Abstract
Background
Since the beginning of the COVID-19 pandemic, multiple changes to the provision of cancer care has been introduced to maximize patient safety and protect staff. We aimed to identify factors influencing clinicians’ decision on treatment modification during the initial phase of the pandemic, and to assess its impact on outcomes in patients with colorectal cancer.
Patients and Methods
Electronic records of patients seen in a large United Kingdom tertiary cancer center was reviewed. The frequency and type of changes to systemic anticancer therapy , as well as the factors predicting clinicians’ decision were assessed.
Results
A total of 418 patients; mean age 63 ± 12 years and 57% male were included. More than half of the patients had modification to their treatment; with treatment delay (21%) or cancellation (10%), being the most common. Majority of patients on neoadjuvant treatment (97%) proceeded with treatment, with some form of treatment modification in 20%. Half of patients on adjuvant treatment had their treatment plan modified. Overall, a change in treatment was more likely in older patients (OR 1.028 [95% CI 1.010-1.047]; P = .002), and in patients who had already received higher number of cycles of systemic anticancer therapy (OR 1.040 [95% CI 1.016-1.065]; P = .001). A change in treatment was less likely further out of the first national lockdown (OR 0.837 [95% CI 0.758-0.925]; P < .001). Patients on third-line treatment were most likely to have alterations to their treatment plan (69%, n=33/48).
Conclusion
During the first wave of COVID-19 in the United Kingdom, clinicians adapted clinical practice in accordance to local and national guidance, especially amongst older patients and those on third-line treatment. Further real-world data are needed to document the important impact of changes to treatment on outcomes in patients with cancer.
Keywords: chemotherapy, treatment modification, COVID-19, colorectal cancer, survival outcomes
Micro-Abstract
We investigated factors predicting clinicians’ decision and type of changes to systemic anticancer therapy during the initial phase of the COVID-19 pandemic. Changes in treatment were more likely in older patients, and those who had already received higher number of cycles of treatment and at the initial weeks of lockdown. These results provide insights which may guide future interventions as the pandemic continues.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has required hospitals and national health systems globally to urgently adopt contemporaneous changes to the delivery of healthcare in order to cope with the increasing pressure on services whilst limiting the spread of the disease.1 In the United Kingdom (UK), the first national lockdown was introduced from 23rd March to 23rd June 2020, as part of the government's strategy to "flatten the curve” and allow the National Health Service (NHS) to continue delivering emergency and other crucial health services including cancer care.2
Since the beginning of the pandemic, multiple changes to the provision of cancer care including adaptations in diagnostic pathways and modifications to treatment (change in therapy, deferral, or omission), have been recommended by professional bodies and commissioners of services globally.3 , 4 Within the Colorectal Oncology group at The Christie NHS Foundation Trust (Manchester, UK), we rapidly developed practical guidance for the management of patients with colorectal cancer in view of the growing concerns during that period especially with the lack of adequate testing, the anticipated redeployment of health professionals to acute hospitals, and remote work to reduced hospital footfall. Amongst others, adjuvant chemotherapy was considered if survival gain was expected to be >10% and 3 months of oral treatment was the preferred option. Treatment breaks for patients on palliative treatment, as well as, delays in the initiation of treatment for second- and third- line treatments were considered unless clinically indicated. This guidance was further reviewed and amended with the publication of the national gui
On 20th March 2020, the National Institute for Health and Care Excellence (NICE) published a COVID-19 rapid guideline for the delivery of systemic anticancer treatments (SACT).4 The purpose of this was to maximize the safety of patients with cancer and make the best use of the NHS resources during the COVID-19 pandemic, while protecting staff from the infection. It also encouraged contingency planning to ensure that patient needs are attended to if services were to become limited due to increasing service demands due to the COVID-19 pandemic. Hence, clinicians were advised to minimize face-to-face contact with patients when possible and reprioritize SACT plans. Additionally, early in the pandemic, there were concerns on whether certain cancer types and potential immunosuppression associated with treatments may risk patients becoming seriously unwell if they contacted COVID‑19. As the pandemic evolved, a universal decrease in and/or temporary cessation of most non-COVID-19 NHS services also became necessary.
A year on from the first wave of COVID-19 in the UK, there is now growing concern of the impact of COVID-19 related practice changes on patient groups requiring time-critical investigations and management. Several reports have now highlighted the impact on patients with cancer for whom timely diagnosis and the prompt initiation of treatment is particularly vital for ensuring optimal outcomes.5, 6, 7, 8, 9, 10 Delays in the diagnostic pathway due to the pandemic is expected to result in 15% increase in colorectal cancer-related deaths,7 and worryingly 36% to 43% decrease in cancer treatments for patients with colorectal cancer have been reported in the initial phase of the pandemic in UK and Scotland.9 , 10
Here, we report experience from a large tertiary cancer center on factors influencing clinicians’ decision on treatment modification during the initial phase of the COVID-19 pandemic and the subsequent impact on the survival outcomes of patients with colorectal and anal cancers, including those who were on 3 or more lines of treatment.
Patients and Methods
Data Collection
Electronic records of consecutive patients seen in the colorectal medical oncology outpatient clinics between 16th March (a week prior to the first UK national lockdown) and 8th May 2020 were reviewed. Patient demographics and salient clinical details including ECOG performance status, disease status, line of treatment, type of chemotherapy, and nature of treatment changes (if any) were extracted. Patients on third-line treatment were prospectively followed until 27th April 2021 when data were censored for final analysis. For this subgroup of patients on third-line treatment, further detailed characteristics including if treatment modifications were due to the COVID-19 pandemic or patient's condition and/or lack of further treatment options were recorded. This study was conducted following approval by The Christie Quality Improvement and Clinical Audit department (reference number 2966).
Study Measures
The primary objective was to identify the frequency and the type of changes to SACT on patients with colorectal cancer during the initial phase of the pandemic. The secondary objectives were (1) to assess if there were factors predicting the clinicians’ decision leading to the changes in treatment, and (2) to perform a subgroup analysis for patients who were on third-line or beyond of palliative SACT and the impact of such treatment modifications on survival outcomes.
Statistical Analysis
A complete descriptive analysis for all the variables was performed using IBM SPSS Statistics for Mac, version 26.0 (IBM Corp, Armonk, NY). An exhaustive evaluation of associations between the different variables was performed using the χ2 test for comparing categorical variables. Normal distribution tests were applied to continuous variables. The non-parametric test was used to compare outcome dichotomous variables with the ordinal variables of the questionnaire. The multivariate analysis was performed using a logistic regression for binary outcomes and odds ratios (OR) with 95% confidence intervals (CIs) were calculated. Kaplan-Meier analyses were used to perform landmark analysis of the impact of decisions on treatment changes (within predetermined study period) on survival outcomes.
Results
Patient Demographics
A total of 418 patients were included in this study (Table 1 ). Majority were male (n = 237, 56.7%), and had favorable performance status (ECOG PS 0 or 1) (n = 399, 95.4%). Almost all patients (n = 414, 99%) had a primary diagnosis of colorectal cancer. During the study period, almost half of the patients assessed (n = 199) were considered for or were on first-line treatment for advanced disease, and around 10% (n = 48) were on third-line treatment or beyond.
Table 1.
Patient Demographics (n = 418)
| Number (%) | ||
|---|---|---|
| Mean age (y) | 63.0 ± 12.0 | |
| Gender | Male | 237 (56.7%) |
| Female | 181 (43.3%) | |
| ECOG performance status | 0 | 128 (30.6%) |
| 1 | 271 (64.8%) | |
| 2 | 17 (4.1%) | |
| 3 | 2 (0.5%) | |
| Primary diagnosis | Colorectcal cancer | 414 (99%) |
| Anal cancer | 4 (1%) | |
| Treatment regime | OxMdG | 63 (15.1%) |
| IrMdG | 85 (20.3%) | |
| MdG | 4 (1%) | |
| Capecitabine | 68 (16.3%) | |
| Lonsurf | 22 (5.3%) | |
| OxMdG + Cetuximab | 7 (1.7%) | |
| OxMdG + Panitumumab | 14 (3.3%) | |
| IrMdG + Cetuximab | 26 (6.2%) | |
| IrMdG + Panitumumab | 25 (6%) | |
| OxCap | 45 (10.8%) | |
| Irinotecan + Capecitabine | 3 (0.7%) | |
| Oxaliplatin + Raltitrexed | 2 (0.5%) | |
| FOLFOXIRI | 4 (1%) | |
| Raltitrexed | 2 (0.5%) | |
| Nivolumab | 4 (1%) | |
| Carboplatin/Paclitaxel | 1 (0.2%) | |
| MCap | 6 (1.4%) | |
| Panitumumab | 6 (1.4%) | |
| Single-agent Irinotecan | 18 (4.3%) | |
| Add-Aspirin Trial | 2 (0.5%) | |
| Encorafenib + Cetuximab | 3 (0.7%) | |
| MdG + Avastin | 1 (0.2%) | |
| Single-agent Cetuximab | 5 (1.2%) | |
| Cape-Bev - Sol Trial | 1 (0.2%) | |
| Encorafenib/Binimetinib + Panitumumab | 1 (0.2%) | |
| Treatment line | Neoadjuvant | 30 (7.2%) |
| Adjuvant | 76 (18.2%) | |
| 1st line | 199 (47.6%) | |
| 2nd line | 65 (15.6%) | |
| 3rd line | 39 (9.3%) | |
| 4th line | 8 (1.9%) | |
| 5th line | 1 (0.2%) | |
| Median number of cycles of treatment patients have already received at decision-making time-point | 4 (1;9) | |
| Nature of change in treatment due to COVID-19 | Change in treatment | 219 (52.4%) |
| Type of chemotherapy | 32 (7.7%) | |
| Dose of chemotherapy | 7 (1.7%) | |
| Delay in chemotherapy | 88 (21.1%) | |
| Lengthening the interval between doses | 26 (6.2%) | |
| Treatment cancelled | 43 (10.2%) | |
| Not commenced on next line of treatment at progression | 23 (5.5%) | |
| Continue as planned | 197 (47.6%) | |
| Decision-making time-point relative to national lockdown (23rd March 2020) (wk) | –1 | 121 (28.9%) |
| 0 | 95 (22.7%) | |
| +1 | 68 (16.3%) | |
| +2 | 43 (10.3%) | |
| +3 | 22 (5.3%) | |
| +4 | 23 (5.5%) | |
| +5 | 21 (5%) | |
| +6 | 21 (5%) | |
| +>6 | 4 (0.8%) |
Nature of Treatment Modifications
More than half of the patients (n = 219, 52.4%) have had some modifications to their treatment plan, as detailed in Table 1. More than 1 in 5 patients (n = 88, 21.1%) had their treatment delayed and 10.2% (n = 43) cancelled. Changes to the type of chemotherapy regime were reported in 7.7% (n = 32) of patients. Meanwhile, there was an in increase in the time interval between treatment doses in 6.2% (n = 26) of patients. In 5.5% (n = 23) of patients, there was a delay in starting the next line of treatment at disease progression.
In the subgroup of patients who were being considered for or undergoing neoadjuvant (n = 30) or adjuvant treatment (n = 76), the majority of patients (n = 61, 57.5%) proceeded with their treatment as initially planned (as per standard of care). In the majority of patients on neoadjuvant treatment, they either carried on with the initial plan (n = 23/30, 76.6%) or had some minor treatment modifications but still carried on with treatment (n = 6, 20%) (Table 2 ). Treatment was prematurely interrupted in one patient on neo-adjuvant chemotherapy, but only after he had already received 5 cycles of treatment. Meanwhile, 1 in 4 patients (n = 19/76) on adjuvant chemotherapy did not start or had their treatment cancelled while half of the patients (n = 38/76) carried on with their treatment as planned.
Table 2.
Nature of Treatment Modifications in Patients Being Considered for or Receiving Neoadjuvant or Adjuvant Treatment (n = 106)
| Neoadjuvant | Adjuvant | ||
|---|---|---|---|
| Nature of change in treatment due to COVID-19 | Change in treatment | 7 (23.4%) | 38 (50%) |
| Type of chemotherapy | 2 (6.7%) | 11 (14.5%) | |
| Dose of chemotherapy | 3 (10%) | 1 (1.3%) | |
| Interval lengthened | 0 | 2 (2.6%) | |
| Delay in chemotherapy | 1 (3.3%) | 5 (6.6%) | |
| Treatment cancelled | 1 (3.3%) | 18 (23.7%) | |
| Not commenced treatment | 0 | 1 (1.3%) | |
| Continue as planned | 23 (76.6%) | 38 (50%) | |
| 30 | 76 |
Factors Predicting Treatment Modifications
A change (any) in treatment was more likely in older patients [OR 1.028 (95% CI 1.010-1.047); P = .002], and in patients who had already received higher number of cycles of treatment at the decision-making time-point [OR 1.040 (95% CI 1.016-1.065); P = .001] (Table 3 ). A change in treatment was less likely further out of the start date of the UK national lockdown [OR 0.837 (95% CI 0.758-0.925); P < .001) (Table 3). Indeed, treatments carried on as per national guidelines in 70% (n = 48) of patients seen in clinic after 20th April 2020 compared to the intitial 4 weeks of lockdown.
Table 3.
Univariate and Multivariate Analyses of Factors Associated With a Change (any) in Treatment Due to COVID-19 (n = 418)
| Variables | Univariate Analysis |
Multivariate Analysis* |
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Age | 1.025 (1.008-1.041) | .004 | 1.028 (1.010-1.047) | .002 |
| Gender | 1.250 (0.848-1.842) | .260 | ||
| ECOG performance status | 1.052 (0.741-1.493) | .779 | ||
| Adjuvant/Neoadjuvant treatment | 1.583 (1.016-2.466) | .042 | 1.047 (0.647-1.693) | .852 |
| Number of lines of treatment for metastatic disease | 0.993 (0.759-1.299) | .960 | ||
| Number of cycles of treatment patients have already received at decision-making time-point | 1.044 (1.020-1.068) | <.001 | 1.040 (1.016-1.065) | .001 |
| Decision-making time-point relative to national lockdown (23rd March 2020) | 0.834 (0.761-0.915) | <.001 | 0.837 (0.758-0.925) | <.001 |
Multivariate analysis of factors with P < .05 in univariate analysis.
In further multivariate analysis, the number of lines of treatment for metastatic disease was a significant predictor of treatment cancellation or not commencing another line of treatment (OR 1.441 (95% CI 1.027-2.020); P = .034) (Table 4 ).
Table 4.
Univariate and Multivariate of Factors Associated With Treatment Cancellation or Not Commencing Next Line of Treatment (Due to Disease Progression) Due to COVID-19 (n = 418).
| Variables | Univariate Analysis |
Multivariate Analysis* |
||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Age | 1.027 (1.003-1.051) | .029 | 1.025 (0.997-1.055) | .080 |
| Gender | 1.040 (0.616-1.756) | .882 | ||
| ECOG performance status | 1.220 (0.760-1.957) | .411 | ||
| Adjuvant/Neoadjuvant treatment | 0.660 (0.376-1.161) | .149 | ||
| Number of lines of treatment for metastatic disease | 1.465 (1.047-2.052) | .026 | 1.441 (1.027-2.020) | .034 |
| Number of cycles of treatment patients have already received at decision-making time-point | 0.981 (0.951-1.012) | .226 | ||
| Decision-making time-point relative to national lockdown (23rd March 2020) | 0.942 (0.833-1.066) | .347 | ||
Multivariate analysis of factors with P < .05 in univariate analysis.
Subgroup Analysis and Outcomes in Patients on Third-Line Treatment or Beyond
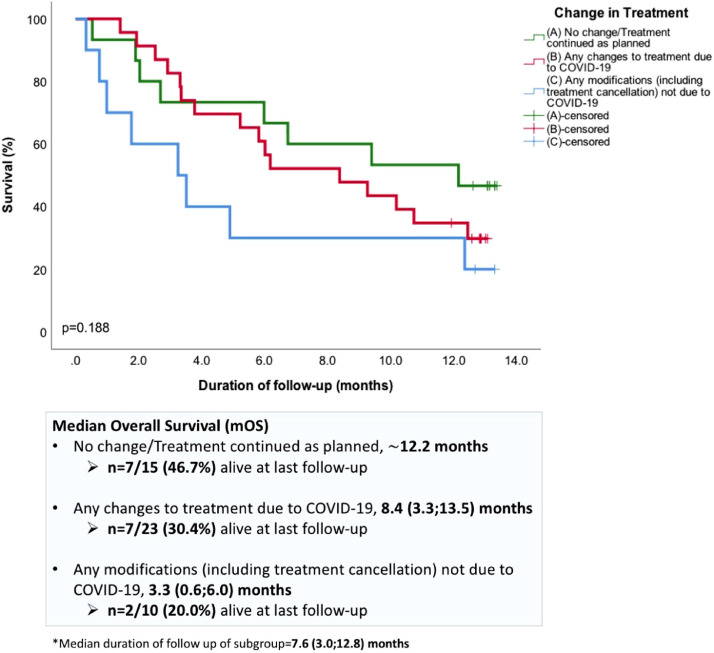
More than two-thirds of patients on third-line treatment have had alterations to their treatment plan (n = 33/48, 68.8%) (Table 5 ). The majority had delay to their treatment (35.4%, n = 17) or cancellation (10.4%, n = 5), and 19% (n = 9) did not commence on the next line of treatment on disease progression (Table 5). For this subgroup of patients who were considered for or on third-line of treatment or beyond, the median duration of follow-up (from decision-making time-point to censor date) was 7.6 (3.0;12.8) months. In the landmark analysis performed, compared to patients who continued on treatment as planned, any treatment modifications due to COVID-19 did not seem to negatively impact on survival outcomes (median overall survival (mOS) 12.2 vs. 8.4 months, not statistically significant, respectively) (Figure 1 ). Meanwhile, the mOS of the cohort of patients in whom treatment was cancelled or stopped due to poor general fitness or no further available treatment options (n = 10) was 3.3 (0.6;6.0) months (Figure 1).
Table 5.
Demographics of Subgroup of Patients Who Were Considered for or Receiving third-Line Treatment or Beyond (n = 48)
| Number (%) | ||
|---|---|---|
| Mean age (y) | 64.4 ± 11.8 | |
| Gender | Male | 30 (62.5%) |
| Female | 18 (37.5%) | |
| ECOG performance status | 0 | 12 (25%) |
| 1 | 32 (66.7%) | |
| 2 | 4 (8.3%) | |
| Primary diagnosis | Colorectcal cancer | 48 (100%) |
| Treatment line | 3rd line | 39 (81.2%) |
| 4th line | 8 (16.7%) | |
| 5th line | 1 (2.1%) | |
| Treatment regime | Lonsurf | 22 (45.8%) |
| OxMdG | 5 (10.4%) | |
| IrMdG | 6 (12.5%) | |
| Irinotecan + Capecitabine | 1 (2.1%) | |
| IrMdG + Cetuximab | 1 (2.1%) | |
| Single-agent Irinotecan | 5 (10.4%) | |
| 5FU + Cetuximab | 1 (2.1%) | |
| Capecitabine | 1 (2.1%) | |
| MCap | 3 (6.3%) | |
| Nivolumab | 2 (4.2%) | |
| Encorafenib + Cetuximab | 1 (2.1%) | |
| Median number of cycles of treatment patients have already received at decision-making time-point, n = 45 | 3 (2;7) | |
| Nature of change in treatment due to COVID-19 | Change in treatment | 33 (68.8%) |
| Dose of chemotherapy | 1 (2.1%) | |
| Interval lengthened | 1 (2.1%) | |
| Delay in chemotherapy | 17 (35.4%) | |
| Treatment cancelled | 5 (10.4%) | |
| Not commenced on next line of treatment at progression | 9 (18.8%) | |
| Continue as planned | 15 (31.3%) | |
| Changes due to COVID-19 | Yes | 22 (66.7%) |
| No | 11 (33.3%) | |
| Not applicable | 15 | |
| Decision-making time-point relative to national lockdown (23rd March 2020) (wk) | –1 | 10 (21.3%) |
| 0 | 12 (25.5%) | |
| +1 | 10 (21.3%) | |
| +2 | 7 (14.9%) | |
| +3 | 2 (4.3%) | |
| +4 | 2 (4.3%) | |
| +5 | 2 (4.3%) | |
| +6 | 3 (6.4%) | |
| Alive at last follow-up | Yes | 22 (45.8%) |
| No | 26 (54.2%) |
Figure 1.
Landmark survival analysis (Kaplan-Meier) of subgroup of patients who were considered for or receiving third-line treatment or beyond (n = 48). Patients who had (A) no change/treatment continued as planned (green line) or (B) any changes to treatment due to COVID-19 (red line) were compared to those who had (C) any modifications not due to COVID-19 for example, treatment cancelled or stopped due to patient's condition or no further lines of treatment available (blue line).
Discussion
The COVID-19 pandemic has resulted in significant changes in the delivery of care in cancer patients in the UK, with a 30% drop in referral of patients with colorectal cancer as well as changes in management plans.11 , 12 In the initial phase of the pandemic, there was lack of evidence on the safety of continuing SACT which were potentially immunosupressive in the face of the risk of rampant community and/or hospital acquired COVID-19 infection. National and international guidance suggested a number of ways to mitigate patient risks including that of nosocomial infections by implementing strategies such as increasing treatment intervals, changing intravenous to oral treatments where possible, deferring treatments when clincally appropriate, or the use of GM-CSF to decrease the incidence of neutropenia.13 However, the adoption of such recommendations were heterogeneous across different service set-up, and it remains unclear how clinicians perceived these recommendations and what were the factors predicting decision-making in this crisis. To our knowledge, this is the first prospective/retropsective study to evaluate this.
It was evident that older patients were more likely to be recommended a change in their treatment. This reflects that age was rapidly identified to be associated with a higher risk of developing COVID-19 complications with disproportionately higher prevalence of hospitalisations and deaths amongst elderly patients.14 We observed that a change in treatment plan was less likely further out from the start date of the first UK national lockdown. The initial changes in SACT delivery reflect the genuine clinical uncertainty faced by clinicians during the first wave of COVID-19 and the subsequent national and international guidelines which were produced. The recovery is unsurprising considering the rapid availability of reassuring safety data related to SACT delivery as the pandemic evolved. These results are concordant with the retrospective analysis on the registered treatments on the NHS England website, where the initial 36% drop in treatments in April 2020 was followed by a gradual increase of treatment in the subsequent 2 months.9 A growing body of evidence suggested that chemotherapy did not adversely affect COVID-19 infection outcomes which helped clinicians to gradually resume standard oncological management plans.15 In addition, patients who had received a high number of treatment lines for metastatic disease prior to the first COVID-19 lockdown were more likely to have their treatment cancelled, or not be offered further treatment at disease progression. This is in agreement with national guidance on chemotherapy "priority groups”3 as the potential benefit of third- or subsequent lines of treatment is small or uncertain and the risk of complications from COVID-19 may outweight any benefit from receiving SACT.
More than half of our patients had a change in their chemotherapy plan in the initial few weeks of the pandemic. In the National Cancer Bowel Audit Survey by Boyle et al., 12 more than 50% of the patients also had a change in the mangement plan in mid-April 2020. However, this survey only reported on changes in the delivery of adjuvant and neoadjuvant chemotherapy and/or chemoradiotherapy, and less than 40% of the centres involved in the survey reported more than 50% changes in adjuvant activity. Our study reports on all consecutive patients seen in the colorectal cancer outpatient clinics in a large tertiary cancer centre, including patients with metastatic disease and those being considered for third-line treatment or beyond, which further complements to the national survey to better reflect the overall experience of the specific changes imposed by the COVID-19 pandemic on the management of all patients with colorectal cancer.
In the initial weeks of COVID-19 outbreak in China, up to 50% of patients with stage II and III colon cancer on adjuvant chemotherapy experienced delays in the initiation of the next cycle of treatment, while 10% had their treatment changed to single agent capecitabine.16 Similarly, in our cohort, half of the patients had a treatment change, reflecting the initial hesitance to offer treatment when it was clinically felt that the risks would outweigh benefits during this period. The importance of timely initiation of adjuvant chemotherapy in patients with colorectal cancer is well described in the literature.17 , 18 The fact that most patients carried on with adjuvant treatment, albeit with some modifications, was in accordance with NICE guidance that this group of patients were in “priority level 3” (benefit from adjuvant chemotherapy 10%-20%) or “priority level 4” (benefit from adjuvant chemotherapy less than 10%)3. On the other hand, most of the neoajuvant treatment carried on as planned reflecting the potential high benefit anticipated with this treatment (“priority level 2” – adds 20%-50% chance of cure to surgery or radiotherapy alone). To our knowledge, this is the first report assessing the changes in neoadjuvant treatment in patients with colorectal cancer.
We specifically interrogated the impact of changes to treatment on the subgroup of patients on third-line treatment or beyond as we hypothesised that any significant effects may be revealed in this subgroup in a relatively short duration of follow-up. Interestingly, irrespective of the nature of treatment changes, there was no survival difference observed in the initial 3 months after a COVID related change in management. Beyond this time-point, those who carried on with treatment subjectively did better but the sample size was too small for robust statistical analysis. Intermittent chemotherapy is an established option in colorectal oncology especially in early lines of treatment19 and this is reflected in our study especially for those patients who are symptomatically/clinically stable enough to have a short treatment break.
Despite the strengths of our study, limitations need to be acknowledged. While this study reports observations in a relatively large sample size of consecutive patients, this is a single centre experience and factors contributing to decision-making need validating in other tertiary cancer or hospital settings. In the subgroup of patients on third-line treatment or beyond, landmark analysis was used to report on survival outcomes based on our prespecified decision-making time-point during the first UK national lockdown. With the relatively small number of patients within this subgroup and the need for longer follow up, the results of this analysis needs to be interpreted with caution. Other factors that could also contribute to survival outcomes, such as burden of disease were not included in the survival analysis.
Clinical Practice Points
-
•
Early in the pandemic, >50% patients with colorectal cancer had modifications to their treatment.
-
•
Almost all patients on neoadjuvant treatment proceeded with treatment.
-
•
Half of patients on adjuvant treatment had their treatment plan modified.
-
•
A change in treatment was more likely in older patients, those who already had more treatment and close to the start of the United Kingdom lockdown.
-
•
Patients on third-line treatment were most likely to have alterations to their treatment plan.
Conclusion
In conclusion, clinicians followed insitutional and national guidance to alter SACT plan in the initial phase of the pandemic, followed by adaptation and rationalisation of clinical practice with increased awareness and understanding of the realtime implications of the COVID-19 pandemic on patients with cancer. Short delays and carefully considered modifications in cancer treatment in the face of COVID-19 did not appear to adversely affect survival outcomes in our cohort. Larger population studies to report real-world outcomes are needed to validate our study and provide vital documentation of the impact of changes to treatment on patients with cancer, in order to make informed care plans as we recover from the pandemic.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. KHJL is currently funded by the Wellcome-Imperial 4i Clinical Fellowship.
Disclosure
KHJL reports speaker honorarium from Janssen, outside the submitted work. JB reports grants and non-financial support from Ipsen, non-financial support from Novartis, personal fees and non-financial support from Pfizer, nonfinancial support from AAA, nonfinancial support from Nanostring, Unites States, and personal fees from Nutricia, Netherlans, outside the submitted work. MS reports personal fees from Servier Global, Merck, UK, and Amgen, UK, outside the submitted work. The remaining authors have stated that they have no conflicts of interest.
References
- 1.Spicer J, Chamberlain C, Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17:329–331. doi: 10.1038/s41571-020-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health England. Coronavirus (COVID-19): guidance. 2020. Available from: https://www.gov.uk/government/collections/coronavirus-covid-19-list-of-guidance (accessed March 16, 2021).
- 3.National Health Service (NHS). Clinical guide for the management of cancer patients during the coronavirus pandemic. Available from: https://www.uhb.nhs.uk/coronavirus-staff/downloads/pdf/CoronavirusCancerManagement.pdf (accessed March 16, 2021).
- 4.National Institute of Health and Care Excellence (NICE). COVID-19 rapid guideline: delivery of systemic anticancer treatments. Available from: https://www.nice.org.uk/guidance/ng161 (accessed March 16, 2021). [PubMed]
- 5.Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tørring ML, Murchie P, Hamilton W, et al. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017;117:888–897. doi: 10.1038/bjc.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer K, Jones CM, Girdler R, et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–320. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark JJ, Dwyer D, Pinwill N, Clark P, Johnson P, Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22:66–73. doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter MA, Murphy J, Cameron D, et al. The impact of COVID-19 on systemic anticancer treatment delivery in Scotland. Br J Cancer. 2021 doi: 10.1038/s41416-021-01262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamposioras K, Saunders M, Lim KHJ, et al. The impact of changes in service delivery in patients with colorectal cancer during the initial phase of the COVID-19 pandemic. Clin Colorectal Cancer. 2020 doi: 10.1016/j.clcc.2020.11.006. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle JM, Kuryba A, Blake HA, et al. The impact of the first peak of the COVID-19 pandemic on colorectal cancer services in England and Wales: a national survey. Color Dis. 2021 doi: 10.1111/codi.15622. codi.15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamposioras K, Mauri D, Papadimitriou K, et al. Synthesis of recommendations from 25 countries and 31 oncology societies: how to navigate through Covid-19 labyrinth. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.575148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Xu Y, Zhang T, Yang Y. Impact of the COVID-19 outbreak on adjuvant chemotherapy for patients with stage II or III colon cancer: experiences from a multicentre clinical trial in China. Curr Oncol. 2020;27:159–162. doi: 10.3747/co.27.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner MC, Farrow NE, Rhodin KE, et al. Delay in adjuvant chemotherapy and survival advantage in stage III colon cancer. J Am Coll Surg. 2018;226:670–678. doi: 10.1016/j.jamcollsurg.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Minicozzi P, Vicentini M, Innos K, et al. Comorbidities, timing of treatments, and chemotherapy use influence outcomes in stage III colon cancer: a population-based European study. Eur J Surg Oncol. 2020;46:1151–1159. doi: 10.1016/j.ejso.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Loree JM, Tan SK, Lafond LM, Speers CH, Kennecke HF, Cheung WY. Real-world effect of maintenance and intermittent chemotherapy on survival in metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:65–72. doi: 10.1016/j.clcc.2017.10.011. [DOI] [PubMed] [Google Scholar]