Abstract
This study aimed to investigate the extract with high antioxidant activity of Tetrastigma hemsleyanum Diels et Gilg and identify the antioxidant components in vitro. α, α-Diphenyl-β-picrylhydrazyl (DPPH) radical assay, Trolox equivalent antioxidant capacity (TEAC) assay, ferric reducing antioxidant power (FRAP), and hydroxyl radical scavenging method were used to screen the extract with high antioxidant activity. The antioxidant capacity of the extracts was evaluated by the free radical scavenging ability of DPPH. The ability of extracts to scavenge 2, 2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical was evaluated by TEAC assay. The FRAP method was used to evaluate the ability of extracts to reduce Fe3+. The ability to scavenge hydroxyl radicals produced by the interaction of hydrogen peroxide and Fe2+ was measured by monitoring the change in the absorbance of the reaction mixture at 536 nm. Then, high-performance liquid chromatography-DPPH (HPLC-DPPH) and HPLC-hydroxyl radical scavenging methods were used to screen the antioxidant components in the extract. The molecular weight of the above antioxidant components was investigated using the qualitative analytical method of high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (HPLC-Q-TOF LC/MS). Based on the concentrations of the samples (0.2–4 mg/mL), the DPPH free radical scavenging ability, ABTS+ free radical scavenging ability, hydroxyl free radical scavenging ability, and Fe3+ reducing ability of the ethyl acetate extract (EAE) were stronger than that of the crude extract (CE), petroleum ether extract (PEE), and n-butanol extract (BE). The EAE has higher antioxidant activity than CE, PEE, and BE. Six antioxidant components, rutin, quercetin, isoquercetin, astragalin, kaempferol, and kaempferol-3-o-rutoside, were identified in the EAE.
1. Introduction
The rhizome of Tetrastigma hemsleyanum Diels et Gilg, a Chinese folk herbal medicine, is used for the treatment of children with high fever, pneumonia, hepatitis, viral meningitis, scrofula, carbuncle, and furuncle [1]. Modern pharmacological studies have shown that Tetrastigma hemsleyanum Diels et Gilg has anti-inflammatory, analgesic [2], antiviral [3], antiliver injury [4, 5], antitumor [6, 7], and other pharmacological effects [8, 9].
A large number of studies are focused on the chemical constituents of Tetrastigma hemsleyanum Diels et Gilg. The aboveground part of Tetrastigma hemsleyanum Diels et Gilg contains β-sitosterol, palmitic acid, pentacosane, gallic acid, ethyl gallate, catechin, 7-O-galloylcatechin, and 3,3'- dimethoxy ellagic acid-4-O-β-D-glucopyranoside [10]. Taraxerone, ergosterol, oleic acid, palmitic acid, myristic acid, hexadecylic acid, heptadecanoic acid, 9,12-octadecadienoic acid, α-linolenic acid, kaempferol, quercetin, and kaempferol-3-o-neohesperidin were found in liposolubility extract of Tetrastigma hemsleyanum Diels et Gilg [11–13]. In addition, Xu analyzed 80% methanol ultrasonic extract of Tetrastigma hemsleyanum by HPLC-Q-TOF-MS and found that it contained kaempferol-7-o-rhamnose-3-o-glucoside, rutin, isoquercetin, kaempferol rutoside, astragaloside, and quercetin [12]. Fan identified 8 flavonoids (isoorientin, orientin, orientin-2”-O-rhamnoside, isoorientin-2”-O-rhamnoside, vitexin, vitexin-2”-O-rhamnoside, isovitexin, and isovitexin-2”-O-rhamnoside) in Tetrastigma hemsleyanum leaves by rapid resolution liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (RRLC-Q-TOF-MS) [14]. These results showed that oleic acid and flavonoids constituted the largest proportion of the plant [15]. Flavonoids have various biological activities, such as the cardiovascular system [16, 17], antioxidant [18–20], anti-tumor [21–23], anti-inflammatory, and analgesic [24], and are one of the most and diverse phenolic groups of natural origin [25]; their antioxidation capability is a significant property.
Free radicals that are produced in the process of life metabolism regulate the signal transmission between cells and cell growth and inhibit the viruses and bacteria [26]. Excessive free radicals attack the cell membrane and leak from the intracellular region, which in turn leads to the denaturation of protein and nucleic acid, thereby, damaging the cells, tissues, and organs, inducing various diseases, and accelerating the aging of the body. The mechanism of inflammation, tumor, aging, and cardiovascular diseases is closely related to the excessive production of free radicals or a decrease in the ability to scavenge free radicals [27]. Antioxidants quench free radicals and form nontoxic ions or molecules. The free radicals were scavenged to achieve antioxidation, and antioxidants can stop or reverse some damage caused by excessive free radicals [28]. Synthetic antioxidants used to prolong the shelf life of food, such as butyl hydroxyanisole (BHA), dibutyl hydroxytoluene (BHT), and propyl gallate (PG) [29–31], have been found to be potential hepatotoxicity and carcinogenicity [32, 33]. Due to restricted use of synthetic antioxidants [34], natural antioxidants with high safety and fewer side effects are favored. Natural antioxidants and active substances mainly originate from plants [31], including tea extract [35, 36], fruit and vegetable extracts [37], balsam pear extracts [38], Chinese herbal extracts [39], raw Rehmanniae extract [40], and spices extracts [41]. Natural antioxidants can eliminate free radicals [42, 43], improve blood circulation, delay cell aging, and prevent cancer [44, 45].
While screening the antitumor extract and active components of Tetrastigma hemsleyanum Diels et Gilg, we found that some of the isolated parts were oxidized [46, 47]. This phenomenon indicated that there might be antioxidant components in Tetrastigma hemsleyanum Diels et Gilg. However, active components responsible for antioxidants in Tetrastigma hemsleyanum Diels et Gilg are yet to be identified. Therefore, by utilizing the antioxidant test, we screened out the extract that harbors high antioxidant activity, and the molecular weight of the potential active components was determined by HPLC-Q-TOF LC/MS.
2. Materials and Methods
2.1. Chemicals and Plant Material
DPPH, ABTS, TPTZ (2,4,6-tri-2-pyridinyl-1,3,5-triazine), and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma (St. Louis, MO, USA), and methanol, ethanol, petroleum ether, ethyl acetate, n-butanol, and other reagents for the antioxidant test were analytically pure and purchased from China National Pharmaceutical Group Co. (Beijing, China). Rutin (lot no. 100080–201805, HPLC ≥98%), astragaloside (lot no. 110781–201908, HPLC ≥98%), isoquercetin (lot no. 111809–201804, HPLC ≥98%), quercetin (lot no. 100081–201610, HPLC ≥98%), kaempferol (lot no. 110861–201703, HPLC ≥98%), and kaempferol-3-o-rutoside (lot no. 112007–201903, HPLC ≥98%) standard reference materials were obtained from National Institutes for Food and Drug Control (Beijing, China). Chromatography grade and LC-MS grade acetonitrile were obtained from Tedia (Fairfield, OH, USA) and Fisher (Waltham, MA, USA), respectively.
The rhizome of Tetrastigma hemsleyanum Diels et Gilg from Guangxi Province, China, was purchased from Guangxi, China (lot no. 180615), and identified by Professor Xia Miao-fen at Zhejiang Pharmaceutical College.
2.2. Extraction, Fractionation, and Antioxidant Evaluation
The extracts were prepared according to the method of Bao et al. [48] and Yong [49] with minor modifications. The dried powder (5 kg) of the rhizomes of Tetrastigma hemsleyanum Diels et Gilg was soaked in three volumes of 60% methanol at room temperature. Subsequently, the soaking solution was filtered, combined, and concentrated under reduced pressure; the crude extract (CE) (364 g) was prepared by vacuum drying. Then, it was suspended in water and extracted successively with petroleum ether, ethyl acetate, and n-butanol to obtain petroleum ether extract (PEE) (28 g), ethyl acetate extract (EAE) (25 g), and n-butanol extract (BE) (68 g), respectively. The antioxidant activities of each extract were measured using the DPPH assay, TEAC assay, ferric reducing antioxidant power (FRAP), and hydroxyl radical scavenging method to screen the extract with high antioxidant activity. The antioxidant components in this extract were detected by HPLC-DPPH and HPLC-hydroxyl radical scavenging methods. The molecular weight of the antioxidant components was deduced by HPLC-Q-TOF LC/MS. The extracts were solubilized in ethanol solution (80% v/v), except for PEE, which was dissolved in dimethyl sulfoxide (DMSO).
2.3. DPPH Assay
The DPPH free radical scavenging activity was assessed according to Leong et al. [50] with a slight improvement. Briefly, 3.0 mL of DPPH 80% ethanol solution (10−4 M) was mixed with 0.1 mL of the sample solution. After incubation for 30 min in the dark at room temperature, the absorbance was measured at 517 nm using a UV-vis spectrophotometer (Shimadzu UV-1800, Kyoto, Japan). The radical scavenging activity (%) was calculated as follows:
| (1) |
where A1 is the absorbance of the sample, and A0 is the absorbance of the blank group.
2.4. TEAC Assay
The antioxidant activity against ABTS+ was measured as described by Strail et al. [51] with some modifications. ABTS+ was formed by reacting 7.0 mM ABTS with 4.95 mM potassium persulfate solution at room temperature in the dark for 12 h. Then, the solution was diluted with 80% ethanol before measuring the absorbance (0.70 ± 0.02) at 734 nm. After incubation of 20 μL sample solution (0.2–4 mg/mL) with 3.0 mL ABTS+ at 30°C for 10 min, the absorbance was measured at 734 nm by the UV-vis spectrophotometer. The antioxidant capacity was expressed as mg of Trolox/g of samples (TEAC), and the TEAC was calculated as follows:
| (2) |
where A1 is the absorbance of the sample, A0 is the absorbance of the blank group, b is the slope of the standard curve, a is the intercept of the standard curve, V is the volume of Trolox (μL), and W is the weight of extracts (g).
2.5. FRAP
The antioxidant activity against Fe3+-TPTZ was performed according to the method established by Benzie et al. [52] with some modifications. Fresh FRAP reagent was prepared by mixing 300 mm acetate buffer (pH 3.6), 10 mm TPTZ solution, and 20 mm FeCl3·6H2O solution at the ratio of 10 : 1:1. A volume of 3.0 mL FRAP reagent was added to 20 L sample solution (0.2–4 mg/mL) and incubated at 37°C for 30 min. The absorbance of the reaction mixture was measured at 593 nm. FRAP was expressed as the number of moles of FeSO4 required to achieve the same absorbance per g of samples and calculated as follows:
| (3) |
where A1 is the absorbance of the sample, A0 is the absorbance of the blank group, b is the slope of the standard curve, a is the intercept of the standard curve, and W is the weight of extracts (g).
2.6. Hydroxyl Radical Scavenging Assay
The hydroxyl radical scavenging capacity was evaluated according to the method described by Jin et al. [53] with some modifications. Briefly, 1.0 mL phosphate-buffered saline (PBS, pH 7.4) was mixed with 0.5 mL (0.148 mg/mL) o-phenanthrene solution, 0.5 mL (0.208 mg/mL) ferrous sulfate solution, and 1.0 mL sample solution (0.2–4 mg/mL). After the mixture was vortexed, 1.0 mL (0.01%) hydrogen peroxide (H2O2) solution was added and incubated at 37°C for 60 min. The absorbance was measured at 536 nm. The hydroxyl radical scavenging capacity was calculated as follows:
| (4) |
where A1 is the absorbance of the sample, A0 is the absorbance of the blank control group, and A2 is the absorbance of the sample and reagent blank group.
2.7. HPLC Conditions
Herein, we used traditional methods to select the extract with high antioxidant activity, and the antioxidant components were screened using the HPLC-DPPH scavenging method and HPLC-hydroxyl radical scavenging method [54]. The chromatography selected optimal chromatographic conditions to separate the components in the extract. Then, the content of the sample components before and after the addition of the free radicals was detected using a UV detector. Next, the components with antioxidant capacity were selected by comparing the changes in the peak area or peak height of the corresponding components in the two chromatograms. Antioxidant components in the extract with high oxidation activity were screened by using an Agilent HPLC system with a photodiode-array detector (1260 series, Santa Clara, CA, USA). The chromatographic separation was carried out using a Shimadzu VP-ODS C18 (150 × 4.6 mm, 5 μm) with solvent A (0.1% formic acid) and solvent B (acetonitrile). The gradient condition was as follows: 0 min, 18% B; 0–5 min, 25% B; 5–10 min, 35% B; 10–15 min, 60% B; 15–20 min, 90% B; and 20–30 min, 18% B. The flow rate was 1.0 mL/min, the injection volume was 20 μL, the column temperature was set at 30°C, and the detection wavelength was 254 nm.
2.8. HPLC-DPPH Scavenging Method
The majority of the phenolic antioxidants can quench DPPH radicals, and hence, DPPH radicals are often used to screen the antioxidant activities of phenolic compounds [55]. In this study, we used the HPLC-DPPH scavenging method to determine the peak area of each component in the sample before and after adding DPPH. The components of the reduced peak area harbored antioxidant activity. Briefly, 250 μL sample solution (4 mg/L) and 250 μL DPPH solution (500 μM) were mixed and incubated at 37°C in the dark for 30 min. The mixture was then filtered through a 0.45 μm membrane and determined according to the chromatographic conditions in 2.2.5.
2.9. HPLC-Hydroxyl Radical Scavenging Method
Briefly, 200 μL H2O2 solution (25 mM) and 200 μL of FeSO4 solution (250 μM) were added to 250 μL sample solution (4 mg/L). The mixture was incubated at room temperature in the dark for 7 min, filtered with 0.45 μm membrane, and measured according to the chromatographic conditions in 2.2.5.
2.10. Identification of Active Antioxidant Ingredients
The identification experiment was carried out using an Agilent HPLC-Q-TOF LC/MS (1260 series, 6520 series) and an Agilent HPLC system with a photodiode array detector (1260 series). The chromatographic separation was conducted using a Extend Agilent C18 (50 × 2.1 mm, 1.8 μm) with solvent A (0.1% formic acid) and solvent B (acetonitrile). The gradient condition was as follows: 0 min, 18% B; 0–5 min, 25% B; 5–10 min, 35% B; 10–15 min, 60% B; and 15–20 min, 90% B. The flow rate was 0.2 mL/min, injection volume was 1 µL, the column temperature was room temperature, the scanning range was 50–1500, and the capillary voltage was 4000 V. Nitrogen was used as dry gas at a flow rate of 10 mL/min, the temperature was 350°C, and the atomizer pressure was 40 psi. The mass spectrogram and chromatogram were recorded under the negative ion source. After acquiring the molecular weight, the antioxidant components in the sample were further identified based on their standard products by HPLC, according to the chromatographic conditions in 2.2.5.
2.11. Statistical Analysis
All experimental data were expressed as mean ± standard deviation (SD) of three independent experiments in triplicate and analyzed by SPSS (version 17.0). One-way analysis of variance (ANOVA) and Tukey multiple comparisons were carried out to test any significant differences between the means. P < 0.05 indicated statistical significance.
3. Results
3.1. Screening of Antioxidant Extracts
3.1.1. Comparison of DPPH Free Radical Scavenging Capacity of Extracts
DPPH has been widely used in the determination of antioxidant free radical scavenging capacity [56]. The scavenging rate of each extract is shown in Figure 1. Subsequently, the DPPH scavenging activity of EAE and CE was similar to the sample concentrations of 0.2 mg/mL (P > 0.05) and 4 mg/mL (P > 0.05), respectively, while DPPH scavenging activity of EAE and all the other extracts is the largest difference at the sample concentration of 1 mg/mL (P < 0.05). The DPPH scavenging activity of EAE was dose-dependent in the concentration range of 0.2–4 mg/mL and occurred in the following order: EAE > CE > BE > PEE (Figure 2).
Figure 1.
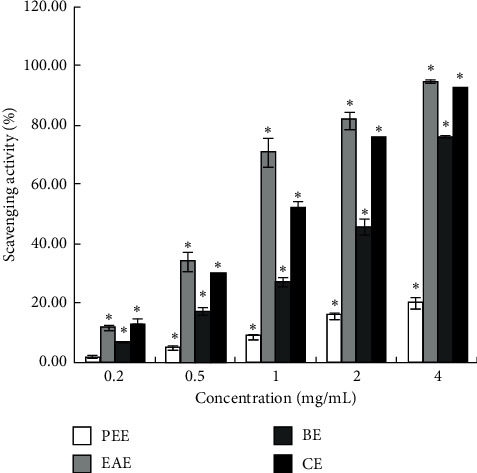
DPPH radical scavenging activity (%) of four extracts. ∗P < 0.05 vs. the blank group.
Figure 2.
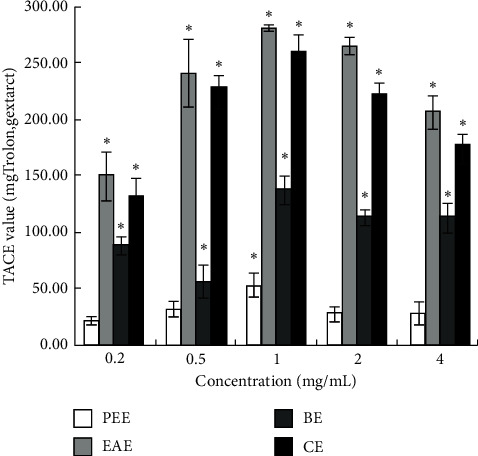
TEAC value (Trolox mg/g extract) of four extracts. ∗P < 0.05 vs. the blank group.
3.1.2. Comparison of ABTS+ Free Radical Scavenging Capacity of Extracts
The TEAC values of different concentrations of each extract are shown in Figure 2. In addition to PPE, the other extracts showed a robust ability to scavenge ABTS+ radicals. However, no significant difference was detected in the TEAC values between EAE and CE at the sample concentrations of 0.2, 0.5, and 1 mg/mL (P > 0.05). On the other hand, in the sample concentration range of 0.2–4 mg/mL, the TEAC values of EAE were 150.78 ± 20.94, 241.72 ± 30.34, 281.36 ± 3.17, 265.54 ± 7.79, and 207.59 ± 14.45 mg Trolox/g extract, respectively. The TEAC value was not dose-dependent because the weight of the extract (W) varied in the five different concentrations of each extract. If calculated according to formula (1), the antioxidant capacity was dose-dependent and expressed in terms of scavenging activity.
3.1.3. Comparison of the Reducing Power of Extracts
In this experiment, the reduction ability of CE and EAE was dissimilar (P < 0.05). At different concentrations, the reduced ability of EAE was significantly higher than that of other extracts, indicating a high reduction ability of Fe3+. In the concentration range of 0.2–4 mg/mL, the FRAP values of EAE were 0.74 ± 0.06, 1.12 ± 0.04, 1.02 ± 0.04, 0.71 ± 0.01, and 0.68 ± 0.00 mM FeSO4/g extract, respectively (Figure 3).
Figure 3.
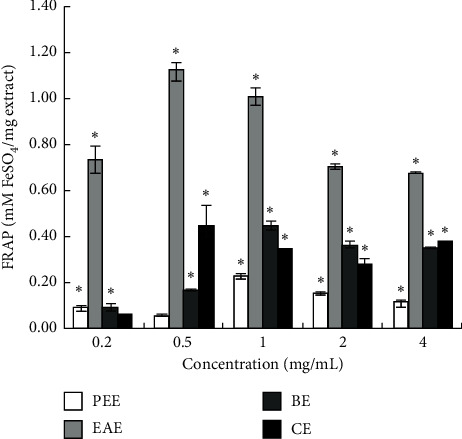
FRAP value (FeSO4 mM/mg extract) of four extracts. ∗P < 0.05 vs. the blank group.
3.1.4. Comparison of Hydroxyl Radicals Scavenging Capacity of Extracts
Figure 4 shows the highest clearance rate of EAE, although the clearance rates of CE of 1 and 2 mg/mL concentrations are close to that of EAE (P > 0.05). At 0.2, 0.5, and 0.4 mg/mL, the clearance rate of EAE was significantly different from that of other extracts (P < 0.05). In the concentration range of 0.2–4 mg/mL, the scavenging activity of hydroxyl radicals in EAE was 32.69 ± 3.66%, 59.73 ± 2.76%, 56.80 ± 1.69%, 51.28 ± 3.47%, and 47.60 ± 1.63%, respectively.
Figure 4.
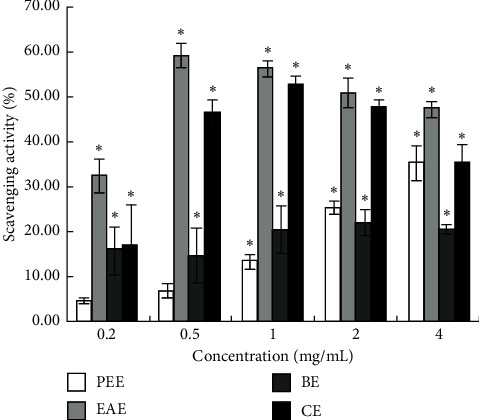
Hydroxyl radicals scavenging activity (%) of four extracts. ∗P < 0.05 vs. the blank group.
3.2. Screening Antioxidant Components in the Extract with High Antioxidant Activity by HPLC
The present study demonstrated that the EAE had the strongest antioxidant activity among the four extracts. Then, we used HPLC to analyze and screen the antioxidant components by HPLC-DPPH and HPLC-hydroxyl radical scavenging methods.
When no reagent was added to the sample solution, nine chromatographic peaks were obtained (Figure 5(a)). After the DPPH solution (Figure 5(b)) or hydroxyl radical reagent (Figure 5(c)) was added to the sample solution, distinct changes were detected in the area and height of the nine peaks. Compared to the chromatograms of the sample before and after the reaction, the amount of free radicals and the antioxidant content decreased with the progress in the antioxidant reaction (Figure 5). A total of nine components were identified as peaks.
Figure 5.
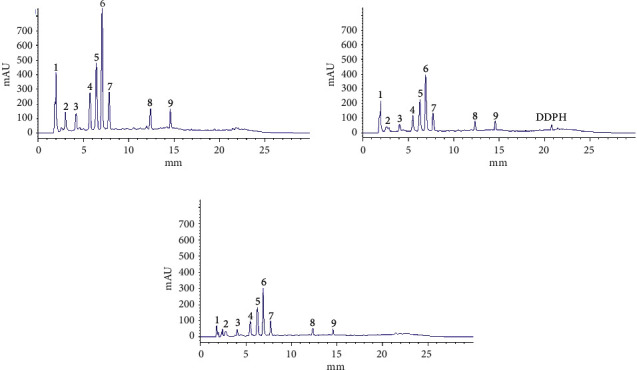
Chromatogram of antioxidant components in EAE (4 mg/mL) screened by HPLC. (a) Chromatogram of the sample without any added reagent. (b) Chromatogram of the sample with added DPPH. (c) Chromatogram of the sample with added hydroxyl radical.
3.3. Identifying Antioxidant Components in the Extract with High Antioxidant Activity
The response of each component in the EAE extract to the negative ion source is better than that of the positive ion source, which is the same as reported previously [57, 58]. Based on the total ion chromatogram (Figure 6) and six-component mass spectrogram (Figure 7) under the negative ion detection mode and the molecular weight and molecular structure (Table 1) analyzed by the data of the workstation, we speculated the potential compounds and compared them with the corresponding standards by HPLC. Finally, we identified six compounds, namely, rutin, isoquercetin, astragaloside, quercetin, kaempferol, and kaempferol-3-o-rutoside (Figure 8).
Figure 6.
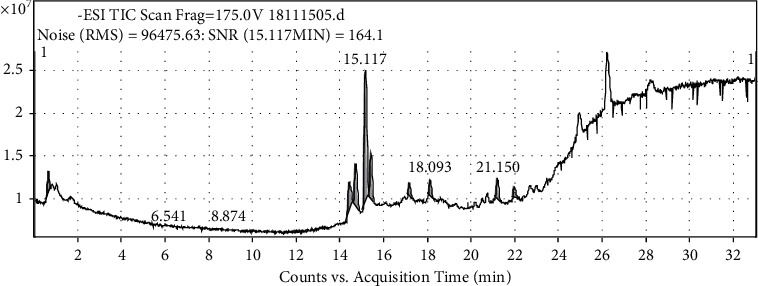
Total ion current diagram of EAE (4 mg/mL).
Figure 7.
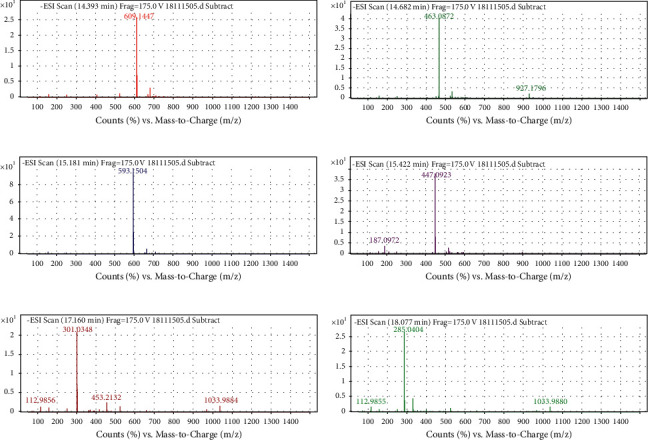
Mass chromatogram of components in EAE (4 mg/mL). (a) Mass chromatogram of rutin. (b) Mass chromatogram of isoquercetin. (c) Mass chromatogram of kaempferol-3-o-rutoside. (d) Mass chromatogram of astragaloside. (e) Mass chromatogram of quercetin. (f) Mass chromatogram of kaempferol.
Table 1.
Molecular weight and formula of six chemical components in EAE (4 mg/mL).
| No. | tR/min | Molecular formula | Measured value (m/z) | Theoretical value (m/z) | Relative error (ppm) |
|---|---|---|---|---|---|
| 1 | 14.393 | C27H30O16 | 610.1494 | 610.1473 | 2.31 |
| 2 | 14.682 | C21H19O12 | 464.0955 | 464.0944 | 2.24 |
| 3 | 15.181 | C27H29O15 | 594.1585 | 594.1576 | 1.41 |
| 4 | 15.422 | C21H19O11 | 448.1006 | 448.0996 | 2.19 |
| 5 | 17.160 | C15H9O7 | 302.0427 | 302.0421 | 1.82 |
| 6 | 18.077 | C15H9O6 | 286.0477 | 286.0477 | 0.09 |
Figure 8.
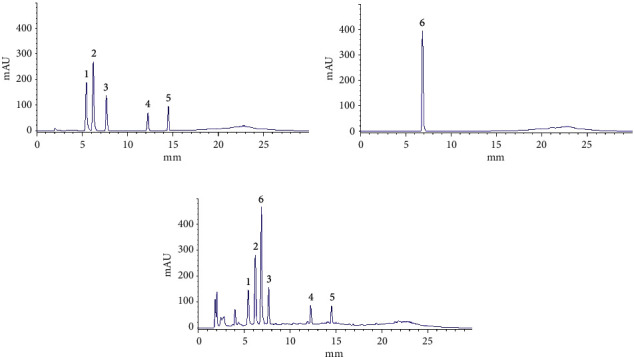
Chromatogram of standard reference and EAE (4 mg/mL). (a) Chromatogram of mixed standard reference. (b) Chromatogram of kaempferol-3-o-rutoside standard reference. (c) Chromatogram of EAE: 1, rutin; 2, isoquercetin; 3, astragaloside; 4, quercetin; 5, kaempferol; 6, kaempferol-3-o-rutoside.
4. Discussion
The current results are different from those of Sun et al. that 80% methanol extract of Tetrastigma hemsleyanum had better scavenging ability to DPPH and ABTS+ free radicals than ethyl acetate extract and hexane extract [49], which could be attributed to different extraction methods, origins of the herb, and concentrations of the samples. In this study, the total antioxidant capacity of CE was not equivalent to that of EAE, although the trend of CE in scavenging DPPH radicals, ABTS+ radicals, hydroxyl radicals, and reducing Fe3+ was consistent with that of EAE (Figures 1–4). This might be ascribed to the lower content of flavonoids in CE than that in EAE.
Figure 5 shows that the content of peaks 1, 2, and 3 also decreased; however, the molecular weight was not determined by HPLC-Q-TOF LC/MS. Furthermore, phenolic acids, such as catechin, epicatechin, gallic acid, procyanidin B1, and procyanidin B2 [59, 60], with strong antioxidant activity, have not been identified in EAE, CE, PEE, and BE, which might be due to the extraction process or the low content of phenolic acids in the extracts. The antioxidative activity of EAE in vivo and the distribution and metabolism of each antioxidant component in the organisms are not clear, and the mechanism of EAE underlying the antioxidative effect remains to be elucidated.
The six identified compounds were flavonoids and polyhydroxy compounds, and their antioxidant activity has been reported. Among these, rutin is a strong antioxidant [57], and its antioxidant activity is based on three mechanisms: the o-dihydroxy structure in B ring, the 2,3 double bond of the 4-oxo-functional group coupling in C ring, and the 5-OH and 7-OH groups of the 4-oxo-functional group in A and C rings. Rutin alleviates early brain injury of rats with subarachnoid hemorrhage by antioxidation and antiapoptosis [58]. Quercetin inhibits the activities of acetylcholinesterase and butyryl cholinesterase and Fe2+ induces lipid peroxidation in the brain homogenates of the rat, which are the putative mechanisms that underlie the quercetin-mediated management of oxidative stress-induced neurodegeneration [61]. Isoquercetin, a potent antioxidative compound, has neuroprotective capacities that are beneficial for the treatment of ischemic stroke and related diseases [62]. Astragalin, a major flavonoid, is a potential beneficial agent that protects the diabetic-induced spermatogenic dysfunction in male mice by increasing the antioxidant enzyme activities and inhibiting inflammation [63, 64]. Kaempferol and kaempferol-3-o-rutoside increases the DPPH and ABTS free radical scavenging activity and inhibits the T cell proliferation induced by concanavalin A and nitric oxide (NO) or reactive oxygen species (ROS) production in LPS-induced 264.7 macrophages cells [65].
5. Conclusions
In this study, for the first time, we used in vitro traditional antioxidant tests (DPPH assay, TEAC assay, FRAP, and hydroxyl radical scavenging assay) combined with HPLC-DPPH and HPLC-hydroxyl radical scavenging methods to screen out EAE with high antioxidant activity from Tetrastigma hemsleyanum and identified six antioxidant components by Q-TOF LC/MS and HPLC. The EAE may be used in food, medicine, and other fields to prevent the disease and assist the treatment of some oxidative stress-related diseases as these six compounds have significant biological activities. However, further studies are needed on the correlation between the content of each antioxidant component in EAE and the antioxidant capacity of EAE and the antioxidant mechanism.
Acknowledgments
This work was supported by the Ningbo Natural Science Foundation (2018A610244) and the science and technology benefiting people technology R&D project of Ningbo Science and Technology Bureau (2015C50063).
Abbreviations
- DPPH:
1,1-Diphenyl-2-picrylhydrazyl radical, 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl
- TEAC:
Trolox equivalent antioxidant capacity
- FRAP:
Ferric reducing antioxidant power
- ABTS:
2, 2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- HPLC:
High-performance liquid chromatography
- HPLC-Q-TOF LC/MS:
High-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry
- EAE:
The ethyl acetate extract
- CE:
The crude extract
- PEE:
Petroleum ether extract
- BE:
n-Butanol extract
- BHT:
Dibutyl hydroxytoluene
- BHA:
Butyl hydroxyanisole
- PG:
Propyl gallate
- TPTZ:
2,4,6-Tri-2-pyridinyl-1,3,5-triazine
- Trolox:
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid.
Data Availability
The datasets generated and analyzed during the present study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Liao S. B., Cai W. W., Chen D., Xie P., Huang J., Mu Z. X. Anti-inflammatory and analgesic effects of the extracts of Tetrastigmais hemsleyanum’s aerial parts form Fujian in vivo. Chinese Journal of Modern Applied Pharmacy . 2017;34(2):319–324. [Google Scholar]
- 2.Dan-Dan W., Rong G., Bin Y. Experimental study on the effects of Radix Tetrastigmae on respiratory syncytial virus. Natural Product Research and Development . 2019;31:1070–1074. [Google Scholar]
- 3.Zhong X. M., Mao Q. Q., Huang Z. Experimental study on the protective effect and acute toxicity of Tetrastigma hemsleyanum extract on CCl4 induced acute liver injury in mice. Chinese Traditional Patent Medicine . 2006;28(3):422–424. [Google Scholar]
- 4.Ping L. I., Weiwei J. I., Xin P., Pathology D. O. Study on the trifolium extract in anit-induced liver injury in mice. China Modern Doctor . 2018;56(30):32–35. [Google Scholar]
- 5.Wu H. Y., Wu Y. T., Chen Y. Y., Luo H. L., Jiang H. F. Effect of Radix Tetrastigma hemsleyani flavones on apoptosis and MAPK signaling pathway in myeloid leukemia NB-4 cells. Chinese Journal of Pathophysiology . 2019;35(8):1451–1456. [Google Scholar]
- 6.Hu T., Feng Z. Q., Zhong L. R. Effect of flavonoids from Tetrastigma hemsleyani Diels et Gilg on myeloid derived suppressive cells in mice bearing Lewis lung cancer. Chinese Journal of Integrated Traditional and Western Medicine . 2018;38(10):1229–1233. [Google Scholar]
- 7.College J. N. M. Dictionary of Traditional Chinese Medicine . Shanghai, China: Shanghai People’s Publishing House; 1977. [Google Scholar]
- 8.Wang X. L. Influence of the water extract of Radix Tetrastigmae on IL-23 and IL-17 in rats of rats of chronic obstructive pulmonary disease. Journal of Practical Traditional Chinese Medicine . 2016;32(9):848–850. [Google Scholar]
- 9.Liu D. D., Cao G., Zhang Q., Ye Y. L., Han L. K., Ge W. H. Inhibition effect of flavonoids from Radix tetrastigmae on acute lung injury of aged mice through p38MAPK and NF-κB pathway. Chinese Pharmacological Bulletin . 2015;12:1725–1729. [Google Scholar]
- 10.Liu D., Ju J. H., Yang J. S. Studies on chemical sonstituents form Tetrastigma hypoglaucum. Chinese Journal of traditional Chinese Medicine . 2004;34(1):4–6. [Google Scholar]
- 11.Liu D., Yang J. S. A Study on chemical components of Tetrastigma hypoglaucum Diels et Gilg. native to China. Chinese Traditional and Herbal Drugs . 1999;24(10):611–612. [PubMed] [Google Scholar]
- 12.Hu Y. J., Cheng L., Pu J. B. GC-MS analysis of petroleum ether extract from Testrastigma hemsleyanum. Chinese Traditional Medicine Science and Technology . 2013;20(1):46–47. [Google Scholar]
- 13.Li Y. Q., Lu W. C., Guo Y. Z. Studies on the chemical constituents of Testrastigma hemsleyanum Diels et Gilg. Chinese Traditional and Herbal Drugs . 2003;34(11):982–983. [Google Scholar]
- 14.Shi-Ming F., Xin-Yue X., Fan-Tian Z., Xiao-Hong Z., Bi-Ya C., Wen X. U. Identification of chemical components and determination of flavonoids in Tetrastigma hemsleyanum leaves. Chinese Journal of Pharmaceutical Analysis . 2017;37(8):1481–1486. [Google Scholar]
- 15.Zeng M. L., Shen N. T., Wu S. W., Li Q. Analysis on chemical constituents in Tetrastigma hemsleyanum by UPLC-Triple-TOF/MS. Chinese Traditional and Herbal Drugs . 2017;4(5):874–883. [Google Scholar]
- 16.Jiang Y., Zheng Q., Zhang A., Cui L., Xia L., Luo M. Flavone from Zhongjiefeng (Herba Sarcandrae Glabrae) inhibits platelet apoptosis in immune-induced bone marrow failure through mitochondrial pathway. Journal of traditional Chinese medicine=Chung i tsa chih ying wen pan . 2017;37(5):643–649. [PubMed] [Google Scholar]
- 17.Peng C. L., Zhang S. M., Zhu Y. Differential inhibition effects of gingkgo flavones and ginkgolide on the proliferation of vascular smooth muscle cell and the mechanism. Central South Pharmacy . 2017;15(10):1351–1356. [Google Scholar]
- 18.Lu L., Mi J., Luo Q. Optimization of extraction process of flavonoids from lycium barbarum L. var. Auranticarpum K. F. Ching and its antioxidant activities in vitro. Science and Technology of Food Industry . 2019;40(24):165–171. [Google Scholar]
- 19.Yan-Bo W., Yan S., Yi-Jun Y. Ultrasonic assisted extraction of polyphenols and flavonoids from Maijishan wild acanthopanax and their antioxidant activities in vitro. Natural Product Research and Development . 2019;31(12):2153–2162. [Google Scholar]
- 20.Diao J. J., Cao R. A., Li C. Y. Antioxidant activity and structure of flavonoid compounds extracted from raspberry root. Chinese Journal of Biologicals . 2019;32(12):1350–1357. [Google Scholar]
- 21.Jin H., Yu J., Wang Z. Y. Mechanism of flavonoids of sophorae fructus in inhibiting proliferation, migration and invasion of hepatocellular carcinoma cells by regulating LncRNA FBXL 19-AS1/miR-342-3p pathway. China Journal of Chinese Materia Medica . 2020;10 doi: 10.19540/j.cnki.cjcmm.20200302.401. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y. S., Li X., Qin X. M. Anti- cancer effect of the flavonoids of Astragalus combined with cisplatin on lewis lung carcinoma-bearing mice. Acta Pharmaceutica Sinica . 2020;8 [Google Scholar]
- 23.Zhang L. H., Wu T. T., Zhao L. G., Wei X., Zhang S. H., Wang C. X. Advances in studies anticancer activity of flavonoids from Ginkgo biloba extract. Chinese Pharmaceutical Journal . 2019;54(6):444–449. [Google Scholar]
- 24.Lv G. G., Qiu Z. K., Chang S. Z., Lin H., Lu J., Ye D. D. Anti-inflammatory, analgesic and analgesic effects of total flavone from Stigma maydis and its effect on acute gouty arthritis. Drug Evaluation Research . 2018;41(2):206–209. [Google Scholar]
- 25.Mecenas A. S., Adão Malafaia C. R., Sangenito L. S., et al. Rutin derivatives obtained by transesterification reactions catalyzed by Novozym 435: antioxidant properties and absence of toxicity in mammalian cells. PLoS One . 2018;13(9) doi: 10.1371/journal.pone.0203159.e0203159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Živković L., Bajić V., Bruić M., et al. Antigenotoxic and antioxidant potential of medicinal mushrooms (Immune Assist) against DNA damage induced by free radicals-an in vitro study. Mutation Research . 2019;845 doi: 10.1016/j.mrgentox.2019.06.008.403078 [DOI] [PubMed] [Google Scholar]
- 27.Rohman R., Kar R. How does the presence of an oxyradical influence the behavior of polyphenolic antioxidant? A case study on gallic acid. Journal of Molecular Modeling . 2018;24(7):p. 165. doi: 10.1007/s00894-018-3701-0. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., Yang K., Cao Q., et al. Homogenate-assisted vacuum-powered bubble extraction of moso bamboo flavonoids for on-line scavenging free radical capacity analysis. Molecules . 2017;22(7) doi: 10.3390/molecules22071156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao P., Huang H., Li X., Chen J., Duan J.-a. Characterization, evaluation of nutritional parameters of Radix isatidis protein and its antioxidant activity in D-galactose induced ageing mice. BMC Complementary and Alternative Medicine . 2019;19(1):p. 297. doi: 10.1186/s12906-019-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mira-Sánchez M. D., Castillo-Sánchez J., Morillas-Ruiz J. M. Comparative study of rosemary extracts and several synthetic and natural food antioxidants. Relevance of carnosic acid/carnosol ratio. Food Chemistry . 2020;309 doi: 10.1016/j.foodchem.2019.125688.125688 [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud A. M., Wilkinson F. L., Sandhu M. A., Dos Santos J. M., Alexander M. Y. Modulating oxidative stress in drug-induced injury and metabolic disorders: the role of natural and synthetic antioxidants. Oxidative Medicine and Cellular Longevity . 2019;2019:5. doi: 10.1155/2019/3206401.3206401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheih I.-C., Wu T.-K., Fang T. J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresource Technology . 2009;100(13):3419–3425. doi: 10.1016/j.biortech.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Chen N., Yang H., Sun Y., Niu J., Liu S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides . 2012;38(2):344–349. doi: 10.1016/j.peptides.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Zhao B. L. Oxygen Free Radicals and Natural Antioxidants . Beijing, China: Science Press; 1999. [Google Scholar]
- 35.Li Z., Caiyun H., Fazheng R. Anti-obesity efficacy of extracts from several kinds of tea in high-fat diet-induced obese mice. Food Science . 2018;39(17):192–198. [Google Scholar]
- 36.Yan-Hui L. I., Miao-Ling L., Si-Hui L., Han-Tao S. U., Hai-Yan S. Effects of tea extracts of different fermentations on proliferation of colorectal cancer cell line HCT116. Guangdong Agricultural Sciences . 2018;45(9):103–108. [Google Scholar]
- 37.Liu L., Yu L., Zhou M. Intervention study on that effect of grape seed extract proanthocyanidins on blood glucose and blood lipid in the patients with type 2 diabetes mellitus. International Journal of Laboratory Medicine . 2019;40(15):1844–1849. [Google Scholar]
- 38.Zhang L., Lei S. Protective effects and mechanism of Momordica Charantia extract on RGCS of type 2 diabetic rats. Chinese Journal of Coal Industry Medicine . 2020;23(1):11–15. [Google Scholar]
- 39.Wang H., Chi Q., Xiong S. L., Zeng C. F., Wan L. Effects of Lonicera japonica extract on lung inflammation in LPS-induced ARDS rats. Journal of Guangdong Pharmaceutical University . 2017;33(3):379–382. [Google Scholar]
- 40.Zhang X., Shi M. Y., Sun M., Zhang Y. B., Zhang Y. L., Liu L. J. Influences of Shengdihuang(Rehmanniae recens Radix) extract on pulmonary index and monocyte chemoattractant protein-1 mRNA expression of pulmonary interstitial fibrosis rats. LiShiZhen Medicine and Materia Medica Research . 2018;29(12):2884–2886. [Google Scholar]
- 41.Tang X. Y., Zhao Y. L. Study on the effect of garlic extracts on preservation and antioxidant activity of chilled meat. Meat Industry . 2017;37(7):435–438. [Google Scholar]
- 42.Huda-Faujan N., Noriham A., Norrakiah A. S., Babji A. S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. African Journal of Biotechnology . 2009;8 [Google Scholar]
- 43.Khan W., Subhan S., Shams D. F., et al. Antioxidant potential, phytochemicals composition, and metal contents of Datura alba. BioMed Research International . 2019;2019:8. doi: 10.1155/2019/2403718.2403718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng-Ru L. I., Yu-Zhi Z., Guan-Hua D. U., Xue-Mei Q. Research progress about the anti-aging effect and mechanism of flavonoids from traditional Chinese medicine. Acta Pharmaceutica Sinica . 2019;54(8):1382–1391. [Google Scholar]
- 45.Wang G. L., Li J. K., Wu X., Wang K. Extraction and purification of Debregeasia orientalis fruit polyphenols and its antioxidant, antitumor activity. Natural Product Research and Development . 2019;31(1):1–9. [Google Scholar]
- 46.Ding L., Xiong J. Q. Study preliminary on the anti-tumor effect of the extract of Tetrastigmatis hemsleyani. Strait Pharmaceutical Journal . 2011;23(12):46–48. [Google Scholar]
- 47.Ding L., Zhang L. X., Qiu Y., Wang Y. H. Chemical constituents in chloroform extraction of Tetrastigmatis hemsleyani Diels et. Gilg and their antitumor activities. Chinese Pharmaceutical Journal . 2016;50(21):1857–1860. [Google Scholar]
- 48.Bao L., Ma X. F., Song X. H. Two new resveratrol tetramers isolated from Cayratia japonica (thunb.) gagn. With strong inhibitory activity on fatty acid synthase and antioxidant activity. Chemistry and Biodiversity . 2011;7(12):2931–2940. doi: 10.1002/cbdv.200900394. [DOI] [PubMed] [Google Scholar]
- 49.Yong S. Chemical composition, activities of anti-oxidation and anticancer of Terastigma hemsleyanum . Nanchang, China: JiangXi: NanChang University; 2018. [Google Scholar]
- 50.Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chemistry . 2002;76(60-75) [Google Scholar]
- 51.Stratil P., Klejdus B., Kubáň V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. Journal of Agricultural and Food Chemistry . 2006;54(3):607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- 52.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry . 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 53.Jin M., Cai Y. X., Li J. R., Zhao H. 1, 10-Phenanthroline-Fe2+ oxidative assay of hydroxyl radical produced by H2O2/Fe2+ Progress in Biochemistry and Biophysics . 1996;23(6):553–555. [Google Scholar]
- 54.Fu J. D. New Method for Rapid Screening of Antioxidants from Nature Products . ZheJiang, China: ZheJiang University; 2010. pp. 14–54. [Google Scholar]
- 55.Brand-Williams W. M., Cuvelier M. E., Berset C. L. W. T. Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology . 1995;28(1) doi: 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- 56.Duan X., Jiang Y., Su X., Zhang Z., Shi J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chemistry . 2007;101(4):1365–1371. [Google Scholar]
- 57.Vu H. T. H., Hook S. M., Siqueira S. D., Anette M., Thomas R., Arlene M. D. Are phytosomes a superior nanodelivery system for the antioxidant rutin? International Journal of Pharmaceutics . 2018;548 doi: 10.1016/j.ijpharm.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Han Y. W., Wang C. C., Ming L. X. Protective effect of rutin on early brain injury in mice with subarachnoid hemorrhage by anti-oxidation and anti-apoptosis. Drugs & Clinic . 2020;35(3):421–425. [Google Scholar]
- 59.Xu W., Fu Z. Q., Lin J., Huang X. C., Fan S. M. Qualitative and quantitative analysis of major constituents in Tetrastigma hemsleyanum by HPLC-Q-TOF-MS and UPLC-QqQ-MS. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica . 2014;39(22):4365–4372. [PubMed] [Google Scholar]
- 60.Wen X. U., Zhi-Qin F. U., Jing L., Xue-Cheng H., Hong-Min Y. U., Ze-Hao H. Rapid simultaneous determination of ten major flavonoids in Tetrastigma hemsleyanum by UPLC-MS/MS. Yao xue xue bao = Acta pharmaceutica Sinica . 2014;49(12):1711–1717. [PubMed] [Google Scholar]
- 61.Ademosun A. O., Oboh G., Bello F., Ayeni P. O. Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. Journal of Evidence-Based Complementary and Alternative Medicine . 2016;21 doi: 10.1177/2156587215610032. [DOI] [PubMed] [Google Scholar]
- 62.Wang C.-P., Shi Y.-W., Tang M., et al. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Molecular Neurobiology . 2017;54(3):2126–2142. doi: 10.1007/s12035-016-9806-5. [DOI] [PubMed] [Google Scholar]
- 63.Xia B. H., Zhou Y. M., Pi S. L., al e. Determination of antioxidant activity of isoquercetin, rutin and astragaloside in mulberry leaves by UPLC. Journal of Chinese Medicinal Materials . 2016;39(3):586–589. [Google Scholar]
- 64.Abarikwu S. O., Pant A. B., Farombi E. O. Dietary antioxidant, quercetin, protects sertoli-germ cell coculture from atrazine-induced oxidative damage. Journal of Biochemical and Molecular Toxicology . 2012;26(11):477–485. doi: 10.1002/jbt.21449. [DOI] [PubMed] [Google Scholar]
- 65.Jingqiu W., Xianying F., Lin G., Fuliang C., Linguo Z., Zhenzhong W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One . 2018;13(5) doi: 10.1371/journal.pone.0197563.e0197563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author upon request.


