Abstract
The Coronavirus Disease 2019 (COVID-19) pandemic has shocked world health authorities generating a global health crisis. The present study aimed to analyze the different factors associated with physical activity that could have an impact in the COVID-19, providing a practical recommendation based on actual scientific knowledge. We conducted a consensus critical review using primary sources, scientific articles, and secondary bibliographic indexes, databases, and web pages. The method was a narrative literature review of the available literature regarding physical activity and physical activity related factors during the COVID-19 pandemic. The main online database used in the present research were PubMed, SciELO, and Google Scholar. COVID-19 has negatively influenced motor behavior, levels of regular exercise practice, eating and nutritional patterns, and the psychological status of citizens. These factors feed into each other, worsening COVID-19 symptoms, the risk of death from SARS-CoV-2, and the symptoms and effectiveness of the vaccine. The characteristics and symptoms related with the actual COVID-19 pandemic made the physical activity interventions a valuable prevention and treatment factor. Physical activity improves body composition, the cardiorespiratory, metabolic, and mental health of patients and enhancing antibody responses in vaccination.
Keywords: Inactivity, Cardiorespiratory fitness, Metabolic health, Mitochondrial fitness, Mental health
1. Introduction
Originated in Wuhan (Hubei, China) on December 31, 2019, an unknown appearance of 27 cases of pneumonia of unknown etiology has led to a global viral pandemic (SARS-CoV-2) [1]. Its exponential and global increase in the rate of infections and the first deaths was the trigger for the World Health Organization (WHO) to declare the beginning of a pandemic, starting on March 11, 2020, affecting worldwide with more than 110 million confirmed cases and more than 2.5 million deaths [2] on its peak. As of June 2021, this epidemiological crisis continues with about 172 million accumulated cases, 3.69 million deaths while 2.020 million vaccines have been administrated, 443 million have been immunized, being this the 5.7% of the worldwide population [3]. The upcoming years will be marked in history as the era of COVID-19, which public health and socioeconomic scenario have produced behavioral changes that have affected mental and general health especially among those who have suffered from COVID19.
There are growing issues that the lockdown and the COVID-19 restrictions have placed limitations on opportunities for people to be physically active [4]. And yet, it is grounded that active work, modifiable conduct, is defensive against non-transmittable infections [5] and that decreased degrees of physical activity (PA) might have a negative impact on the management of chronic health issues as well as metabolic, pulmonary, and medical specialty conditions [6].
The exchange between protection from COVID-19 and accumulated risk of inactivity presents already vulnerable populations with a possible “no-win” situation; for example, wherever the consequence of protection from effort SARS-CoV-2 infection is accumulated inactivity and associated downstream health impacts [7]. Yet, in the long term, modifications in terms of physical activity may have consequences in the increase of the population sensitive to suffering severe complications from COVID-19 in subsequent epidemic waves. Interestingly, a recent study attributes that physical inactivity is one of the lifestyle-related risk issues for severe COVID-19 requiring hospital admission [8]. Indeed, the authors conclude that a baseline sedentary lifestyle increases the mortality of hospitalized patients with COVID-19.
However, the different elements related to physical activity are multifactorial, and several factors influence the protective effect of PA. Then in the present study, the main finding in physical activity and sport sciences associated with COVID-19 in the literature are discussed. For this aim, we conducted the present consensus critical review with the objective of to analyze the different factors associated with physical activity that could have an impact in the COVID-19, providing a practical recommendation based on actual scientific knowledge.
2. Search methods and strategies for research identification
The protocol used consisted of a literature search using primary sources, like scientific manuscript, and secondary like bibliographic indexes, databases, and web pages. We used PubMed, SciELO, Embase, Science Direct Scopus, and Web of Science employing MeSH-compliant keywords including COVID-19, Coronavirus 2019, SARS-CoV-2, 2019-nCoV, behaivour, inactivity, obesity, cardiorespiratory fitness, metabolic health, mitochondrial fitness, mental health, physical condition, and physical activity. We used articles published from 1 January 2020 till 5 October 2021, although previous studies were included to explain some information in several points of the review. The following exclusion criteria were used: i. studies with old data out of the COVID-19, ii. present inappropriate topics, being not pertinent to the main focus of the review, iii. PhD dissertations, abstracts, conference proceedings, unpublished studies and books. We included all the articles that met the scientific methodological standards and had implications with any of the subsections in which this review article is distributed. The information treatment was performed by all the authors of the review. Finally, articles were discussed by the authors to write the present review.
3. Behavioral changes and consequences of the COVID-19 pandemic
Without a doubt, one of the main consequences of the pandemic has been a strong modification of the habits, attitudes, and behaviors of population. COVID-19 has negatively influenced motor behavior, levels of regular exercise practice, eating and nutritional patterns, and even the educational process of students at all age stages worldwide.
From a pedagogical and educational point of view, authors suggest the need to transform educational methodologies and pedagogies towards the use of online teaching, through videos, using emerging pedagogical models such as the “flipped classroom”, involving families in the production of learning resources and training of teachers in digital didactic resources [9], [10], [11].
At nutritional level, severe COVID-19, like other critical illnesses, results in catabolic muscle wasting, feeding difficulties, and frailty, each of which is associated with an increased likelihood of poor outcome [12]. Authors state that as a consequence of prolonged immobilization in SARS-CoV-2 respiratory syndrome, a reduction in muscle functions leading to sarcopenia could be provoked [13]. This sarcopenia is associated with an increased risk of malnutrition, disability, and, in general, a worsening of quality of life. Indeed, recovered SARS-CoV-2 patients, continued to experience poor quality of life months after the infection, consequent with significant nutritional deficits, even at 6 months post medical discharge [14]. Yet, nutrition appears to be a determining factor for health maintaining and recovery, so nutritional support is paramount alongside rehabilitation to improve the chances of recovery for COVID-19 patients [13].
Regarding the levels of physical exercise and physical-sports practice, numerous studies have examined how it has affected by the confinement measures [15], the post-COVID physical sequelae [16], the emotional effects [17], as well as the motivation towards sports practice in practitioners to maintain and/or improve their health [18] and in high-performance athletes [19]. The reduction of mobility, the restrictions on outdoor activities, due to the decrees of the state of alarm, in more than a third of the world's population, has led to a reorganization of the way of doing physical exercise at home or in green areas nearby [20] and has affected the practice of physical exercise regularly [21]. Authors propose that exercise at home using several safe, simple, and easy-to-implement exercises is well suited to avoid “aerial coronaviruses” and maintain physical fitness levels. Such forms of exercise may include, but are not limited to, strengthening exercises, activities for balance and control, stretching exercises, or a combination thereof (eg: walking around the house and going to the store if necessary, lift and carry the groceries, do "lunges" with the legs, climb stairs, do squats, exercises to strengthen the abdominal muscles, arm dips, etc.). In this sense, review studies [22] focus on providing data and providing answers to the return to aerobic-type physical exercise as a potential tool to minimize the practical restriction of blood flow (due to sedentary lifestyle physical and mobility reduction), as well as no-load resistance training such as proposing possible resistance exercise strategies that could be performed during the current COVID-19 pandemic.
Regarding the mental health of the population, scientific evidence shows different psychological behaviors and emotional consequences [23]. One of the factors that have most influenced the psychological and emotional health of the population is the restrictions on the enjoyment and habitual use of the outdoor space. Time spent outdoors is associated with positive mental health. In this sense, the study carried out by Cindrich et al. (2021) [22] analyzed the impact of COVID-19 acute public health restrictions on time outdoors in April 2020, and quantified the association between time outdoors, stress, positive mental health, using secondary analyzes of cross-sectional data from the COVID study and well-being. Varga et al. (2020) [23] indicate that younger individuals and people with a history of mental illness experienced higher levels of loneliness compared to other subgroups during the first four months of social closures related to the COVID-19 pandemic. The study by Pirkis et al. (2021) [24] emphasizes the importance of monitoring the profound consequences of the pandemic on mental health for the prevention of autolytic behaviors. It appears that, in high-income and upper-middle-income countries, the number of suicides has remained practically unchanged or has decreased in the first months of the pandemic compared to the levels expected according to the pre-pandemic period.
It is worth highlighting the importance of future research integrating and analyzing information along multiple axes (for example, clinical and socioeconomic axes, resource deficits, and external stressors) to avoid imprecise contextualization [27] and offer operational solutions to the psychological, emotional, and maintenance of healthy lifestyle consequences that have been diminished in the world population by SARS-CoV-2 [25,26]
4. Inactivity, obesity and COVID 19
Physical inactivity has been defined as "doing no or little PA at work, at home, for transport or in discretionary time" [28]. With regards to the relationship between PA and COVID-19, it has been shown that Brazilian adults who had symptoms of this virus had a low level of PA, whereas those who showed no symptoms had light-to-moderate levels of PA [29]. During the lockdown, PA decreased compared to a normal week and this decrease was larger in men than in women [30]. Interestingly, PA decreased when the lockdown started and gradually increased during lockdown without reaching levels of a normal week [31]. Considering the specific domains of PA, daily occupational, transportation, and sporting activities decreased in lockdown, whereas leisure-time activities increased in Greek adults [32]. During the lockdown, physical activities such as jogging and sports decrease, whereas watching TV, using electronics, and social media increase [33]. In a study of nine European countries, physical inactivity and time in front of a screen increased, whereas walking and sports time decreased [34]. In addition to the general population, a similar trend was observed in chess players [35, 36] and patients with automatic implantable cardioverter-defibrillators for primary prevention of sudden death [37]. Considering PA as a spectrum ranging from physical inactivity to high PA, the abovementioned studies highlighted a decrease in PA during the lockdown.
Physical inactivity influences all aspects of human function including neuromuscular, cardiorespiratory, and metabolic systems [38]. Physical inactivity is associated with positive energy balance, fat deposition, and low-grade systemic inflammation [38]. This positive energy balance might further increase considering overeating during home confinement [39]. The increased caloric intake and reduced PA energy expenditure induced weight gain during lockdown [40]. During a seven-week lockdown, obese adults reported weight gain 1.6 kg with those with regular exercise presenting lower weight gain [41]. Weight gains were attributed partially to meal size, unhealthy food consumption, and screen time [34]. With regards to caloric intake, it has been suggested that lockdown-related frustration links with several psychological problems including depression, which, in turn, push people to consume high sugar foods increasing the risk for obesity [42] (Mediouni, Madiouni, & Kaczor-Urbanowicz, 2020). With regards to childhood obesity, decreased PA has been identified as a risk factor combined with other biological, psychosocial, and behavioral aspects [43]. In a study of Sicilian adults, overweight showed lower PA than normal-weight adults [30]. Individuals with the highest decrease in PA reported lower physical and mental health, while those with the highest increase in PA reported a significantly higher increase in sleep and lower weight gain [44]. Adolescents who did less PA were more likely to be overweight or obese and less likely to have strong prior PA habits [45]. With regards to the methodological approaches of the relevant studies presented in this section, it should be highlighted that they relied mostly on online questionnaires to evaluate PA levels [30, 32, 46] and less accelerometer [37] and count of steps [31].
5. Physical activity and COVID-19
Subjects with chronic diseases such as hypertension, diabetes, obesity, and systemic diseases as heart, pulmonary, renal, liver, vas sickness, or medical specialty pathologies, have shown a poorer prognosis with coronavirus infection [47], [48], [49]. Yet, within an effective treatment and with only 5.7% of the worldwide population [3] vaccinated, the management of those pathologies is crucial to cut back COVID-19 mortality. In this line, physical exercise has been postulated as the true “polypill” for all increased-risk- mortality causes [50]. Large observational studies also suggest that exercise itself can reduce the risk of all-cause and disease-specific mortality [51, 52]. Indeed, a baseline sedentary lifestyle is an independent risk factor for mortality in hospitalized patients with COVID-19 [52]. Yet, restrictions and public health recommendations such as stay-at-home orders, closures of parks, gymnasiums, and fitness centers to prevent the spread of the virus have had an impact, reducing daily physical activity (PA) [53]. These recommendations are unfortunate because daily PA has a strong impact boosting the immune system response, counteracting with most of the chronic diseases that increase the COVID-19 fatality risk.
Evidence suggests that the main reason why chronic diseases predispose to worst COVID-19 prognosis and symptomatology is that they are linked with a pro-inflammatory state and an imbalance between the pro-inflammatory angiotensin-converting enzyme-1 (ACE1) and anti-inflammatory ACE2 axes [54]. In this line, current studies show how PA could reverse this pro-inflammatory link, although it would not attenuate the risk of getting infected with SARS-CoV-2, it would reduce one's risk of getting severe symptomatology [55]. Furthermore, the quarantine and pandemic are leading to an increase of psychological disorders like fear, anxiety, post-traumatic stress disorder [56]. Likewise, nutritional habits have changed significantly, showing unhealthy dietary changes and increases in the body weight of the population [57]. These psychological and nutritional changes have a direct negative effect on the immune system [58]. In this line, PA increases endorphin, dopamine, and serotonin, and counteracts the effects of a caloric super habit and poor nutrition, enhancing immune function [59].
Mainly because of its impact on the inflammation process, since during and after physical exercise, pro-and anti-inflammatory cytokines are released, preventing inflammation, and acting as an adjuvant booster for the immune cells, improving the performance of natural killer cells, neutrophils, and macrophages, thus avoiding damage caused by COVID-19 symptoms [60]. In this line, recent studies suggest that PA due its effects, may boost vaccination programs due to an increase in T-cells and neutralizing antibodies. Yet, further research is needed in the case of acute exercise to boost vaccine responses, where there have been inconsistent findings in terms of the benefit to add exercise bouts to vaccines to frail adults [61].
Regarding PA considerations, authors conclude that following up the daily PA guidelines of 150 min of moderate or 75 min of high-intensity PA, with a frequency of 3 to 5 days, is enough for a better mental and physical well-being and a lower prevalence of COVID-19 symptomatology, better prognosis and recuperation [62]. However, the authors clarify that in certain countries or areas in which situations or contexts of restriction of movement continue to be imposed, a weekly increase in physical activity from 150 min to 200–400 min needs to be done in order to compensate for the less active lifestyle [63]. Likewise, overtraining, high-intensity physical exercise while suffering from a respiratory tract disease, has been associated with immune dysfunction and an elevated risk for respiratory illness. Therefore, this can be extrapolated to COVID-19, and precautions must be taken. Independently, level of physical fitness, the characteristics and type of training, or the individual immunological characteristics pre-existing need to be considered to provide tailored immunological and anti-inflammatory responses [64]. Since not only responses to diseases are heterogeneous but also responses to exercise may be different.
In general terms, during the COVID-19 pandemic, the most recommended aerobic activities are walking, climbing stairs, running among others [65]. Depending on the type of population, previous training and experience, training frequency and intensity should be adapted. Yet, authors conclude that frequency may vary from one time per day to healthy young adults and 2–3 times a week. Suggested workload may vary from 150 to 300 min per week, achieving a medium intensity (effort ranging from 5 in a scale of 1 to 10), or a high intensity of 75 min (effort ranging from 8 in a scale of 1 to 10), while teenagers or preschool children should perform between 60 and 180 min of medium intensity PA [66]. Regarding strength training, recommendations are of 2 to 3 sessions per week [63]. Exercises may vary from: lifting and carrying, lunges, stair climbing, stand-to-sit and sit-to-stand using house items, squats, or sit-ups [63]. Authors highlight the importance of strength training for the breathing muscles [67], during the recovery period of patients after acute COVID-19 infection [68]. In this line, 50 to 100 resisted breaths daily, 5 days a week seems an optimal approach [59]. Another type of PA interventions, such as combining both, either aerobic and strength exercise (concurrent training), seems less effective than only aerobic or resistance training, although it is also capable of improving immune function [59].
What is indisputable is that the COVID-19 is seriously affecting psychological and physical health, especially in sensitive population groups. Given both the acute and chronic benefits of PA, following the recommendations in terms of strength and aerobic PA is a contributing factor in the prevention and treatment of COVID-19.
Finally, in the actual global vaccine administration also PA could present a critical role since previous authors highlighted how the acute stressor of PA could enhance the response to delayed-type hypersensitivity and antibody response to vaccination [69]. In this line, the high-intensity exercise might enhance antibody responses in influenza vaccination [70, 71]. Then, beyond vaccination, PA is presented as a safe and potential preventive measure, especially for the most vulnerable groups [61].
6. Cardiorespiratory fitness and COVID-19
COVID-19 is a disease caused by the SARS-CoV-2 coronavirus that induces a systemic inflammatory response and causes cough, fever, and respiratory distress, which places a great burden on the cardiopulmonary system [72]. The most serious forms are manifested by impaired respiratory function, which in many cases requires mechanical ventilation, and by the systemic inflammatory response syndrome, which can lead to septic shock with multi-organ failure, and high mortality rates [73]. Among the factors most widely related to a worse prognosis of this disease are age and certain comorbidities such as for overweight/obesity [74], insulin resistance and diabetes [75], hypertension, and respiratory and cardiovascular system disease [76]. These comorbidities are characterized by low-grade chronic inflammation with high levels of various pro-inflammatory cytokines and the inflammasome, which, as everything seems to indicate, predisposes to a higher risk of infection and hospitalization, as well as mortality [73]. These are related in many cases to lifestyle, supported by poor eating habits and especially by a sedentary pattern and lack of physical exercise [77].
In this sense, the regular practice of moderate to intense intensity physical exercise is well known, it significantly improves cardiorespiratory capacity [78]. A higher CRF is associated with a greater cardiopulmonary reserve and causes powerful and wide-ranging effects on the immune system, among which are mainly its anti-inflammatory effects, giving the body a better ability to respond to any aggression [73]. CRF reflects the integrated function of multiple organ systems and is an important parameter of general health and the body's ability to respond to both internal and external stressors such as COVID-19 itself [79]. In this sense, an inverse association has been described between the level of CRF and hospitalization for COVID-19. Women in the lowest CRF quartile have shown hospitalization rates approximately twice as high as those in the CRF highest quartile. The effect in men has been even more pronounced [80]. High levels of CRF may enhance a stronger host immune defense against SARS-CoV-2 by decreasing susceptibility to viral infections and better controlling pro-inflammatory responses and enhancing post-infection host antiviral responses. Therefore, CRF is not only an objective measure of habitual physical activity but also a useful diagnostic and prognostic health indicator for patients who contract SARS-CoV-2 [81].
On the other hand, it has been determined that people who have seriously suffered from the disease, many of whom have had to be hospitalized, experience deconditioning, that is, a reduction in CRF, muscle strength, or aerobic capacity due to the impossibility of mobility and restrictions in activities of daily living [82]. In addition, for those patients who have been in the ICU (> 30% of hospitalizations), this deterioration is maintained in the long term [83]. Current evidence indicates that while an increase in physical function and CRF is observed within the first 6 months post-COVID-19 [84], recovery is incomplete and people who have suffered from SARS-CoV-2 infection may experience residual alterations in physical function and CRF at one year or 2 years post-infection [85]. Therefore, the need to plan targeted interventions to promote the physical recovery of people after SARS-CoV-2 infection [86] is demonstrated. In this sense, it has been shown that a physical exercise program that combines aerobic and resistance training can improve physical function and CRF in the first 2 months after discharge from hospital post-SARS-CoV-2 [87]. However, more studies are required to determine the effectiveness of interventions with exercise, to promote the improvement of CRF and which would suggest that the sequelae left by the virus [86] have already been overcome.
In summary, based on the current evidence, everything seems to indicate the relationship between CRF and the severity of the prognosis in the current COVID-19 pandemic. In the same way, recovering the CRF lost during the infection or hospitalization period as soon as possible seems essential to leave behind the sequelae produced by SARS-CoV-2.
7. Metabolic health and COVID-19
The association between individual components of metabolic health (hypertension, diabetes, systemic inflammation, and dyslipidemia) and COVID-19 infection, severity and death has been demonstrated with mounting evidence since the beginning of the COVID-19 pandemic [88], [89], [90], [91], [92], [93]. Similarly, higher risk of SARS-CoV-2 infection has been linked to an excess of adipose tissue and obesity [94].
Concerning hypertension, this pathology is treated with angiotensin-converting enzyme (ACE) and angiotensin II type-I receptor blockers (ARBs) [95] that produce an upregulation of ACE2 [96]. This enzyme (ACE2), expressed by epithelial cells of the lung, blood vessels, kidneys and intestine [97], plays an essential role in the human pathogenic of the SARS-Cov-2 because ACE2 acts as a coreceptor for SARS-Cov-2 to enter into cells [98]. Consequently, the increased expression of ACE2 would facilitate infection with COVID-19. Therefore, hypertension management must be a principal goal during the COVID-19 pandemic. In this way, physical exercise has been proposed as an alternative to pharmacologic therapies used to reduce blood pressure [99]. Specifically, aerobic exercise has traditionally been the most prescribed to treat this disease [99]. Moreover, other training proposals based on strength exercises have demonstrated their effectiveness in lowering blood pressure [100]. In this way, training programs performed at a moderate intensity and with a frequency of three times per week, seem to be optimal in order to reduce blood pressure [100].
Additionally, there is evidence of increased incidence and severity of COVID-19 in patients with diabetes [101] or uncontrolled hyperglycemia showing that diabetes or poor glycaemic control is clearly one of the most important comorbidities linked to COVID-19 [101]. Previous studies have found a link between COVID-19 and the pathophysiology of diabetes [101]. In this way, some studies reported an increased ACE2 expression in diabetic mice [102] and others found a causally related ACE2 expression and diabetes [103]. Hence, increased ACE2 expression may increase the risk of SARS-CoV2 infection in people with diabetes [101]. Furthermore, diabetes patients showed an increase of furin which facilitates viral replication [104], an impaired T-Cell function [105] and an increased interleukin-6 (IL-6) which plays a role in COVID-19 [106]. Therefore, blood glucose control is important for all patients who have diabetes and COVID-19 infection. Interestingly, exercise is widely perceived to be beneficial for glycaemic control in patients with type diabetes [107]. Regarding the type of exercise, resistance and aerobic exercises are both recommended as effective treatments for people with diabetes [108].
Remarkably, obesity is one of the strongest predictors of hospitalization in COVID-19 patients [109]. In particular, visceral adipose tissue that could be related with waist circumference is associated to the risk of hospitalization in an intensive care unit in COVID-19 patients [110]. In addition, among all groups affected by the COVID-19 lockdown, overweight and obese people are particularly impairing their lifestyles (e.g., reducing physical activities) and dietary patterns, increasing their body mass and fat mass [57]. Moreover, a recent meta-analysis has shown that dyslipidemia increases COVID-19 severity and mortality [111], highlighting that metabolic and lipid profile may even worsen due to less activity and an imbalanced diet during the pandemic. Hence, diet and exercise interventions must be implemented, specifically during situations of lockdown as a treatment for obesity.
Another metabolic health factor that has been related to poor COVID-19 outcomes and death is systemic inflammation [112, 113]. Additionality, IL-6 has been demonstrated to play a role in the pathogenesis and therapy of COVID-19 [114]. In this way, it has been previously argued that obesity and specifically visceral adipose tissue increased systemic and local inflammation [115] due to the enhancement of expression of pro-inflammatory cytokines [113,116, 117] Moreover, the increase of the expression of ACE2 together with other dysfunctions such as the activation of the renin-angiotensin-aldosterone system in obese people with hypertension or the microvascular dysfunction in obese people with diabetes or hyperglycemia [113, 116, 117] may play an essential role in the contribution of COVID-19 mortality. Of note, exercise training has been clearly demonstrated its effectiveness to reduce systemic inflammation with and without weight loss in the patients [118]. In this way, aerobic training combined with resistance exercise training has been proposed as an effective program to improve systemic inflammation, showing a greater decrease in C-reactive protein when patients decrease body weight and adiposity [118]. Thus, a combination of diet and the encouragement that individuals to become physically active can lead to favorable changes in systemic inflammation. This fact could avoid worse outcomes in COVID-19 because systemic inflammation is associated with adiposity and obesity and they can delay immune response to pathogens [112, 113, 115, 116].
Current exercise recommendations to obesity management suggest that individuals with obesity should perform 30–60 min of moderate-intensity physical activity on most (if not all) days of the week [119]. In addition, strength training (e.g., circuit resistance training) [120] and high-intensity training [121] have been demonstrated their effectiveness to reduce fat mass in obese people. Therefore, a combination of training could enhance the management of obesity as a risk factor during COVID-19. Recently, it has been demonstrated the additive effect of hypoxic conditioning to exercise in order to reduce body mass and fat mass [122]. In addition, it has been hypothesized that hypoxic preconditioning appears to be a promising strategy to decrease inflammation, improve the immune system and maintain tissue oxygenation, which is the main goal of respiratory pathologies, including COVID-19 [123]. Moreover, this hypoxic intervention has been shown effective in other aforementioned metabolic health factors (i.e., anti-hypertensive) [124] associated to COVID-19 severity. Thus, future studies that analyze the effect of hypoxic preconditioning on COVID-19 are needed to confirm or refute the hypothesis.
To summarize, metabolic health factors are linked to COVID-19 severity and mortality and they may even worsen due to the decrease of physical activity and imbalanced diet during the pandemic. In this way, an individual and optimal training program seem to be an effective tool to fight with the virus, improving the immune response and improving metabolic health.
8. Mitochondrial fitness and COVID-19
To achieve optimal body homeostasis and health, cells must be healthy, and this can only be achieved if mitochondria are healthy [125]. Among functions that mitochondria have, energy is one of the main ones, using oxygen to burn fat, glucose, and amino acids to create ATP. Yet, a damaged power-energy plant, produces less energy and more pollution, similarly occurs to the mitochondrial function, a damaged mitochondrion is not energy efficient and generates more free radicals not performing the rest of its functions correctly [126]. Although producing energy is the main work of the mitochondria, they participate in many other processes, such as synthesis of steroid hormones (testosterone and estradiol), regulation of cellular calcium, detoxification of ammonia in the liver, and apoptosis [127]. When a disparity is presented between energy and cellular communication, illness may appear. As for example chronic fatigue [128, 129], diabetes [130], cardiovascular disease [131], neurodegenerative diseases [132], migraines [133], cancer [134] and even infertility [135], as well as premature aging [136].
Despite its potential role in maintaining oxidative homeostasis, ROS generation and its link with physiopathology, the mitochondria have yet received limited attention on its role on COVID-19 pathogenesis, being unknown its role during the inflammatory cytokine storm (-). Being information scare, associations and hypotheses can only be made by now. In this line, the mitochondrial antiviral signaling complex (MAVS), plays an important role on the immune defense system. MAVS are activated by the retinoic acid-inducible gene-like receptors (RLRs), leading to the transcription of class 1 interferons, which serve as central molecules in the cellular defense against viruses [137]. Yet, to impair cellular antiviral defense mechanisms, many viruses evolved mechanisms to evade cellular detection by RLR, or to reduce mitochondrial efficiency and thereby inhibit the antiviral host response [137]. Thus, it is likely to think that COVID-19 may cause mitochondrial dysfunctions through viral invasion of host mitochondria.
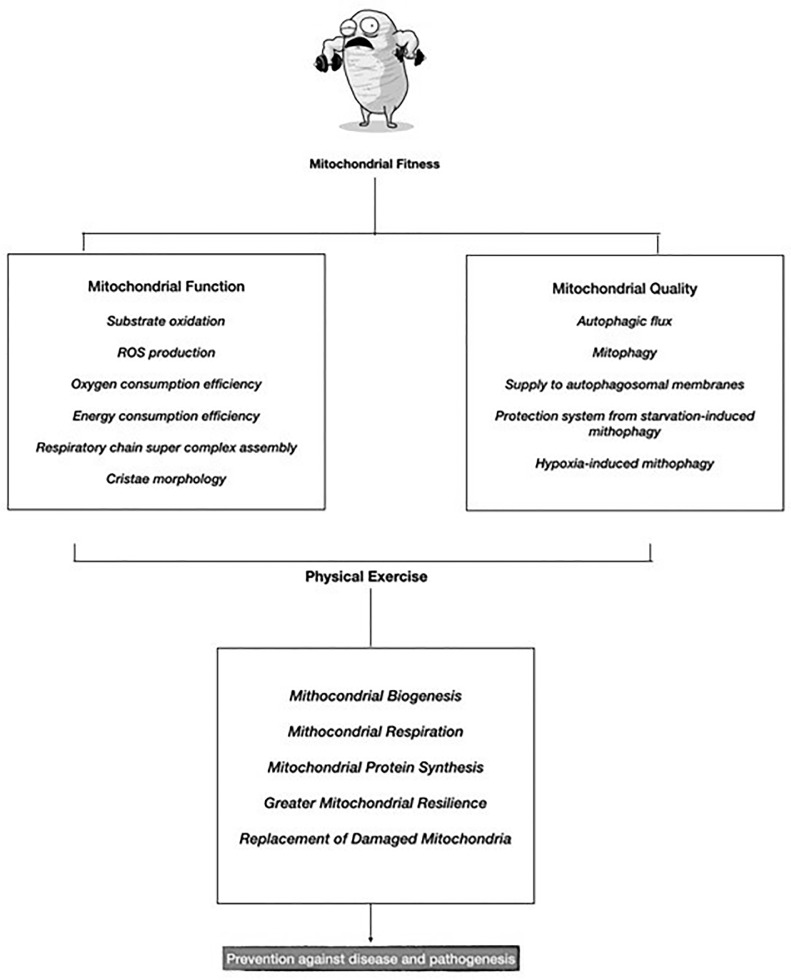
Consequently, it is appropriate to think that mitochondrial health is a preventive factor against the pathogenesis of COVID-19. In this line, the concept of mitochondrial fitness is born, which refers to the biological efficiency and adequacy of the mitochondria. Following David-Sebastián et al., 2017, this mitochondrial fitness would be divided into function and mitochondrial quality (Fig. 1 ). Yet, both can be achieved via physical exercise, highlighting its effects on mitochondrial biogenesis, mitochondrial respiration, mitochondrial protein synthesis, higher reliance of mitochondria on fatty acid substrates and better oxidative stress handling [137]. This will allow to generate new mitochondria, thus, more energy, improved physical performance [139], and replacement of damaged mitochondria, preventing from associated diseases [140], and slowing aging [141]. Therefore, the boost on mitochondrial fitness via physical exercise, will also enhance the immune system and its response, with acute and chronic anti-inflammatory effects, improving hosts immune defense efficiency preventing from the cytokine responses of COVID-19 that can result in sepsis and COVID-19-related death.
Fig. 1.
Mitochondrial Fitness, COVID-19 and Physical Exercise.
Among different physical activity interventions, traditional endurance exercise [142] has proven great efficiency on mitochondrial fitness. Yet, if resisted training is added to the equation [143], benefits are magnified. Furthermore, new training methodologies based on low volume and high intensity as High Intensity Interval Training (HIIT) have shown great efficacy in generating new mitochondria and optimizing their functioning [144]. In addition, if physical exercise is performed in fasting conditions (low muscle glycogen), the body is forced to burn more fat, tripling mitochondrial biogenesis compared to training with high glycogen [145].
Yet, other habits apart from physical exercise which may enhance mitochondrial biogenesis and fitness. The practice of intermittent fasting, triggers processes of mitophagy (autophagy of the mitochondria) that rejuvenate them and reduce disease [146]. Furthermore, it helps in regulating blood glucose and HbA1c levels [147], which according to recent studies well-controlled blood glucose may lead to an improved outcome of patients with COVID-19 since glucose control helps to prevent and control infections and complications, especially among patients with type 1 and 2 diabetes [148], death risk factor for COVID-19 symptomatology [101]. Furthermore, the habituation and respect of the circadian rhythm. Mitochondria have their own circadian rhythms, essential for maintaining a healthy a metabolism [149]. This optimizes, the secretion of melatonin, which is closely linked to mitochondrial function [150] and has proven to inhibit COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells [151].
Furthermore, the work of the mitochondria is complex, requiring a large number of vitamins, minerals, coenzymes, and antioxidants. Among them, Coenzyme Q-10 (CoQ10) is an essential molecule for mitochondrial health, showing reduced risk of associated with inefficient mitochondria: coronary health [152], fibromyalgia [153], neuromuscular and neurodegenerative diseases [154], metabolic syndrome [155]. Yet, alpha lipoic acid is an antioxidant, especially relevant for slowing down the deterioration caused by free radicals resulting from energy production [156]. Magnesium and B vitamins are also key micronutrients since both are required in mitochondrial biogenesis processes [156, 157]. Among B group vitamins specially B1 Thiamine, B2 Rivoflavin, B3Niacin, B9 Folate & B12, which participate in making energy production efficient [158], detoxification of mitochondria [159] and preventing their degeneration [160]. Furthermore, endogenous acetylcarnitine has beneficial effects in elderly animals and humans, including restoration of mitochondrial content and function. Authors suggest that acetylation of mitochondrial proteins leads to a specific increase in mitochondrial gene expression and mitochondrial protein synthesis [161]. It even seems that when is combined with CoQ10 and alpha-lipoic acid the beneficial effects on mitochondrial biogenesis are enhanced, surrogating markers of cellular energy dysfunction [162], and inducing reduced lactate and markers of oxidative stress in subjects with mitochondrial cytopathies [163].
9. Physical activity and mental health in COVID-19
Numerous health measures have been taken since the emergence of COVID-19 to prevent global transmission of the virus. Economic and social measures aimed at protecting the population and minimizing the risks of the spread of the virus have also been implemented [164]. Of all these measures, the most drastic and the one with the best health results have been the home confinement of the population [165].
However, it is known that the effects of prolonged low physical activity have detrimental effects on health. Regular physical exercise helps to maintain physical and mental well-being, which facilitates an improvement in the quality of life of people [166, 167].
Following the OMS declaration in January 2020 of a global public health emergency, prolonged confinement occurred in much of Europe. This drastically prevented people's lives from continuing as normal [168]. Travel, the daily routine of work, social and family contacts, and the pursuit of any type of recreational activity both indoors and outdoors were disrupted [169].
This disruption of people's lives forced them to lead a more sedentary routine, even making it impossible to commute to the workplace. This made it significantly more difficult to continue an adequate level of physical activity, which can negatively affect not only physical health but also mental health [170]. Even more so in the case of those infected persons who have had to remain in the hospital in a state of absolute repose [171].
In relation to physical activity, recent studies demonstrate the importance of maintaining healthy habits that benefit people's general well-being and combat the effects of prolonged social isolation [172]. Physical deterioration due to a very low caloric consumption is associated with a gradual loss of muscle mass and also with the appearance of various chronic physiological pathologies such as diabetes, cardiovascular diseases, and obesity, as well as other pathologies of a psychological nature such as increased anxiety [173]. In this line, sedentary behaviors can increase the consumption of foods high in fats and sugars, which will also lead to an increase in body fat and the appearance of oral health problems [174].
In an attempt to reduce the negative impact of sedentary routines following the onset of COVID-19, experts recommend spending part of the day engaged in moderate physical activity to reduce the body's pro-inflammatory state and strengthen the immune system, hindering the increase of diseases associated with the inflammatory process and elevated cortisol levels [175, 176].
Regarding mental health, since the beginning of the pandemic, there has been a significant increase in the presence of mental disorders related to mood [177]. From the first months, the population began to show signs of alarm, fear and confusion related to the virus, the lack of information and the seriousness of the health situation [178] Subsequently, during the time of home confinement, the risk of psychological problems derived from the lack of interaction and a life ordered by previously established patterns and routines increased considerably [54].
For these factors, in the following months, the presence of mental disorders such as anxiety and depression has increased, which have also been exacerbated by the lack of resources available for correct treatment due to the collapse of the health system, whose priority was to save lives [180].
Regarding anxiety, its prevalence has been associated with fear of contagion, perceptions of international catastrophe, uncertainty in relation to employment and the availability of a sufficient economy, and other factors such as the illness of a close relative. People with continued high levels of anxiety will suffer psychically and physically and will exhibit maladaptive and disruptive behaviors that may hinder recovery [125].
Regarding depressive symptomatology, recent studies indicate that, during the first months of the pandemic, more than 25% of the participants showed mild to severe depressive symptoms. The most common causes were the loss of family members to COVID-19, the feeling of loneliness during confinement, and the impossibility of accompanying sick family members. Among the most common physical symptoms were persistent headaches, fatigue, sleep disturbances or anhedonia [169].
10. Practical recommendations
COVID-19 is seriously affecting psychological and physical health, especially in sensitive population groups. Physical activity is a contributing factor in the prevention and treatment of COVID-19. Thus, 150 min of moderate or 75 min of high-intensity physical activity, with a frequency of 3 to 5 days, is enough for a better mental and physical well-being and a lower prevalence of COVID-19 symptomatology.
Cardiovascular physical activity as walking, climbing stairs, running among others [39] is encouraged with a strength training frequency of 2 to 3 sessions per week [118].
Healthy young adults should exercise 2–3 times a week between 150 and 300 min per week, achieving a medium intensity (effort ranging from 5 in a scale of 1 to 10), or a high intensity of 75 min (effort ranging from 8 in a scale of 1 to 10).
Teenagers or preschool children should perform between 60 and 180 min of medium-intensity physical activity.
Higher levels of respiratory muscle strength are associated with a better prognosis and rehabilitation after COVID-19 [47], especially if the subject has received artificial respiration by intubation [48]. Thus, 50 to 100 resisted breaths daily, 5 days a week seems an optimal approach [156] either for prevention and rehabilitation.
11. Future research lines
The multifactorial factors that are involved in the COVID-19 and the interconnexion of them allow us to propose different future research lines. For example, to analyze the effect of physical condition in the vaccines efficiency and the heard immunity success, since the impact of physical condition on immunity system and its importance for vaccines effects [174]. Taking into account the large impact of COVID-19 in mental health [33, [175], [176], [177], [178]], it would be interesting how physical activity could modulate these impact in population.
12. Conclusion
The characteristics and symptoms related with the actual COVID-19 pandemic made the physical activity interventions a valuable prevention and treatment factor. Physical activity improves body composition, the cardiorespiratory, metabolic, and mental health of patients and enhancing antibody responses in vaccination.
Funding
This research received no external funding
Availability of data and material
Not applicable
Code availability
Not applicable.
Ethics approval
Not applicable.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Platto S., Wang Y., Zhou J., Carafoli E. History of the COVID-19 pandemic: origin, explosion, worldwide spreading. Biochem. Biophys. Res. Commun. 2021;538:14–23. doi: 10.1016/j.bbrc.2020.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Who.int. [citado el 5 de julio de 2021]. Disponible en: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200327-sitrep-67-covid-19.pdf?sfvrsn=b65f68eb_4.
- 3.Coronavirus (COVID-19) vaccinations [Internet]. Ourworldindata.org. [citado el 5 de julio de 2021]. Disponible en: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL.
- 4.Lippi G., Henry B.M. Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19) Eur. J. Prev. Cardiol. 2020;27(9):906–908. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chekroud S.R., Gueorguieva R., Zheutlin A.B., Paulus M., Krumholz H.M., Krystal J.H., et al. Association between physical exercise and mental health in 1•2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry. 2018;5(9):739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 6.Durstine J.L., Gordon B., Wang Z., Luo X. Chronic disease and the link to physical activity. J. Sport Health Sci. 2013;2(1):3–11. [Google Scholar]
- 7.Rogers N.T., Waterlow N.R., Brindle H., Enria L., Eggo R.M., Lees S., et al. Behavioral change towards reduced intensity physical activity is disproportionately prevalent among adults with serious health issues or self-perception of high risk during the UK COVID-19 lockdown. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.575091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer M., Kivimäki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varea V., González-Calvo G., García-Monge A. Exploring the changes of physical education in the age of Covid-19. Phys. Educ. Sport Pedagogy. 2020; 1–11.
- 10.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat. Rev. Dis. Primers. 2020;6(1):60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermúdez Escallón J.R., Aldana Herrán A.C., Arra Párraga D.L.P., Salim Torres Y.Y., Tolosa Cubillos J.M. Rehabilitación pulmonar ambulatoria en pacientes con Covid-19: un reto en épocas de pandemia. Rev. Colomb Méd. Fís. Rehabil. 2020;30(Supl):130. [Google Scholar]
- 12.Carfì A., Bernabei R., Landi F. Gemelli Against COVID-19 post-acute care study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castañeda-Babarro A., Arbillaga-Etxarri A., Gutiérrez-Santamaría B., Coca A. Physical activity change during COVID-19 confinement. Int. J. Environ. Res. Public Health [Internet] 2020;17(18) doi: 10.3390/ijerph17186878. Disponible en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14.Tison G.H., Avram R., Kuhar P., Abreau S., Marcus G.M., Pletcher M.J., et al. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann. Intern. Med. 2020;173(9):767–770. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seçer İ., Ulaş S. An investigation of the effect of COVID-19 on OCD in youth in the context of emotional reactivity, experiential avoidance, depression and anxiety. Int. J. Ment. Health Addict. 2020;1–14. [DOI] [PMC free article] [PubMed]
- 16.Sonza A., Da Cunha de Sá-Caputo D., Bachur J.A., Rodrigues de Araújo M das G., Valadares Trippo K.V.T., Ribeiro Nogueira da Gama DRN da G., et al. Brazil before and during COVID-19 pandemic: impact on the practice and habits of physical exercise. Acta. Biomed. 2020;92(1) doi: 10.23750/abm.v92i1.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washif J.A., Mohd Kassim S.F.A., Lew P.C.F., Chong C.S.M., James C. Athlete's perceptions of a “quarantine” training camp during the COVID-19 lockdown. Front. Sports Act Living. 2020;2 doi: 10.3389/fspor.2020.622858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latorre-Román P.Á., Guzmán-Guzmán I.P., Delgado-Floody P., Herrador Sanchez J., Aragón-Vela J., García Pinillos F., et al. Protective role of physical activity patterns prior to COVID-19 confinement with the severity/duration of respiratory pathologies consistent with COVID-19 symptoms in Spanish populations. Res. Sports Med. 2021;1–12. [DOI] [PubMed]
- 19.Chen P., Mao L., Nassis G.P., Harmer P., Ainsworth B.E., Li F. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020;9(2):103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira-Júnior J.B., Freitas E.D.S., Chaves S.F.N. Exercise: a protective measure or an “open window” for COVID-19? A mini review. Front. Sports Act Living. 2020;2:61. doi: 10.3389/fspor.2020.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammar A., Brach M., Trabelsi K., Chtourou H., Boukhris O., Masmoudi L., et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):1583. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cindrich S.L., Lansing J.E., Brower C.S., McDowell C.P., Herring M.P., Meyer J.D. Associations between change in outside time pre-and post-COVID-19 public health restrictions and mental health: brief research report. Front. public health. 2021;9:8. doi: 10.3389/fpubh.2021.619129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga T.V., Bu F., Dissing A.S., Elsenburg L.K., Bustamante J.J.H., Matta J., et al. Loneliness, worries, anxiety, and precautionary behaviours in response to the COVID-19 pandemic: a longitudinal analysis of 200,000 Western and Northern Europeans. Lancet Reg Health Eur. 2021;2(100020) doi: 10.1016/j.lanepe.2020.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirkis J., John A., Shin S., DelPozo-Banos M., Arya V., Analuisa-Aguilar P., et al. Suicide trends in the early months of the COVID-19 pandemic: an interrupted time-series analysis of preliminary data from 21 countries. The Lancet Psychiatry. 2021. [DOI] [PMC free article] [PubMed]
- 25.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull F.C., Armstrong T.P., Dixon T., Ham S., Neiman A., Pratt M. Comparative Quantification of Health Risks Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. World Health Organization; Geneva: 2004. Physical inactivity. [Google Scholar]
- 27.Vancini R.L., Camargo-Neto L., Lira C.A.B., Andrade M.S., Viana R.B., Nikolaidis P.T., et al. Physical activity and sociodemographic profile of brazilian people during COVID-19 outbreak: an online and cross-sectional survey. Int. J. Environ. Res. Public Health. 2020;17(21):1–9. doi: 10.3390/ijerph17217964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giustino V., Parroco A.M., Gennaro A., Musumeci G., Palma A., Battaglia G. Physical activity levels and related energy expenditure during COVID-19 quarantine among the sicilian active population: a cross-sectional online survey study. Sustainability (Switzerland [Internet] 2020;12(11) Disponible en: http://dx.doi.org/10. [Google Scholar]
- 29.Ding D., Cheng M., Pozo Cruz B., Lin T., Sun S., Zhang L., et al. How COVID-19 lockdown and reopening affected daily steps: evidence based on 164,630 person-days of prospectively collected data from Shanghai, China. Int. J. Behav. Nutrition Phys. Activity. 2021;18(1):1186. doi: 10.1186/s12966-021-01106-x. 12966–021–01106–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourdas D.I., Zacharakis E.D. Impact of COVID-19 lockdown on physical activity in a sample of Greek adults. Sports. 2020;8(10):1–13. doi: 10.3390/sports8100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alomari M.A., Khabour O.F., Alzoubi K.H. Changes in physical activity and sedentary behavior amid confinement: the bksq-covid-19 project. Risk Manag. Healthc Policy. 2020;13:1757–1764. doi: 10.2147/RMHP.S268320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuentes-García J.P., Martínez Patiño M.J., Villafaina S., Clemente-Suárez V.J. The effect of COVID-19 confinement in behavioral, psychological, and training patterns of chess players. Front Psychol. 2020;11:1812. doi: 10.3389/fpsyg.2020.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemente-Suárez V.J., Fuentes-García J.P., de la Vega Marcos R., Martínez Patiño M.J. Modulators of the personal and professional threat perception of Olympic athletes in the actual COVID-19 crisis. Front Psychol. 2020;11:1985. doi: 10.3389/fpsyg.2020.01985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sassone B., Mandini S., Grazzi G., Mazzoni G., Myers J., Pasanisi G. Impact of COVID-19 pandemic on physical activity in patients with implantable cardioverter-defibrillators. J. Cardiopulm Rehabil. Prev. 2020;40(5):285–286. doi: 10.1097/HCR.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narici M., Vito G.D., Franchi M., Paoli A., Moro T., Marcolin G., et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. EJSS (Champaign) 2021;21(4):614–635. doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- 36.Khan M.A., Moverley Smith J.E. Covibesity,” a new pandemic. Obesity Med. 2020;19 doi: 10.1016/j.obmed.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luis D., Izaola O., Primo D., Gómez E., Torres B., López Gómez J.J. Effect of lockdown for covid-19 on self-reported body weight gain in a sample of obese patients. Nutricion Hospitalaria. 2020;37(6):1232–1237. doi: 10.20960/nh.03307. [DOI] [PubMed] [Google Scholar]
- 38.Mediouni M., Madiouni R., Kaczor-Urbanowicz K.E. COVID-19: how the quarantine could lead to the depreobesity. Obesity Med. 2020;19 doi: 10.1016/j.obmed.2020.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsenoli M., Moverley Smith J.E., Khan M.A. A community perspective of COVID-19 and obesity in children: causes and consequences. Obesity Med. 2021;22 doi: 10.1016/j.obmed.2021.100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz M.C., Devonport T.J., Chen-Wilson C.-.H.J., Nicholls W., Cagas J.Y., Fernandez-Montalvo J., et al. A cross-cultural exploratory study of health behaviors and wellbeing during COVID-19. Front Psychol. 2020;11 doi: 10.3389/fpsyg.2020.608216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng K., Cooper J., McHale F., Clifford J., Woods C. Barriers and facilitators to changes in adolescent physical activity during COVID-19. BMJ Open Sport and Exercise Med. 2020;6(1) doi: 10.1136/bmjsem-2020-000919. 1136–2020–000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musumeci G., Palma A., Battaglia G. Physical activity levels and related energy expenditure during COVID-19 quarantine among the sicilian active population: a cross-sectional online survey study. Sustainability Switzerland. 2020;12(11):3390. 12114356. [Google Scholar]
- 43.Silva L.R.B., Seguro C.S., Oliveira C.G.A., Santos P.O.S., Oliveira J.C.M., Souza Filho L.F.M., et al. Physical Inactivity Is Associated With Increased Levels of Anxiety, Depression, and Stress in Brazilians During the COVID-19 Pandemic: a Cross-Sectional Study. Front. Psychiatry. 2020;11:3389. doi: 10.3389/fpsyt.2020.565291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sassone B., Mandini S., Grazzi G., Mazzoni G., Myers J., Pasanisi G. Impact of COVID-19 Pandemic on Physical Activity in Patients with Implantable Cardioverter-Defibrillators. J. Cardiopulm. Rehabil. Prev. 2020;40(5):285–286. doi: 10.1097/HCR.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y.-.Y., Ma Y.-.T., Zhang J.-.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiuza-Luces C., Garatachea N., Berger N.A., Lucia A. Exercise is the real polypill. Physiology (Bethesda) 2013;28(5):330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 49.Nyberg S.T., Singh-Manoux A., Pentti J., Madsen I.E.H., Sabia S., Alfredsson L., et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern. Med. 2020;180(5):760–768. doi: 10.1001/jamainternmed.2020.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salgado-Aranda R., Pérez-Castellano N., Núñez-Gil I., Orozco A.J., Torres-Esquivel N., Flores-Soler J., et al. Influence of baseline physical activity as a modifying factor on COVID-19 mortality: a single-center, retrospective study. Infect. Dis. Ther. 2021;10(2):801–814. doi: 10.1007/s40121-021-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphreys H., Kilby L., Kudiersky N., Copeland R. Long COVID and the role of physical activity: a qualitative study. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-047632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao X., Song Ll-N, Yang J.-.K. ACE2 and energy metabolism: the connection between COVID-19 and chronic metabolic disorders. Clin. Sci. (Lond) 2021;135(3):535–554. doi: 10.1042/CS20200752. [DOI] [PubMed] [Google Scholar]
- 53.Kenyon C. The Forrest Gump approach to preventing severe COVID-19 – reverse the predisposing pro-inflammatory state with exercise. Microbes Infect. 2020;22(4–5):151–153. doi: 10.1016/j.micinf.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clemente-Suárez V.J., Navarro-Jiménez E., Jimenez M., Hormeño-Holgado A., Martinez-Gonzalez M.B., Benitez-Agudelo J.C., et al. Impact of COVID-19 pandemic in public mental health: an extensive narrative review. Sustainability. 2021;13(6):3221. [Google Scholar]
- 55.Clemente-Suárez V.J., Ramos-Campo D.J., Mielgo-Ayuso J., Dalamitros A.A., Nikolaidis P.A., Hormeño-Holgado A., et al. Nutrition in the actual COVID-19 pandemic. A narrative Rev. Nutrients. 2021;13(6):1924. doi: 10.3390/nu13061924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yousfi N., Bragazzi N.L., Briki W., Zmijewski P., Chamari K. The COVID-19 pandemic: how to maintain a healthy immune system during the lockdown–a multidisciplinary approach with special focus on athletes. Biol. sport. 2020;37(3):211. doi: 10.5114/biolsport.2020.95125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khoramipour K., Basereh A., Hekmatikar A.A., Castell L., Ruhee R.T., Suzuki K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2021;39(1):101–107. doi: 10.1080/02640414.2020.1807089. [DOI] [PubMed] [Google Scholar]
- 58.Silveira M.P., Silva Fagundes K.K., Bizuti M.R., Starck É., Rossi R.C., Silva D.T.D.R. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin. Experimental Med. 2020;1–14. [DOI] [PMC free article] [PubMed]
- 59.Valenzuela P.L., Simpson R.J., Castillo-García A., Lucia A. Physical activity: a coadjuvant treatment to COVID-19 vaccination? Brain Behav. Immun. 2021;94:1–3. doi: 10.1016/j.bbi.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sallis R., Young D.R., Tartof S.Y., Sallis J.F., Sall J., Li Q., et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br. J. Sports Med. 2021;bjsports-2021-104080. [DOI] [PubMed]
- 61.Jiménez-Pavón D., Carbonell-Baeza A., Lavie C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: special focus in older people. Prog. Cardiovasc. Dis. 2020;63(3):386–388. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Codella R., Chirico A., Lucidi F., Ferrulli A., La Torre A., Luzi L. The immune-modulatory effects of exercise should be favorably harnessed against COVID-19. J. Endocrinol. Invest. 2021;44(5):1119–1122. doi: 10.1007/s40618-020-01403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polero P., Rebollo-Seco C., Adsuar J.C., Pérez-Gómez J., Rojo-Ramos J., Manzano-Redondo F., et al. Physical Activity Recommendations during COVID-19: narrative Review. Int. J. Environ. Res. Public Health. 2020;18(1):65. doi: 10.3390/ijerph18010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alschuler L., Chiasson A.M., Horwitz R., Sternberg E., Crocker R., Weil A., et al. Integrative medicine considerations for convalescence from mild-to-moderate COVID-19 disease. Explore (NY) [Internet]. 2020; Disponible en: 10.1016/j.explore.2020.12.005. [DOI] [PMC free article] [PubMed]
- 65.Curci C., Pisano F., Bonacci E., Camozzi D.M., Ceravolo C., Bergonzi R., et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2020;56(5):633–641. doi: 10.23736/S1973-9087.20.06339-X. [DOI] [PubMed] [Google Scholar]
- 66.Silberman D.M., Wald M.R., Genaro A.M. Acute and chronic stress exert opposing effects on antibody responses associated with changes in stress hormone regulation of T-lymphocyte reactivity. J. Neuroimmunol. 2003;144(1–2):53–60. doi: 10.1016/j.jneuroim.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 67.Edwards K.M., Burns V.E., Allen L.M., McPhee J.S., Bosch J.A., Carroll D., et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 2007;21(2):209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- 68.Edwards K.M., Burns V.E., Reynolds T., Carroll D., Drayson M., Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav. Immun. 2006;20(2):159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Brawner C.A., Ehrman J.K., Bole S., Kerrigan D.J., Parikh S.S., Lewis B.K., et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to Coronavirus disease 2019. Mayo Clin. Proc. 2021;96(1):32–39. doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zbinden-Foncea H., Francaux M., Deldicque L., Hawley J.A. Does high cardiorespiratory fitness confer some protection against proinflammatory responses after infection by SARS-CoV-2? Obesity (Silver Spring) 2020;28(8):1378–1381. doi: 10.1002/oby.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y., Lu Y., Huang Y.-.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113(154378) doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erener S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020;39(101044) doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;10 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pedersen B.K., Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scandinavian J. Med. Sci. Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 75.Myers J., Kokkinos P., Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. doi: 10.3390/nu11071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross R., Blair S.N., Arena R., Church T.S., Després J.-.P., Franklin B.A., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American heart association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 77.Brawner C.A., Ehrman J.K., Bole S. Maximal exercise capacity is inversely related to hospitalization secondary to coronavirus disease 2019. Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee Dd-C, Artero E.G., Sui X., Blair S.N. Mortality trends in the general population: the importance of cardiorespiratory fitness. J. Psychopharmacol. 2010;24(4 Suppl):27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herridge M.S., Moss M., Hough C.L., Hopkins R.O., Rice T.W., Bienvenu O.J., et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 80.Herridge M.S., Tansey C.M., Matté A., Tomlinson G., Diaz-Granados N., Cooper A., et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 81.Ong K.-.C., Ng A.W.-.K., Lee L.S.-.U., Kaw G., Kwek S.-.K., Leow M.K.-.S., et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur. Respir. J. 2004;24(3):436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 82.Li T.S., Gomersall C.D., Joynt G.M., Chan D.P.S., Leung P., Hui D.S.C. Long-term outcome of acute respiratory distress syndromecaused by severe acute respiratory syndrome (SARS): an observational study. Crit. Care Resusc. 2006;8:302–308. [PubMed] [Google Scholar]
- 83.Rooney S., Webster A., Paul L. Systematic review of changes and recovery in physical function and fitness after Severe Acute Respiratory Syndrome-related Coronavirus infection: implications for COVID-19 rehabilitation. Phys. Ther. 2020;100(10):1717–1729. doi: 10.1093/ptj/pzaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lau H.M.-.C., Ng G.Y.-.F., Jones A.Y.-.M., Lee E.W.-.C., Siu E.H.-.K., Hui D.S.-.C. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust. J. Physiother. 2005;51(4):213–219. doi: 10.1016/S0004-9514(05)70002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao F., Zheng K.I., Wang X.-.B., Sun Q.-.F., Pan K.-.H., Wang T.-.Y., et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43(7):e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 87.Zhao X., Gang X., He G., Li Z., Lv Y., Han Q., et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 2020;11 doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yates T., Razieh C., Zaccardi F., Davies M.J., Khunti K. Obesity and risk of COVID-19: analysis of UK biobank. Prim Care Diabetes. 2020;14(5):566–567. doi: 10.1016/j.pcd.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen Q., Dominguez J., Nguyen L., Gullapalli N. Hypertension management: an update. Am. Health Drug Benefits. 2010;3(1):47–56. [PMC free article] [PubMed] [Google Scholar]
- 90.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decadelong structural studies of SARS. J. Virology. 2020. [DOI] [PMC free article] [PubMed]
- 92.Lan J. Structure of the SARS- CoV-2 spike receptor- binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 93.Weber M.A., Schiffrin E.L., White W.B., Mann S., Lindholm L.H., Kenerson J.G., et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension: a statement by the American society of hypertension and the international society of hypertension. J. Clin. Hypertens (Greenwich) 2014;16(1):14–26. doi: 10.1111/jch.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliver-Martínez P.A., Ramos-Campo D.J., Martínez-Aranda L.M., Martínez-Rodríguez A., Rubio-Arias J.Á. Chronic effects and optimal dosage of strength training on SBP and DBP: a systematic review with meta-analysis: a systematic review with meta-analysis. J. Hypertens. 2020;38(10):1909–1918. doi: 10.1097/HJH.0000000000002459. [DOI] [PubMed] [Google Scholar]
- 95.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017;18(3):563. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao S., Lau A., So H.-.C. Exploring diseases/traits and blood proteins causally related to 284 expression of ACE2, the putative receptor of 2019-nCov: a Mendelian Randomization analysis. Vol. 285 medRxi. 2003. p. 2020. [DOI] [PubMed]
- 98.Fernandez C., Rysä J., Almgren P., Nilsson J., Engström G., Orho-Melander M., et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J. Intern. Med. 2018;284(4):377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan W.-.J., Ni Z.-.Y., Hu Y., Liang W.-.H., Ou C.-.Q., He J.-.X., et al. Clinical characteristics of Coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res. Rev. 2020 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boule N.G., Haddad E., Kenny G.P., Wells G.A., Sigal R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. Scand J. Med. Sci. Sports. 2002;12(1):60–61. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 102.Yang Z., Scott C.A., Mao C., Tang J., Farmer A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44(4):487–499. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 103.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G., et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43(10):e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 105.Atmosudigdo I.S., Pranata R., Lim M.A., Henrina J., Yonas E., Vania R., et al. Dyslipidemia increases the risk of severe COVID-19: a systematic review, meta-analysis, and meta-regression. J. Clin. Exp. Hepatol. [Internet]. 2021; Disponible en: 10.1016/j.jceh.2021.01.007. [DOI] [PMC free article] [PubMed]
- 106.Rg D.G.R.S., Wa B. Metabolic Syndrome and COVID-19: An update On the Associated Comorbidities and Proposed Therapies. En. 2021. [DOI] [PMC free article] [PubMed]
- 107.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morys F., Dagher A. Poor metabolic health increases COVID-19-related mortality in the UK Biobank sample. Front. Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.652765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bansal R., Gubbi S., Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology [Internet] 2020;161(10) doi: 10.1210/endocr/bqaa112. Disponible en: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohammad S., Aziz R., Al Mahri S., Malik S.S., Haji E., Khan A.H., et al. Obesity and COVID-19: what makes obese host so vulnerable? Immun. Ageing. 2021;18(1):1. doi: 10.1186/s12979-020-00212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fedewa M.V., Hathaway E.D., Ward-Ritacco C.L. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017;51(8):670–676. doi: 10.1136/bjsports-2016-095999. [DOI] [PubMed] [Google Scholar]
- 112.Costa F.F., Rosário W.R., Ribeiro Farias A.C., de Souza R.G., Duarte Gondim R.S., Barroso W.A. Metabolic syndrome and COVID-19: an update on the associated comorbidities and proposed therapies. Diabetes Metab Syndr. 2020;14(5):809–814. doi: 10.1016/j.dsx.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsigos C., Hainer V., Basdevant A. Management of obesity in adults: european clinical practice guidelines. Obes. Facts. 2008;1(2):106–116. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramos-Campo D.J., Andreu Caravaca L., Martínez-Rodríguez A., Rubio-Arias J.Á. Effects of resistance circuit-based training on body composition, strength and cardiorespiratory fitness: a systematic review and meta-analysis. Biology (Basel) [Internet] 2021;10(5) doi: 10.3390/biology10050377. Disponible en: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Batacan Jr R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017;51(6):494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 116.Hertzog R.G., Bicheru N.S., Popescu D.M., Călborean O., Catrina A.M. Hypoxic preconditioning—A nonpharmacological approach in COVID-19 prevention. Int. J. Infect. Diseases. 2021;103:415–419. doi: 10.1016/j.ijid.2020.11.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Serebrovskaya T.V., Manukhina E.B., Smith M.L., Downey H.F., Mallet R.T. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp. Biol. Med. (Maywood) 2008;233(6):627–650. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- 118.Liesa M., Palacín M., Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009;89(3):799–845. [DOI] [PubMed]
- 119.Memme J.M., Erlich A.T., Phukan G., Hood D.A. Exercise and mitochondrial health. J. Physiol. 2021;599(3):803–817. [DOI] [PubMed]
- 120.Osellame L.D., Blacker T.S., Duchen M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26(6):711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Myhill S., Booth N.E., McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2009;2(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 122.Filler K., Lyon D., Bennett J., McCain N., Elswick R., Lukkahatai N., et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sivitz W.I., Yorek M.A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12(4):537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ballinger S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005;38(10):1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 125.Beal M.F. Bioenergetic approaches for neuroprotection in Parkinson's disease. Ann. Neurol. 2003;53(S3):S39–S47. doi: 10.1002/ana.10479. Suppl 3discussion S47-8. [DOI] [PubMed] [Google Scholar]
- 126.Stuart S., Griffiths L.R. A possible role for mitochondrial dysfunction in migraine. Mol. Genet Genomics. 2012;287(11–12):837–844. doi: 10.1007/s00438-012-0723-7. [DOI] [PubMed] [Google Scholar]
- 127.Boland M.L., Chourasia A.H., Macleod K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Demain L.A.M., Conway G.S., Newman W.G. Genetics of mitochondrial dysfunction and infertility: genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017;91(2):199–207. doi: 10.1111/cge.12896. [DOI] [PubMed] [Google Scholar]
- 129.Bratic A., Larsson N.-.G. The role of mitochondria in aging. J. Clin. Invest. 2013;123(3):951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burtscher J., Millet G.P., Burtscher M. Low cardiorespiratory and mitochondrial fitness as risk factors in viral infections: implications for COVID-19. Br. J. Sports Med. 2021;55(8):413–415. doi: 10.1136/bjsports-2020-103572. [DOI] [PubMed] [Google Scholar]
- 131.Sebastián D., Palacín M., Zorzano A. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol. Med. 2017;23(3):201–215. doi: 10.1016/j.molmed.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 132.Nieman D.C., Williams A.S., Shanely R.A., Jin F., McAnulty S.R., Triplett N.T., et al. Quercetin's influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010;42(2):338–345. doi: 10.1249/MSS.0b013e3181b18fa3. [DOI] [PubMed] [Google Scholar]
- 133.López-Lluch G., Irusta P.M., Navas P., de Cabo R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008;43(9):813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eluamai A., Brooks K. Effect of aerobic exercise on mitochondrial DNA and aging. J. Exerc. Sci. Fit. 2013;11(1):1–5. [Google Scholar]
- 135.Little J.P., Safdar A., Cermak N., Tarnopolsky M.A., Gibala M.J. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am. J. Physiol.Regulatory, Integrative and Comparative Physiol. 2010;298(4):912–917. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- 136.Wang L., Mascher H., Psilander N., Blomstrand E., Sahlin K. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J. Appl. Physiol. 2011;111(5):1335–1344. doi: 10.1152/japplphysiol.00086.2011. [DOI] [PubMed] [Google Scholar]
- 137.Psilander N. The effect of different exercise regimens on mitochondrial biogenesis and performance. Inst För Fysiologi Och Farmakologi/Dept of Physiology and Pharmacology. 2014.
- 139.Dutta D., Calvani R., Bernabei R., Leeuwenburgh C., Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities: mechanisms and therapeutic opportunities. Circ. Res. 2012;110(8):1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bozkaya G., Ozgu E., Karaca B. The association between estimated average glucose levels and fasting plasma glucose levels. Clinics (Sao Paulo) 2010;65(11):1077–1080. doi: 10.1590/S1807-59322010001100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Q., Chen H., Li J., Huang X., Lai L., Li S., et al. Fasting blood glucose predicts the occurrence of critical illness in COVID-19 patients: a multicenter retrospective cohort study. J. Infect. 2020;81(3):e20–e23. doi: 10.1016/j.jinf.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bailey S.M., Udoh U.S., Young M.E. Circadian regulation of metabolism. J. Endocrinol. 2014;222(2):R75–R96. doi: 10.1530/JOE-14-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Escames G., Guerra-Librero A., Shen Y., Florido J., Sayed R., Molina-Navarro M., et al. PO-090: oncostatic effect of melatonin in head and neck cancer: role of mitochondrial function. Radiother. Oncol. 2017;122:43–44. [Google Scholar]
- 144.Reiter R.J., Sharma R., Ma Q., Dominquez-Rodriguez A., Marik P.E., Abreu-Gonzalez P. Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med. Drug Discov. 2020;6(100044) doi: 10.1016/j.medidd.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mortensen S.A., Rosenfeldt F., Kumar A., Dolliner P., Filipiak K.J., Pella D., et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 146.Cordero M.D., Alcocer-Gómez E., de Miguel M., Culic O., Carrión A.M., Alvarez-Suarez J.M., et al. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal. 2013;19(12):1356–1361. doi: 10.1089/ars.2013.5260. [DOI] [PubMed] [Google Scholar]
- 147.Mancuso M., Orsucci D., Volpi L., Calsolaro V., Siciliano G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr. Drug Targets. 2010;11(1):111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- 148.Casagrande D., Waib P.H., Júnior A.A.J. Mechanisms of action and effects of the administration of Coenzyme Q10 on metabolic syndrome. J. Nutrition & Intermediary Metab. 2018;13:26–32. [Google Scholar]
- 149.Ames B.N. Prevention of mutation, cancer, and other age-associated diseases by optimizing micronutrient intake. J. Nucleic Acids. 2010;2010:1–11. doi: 10.4061/2010/725071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.George G.A., Heaton F.W. Changes in cellular composition during magnesium deficiency. Biochem. J. 1975;152(3):609–615. doi: 10.1042/bj1520609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kucharská J. En: Mitochondrial Medicine. Springer Netherlands; Dordrecht: 2008. Vitamins in Mitochondrial Function; pp. 367–384. [Google Scholar]
- 152.Depeint F., Bruce W.R., Shangari N., Mehta R., O'Brien P.J. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006;163(1–2):94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 153.Marriage B., Clandinin M.T., Glerum D.M. Nutritional cofactor treatment in mitochondrial disorders. J. Am. Diet Assoc. 2003;103(8):1029–1038. doi: 10.1016/s0002-8223(03)00476-0. [DOI] [PubMed] [Google Scholar]
- 154.Rosca M.G., Lemieux H., Hoppel C.L. Mitochondria in the elderly: is acetylcarnitine a rejuvenator? Adv. Drug Deliv. Rev. 2009;61(14):1332–1342. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodriguez M.C., MacDonald J.R., Mahoney D.J., Parise G., Beal M.F., Tarnopolsky M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35(2):235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 156.Tarnopolsky M.A. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv. Rev. 2008;60(13–14):1561–1567. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 157.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Güner R., Hasanoğlu I., Aktaş F. COVID-19: prevention and control measures in community. Turk J. Med. Sci. 2020;50(SI–1):571–577. doi: 10.3906/sag-2004-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pieh C., Budimir S., Probst T. The effect of age, gender, income, work, and physical activity on mental health during coronavirus disease (COVID-19) lockdown in Austria. J. Psychosom. Res. 2020;136(110186) doi: 10.1016/j.jpsychores.2020.110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duncan G.E., Avery A.R., Seto E., Tsang S. Perceived change in physical activity levels and mental health during COVID-19: findings among adult twin pairs. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jarvis C.I., Van Zandvoort K., Gimma A., Prem K., CMMID COVID-19 working group. Klepac P., et al. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18(1):124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kraemer M.U.G., Yang C.-.H., Gutierrez B., Wu C.-.H., Klein B., Pigott D.M., et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salman D., Vishnubala D., Le Feuvre P., Beaney T., Korgaonkar J., Majeed A., et al. Returning to physical activity after covid-19. BMJ. 2021;372:m4721. doi: 10.1136/bmj.m4721. [DOI] [PubMed] [Google Scholar]
- 164.Amini H., Habibi S., Islamoglu A.H., Isanejad E., Uz C., Daniyari H. COVID-19 pandemic-induced physical inactivity: the necessity of updating the Global Action Plan on Physical Activity 2018-2030. Environ. Health Prev. Med. 2021;26(1):32. doi: 10.1186/s12199-021-00955-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tomanek M., Lis A. Physical activity in the context of the COVID-19 pandemic: research profiling and mapping. Phys. Educ. Stud. 2021;25(3):136–148. [Google Scholar]
- 166.Dwyer M.J., Pasini M., De Dominicis S., Righi E. Physical activity: benefits and challenges during the COVID-19 pandemic. Scand J. Med. Sci. Sports. 2020;30(7):1291–1294. doi: 10.1111/sms.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Robinson E., Boyland E., Chisholm A., Harrold J., Maloney N.G., Marty L., et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite. 2021;156(104853) doi: 10.1016/j.appet.2020.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pfefferbaum B., North C.S. Mental health and the covid-19 pandemic. N. Engl. J. Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 169.Talevi D., Socci V., Carai M., Carnaghi G., Faleri S., Trebbi E., et al. Mental health outcomes of the CoViD-19 pandemic. Riv. Psichiatr. 2020;55(3):137–144. doi: 10.1708/3382.33569. [DOI] [PubMed] [Google Scholar]
- 170.Kola L., Kohrt B.A., Hanlon C., Naslund J.A., Sikander S., Balaji M., et al. COVID-19 mental health impact and responses in low-income and middle-income countries: reimagining global mental health. Lancet Psychiatry. 2021;8(6):535–550. doi: 10.1016/S2215-0366(21)00025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Torales J., O'Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry. 2020;66(4):317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 172.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I. COVID-19 BioB Outpatient Clinic Study group. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Clemente-Suárez V.J., Hormeño-Holgado A., Jiménez M., Benitez-Agudelo J.C., Navarro-Jiménez E., Perez-Palencia N., Tornero-Aguilera J.F. Dynamics of population immunity due to the herd effect in the COVID-19 pandemic. Vaccines (Basel) 2020;8(2):236. doi: 10.3390/vaccines8020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clemente-Suárez V.J., Navarro-Jiménez E., Moreno-Luna L., Saavedra-Serrano M.C., Jimenez M., Simón J.A., Tornero-Aguilera J.F. The Impact of the COVID-19 Pandemic on Social, Health, and Economy. Sustainability. 2021;13(11):6314. [Google Scholar]
- 176.Martín-Rodríguez A., Tornero-Aguilera J.F., López-Pérez P.J., Clemente-Suárez V.J. Gender differences in nutritional, odontological and psychological patterns of adolescent students during COVID-19 pandemic. Appl. Sci. 2021;11(18):8499. [Google Scholar]
- 177.Clemente-Suárez V.J., Martínez-González M.B., Benitez-Agudelo J.C., Navarro-Jiménez E., Beltran-Velasco A.I., Ruisoto P.…Tornero-Aguilera J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health. 2021;18(19):10041. doi: 10.3390/ijerph181910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rodriguez-Besteiro S., Tornero-Aguilera J.F., Fernández-Lucas J., Clemente-Suárez V.J. Gender differences in the covid-19 pandemic risk perception, psychology and behaviors of spanish university students. Int. J. Environ. Res. Public Health. 2021;18(8):3908. doi: 10.3390/ijerph18083908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Clemente-Suárez, V. J., Navarro-Jiménez, E., Ruisoto, P., Dalamitros, A. A., Beltran-Velasco, A. I., Hormeño-Holgado, A., Tornero-Aguilera, J. F. (2021). Performance of Fuzzy Multi-Criteria Decision Analysis of Emergency System in COVID-19 Pandemic. An Extensive Narrative Review. International Journal of Environmental Research and Public Health, 18(10), 5208. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable