Abstract
Introduction
Prospective and longitudinal data on pulmonary injury over one year after acute coronavirus disease 2019 (COVID-19) are sparse. We aim to determine reductions in pulmonary function and respiratory related quality of life up to 12 months after acute COVID-19.
Methods
Patients with acute COVID-19 were enrolled into an ongoing single-centre, prospective observational study and prospectively examined 6 weeks, 3, 6 and 12 months after onset of COVID-19 symptoms. Chest CT-scans, pulmonary function and symptoms assessed by St. Georges Respiratory Questionnaire were used to evaluate respiratory limitations. Patients were stratified according to severity of acute COVID-19.
Results
Median age of all patients was 57 years, 37.8% were female. Higher age, male sex and higher BMI were associated with acute-COVID-19 severity (p < 0.0001, 0.001 and 0.004 respectively). Also, pulmonary restriction and reduced carbon monoxide diffusion capacity was associated with disease severity. In patients with restriction and impaired diffusion capacity, FVC improved over 12 months from 61.32 to 71.82, TLC from 68.92 to 76.95, DLCO from 60.18 to 68.98 and KCO from 81.28 to 87.80 (percent predicted values; p = 0.002, 0.045, 0.0002 and 0.0005). The CT-score of lung involvement in the acute phase was associated with restriction and reduction in diffusion capacity in follow-up. Respiratory symptoms improved for patients in higher severity groups during follow-up, but not for patients with initially mild disease.
Conclusion
Severity of respiratory failure during COVID-19 correlates with the degree of pulmonary function impairment and respiratory quality of life in the year after acute infection.
Keywords: SARS-CoV-2, COVID-19, Post-acute COVID, Post-COVID, Long-COVID, Pneumonia, Pulmonary function, Pulmonary outcome, Pulmonary sequelae, Pulmonary restriction, Quality of life
Graphical abstract
1. Introduction
Severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) causes acute viral respiratory tract infections including pneumonia. After initial infection with SARS-CoV-2 in the upper respiratory tract, viral replication continues in lower airways and alveolar epithelial cells [1], leading to a hyper-inflammatory immune response causing alveolar damage and vascular leakage [2,3]. Chronic lung injury was observed in 25–63% patients three months post-acute COVID-19 [4,5]. Known pathomechanisms of chronic lung injury and fibrosis such as a TGF-beta dominated adaptive immune response [6], fibroblast activation [7], alveolar epithelial cell death and distortion of the basal lamina leading to alveolar collapse induration [8] have been observed in COVID-19. Moreover, viral pneumonia following severe acute respiratory syndrome corona virus (SARS), Middle East Respiratory Syndrome Coronavirus (MERS) and Influenza-A-Virus H1N1 (H1N1) infections have been associated with pulmonary restriction and reduced diffusion capacity and pathological chest CT findings [[9], [10], [11]]. First data of the early post-acute COVID-19 phase revealed that up to six months post infection, COVID-19 patients show a pattern of pulmonary restriction and abnormal carbon monoxide diffusion capacity in lung function testing [4,5,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. Similar results were seen for month 6 and 12 after symptom onset in a Chinese prospective cohort study [[22], [23], [24]]. Another group of patients that is not necessarily characterized by its initial disease severity, but by long-term symptoms are patients suffering from chronic COVID-19 that is frequently referred to as long COVID [25]. The pathophysiology, however, is still poorly understood and treatment options are very limited [26].
So far, prospective and longitudinal data on pulmonary injury over one year after acute coronavirus disease 2019 (COVID-19) are sparse, particularly no data from European patients are available. Further, disease severity is commonly classified according to WHO groups into mild/moderate and severe and critical. With this prospective study, we aim to provide data on pulmonary function, symptom burden, patient reported outcomes and radiological characteristics of SARS-CoV-2 infection in more detail. Moreover, this study aims to describe different patterns of pulmonary injury and their relation to subjective limitations in hospitalized and non-hospitalized patients with COVID-19.
2. Material and methods
2.1. Patients
This analysis included participants of the Pa-COVID-19 study at Charité Universitätsmedizin Berlin, an academic tertiary care medical centre. Pa-COVID-19 is a prospective observational study registered at the German clinical trials registry (DRKS 00021688) aiming to provide a platform for clinical characterisation of acute and post-acute COVID-19 [27]. The study was approved by Charité ethical committee (EA2/066/20). Patients with a positive SARS-CoV-2 PCR test are offered participation at first contact during hospitalization or follow-up anytime after disease onset and included after giving informed consent. Exclusion criteria are refusal to participate by the patient or legal representative, or a patient condition making additional blood sampling impossible. Medical data is collected prospectively in a purpose-built database. Due to the explorative design of the Pa-COVID-19 study and study initiation at the early beginning of the pandemic, no specific endpoints were defined a priori, and no sample size based on specific outcomes was calculated.
This analysis includes all patients of Pa-COVID-19 who were followed-up as outpatients between 01.05.2020 and 30.06.2021 and in whom lung function testing was performed. Patients were examined 6 weeks as well as 3, 6 and 12 months after onset of first symptoms of SARS-CoV-2 infection. During follow-up, study participants received in addition to lung function testing a structured patient interview with detailed symptom evaluation and standardized patient reported outcome assessment.
2.2. COVID-19 severity groups
Patients were stratified by acute COVID-19 severity in analogy to the WHO ordinal scale of clinical improvement [28], into i) non-hospitalized patients without supplemental oxygen therapy (NOO), ii) hospitalized patients without supplemental oxygen therapy (NOH), iii) with supplemental low-flow oxygen therapy (LFO), iv) high-flow oxygen therapy with or without temporary non-invasive ventilation (HFO), v) invasive mechanical ventilation (IMV) and vi) extracorporeal membrane oxygenation (ECMO). Patients were categorized according to the highest severity of respiratory failure as expressed by the level of respiratory support which occurred during acute COVID-19. Treatment allocation with regard to type of respiratory support was not limited by available medical resources during the study period, but was guided by current clinical guidelines for patients with need for ECMO.
2.3. Pulmonary function tests
Pulmonary function was examined using Ganshorn PowerCube Body+ and Diffusion+ (Schiller Group, Niederlauer, Germany) and performed according to the German, European and American recommendations for pulmonary function testing [[29], [30], [31]]. Reference values were calculated based on the Global Lung Function Initiative (GLI) reference equations (GLI-2012) and results are expressed as percent predicted value (ppv) [32]. Interpretation and grading of diffusing capacity values was adapted from the ERS/ATS official technical standards and the subsequent correspondence [33,34]. A DLCO <80% and ≥60% and <LLN was defined as mildly reduced, a DLCO ≥40% and <60% as moderately reduced and a DLCO <40% as severely reduced. Pulmonary restriction or obstruction was defined according to the “ATS/ERS Task Force: Standardisation of Lung Function Testing” as TLC <5th percentile of the lower limit of normal (LLN) and FEV1/FVC < LLN [35]. Complex restriction was defined according to Clay et al. as difference between ppv TLC and FVC >10% [36]. No further breakdown into severity grades was performed for categorical analysis.
2.4. Chest computed tomography (CT)
CT-scans were performed during acute COVID-19 on the basis of clinical guideline recommendations. If available, we analysed the first CT-scan performed in due course of COVID-19, and defined a maximum time span of 30 days post symptom onset for the CT-chest to be performed to account for the parenchymal chest pathologies obtained in the early phase of SARS-CoV-2 infection. Chest-CT scans were reviewed by two senior thoracic radiologists. All images were reviewed blinded to the patient's clinical characteristics and disease severity. Pulmonary involvement during the acute phase was assessed using a visual score ranging from 0 (no involvement) to 5 (>75% involvement) for each lung lobe as described in more detail by Pan et al. [37].
2.5. Symptom assessment and health related quality of life
A standardized list of 43 symptoms was evaluated at each study visit at baseline and during follow-up in a patient interview (Table S3). To capture overall impact on health, daily life and wellbeing in patients, the St. George's Respiratory Questionnaire (SGRQ) was measured [38]. A total score of 25 or higher, as suggested by the Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, was used as threshold for limitations in health and wellbeing.
2.6. Data analysis
Descriptive statistics were used to calculate median, inter-quartile range (IQR), mean and standard deviations (SD). Difference in continuous variables between three or more groups were analysed by one-way ANOVA or Kruskal-Wallis test. Fisher's exact test (for sample size <5 per group) or Chi-square test were used for analysis of categorical variables. The correlation between lung function and patient reported outcomes from SGRQ was calculated using Pearson correlation coefficients with a two-sided 95% confidence interval. Linear regression analysis was performed for pulmonary function and initial radiological chest CT-score. Logistic regression was performed to assess association of clinical variables, radiological findings and patient reported outcomes with pulmonary restriction and reduced DLCO in post-acute COVID-19. For univariate and multivariate analysis of risk factors for pulmonary restriction and diffusion capacity, patient characteristics and comorbidities recorded at the study inclusion visit were used and for pulmonary function and SGRQ, the lowest values observed during follow-up was used. Variables were adjusted for confounders as determined by clinical evidence (age, BMI) [39] or due to a strong relationship (p < 0.1) in univariate testing (sex, disease severity). Following the design of the underlying Pa-COVID study, this analysis is explorative in nature; we did not adjust for multiple testing and resulting p-values should be interpreted descriptively. The level of significance is marked with asterisks; * for p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. IBM SPSS (IBM SPSS Statistics 27.0), JMP (version 14.2.0) and GraphPad PRISM (Version 9.0.0) were used for statistical analysis and graphical processing.
3. Results
3.1. Baseline characteristics
180 patients with at least one complete pulmonary function dataset at 6 weeks, 3-, 6-, and 12 months follow-up were included in this analysis. Patients were included either at the time of hospital admission for acute SARS-CoV-2 infection (138; 76.7%) or during follow-up (42; 23.3%). A smaller number of patients presented at 6 week follow-up as many patients were either still in inpatient treatment or at rehabilitation at this point of time. At the time of analysis, 73/180 patients (40.5%) participated at follow-up visits at week 6, 118/180 (65.5%) at month 3, 139/180 (77.2%) at month 6 and 72/180 (40.0%) at month 12 of follow-up. 13/180 patients (7.2%) were lost to follow-up.
138/180 (76.6%) patients were initially hospitalized. 42/180 (23.3%) were never hospitalized (NOO), 29/180 (16.1%) were treated on a normal ward without need for oxygen (NOH), 43/180 (23.9%) received low-flow oxygen treatment via nasal cannula (LFO), 24/180 (13.3%) received high-flow oxygen treatment (HFO) including 3 patients with temporary non-invasive ventilation, 26/180 (14.4%) patients required invasive mechanical ventilation (IMV) and 16/180 (8.8%) patients were treated with ECMO (determined by the highest level of respiratory support, Table 1 ).
Table 1.
Patient characteristics stratified by level of respiratory support during acute phase of COVID-19. P-values of less than 0.05 were obtained for age, BMI, chronic heart disease, diabetes, pulmonary restriction and DLCO reduction (bold). P-values are based on Chi2, Fischer's exact test or Kruskal-Wallis-test where applicable. Abbreviations: NOO – no oxygen, outpatient; NOH – no oxygen hospitalized; LFO – low-flow oxygen supply; HFO – high-flow oxygen (including termporary non-invasive ventilation); IMV – invasive mechanical ventilation; ECMO – extracorporeal membrane oxygenation; CCI – Charlson Comorbidity Index. *missing values: Smoking history n = 3; Immunosuppression: n = 1.
| All | Level of respiratory support | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 180) | NOO (n = 42) | NOH (n = 29) | LFO (n = 43) | HFO (n = 24) | IMV (n = 26) | ECMO (n = 16) | p | |
| Age (median (IQR)) | 56.50 (43.25–65.75) | 44.00 (35.00–60.00) | 62.00 (40.00–70.00) | 56.00 (48.00–65.00) | 60.00 (52.00–65.50) | 60.50 (52.00–71.00) | 56.00 (49.00–63.50) | <0.0001 |
| Sex female (n/%) | 68 (37.78%) | 26 (61.90%) | 15 (51.72%) | 10 (23.26%) | 5 (20.83%) | 7 (26.92%) | 5 (31.25%) | 0.001 |
| Sex male (n/%) | 112 (62.22%) | 16 (38.10%) | 14 (48.28%) | 33 (76.74%) | 19 (79.17%) | 19 (65.52%) | 11 (68.75%) | 0.001 |
| BMI (median (IQR)) | 26.72 (23.88–31.30) | 24.02 (22.45–26.47) | 25.1 (23.46–27.34) | 27.76 (24.33–32.72) | 29.52 (25.95–33.36) | 29.39 (26.12–32.14) | 29.35 (27.56–35.51) | 0.004 |
| Smoking | ||||||||
| Smoking history* (n/%) | 62 (34.44%) | 12 (29.27%) | 8 (27.59%) | 16 (37.21%) | 8 (33.33%) | 14 (56.00%) | 4 (26.67%) | 0.246 |
| Comorbidities | ||||||||
| CCI 0 (n/%) | 51 (28.33%) | 21 (50.00%) | 8 (27.59%) | 10 (23.26%) | 5 (20.83%) | 3 (11.54%) | 4 (25.00%) | 0.016 |
| CCI 1–2 (n/%) | 65 (36.11%) | 13 (30.95%) | 7 (24.14%) | 20 (46.51%) | 9 (37.50%) | 10 (38.46%) | 6 (37.50%) | 0.502 |
| CCI 3–4 (n/%) | 46 (25.56%) | 8 (19.05%) | 14 (48.28%) | 8 (18.60%) | 4 (16.67%) | 8 (30.77%) | 4 (25.00%) | 0.063 |
| CCI >5 (n/%) | 18 (10.00%) | 0 | 0 | 5 (11.63%) | 6 (25.00%) | 5 (19.23%) | 2 (12.50%) | 0.001 |
| Chronic lung disease (n/%) | 26 (14.44%) | 6 (14.29%) | 4 (13.79%) | 2 (4.65%) | 5 (20.83%) | 6 (23.08%) | 3 (18.75%) | 0.224 |
| Asthma (n/%) | 13 (7.22%) | 5 (11.90%) | 1 (3.45%) | 1 (2.33%) | 2 (8.33%) | 4 (15.38%) | 0 | 0.205 |
| COPD (n/%) | 11 (6.11%) | 0 | 2 (6.90%) | 2 (4.65%) | 2 (8.33%) | 3 (11.54%) | 2 (12.50%) | 0.166 |
| Chronic heart disease (n/%) | 78 (43.33%) | 9 (21.43%) | 10 (34.48%) | 19 (44.19%) | 14 (58.33%) | 15 (57.69%) | 11 (68.75%) | 0.003 |
| Chronic kidney disease (n/%) | 21 (11.67%) | 2 (4.76%) | 6 (20.69%) | 6 (13.95%) | 2 (8.33%) | 3 (11.54%) | 2 (12.50%) | 0.429 |
| Diabetes (n/%) | 30 (16.67%) | 4 (9.52%) | 1 (3.45%) | 6 (13.95%) | 7 (29.17%) | 5 (19.23%) | 7 (43.75%) | 0.006 |
| Chronic liver disease (n/%) | 9 (5.00%) | 1 (2.38%) | 2 (6.90%) | 2 (4.65%) | 2 (8.33%) | 2 (7.69%) | 0 | 0.777 |
| Chronic immunological disease (n/%) | 4 (2.22%) | 2 (4.76%) | 4 (13.79%) | 2 (4.65%) | 2 (8.33%) | 0 | 3 (18.75%) | 0.139 |
| Chronic neurological disease (n/%) | 23 (12.22%) | 4 (9.52%) | 3 (10.34%) | 8 (18.60%) | 5 (20.83%) | 1 (3.85%) | 2 (12.50%) | 0.405 |
| Psychiatric disease (n/%) | 12 (6.67%) | 3 (7.14%) | 1 (3.45%) | 3 (6.98%) | 2 (8.33%) | 0 | 3 (18.75) | 0.290 |
| Active cancer (n/%) | 5 (2.78%) | 2 (4.76%) | 1 (3.45%) | 0 | 0 | 0 | 0 | 0.756 |
| Chronic haematological disease (n/%) | 4 (2.22%) | 1 (2.38%) | 2 (7.14%) | 3 (6.98%) | 4 (16.67%) | 0 | 1 (6.25%) | 0.188 |
| Immunosuppression* (3 M pre-COVID) (n/%) | 11 (6.11%) | 1 (2.38%) | 2 (7.14%) | 3 (6.98%) | 4 (16.67%) | 0 | 1 (6.25%) | 0.188 |
| Organ transplanted (n/%) | 5 (2.78%) | 1 (2.38%) | 2 (6.90%) | 1 (2.33%) | 1 (4.17%) | 0 | 0 | 0.779 |
| Pulmonary function | ||||||||
| Simple Restriction (n/%) | 59 (32.8%) | 5 (11.90%) | 5 (17.24%) | 13 (30.95%) | 11 (45.83%) | 16 (61.54%) | 9 (56.25%) | <0.0001 |
| Complex Restriction (n/%) | 92 (51.10%) | 25 (59.52%) | 19 (65.52%) | 19 (45.24%) | 12 (50.00%) | 10 (38.46%) | 7 (43.75%) | 0.337 |
| Obstruction (n/%) | 8 (4.40%) | 0 | 1 (3.45%) | 2 (4.76%) | 2 (8.33%) | 3 (11.53%) | 0 | 0.185 |
| DLCOreduced (n/%) | 109 (60.60%) | 18 (42.86%) | 15 (51.72%) | 26 (61.90%) | 26 (61.90%) | 17 (65.38%) | 19 (76.00%) | 0.010 |
Median age of all patients was 56.5 years (IQR 43.25–65.75). Median age of patients increased continuously with the level of respiratory support from 44 years (35–60) in the NOO group to 61 years (52–71) in the MV group, median age in the ECMO group was 56 years (49–64) (Table 1). Median (IQR) body-mass index (BMI) of study participants increased with level of respiratory support. Overall, 68/180 (37.8%) of all study participants were female, and the proportion of female patients was reduced with increasing level of care. Of all patients, 62/180 (34.4%) were former or current smokers. COVID-19 severity correlated particularly with chronic heart disease and diabetes (Table 1).
3.2. Body plethysmography, carbon monoxide diffusion capacity and respiratory muscle strength
55/180 (32%) study participants showed pulmonary restriction and 104/180 (61%) showed reduced carbon monoxide diffusion capacity (DLCO) (lowest value at any point of time during follow-up). Pulmonary restriction and impairment of DLCO was associated with increasing severity of lung failure expressed as the level of respiratory support during the acute phase of SARS-CoV-2 infection (p < 0.0001 and 0.01 respectively; Table 1). In contrast, complex restriction, as defined by Clay et al. [36], was not associated with severity of respiratory failure during acute SARS-CoV-2 infection, but was slightly more common among patients with mild disease.
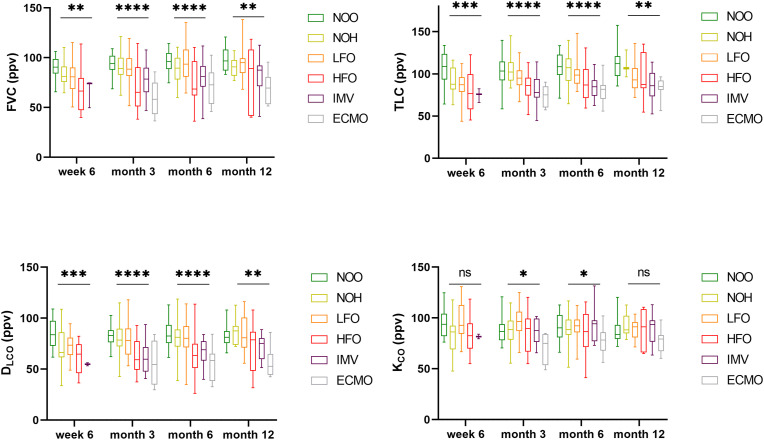
Pronounced differences in restrictive ventilation patterns were seen between groups of different disease severity during acute COVID-19. (Fig. 1 , Table s1). Median (IQR) of TLC and FVC was lower in patients with higher level of respiratory support during acute COVID-19. This difference persisted until 12-months after acute COVID-19 (Table s1).
Fig. 1.
Pulmonary restriction increased with disease severity. Bodyplethysmography 6 weeks, 3, 6 and 12 months post SARS-CoV-2 infection showed marked differences for FVC and TLV for all time points. Diffusion capacity is reduced in the early reconvalescent phase post COVID-19. DLCO strongly correlates with disease severity in the follow-up phase for all time points, whereas this trend is less remarkably seen for KCO after acute COVID-19. (Abbreviations: ppv – percent predicted value; NOO – no oxygen outpatient; NOH – no oxygen hospitalized, LFO – low-flow oxygen; HFO – high-flow oxygen; IMV – invasive mechanical ventilation; ECMO – extracorporeal membrane oxygenation; ns = P ≥ 0.05; * = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** < P ≤ 0.0001).
Likewise, impaired DLCO was associated with disease severity. A strong association was seen in patients stratified by level of respiratory support as a proxy of acute COVID-19 severity and DLCO (Fig. 1) for all time points during follow-up. With regard to KCO (DLCO/VA, Krogh-Index), that represents diffusion capacity per unit of ventilated alveolar volume, differences between severity groups were less pronounced (Fig. 1).
There was no association between pulmonary obstruction and disease severity after acute COVID-19 (Table 1). Reduced FEV1 was attributable to concurrent reduced FVC (Figure s1a). There were individual cases with reduced airway occlusion pressure (P0.1) and inspiratory muscle strength (Pimax), no association with regard to disease severity was observed (Figure s1b).
3.2.1. Pulmonary function during follow-up
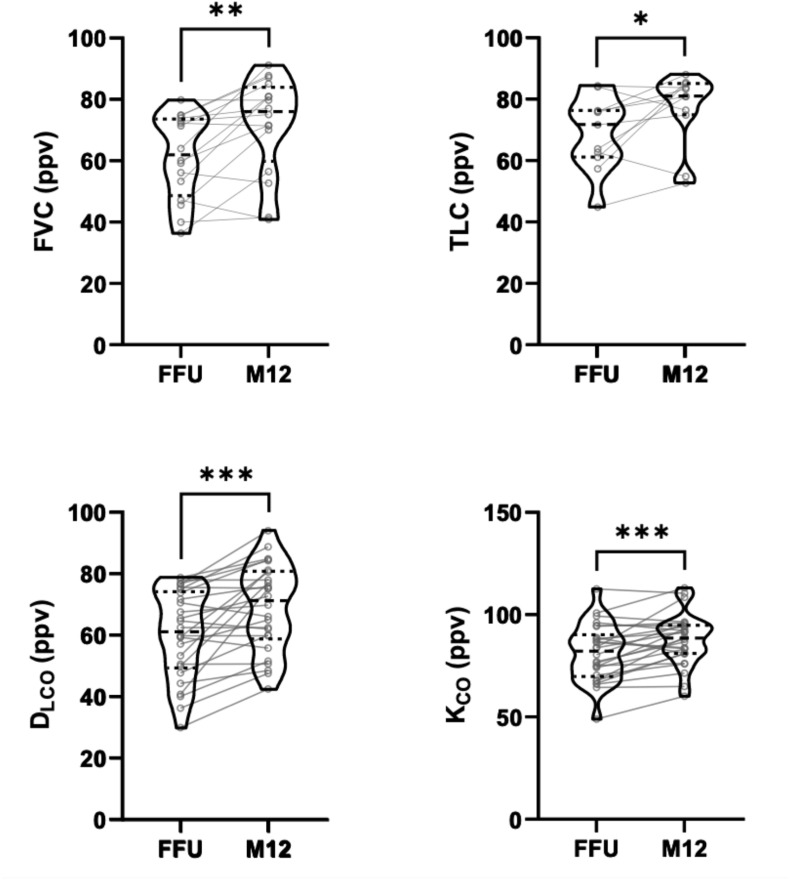
Patients with pathological pulmonary function in the early post-acute phase (defined as TLC/FVC/DLCO < Lower Limit of Normal (LLN) at first follow-up) showed improvement up to month 12 for pulmonary restriction or reduced DLCO (Fig. 2 ). When all patients were analysed over time, median TLC, FVC, DLCO and KCO increased up to month 6, with no further improvement seen between month 6 and 12 (Figure s2, Table s1).
Fig. 2.
Pulmonary restriction and impaired diffusion capacity improves over time. FVC, TLC, DLCO and KCO during follow up showed improvements in patients with initially reduced pulmonary function test results. Changes in relevant pulmonary function parameters are shown between first follow-up and month 12 follow up for every single individual, represented by a connecting line. (Abbreviations: FFU – first follow-up, M12 – month 12 follow-up).
3.3. Chest CT
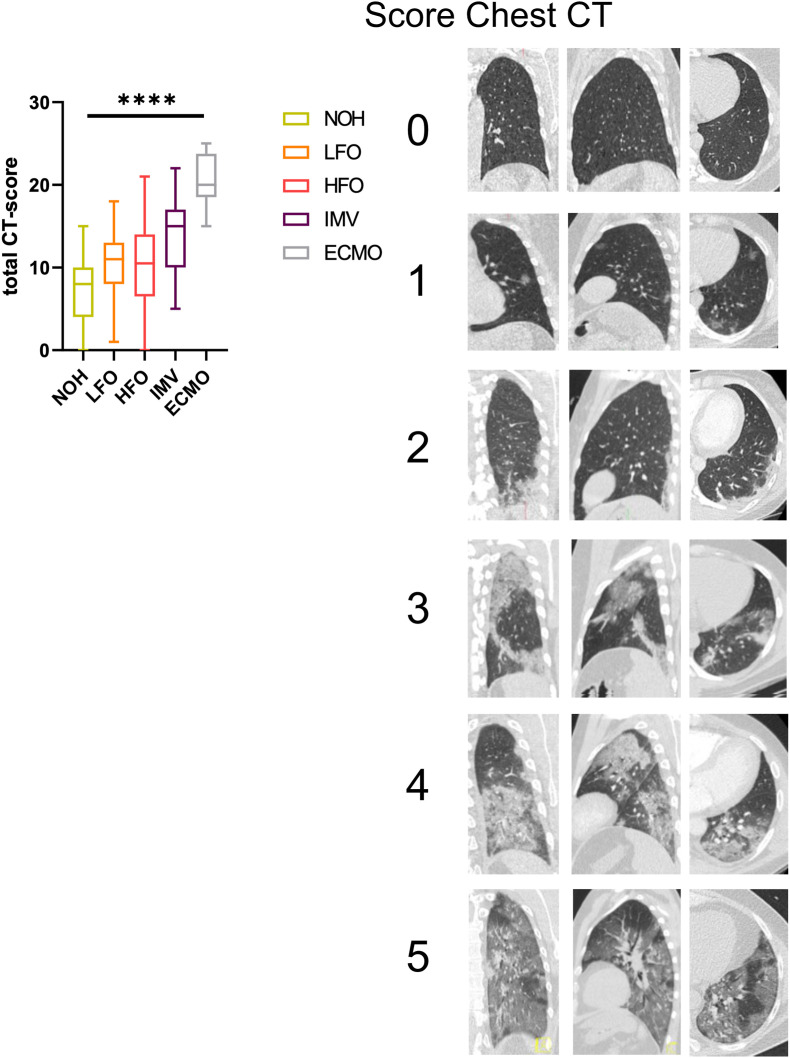
Chest-CT was performed in 88 (51.2%) patients during acute-COVID-19 infection. Median time of first chest CT was 9 days (IQR 6–14) post symptom onset. The CT score assessing lung involvement as suggested by Pan et al. increased with acute COVID-19 severity (p < 0.0001) (Fig. 3 ). Impaired pulmonary function after acute COVID-19 (TLC, FVC, DLCO) correlated well with initial pulmonary involvement during acute phase (p < 0.0001; 0.005; 0.006 respectively) (Fig. 4 ). Linear regression analysis showed a 15 %-points decrease in TLC and 10 %-points decrease in FVC and DLCO (ppv) for every 10 point increase in CT-chest score during acute SARS-CoV-2 infection.
Fig. 3.
a) CT-score as suggested by Pan et al. at time of hospital admission (median 9 days post symptom onset) showed a increase in pulmonary involvement with higher disease severity determined be level of respiratory support. b) Representative CT-chest scans assessed using the 6-point scale of Pan et al. This figure shows left lower lobe involvement of 0% (score 0), <5% (score 1), 5–25% (score 2), 26–50% (score 3), 51–75% (score 4), and >75% (score 5) on axial, coronal, and sagittal CT sections.
Fig. 4.
The proportion of pulmonary involvement during acute phase negatively correlates with first pulmonary function test post-acute COVID-19 for TLC, FVC and DLCO. Linear regression analysis reveals for every 10 points increase in CT-score an estimated decrease of 15 %-points TLC and 10 %-points FVC and DLCO post-acute COVID-19.
3.4. Symptom assessment and health related quality of life
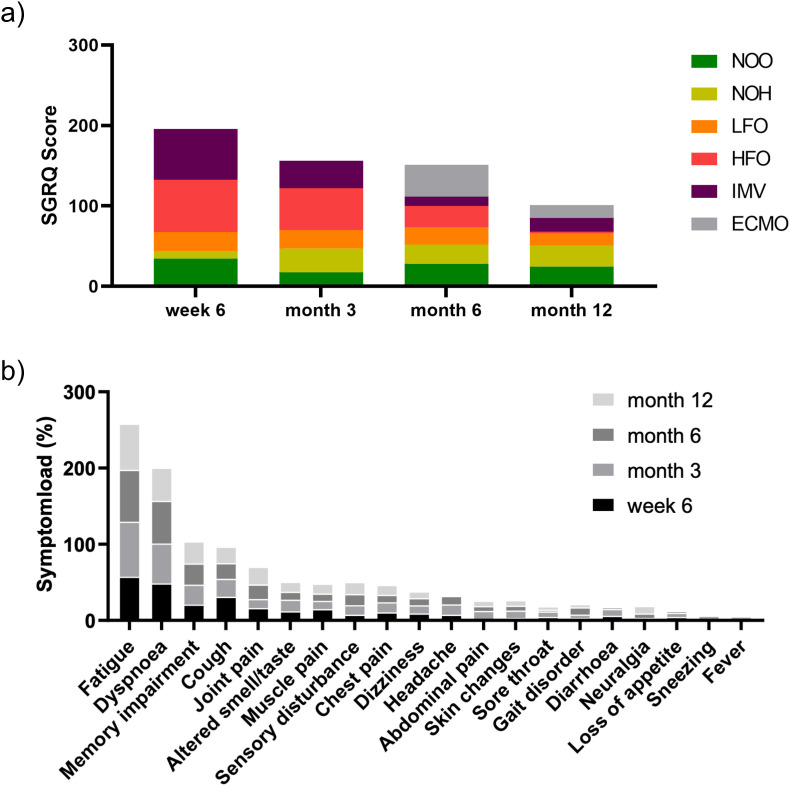
The five most common reported symptoms during follow up were fatigue, dyspnea, cough, cognitive impairment and joint pain for all time-points. Symptom load was still high at month 12, with 60.87% of all patients reporting fatigue, 43.48% reporting shortness of breath and 23.19% claiming persistent cognitive impairment (Fig. 5 b).
Fig. 5.
Symptom load and patient reported health outcome 6 weeks, 3, 6, and 12 months post-acute COVID: a) Cumulative median SGRQ-Score develops divergently over 12 months for different disease severity groups. Median total SGRQ is higher after HFO, IMV and ECMO treatment initially and decreases until month 12, whereas for LFO, NOH and NOO it remains constant over time of follow-up over 12 months. b) Cumulative symptom load for most frequently reported symptoms (as %) during follow up. Fatigue and respiratory symptoms score highest during follow up over 12 months. The proportion of patients reporting fatigue, pulmonary- and neurocognitive sequelae in our cohort remains high 12 months post-acute COVID-19.
Standardized assessment of respiratory symptoms by SGRQ showed an improvement over 12 months post symptom onset in patients with higher disease severity in the acute phase (ECMO, IMV, HFO), whereas SGRQ scores in patients in lower severity categories (LFO, NOH and NOO) remained almost constant during 12 months of follow-up. (Fig. 5a).
In general, total SGRQ score was strongly associated with FVC (p < 0.0001), DLCO (p < 0.0001) and KCO (p < 0.0001), but less pronounced for TLC (p = 0.091) (Table 2 , Figure s5). The contribution of SGRQ sub-scores for symptoms, activity and impact are shown in Table 2.
Table 2.
Patient reported outcome post-acute COVID-19 correlates with pulmonary function outcome. SGRQ results of all time-points were matched with respective pulmonary function tests. Total and SGRQ sub-scores negatively correlate with DLCO, KCO and FVC.
| SGRQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | Symptoms | Activity | Impact | |||||
| PFT | R2 | p | R2 | p | R2 | p | R2 | p |
| DLCO | 0.22 | <0,0001 | 0.14 | <0.0001 | 0.24 | <0.0001 | 0.14 | <0.0001 |
| KCO | 0.12 | <0,0001 | 0.08 | 0.0002 | 0.11 | <0.0001 | 0.12 | <0.0001 |
| FVC | 0.17 | <0,0001 | 0.06 | 0.0011 | 0.13 | <0.0001 | 0.12 | <0.0001 |
| TLC | 0.02 | 0.0911 | 0.01 | 0.3694 | 0.02 | 0.0519 | 0.01 | 0.2130 |
3.5. Risk factors for pulmonary restriction and reduced diffusion capacity
Univariate logistic regression showed an association of pulmonary restriction and reduced DLCO with initial disease severity, sex, SGRQ outcome (score>25), Charlson Comorbidity Index and cardiovascular disease (Table 3 ). The odds of restriction and reduced DLCO increased with acute COVID-19 disease severity (Table 3). When adjusted for age, sex and BMI, the odds of adverse pulmonary outcome was still remarkably higher in patients with HFO, IMV and ECMO compared to NOO. For reduced DLCO, logistic regression showed no association with initial pulmonary involvement as determined by CT-score (p = 0.11). All other effect sizes were also adjusted for age, sex, BMI and disease severity (Table 3).
Table 3.
Association of demographic characteristics, clinical indicators and comorbidities with pulmonary restriction and impaired DLCO post-acute COVID-19. Univariate analysis revealed male sex, disease severity, SGRQ score >25, Charlson Comorbidity Index and cardiovascular disease to be associated with pulmonary restriction and reduced DLCO. A relationship between CT-chest score was only seen for patients developing restriction. In multivariable analysis, adjustment for age, BMI, sex was performed for disease severity and age, BMI, sex and disease severity for all other variables. SGRQ outcome over the threshold of 25 showed to be associated with both pulmonary restriction and impaired DLCO. Patient characteristics and comorbidities were collected at study inclusion. Worst SGRQ outcome independent of follow-up was used for univariate and multivariate analysis.
| Pulmonary restriction |
Impaired DLCO |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | aOR (95% CI) | p | OR (95% CI) | p | aOR (95% CI) | p | |
| Age | 1.02 (0.99–1.04) | 0.081 | 1.01 (0.99–1.03) | 0.213 | ||||
| BMI>30 | 1.55 (0.79–3.04) | 0.201 | 0.73 (0.38–1.49) | 0.337 | ||||
| Sex (male) | 4.52 (2.11–9.68) | <0.0001 | 2.14 (1.15–3.96) | 0.016 | ||||
| Disease severity NOO | Reference group | Reference Group | ||||||
| NOH | 1.15 (0.39–5.75) | 0.554 | 1.36 (0.34–5.56) | 0.662 | 1.43 (0.55–3.70) | 0.462 | 1.63 (0.60–4.40) | 0.336 |
| LFO | 3.23 (1.03–10.11) | 0.044 | 2.26 (0.66–7.71) | 0.194 | 2.17 (0.91–5.19) | 0.082 | 2.45 (0.93–6.47) | 0.071 |
| HFO | 6.09 (1.77–20.90) | 0.004 | 4.23 (1.09–16.40) | 0.037 | 3.24 (1.11–9.46) | 0.032 | 4.23 (1.25–14.32) | 0.020 |
| IMV | 12.80 (3.70–44.31) | <0.0001 | 10.46 (2.68–40.83) | 0.001 | 4.22 (1.40–12.72) | 0.010 | 5.35 (1.56–18.40) | 0.008 |
| ECMO | 9.26 (2.38–36.08) | 0.001 | 7.77 (1.81–33.46) | 0.006 | 9.33 (1.88–46.35) | 0.006 | 12.10 (2.24–65.33) | 0.002 |
| CT-chest (scale) | 1.80 (1.19–2.74) | 0.006 | 1.42 (0.85–2.36) | 0.176 | 1.42 (0.92–2.19) | 0.111 | 0.91 (0.50–1.64) | 0.743 |
| SGRQ>25 | 2.63 (1.22–5.67) | 0.021 | 2.80 (1.14–6.91) | 0.025 | 4.91 (2.28–10.58) | <0.0001 | 5.99 (2.47–14.53) | <0.0001 |
| CCI | 1.48 (1.06–2.07) | 0.023 | 1.25 (0.71–2.18) | 0.437 | 1.54 (1.10–2.15) | 0.013 | 2.07 (1.11–3.85) | 0.021 |
| Cardiovascular dis.. | 2.06 (1.09–3.88) | 0.026 | 1.21 (0.55–2.67) | 0.644 | 1.51 (0.82–2.79) | 0.190 | 1.14 (0.53–2.46) | 0.736 |
| Pulmonary dis.. | 1.40 (0.58–3.43) | 0.447 | 1.25 (0.45–3.46) | 0.663 | 0.70 (0.20–1.62) | 0.700 | 0.62 (0.24–1.58) | 0.312 |
| Renal dis.. | 1.75 (0.68–4.50) | 0.244 | 1.38 (0.47–4.02) | 0.561 | 2.03 (0.71–5.90) | 0.187 | 1.85 (0.59–5.77) | 0.293 |
| Diabetes | 1.80 (0.85–3.78) | 0.121 | 0.90 (0.39–2.07) | 0.798 | 1.78 (0.78–4.07) | 0.172 | 1.29 (0.53–3.17) | 0.578 |
| Liver dis.. | 0.83 (0.42–1.64) | 0.584 | 0.74 (0.68–1.95) | 0.536 | 1.17 (0.68–2.01) | 0.578 | 1.01 (0.80–1.28) | 0.907 |
| Organ transplant | 0.06 (0.07–6.49) | 0.722 | 0.67 (0.06–7.68) | 0.745 | 1.93 (0.20–18.88) | 0.574 | 1.90 (0.19–19.27) | 0.588 |
| Autoimmune dis.. | 0.89 (0.53–1.48) | 0.639 | 0.86 (0.44–1.71) | 0.676 | 1.40 (0.55–3.57) | 0.485 | 1.29 (0.57–2.80) | 0.574 |
| Immunosuppression | 3.20 (.086–11.81) | 0.081 | 2.59 (0.64–10.48) | 0.182 | 1.49 (0.37–5.96) | 0.575 | 1.05 (0.25–4.40) | 0.945 |
| Smoking | 1.63 (0.85–3.15) | 0.142 | 1.31 (0.62–2.77) | 0.480 | 0.99 (0.52–1.89) | 0.981 | 0.74 (0.36–1.53) | 0.981 |
4. Discussion
In this study of COVID-19 survivors, we longitudinally analysed pulmonary function, respiratory symptoms and health related quality of life and studied CT chest morphology at acute phase of 180 patients during 12 months after SARS-CoV-2 infection. We identified demographic characteristics, clinical indicators and comorbidities that increase the risk and severity of pulmonary injury. The detailed data on pulmonary function presented gives first insight into different patterns of pulmonary impairment according to clinical severity in the acute phase and its sequelae up to 12 months post SARS-CoV-2 infection.
Reduced FVC, TLC and DLCO were associated with severe and critical COVID-19 in the literature, representing patients with LFO, HFO, IMV and ECMO in our cohort [22,23,39]. This study demonstrates that the degree of pulmonary functional impairment correlates with clinical severity during acute COVID-19 and that these differences in pulmonary function were still apparent after 12 months of follow-up.
Pulmonary restriction was associated with the degree of lung parenchymal involvement seen on CT scans during acute COVID-19, reflecting inflammation and fibrotic transformation following SARS-CoV-2 infection. Increasing evidence suggests a profibrotic phenotype following SARS-CoV-2 infection [[6], [7], [8],40], in line with other viral causes of pneumonia such as SARS, MERS and influenza infections [[9], [10], [11]]. Post mortem analysis of lung tissue in lethal COVID-19 were reported to show ultrastructural alteration including alveolar collapse and fibrosis [8,40]. Also, similarities in gene expression between idiopathic pulmonary fibrosis and COVID-19 in explanted lungs of patients undergoing lung transplantations or post mortem analysis were found using single-cell RNA sequencing, including Keratin-17 expressing epithelial cells, profibrotic macrophages and myofibroblasts [40,41]. Thus, analysis of CT scans during the acute phase may have prognostic relevance for patients.
It could be argued that pulmonary restriction after acute COVID-19 is caused by ventilator-induced lung injury (VILI), a common observation in ARDS patients [42,43] including subsequent pulmonary restriction and reduced DLCO [44,45]. In this study however, there was no obvious difference in FVC, TLC and DLCO at all follow-up visits between patients who needed mechanical ventilation and those who received high-flow oxygen therapy. Although more data is needed to confirm this hypothesis, our data indicate that post COVID-19 pulmonary restriction is probably not caused by VILI, but rather by consequences of viral infection.
Two different types of restriction were discernible in this study population: the pattern of simple pulmonary restriction was more frequently observed in patients with higher initial disease severity, whereas complex pulmonary restriction was seen predominantly in patients with less severe COVID-19. Complex restriction, according to the definition by Clay et al. [36], describes a restriction pattern where FVC is disproportionally reduced compared to the reduction of TLC, combined with an increased RV and RV/TLC ratio, and usually without evidence of obstruction. Complex restriction occurs in obesity or may indicate occult obstruction, but also typically can be observed in neuromuscular diseases. Whether particularly the latter condition is related to the complex restriction pattern after acute COVID-19 in this study population warrants further investigation. The simple restriction pattern observed in severely ill patients may reflect fibrotic changes in the lung interstitium typically associated with ARDS.
In line with previous observations of impaired carbon monoxide diffusion capacity post-acute COVID-19 at time of hospital discharge [13,17,46], DLCO was reduced with increasing acute COVID-19 severity whereas KCO (DLCO/VA) was not reduced except for those patients with most severe pulmonary injury. In the latter patient group however, patients had both reduced DLCO as well as reduced KCO 3, 6 and 12 months post infection. This indicates that in patients with the highest pulmonary injury during the acute phase, diffusion impairment is predominant whereas in milder disease loss of ventilated area is the predominant mechanism of pulmonary function impairment.
Analysis of pulmonary function over time revealed three groups: (i) those less severely affected during acute COVID-19 who did not show alterations in pulmonary function during follow-up, (ii) those with compromised pulmonary function at first follow-up who showed improvement over time and (iii) those with severely compromised pulmonary function at first follow-up who did not show relevant improvement over time. In particular, many patients with the most severe respiratory failure who needed ECMO treatment still have pulmonary impairment one year after the acute disease.
The symptom cluster including fatigue, dyspnoea and cognitive deficits as described for the early convalescent phase [26] persisted over 12 months of follow-up in our study population. Respiratory health related quality of life as captured by total SGRQ improved over time, but with a relevant proportion of patients remaining above the threshold value of 25 one year after acute COVID-19. TLC however did not correlate with SGRQ score, likely due to the high proportion of patients with complex restriction and preserved TLC.
Limitations of this study were the availability of data from a single centre at this point of time, and the reduced number of patients available in the first and the last 12 month follow-up visit, particularly in the group of patients after invasive mechanical ventilation and ECMO treatment. As we present pulmonary function data as ppv, age and sex might not act as a confounder in this case, however, this might be the case for patient reported outcome. With respect to the SGRQ outcome, studies show that no relevant differences were found between patients of similar disease severity with respect to age and sex [47,48]. Further, as more evidence on cardiovascular events during acute and post-acute COVID-19 becomes available, the occurrence of pulmonary embolism or cardiac involvement might affect the outcome of patient reported outcome. Chest CT-scans were only performed on the basis of clinical indication. This may lead to a selection bias towards cases of more severe COVID-19. Patients who initially were not hospitalized (NOO group) presented to our outpatient department due to specific respiratory symptoms and may not be representative for all non-hospitalized COVID-19 patients.
By summarizing the results from pulmonary function tests with assessment of respiratory symptoms and the evolution of findings over time, we hypothesize that two main patterns of pulmonary involvement are discernible after COVID-19: in patients with severe disease and particularly those with respiratory failure requiring ECMO treatment, a pattern of interstitial lung involvement characterized by simple restriction and reduction of diffusion capacity predominates. This pattern has potential for functional and subjective improvement over time during the first year of follow-up. In patients with mild to moderate initial disease however, a disease pattern characterized by a loss of ventilated area and symptom persistence over one year after follow-up predominates. Particularly for the latter pattern, potential underlying mechanisms are unknown, and these patterns of pulmonary injury will need to be confirmed and further characterized in larger and multi-centric studies.
In conclusion, this study demonstrated the relevance of initial disease severity and results of thoracic CT for pulmonary functional impairment and respiratory symptoms in the first year after SARS-CoV-2 infection in hospitalized patients.
Funding
The Pa-COVID-19 study is supported by grants from the Berlin Institute of Health (BIH) and the German Federal Ministry of Education and Research (01KX2021 and 01KI20160A). The section you refere report conflict of interest / competing interests.
CRediT authors contribution statement
Fridolin Steinbeis: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, developed the study design, performed data analysis and interpretation and wrote the manuscript. Charlotte Thibeault: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Felix Doellinger: performed data analysis and interpretation of radiological data. Raphaela Maria Ring: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Mirja Mittermaier: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Christoph Ruwwe-Glösenkamp: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Florian Alius: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Philipp Knape: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Lena Johanna Lippert: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Elisa Theresa Helbig: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Daniel Grund: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Bettina Temmesfeld-Wollbrück: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Norbert Suttorp: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Leif Erik Sander: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Florian Kurth: contributed substantially to the study design data analysis and interpretation, and the writing of the manuscript. Tobias Penzkofer: performed data analysis and interpretation of radiological data. Martin Witzenrath: developed the study design, performed data analysis and interpretation and wrote the manuscript. Thomas Zoller: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, developed the study design, performed data analysis and interpretation and wrote the manuscript.
Declaration of competing interest
M.W. received funding for research from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Actelion, Bayer Health Care, Biotest, Boehringer Ingelheim, Noxxon, Pantherna, Quark Pharma, Silence Therapeutics, Takeda Pharma, Vaxxilon, and for lectures and advisory from Actelion, Alexion, Aptarion, Astra Zeneca, Bayer Health Care, Berlin Chemie, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Insmed, Novartis, Teva and Vaxxilon. T.Z. received funding for research from Bundesministerium für Bildung und Forschung, Else Kröner-Fresenius Stiftung and Gesellschaft für Internationale Zusammenarbeit.
Acknowledgement
We thank the Pa-COVID-19 study group with Stefan Hippenstiel, Pinkus Tober-Lau, Sascha S. Haenel, David Hillus, Tilman Lingscheid, Holger Müller-Redetzky, Alexander Uhrig, Miriam S. Stegemann, Ralf H. Hübner, Kai-Uwe Eckardt, Martin Möckel, Felix Balzer, Claudia Spies, Steffen Weber-Karstens, Frank Tacke, Chantip Dang-Heine, Michael Hummel, Georg Schwanitz, Uwe D. Behrens, Maria Rönnefarth, Sein Schmidt, Alexander Krannich, Christof von Kalle, Victor M. Corman and Christian Drosten that contributed to set up and realization of the study platform; Linda Jürgens, Malte Kleinschmidt, Sophy Denker, Moritz Pfeiffer, Belén Millet-Pascual-Leone, Felix Machleidt, Sebastian Albus, Felix Bremer, Jan-Moritz Doehn, Tim Andermann, Carmen Garcia, Philipp M. Krause, Liron Lechtenberg, Yaosi Li, Panagiotis Pergantis, Till Jacobi, Teresa Ritter, Berna Yedikat, Lennart Pfannkuch, Christian Zobel, Ute Kellermann, Susanne Fieberg, Laure Bosquillon de Jarcy, Anne Wetzel, Christoph Tabeling, Markus Brack, Jan M. Kruse, Daniel Zickler, Andreas Edel, Britta Stier, Roland Körner, Nils B. Mueller and Philipp Enghard to obtaining informed consent and biosamples and Nadine Olk, Willi M. Koch, Saskia Zvorc, Lucie Kretzler, Lil A. Meyer-Arndt, Linna Li and Isabelle Wirsching, Paula Stubbemann, Jo Bagli, Olivia Zielonka and Vivien Schreiber to data collection. We also thank Theresa Keller and Lukas Mödl from Charité - Institute of Biometry and Clinical Epidemiology for their statistical expertise and critical review of the manuscript.
M.M. is participant in the BIH-Charité Digital Clinician Scientist Program funded by the Charité –Universitätsmedizin Berlin, the Berlin Institute of Health, and the German Research Foundation (DFG). M.W. is supported by grants from the German Research Foundation, SFB-TR84 C6 and C9, SFB 1449 B2, by the German Ministry of Education and Research (BMBF) in the framework of CAPSyS (01ZX1304B), CAPSyS-COVID (01ZX1604B), SYMPATH (01ZX1906A), PROVID (01KI20160A), P4C (16GW0141), MAPVAP (16GW0247), NUM-NAPKON (01KX2021), and by the Berlin Institute of Health (CM-COVID).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106709.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wiersinga W.J., Rhodes A., Cheng A.C., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Polak S.B., Van Gool I.C., Cohen D., et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 4.Lerum T.V., Aaløkken T.M., Brønstad E., et al. Dyspnoea, lung function and CT findings 3monthsafter hospital admission for COVID -19. Eur. Respir. J. 2021;57(4):2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur. Respir. J. 2021;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira-Gomes M., Kruglov A., Durek P., et al. In severe COVID-19, SARS-CoV-2 induces a chronic, TGF-β-dominated adaptive immune response. medRxiv. 2020 p. 2020.09.04.20188169. [Google Scholar]
- 7.Gralinski L.E., Bankhead A., 3rd, Jeng S., et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4(4) doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochs M., Timm S., Elezkurtaj S., et al. Collapse induration of alveoli is an ultrastructural finding in a COVID-19 patient. Eur. Respir. J. 2021:2004165. doi: 10.1183/13993003.04165-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh M.-J., Lee W.-C., Cho H.-Y., et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza and other respiratory viruses. 2018;12(5):643–648. doi: 10.1111/irv.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park W.B., Jun K.I., Kim G., et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J. Kor. Med. Sci. 2018;33(24):e169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Tan C., Wu J., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y-m, Shang Y-m, Song W-b, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guler S.A., Ebner L., Beigelman C., et al. Pulmonary function and radiological features four months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R., Gao Y., Chen M., et al. Impaired pulmonary function in discharged patients with COVID-19: more work ahead. Eur. Respir. J. 2020;56(1):2002194. doi: 10.1183/13993003.02194-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nusair S. Abnormal carbon monoxide diffusion capacity in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;56(1):2001832. doi: 10.1183/13993003.01832-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(1):129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damanti S., Ramirez G.A., Bozzolo E.P., et al. 6-Month respiratory outcomes and exercise capacity of COVID-19 acute respiratory failure patients treated with CPAP. Intern. Med. J. 2021;51(11):1810–1815. doi: 10.1111/imj.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González J., Benítez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasio F., Barbuto S., Scarnecchia E., et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir. J. 2021;58(3) doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. 10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X., Liu X., Zhou Y., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. 10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedor C., Freitag H., Meyer-Arndt L., et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany – a first analysis of a prospective observational study. medRxiv. 2021 [Google Scholar]
- 26.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurth F., Roennefarth M., Thibeault C., et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19) Infection. 2020;48(4):619–626. doi: 10.1007/s15010-020-01464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO WHO R&D blueprint novel coronavirus COVID-19 therapeutic trial synopsis. R&D Blueprint. 2020 [Google Scholar]
- 29.Graham B.L., Steenbruggen I., Miller M.R., et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir. Crit. Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrino R., Viegi G., Brusasco V., et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 31.Criée C.-P., Baur X., Berdel D., et al. Leitlinie zur Spirometrie. Pneumologie. 2015;69(3):147–164. doi: 10.1055/s-0034-1391345. [DOI] [PubMed] [Google Scholar]
- 32.Stanojevic S., Quanjer P., Miller M.R., et al. The Global Lung Function Initiative: dispelling some myths of lung function test interpretation. Breathe. 2013;9(6):462–474. [Google Scholar]
- 33.Graham B.L., Brusasco V., Burgos F., et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017;49(1):1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 34.Graham B.L., Brusasco V., Burgos F., et al. DLCO: adjust for lung volume, standardised reporting and interpretation. Eur. Respir. J. 2017;50(2):1701144. doi: 10.1183/13993003.01144-2017. [DOI] [PubMed] [Google Scholar]
- 35.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61(9):744–746. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clay R.D., Iyer V.N., Reddy D.R., et al. The "complex restrictive" pulmonary function pattern: clinical and radiologic analysis of a common but previously undescribed restrictive pattern. Chest. 2017;152(6):1258–1265. doi: 10.1016/j.chest.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelpi M., Argentiero J., Jones P.W., et al. A scoring application for the St. George's respiratory Questionnaire. Chest. 2016;150(3):747–748. doi: 10.1016/j.chest.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 39.Guler S.A., Ebner L., Aubry-Beigelman C., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021;57(4):2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020;12(574):eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyonaga K., Miyajima M., Suga M., et al. Alterations in cytokeratin expression by the alveolar lining epithelial cells in lung tissues from patients with idiopathic pulmonary fibrosis. J. Pathol. 1997;182(2):217–224. doi: 10.1002/(SICI)1096-9896(199706)182:2<217::AID-PATH833>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 42.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 43.Ricard J.D., Dreyfuss D., Saumon G. Ventilator-induced lung injury. Eur. Respir. J. 2003;22(42 suppl):2s–9s. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 44.Neff T.A., Stocker R., Frey H.-R., et al. Long-term assessment of lung function in survivors of severe ARDSa. Chest. 2003;123(3):845–853. doi: 10.1378/chest.123.3.845. [DOI] [PubMed] [Google Scholar]
- 45.von Bahr V., Kalzén H., Frenckner B., et al. Long-term pulmonary function and quality of life in adults after extracorporeal membrane oxygenation for respiratory failure. Perfusion. 2019;34(1_suppl):49–57. doi: 10.1177/0267659119830244. [DOI] [PubMed] [Google Scholar]
- 46.Chapman D.G., Badal T., King G.G., et al. European Respiratory Journal; 2020. Caution in Interpretation of Abnormal Carbon Monoxide Diffusion Capacity in COVID-19 Patients; p. 2003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones P.W., Quirk F.H., Baveystock C.M., et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 48.Quirk F.H., Jones P.W. Patients' perception of distress due to symptoms and effects of asthma on daily living and an investigation of possible influential factors. Clin. Sci. 1990;79(1):17–21. doi: 10.1042/cs0790017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.