receiving therapy, the 32 patients who received BTKi’s experienced a lower response rate (16%; 5 of 32).
After the first dose, patients who had a weak titer (below the manufacturer’s positivity threshold of 50 arbitrary units [AU]/mL but higher than the detection limit of 6.8 AU/mL) were more likely to present an antibody response after the second dose (94%; 16 of 17) than those with a titer <6.8 AU/mL (29%; 19 of 65; odds ratio [OR], 38.74, 95% confidence interval [CI], 6.27-414.80; P < .001).
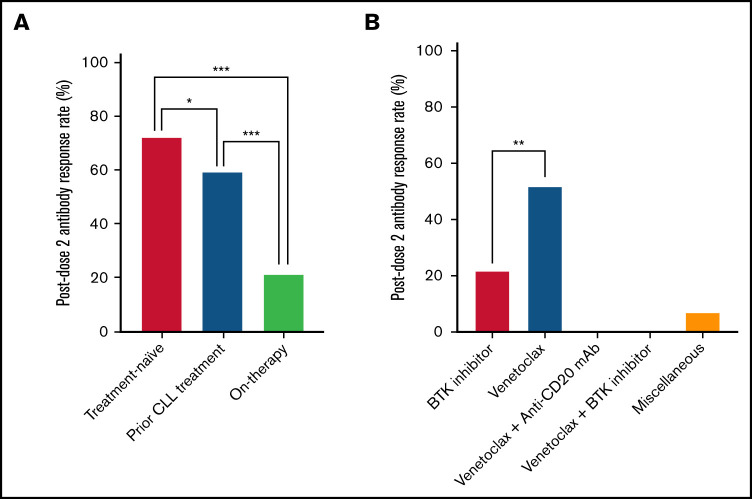
After dose 2, the global response rate was 52% (265 of 506). TN patients had the highest response rate (72%; 151 of 210) compared with patients who had been previously treated, mostly by chemoimmunotherapy (60%; 78 of 130; P = .02) and with patients receiving therapy (22%; 36 of 166; P < .001) (Figure 1A). The lower response rate of the TN patients previously reported by Herishanu et al6 (55.2%; 23 of 58) could be related to different clinical and biological characteristics of the TN groups in the 2 studies. The large majority of the 166 patients receiving therapy were given targeted agents. Those receiving venetoclax monotherapy achieved a significantly higher response rate (52%; 12 of 23) compared with those receiving ibrutinib or acalabrutinib (22%; 23 of 104; P < .001). However, the group of patients receiving venetoclax monotherapy was smaller than the group treated with BTKi’s. All of the patients treated with venetoclax plus anti-CD20 monoclonal antibodies (mAbs) (n = 19) or venetoclax plus BTKi’s (n = 6) were seronegative after the second dose of vaccine. This difference was not reported in the Israeli cohort, because patients receiving venetoclax monotherapy were not segregated from those receiving venetoclax plus anti-CD20 mAbs (Figure 1B).6 The negative impact of BTKi’s or anti-CD20 mAbs on antibody production is not surprising and is in line with other vaccination efficacy reports.4,5,8
Figure 1.
Antibody response rates in patients with CLL after 2 doses of COVID-19 vaccine. (A) Response rates by treatment history. TN patients had a response rate of 72% (151 of 210), significantly higher than that of previously treated patients (60%; 78 of 130; P = .02) and patients receiving therapy (22%; 36 of 166; P < .001). (B) Response rates for patients receiving therapy (n = 166) by treatment type. Patients receiving venetoclax monotherapy had a significantly higher response rate (52%; 12 of 23) than patients who were treated with BTKi’s (22%; 23 of 104; P < .001). All patients treated with venetoclax plus anti-CD20 mAb’s (n = 19) and venetoclax plus BTKi’s (n = 6) were seronegative after the second dose of vaccine. *P < .01; **P < .001; ***P < .0001.
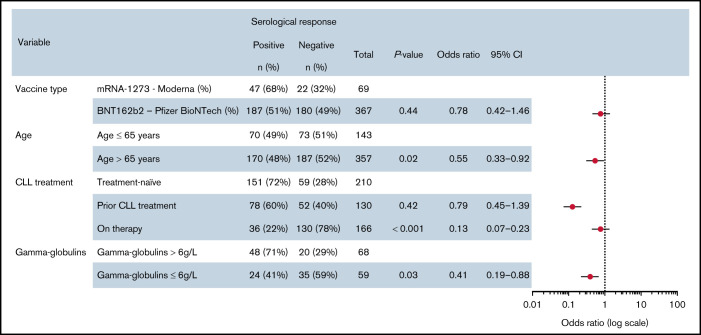
In a univariable analysis of humoral responses after dose 2, the variables found to be significantly associated with seroconversion were age older than 65 years (OR, 0.49; 95% CI, 0.33-0.73; P < .001), BNT162b2 vaccine type (OR, 0.49; 95% CI, 0.28-0.84; P = .001), previous treatment for CLL (OR, 0.59; 95% CI, 0.37-0.93; P = .02), ongoing treatment for CLL (OR, 0.11; 95% CI, 0.07-0.17; P < .001), and gamma globulin ≤6 g/L (OR, 0.33; 95% CI, 0.16-0.69; P < .01). The unexpected higher seroconversion rate with mRNA-1273 vaccine compared with BNT162b2 has been recently reported in hematologic malignancies in contrast with an equivalence in normal patients.9 The factors that were significant by univariable analysis were incorporated into a multivariable analysis (Figure 2), which identified age older than 65 years (OR, 0.55; 95% CI, 0.33-0.92; P = .02), ongoing treatment for CLL (OR, 0.13; 95% CI, 0.07-0.23; P < .001), and gamma globulin ≤6 g/L (OR, 0.41; 95% CI, 0.19-0.88; P = .03) as independent predictors of the absence of seroconversion. The impact of gamma globulin levels is in line with the slightly discrepant data on the impact of low IgG and IgM in the Israeli cohort,6 and low IgA in the British cohort.7
Figure 2.
Multivariable analysis of serologic response in patients with CLL. ORs for antibody response after double-dose vaccination in a multivariable logistic regression analysis. Data points represent the ORs and 95% CIs.
A third dose was proposed only for patients who were seronegative after dose 2 and, so far, 95 have been tested for their response after dose 3. After dose 3, the global response rate was 35% (33 of 95), which seems lower than that in a cohort of patients who received kidney transplants, the only one reported to date (35% vs 49%). The rate is similar to that of a subgroup of patients who received transplants and were given tacrolimus, mycophenolate, or steroids (35% vs 34%).10
When considering the results after dose 3, the group of TN and previously treated patients had a significantly higher response rate (56%; 18 of 32) compared with patients receiving therapy (24%; 15 of 63; P < .01). The majority of these patients who were receiving therapy were being given BTKi’s (73%; 46 of 63) and had a response rate of 28% (13 of 46). No clear assumption can be drawn from the patients receiving other therapies because all of them had previously received several lines of treatment.
We analyzed the post-dose 2 antibody titers tested with the Abbott SARS-CoV-2–IgG II Quant anti-Spike assay for patients who had results after dose 3 (n = 39). Patients who had weak responses after dose 2 (<50 AU/mL but higher than the detection limit of 6.8 AU/mL) were more likely to mount an adequate antibody response after the third dose (89%; 8 of 9) compared with those who remained negative (<6.8 AU/mL) (20%; 6 of 30; OR, 32.00; 95% CI, 3.86-364.8; P < .001).
An additional cohort of 40 patients with CLL who presented with COVID-19 before they received a vaccination was analyzed independently. COVID-19 was confirmed by polymerase chain reaction, and the median time from positive assay result to vaccination was 6 months (range, 3-15 months). All patients achieved seroconversion after a single dose of vaccine, even though 30% (n = 12) were receiving ongoing treatment for CLL. Moreover, IgG anti-spike titers were significantly higher after dose 1 in patients who had previously contracted COVID-19 (n = 13; median, 40 000 AU/mL; interquartile range, 3497-40 000) compared with titers of patients with CLL who seroconverted after dose 2 (n = 93; median, 2184 AU/mL; interquartile range, 313-5799 AU/mL; P < .001), suggesting that the strongest boost of immune response against the virus is the SARS-CoV-2 infection itself.
A limitation of our work is that it lacked an assessment of the cellular immune response. However, a recent report by Ehmsen et al11 suggested that the majority of the seronegative patients with CLL are not protected by a good cellular immune response because they do not elicit CD8+/CD4+ T-cell–specific responses.
In conclusion, mRNA vaccination generated humoral responses in 52% of our CLL cohort. An extra 35% of seronegative patients seroconverted after a third dose. The major independent predictor of negative humoral response was ongoing treatment with BTKi’s. Conversely, patients receiving venetoclax monotherapy experienced a seroconversion rate close to that of TN patients. Moreover, we observed that the majority of post-vaccination COVID-19 infections occurred in patients treated with BTKi’s (data not shown). Consequently, we suggest either postponing the start of BTKi treatment until after completion of the vaccination program or considering venetoclax monotherapy. Why some TN patients also remained seronegative after vaccination is currently under investigation. In light of these national-level data, patients who remain seronegative after a third dose will soon be offered the possibility of receiving SARS-CoV-2 neutralizing mAbs.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all the patients who participated in this study as well as the French Association de Soutien et d’Information à la Leucémie Lymphoïde Chronique et la Maladie de Waldenström (SILLC).
Authorship
Contribution: F.C. was principal investigator and C.B. and A.-S.M. were co-investigators; F.C., C.B., A.-S.M., R.L., and V. Levy conceptualized the study; R.L. and S.B. analyzed and validated laboratory results; C.B. and N.S. performed statistical analyses; C.B., C.A.-N., V. Levy, A.D., C.D., V. Leblond, D.R.-W., A.C., D.C., P.G., R.G., K.L., B.D., L.W., C.T., F.M., H.L., X.T., S.M., F.C., and A.-S.M. evaluated the patients on the study; M.C.B. provided research support and coordination for online data collection; C.B., F.C., A.-S.M., R.L., and V. Levy prepared the first draft of the manuscript; and all authors reviewed the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne-Sophie Michallet, Centre Léon Bérard, 28 Rue Laennec, 69008 Lyon, France; e-mail: anne-sophie.michallet@lyon.unicancer.fr; and Florence Cymbalista, Hôpital Avicenne, AP-HP, 125 Rue de Stalingrad, 93000 Bobigny, France; e-mail: florence.cymbalista@aphp.fr.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauro FR, Giannarelli D, Galluzzo CM, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021;35(3):737-746. [DOI] [PubMed] [Google Scholar]
- 4.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397-e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26):6769-6771. [DOI] [PubMed] [Google Scholar]
- 9.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.