Abstract
We report a case of thyroid storm precipitated by SARS-CoV-2 infection in an adolescent girl with a history of Graves disease and dilated cardiomyopathy. This case highlights that SARS-CoV-2 infection can potentially trigger a thyrotoxicosis crisis and acute decompensated heart failure in a patient with underlying thyroid disease and myocardial dysfunction even in the absence of multi-system inflammatory syndrome in children. We systematically reviewed the thyrotoxicosis cases with SARS-CoV-2 infection and described its impact on pre-existing dilated cardiomyopathy.
Keywords: SARS-CoV-2 infection in children, thyroid storm, graves disease, dilated cardiomyopathy
As of April 26, 2021, more than 147 million confirmed cases of coronavirus disease 2019 (COVID-19) had been established worldwide, with more than 3.1 million deaths. More than 42 million cases have been confirmed in the United States, with more than 563,000 deaths. 1 The COVID-19 infection incidence is highest among persons aged ≥80 years and lowest among children aged ≤9 years. 2 The infection with SARS-CoV-2 is usually mild in children, 3 however, children may develop multisystem inflammatory syndrome in children 2–4 weeks after SARS-CoV-2 infection. There are >800 cases of multisystem inflammatory syndrome in children that have been reported so far. 4 The clinical presentation of the multisystem inflammatory syndrome in children is usually vascular (vasodilation), cardiac, or a combination and less commonly pulmonary disease. Left ventricular dysfunction is the most common cardiac manifestation of the multisystem inflammatory syndrome in children, followed by coronary artery aneurysm and cardiac arrhythmia. 4 Most children recover from the multisystem inflammatory syndrome in children, but long-term sequelae are unknown. Furthermore, it is not known how SARS-CoV-2 infection affects children who have pre-existing left ventricular dysfunction. We present a case of thyroid storm precipitated by SARS-CoV-2 infection in an adolescent girl with a history of Graves disease and dilated cardiomyopathy. This case highlights that SARS-CoV-2 infection can be a potential trigger of thyrotoxicosis and acute decompensated heart failure in a patient with underlying thyroid disease and dilated cardiomyopathy with stable cardiac function, even in the absence of multi-system inflammatory syndrome in children.
Case presentation
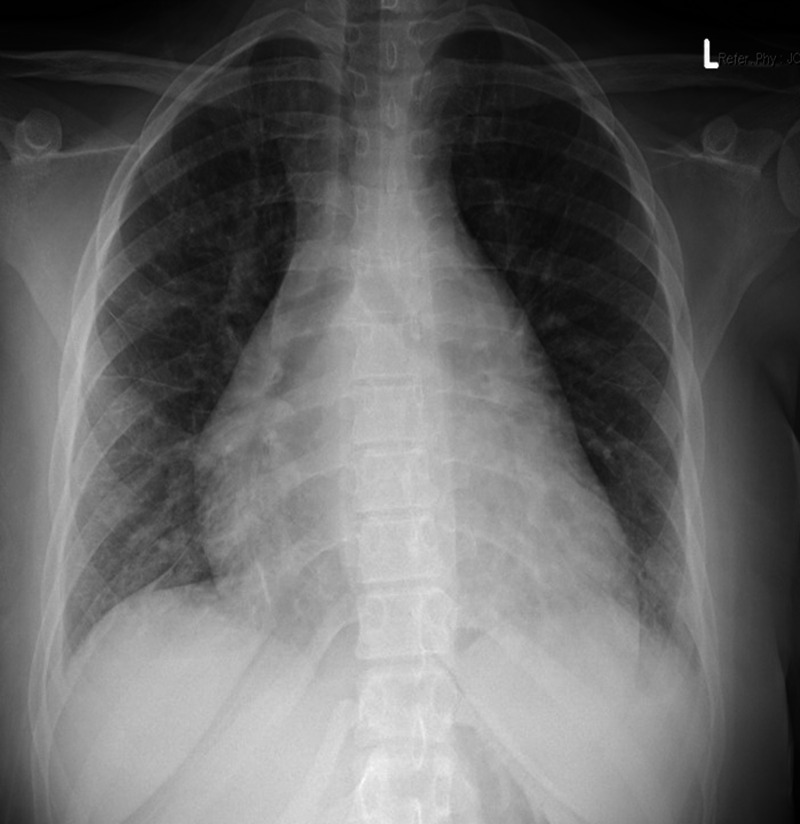
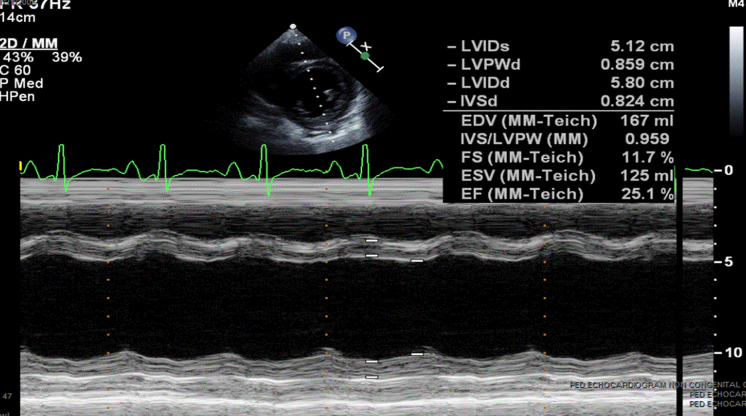
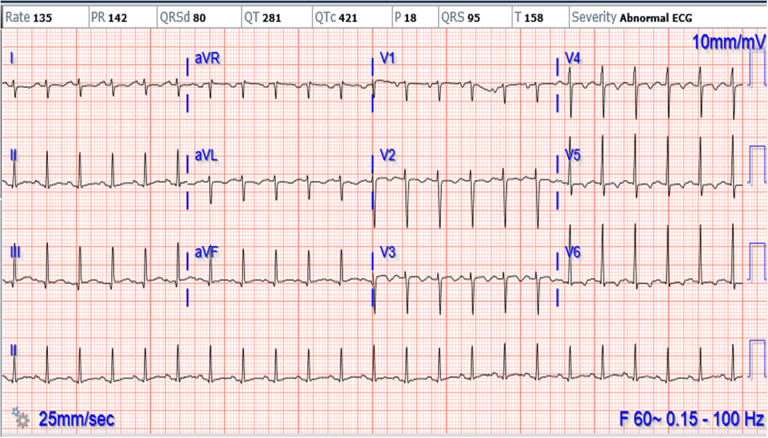
A 16-year-old female with cough and difficulty breathing for 3 days presented to an emergency department and was found positive for SARS-CoV-2 by rapid test. She was afebrile, tachycardic and hypertensive. She had exophthalmos, thyromegaly and a gallop on physical exam. Chest X-ray showed cardiomegaly with pulmonary oedema (Fig 1). She was treated with intravenous Furosemide 40 mg in the emergency room. Her echocardiogram showed severely decreased left ventricular systolic function (ejection fraction 14%), moderate to severe left ventricular dilation, mildly decreased right ventricular systolic function without any evidence of coronary artery dilation or pericardial effusion. (Fig 2). A 12-lead electrocardiogram showed sinus tachycardia and non-specific ST changes (Fig 3). The nasopharyngeal swab specimen was positive for SARS-CoV-2 by reverse transcription-polymerase chain reaction. Her thyroid-stimulating hormone level was extremely low (0.01 mICU/mL) with markedly elevated triiodothyronine (1070 pg/dL) and free thyroxine levels (3760 ng/dL). Additional work to rule out multisystem inflammatory syndrome in children, including ferritin, C-reactive protein, erythrocyte sedimentation rate, troponin, was within normal range except for elevated D-dimer (1152 ng/mL). Infectious workup for respiratory pathogens (influenza viruses A and B, parainfluenza virus 1–4, respiratory syncytial virus, rhinovirus, adenovirus, enterovirus, metapneumovirus, Mycoplasma pneumonia and Chlamydophila pneumoniae) were negative. Serologic tests for cytomegalovirus, Epstein–Barr virus, parvovirus B19 and polymerase chain reaction analyses in blood for enterovirus, parvovirus, human herpes simplex virus 6 and adenovirus were negative. Blood and stool cultures were negative for bacterial pathogens. The stool for campylobacter jejuni and Shiga toxin were negative. All other clinical laboratory test results are summarised in Table 1.
Figure 1.
Chest X-Ray showing cardiomegaly and interstitial oedema.
Figure 2.
M-mode images displaying left ventricular dimension and function.
Figure 3.
ECG showing sinus tachycardia and non-specific ST changes.
Table 1.
Summary of laboratory test results
| Measure | Reference range | Results |
|---|---|---|
| White-cell count (per μL) | 3,900–10,200 | 6700 |
| Platelet count (per μL) | 150,000–370,000 | 392,000 |
| Haemoglobin (g/dL) | 12.0–15.6 | 11.1 |
| Haematocrit (%) | 35.5–45.5 | 32.8 |
| Sodium (mmol/L) | 136–145 | 140 |
| Potassium (mmol/L) | 3.5–5.1 | 3.4 |
| Chloride (mmol/L) | 99–109 | 109 |
| Glucose (mg/dL) | 74–106 | 96 |
| Creatinine (mg/dL) | 0.50–1.10 | 0.63 |
| Total protein (g/dL) | 6.4–8.3 | 7.4 |
| Albumin (g/Ll) | 2.9–5.2 | 4.5 |
| Total bilirubin (mg/dL) | 0.30–1.20 | 1.31 |
| Alanine aminotransferase (U/L) | <40 | 64 |
| Aspartate aminotransferase (U/L) | <35 | 104 |
| Lactate dehydrogenase (U/L) | 100–190 | 139 |
| Troponin I (ng/mL) | <0.03 | <0.01 |
| Creatine kinase (U/L) | 35–210 | 36 |
| C-reactive protein (mg/L) | 0.0–5.0 | 1.2 |
| Erythrocyte sedimentation rate (mm/h) | 0–20 | 9 |
| ProBNP (pg/mL) | 5–125 | 8,859 |
| D-dimer (ng/mL) | 0–500 | 1152 |
| Fibrinogen (mg/dL) | 150–450 | 294 |
| Prothrombin time (s) | 70–120 | 13.9 |
| Ferritin (ng/mL) | 20–250 | 13 |
| Free thyroxine (ng/dL) | 0.9–1.6 | >7.7 |
| T3 level (ng/mL) | 0.83–2.15 | 3.7 |
| Thyroid stimulating hormone (mcIU/mL) | 0.53–3.59 | 0.01 |
| Anti-thyroglobulin antibody (IU/mL) | ≤4.11 IU/ml | 20.38 |
| Thyroid peroxidase antibody (IU/mL) | ≤5.61 IU/mL | >2000 |
| Thyrotropin receptor antibody (IU/mL) | 0–1.75 | 11 |
| Thyroid-stimulating Ig TSI index | ≤1.3 | 6.4 |
She required higher doses of Methimazole up to 20 mg twice daily and beta-blockade with Metoprolol. In addition, oral heart failure therapy with Lasix, Lisinopril and Aldactone alleviated her cardiac symptoms. She responded well with improvement in tachycardia, hypertension and pulmonary oedema. Her triiodothyronine and free thyroxine levels gradually declined. Follow-up echocardiograms (performed on days 3, 5 and 10) showed stabilisation of severely decreased left ventricular systolic function with an ejection fraction between 14 and 18%. She was started on prophylactic Lovenox therapy during hospitalisation, which was stopped after her D-dimer decreased from 1152 to 422 ng/mL. Her pro-BNP also trended down from 8859 to 2,200 pg/ml. She was discharged home on methimazole, aspirin and oral heart failure therapy.
She was followed as an out-patient, and further workup to rule out other causes of dilated cardiomyopathy, including serum amino acids and urine organic acids, were negative. The whole-genome chromosomal microarray showed a duplication of a region within cytogenetic band 12q 24.21, uncertain clinical significance. Three months later from her initial SARS-CoV-2 infection, a cardiac magnetic resonance was performed, which showed severely decreased left ventricular systolic function (ejection fraction 25%), severe dilation with the left-ventricular end-diastolic volume of 167 ml/m2, and mildly depressed right ventricular systolic function (ejection fraction 47%). She did not meet Lake Louise’s imaging criteria for myocarditis diagnosis (no evidence of early or late gadolinium enhancement, no hyperintense signal noted in the myocardium). 5 Myocardial T2 relaxation time was within the normal limit (44–49 ms), suggesting no evidence of myocardial oedema. There was a prolonged myocardial native T1 relaxation time, 1079 ms (Normal, 995.8 ± 30.9 ms), suggesting diffuse myocardial fibrosis secondary to cardiomyopathy (Fig 4).
Figure 4.

Cardiac magnetic resonance imaging showing myocardial fibrosis on T1 mapping.
Discussion
To our knowledge, we describe the first case of thyrotoxicosis precipitated after SARS-CoV-2 infection in a paediatric patient. A literature review showed a 45-year-old female with known thyroid disease who had a thyroid storm and responded well to Atenolol and Methimazole. 6 In another report, an 18-year-old-female was diagnosed with subacute thyroiditis 15 days after SARS-CoV-2 but no evidence of thyrotoxicosis. 7
Any infection can trigger a thyroid storm, but we have limited knowledge about whether SARS-CoV-2 can precipitate a thyroid storm. Patients with Graves’ disease may be at high risk for precipitation of thyrotoxicosis crisis with SARS-CoV2 infection due to its propensity to cause multi-organ system dysfunction. The cytokine response described in COVID-19, specifically, a hyperactivation of T-helper cell response and an increase in interleukin 6, can alter thyroid function and structure. There is some evidence that the virus may have a particular affinity for the thyroid gland. 8 It is known that the SARS-CoV-2 virus produces its effects through its spike protein after binding with the angiotensin-converting enzyme 2 receptors found in the alveolar lung cells, the vascular endothelium and cardiac cardiomyocytes. 9 As a matter of fact, several endocrine organs express angiotensin-converting enzyme 2, namely, the pancreas, thyroid, testis, ovary, adrenal glands and pituitary. 10 Our patient did not have multi-system inflammatory syndrome in children according to criteria laid out by the Center for Disease Control. 11 She presented with a thyroid storm, which further caused acute exacerbation of heart failure. During the COVID-19 pandemic, it has been a challenge to decide whether the patient has multi-system inflammatory syndrome in children or not, as its management requires intravenous gamma globulin and steroids. 12 Our case highlights that history and thorough physical examination help narrow the differential diagnosis and obtain appropriate clinical laboratory tests to reach a final diagnosis. These are important considerations, especially in patients with severe left ventricular dysfunction who may not tolerate the volume overload associated with intravenous gamma globulin infusion.
Our patient was diagnosed with acute decompensated heart failure when she was admitted to the emergency department for a thyroid storm. She had a remote history of dilated cardiomyopathy, but her cardiac function was stable, and no prior documents available to know details about her cardiomyopathy. From history, parents informed that, she had no heart failure symptoms and was not on any cardiac medications. We have limited knowledge on COVID-19 presentation and disease severity in patients with pre-existing dilated cardiomyopathy infected with SARS-CoV-2. A single case report of an infant with enterovirus-induced myocarditis and cardiomyopathy had SARS-CoV-2 infection and subsequently required heart transplantation has been reported. 13 Cardiac involvement in children due to SARS-CoV-2 is still scarce but rapidly emerging in the literature. However, cardiac manifestations are frequent in adults. Puntmann et al. evaluated adults at a mean of 71 days after confirmed COVID-19 diagnosis. 78% had demonstrable cardiac involvement via cardiac magnetic resonance, 76% had detectable high-sensitivity troponin and 60% had evidence of active myocardial inflammation by cardiac magnetic resonance. 14 The cardiac magnetic resonance data revealed that adults infected with SARS-CoV-2 had some myocardial involvement regardless of any preexisting conditions, the severity or course of their infection, the time from their original diagnosis or the presence of any specific heart-related symptoms. Recently, Lindner et al. published that older adults, who had tested positive for COVID-19, had the SARS-CoV-2 virus in their myocardial tissue samples but did not show signs of inflammation or myocarditis. 15 In our patient, there was no significant cardiac magnetic resonance evidence of myocardial inflammation. There was no evidence of myocardial delayed Gadolinium contrast agent enhancement, which rules out focal myocardial scarring. Prolonged T1 relaxation time, as noted in our patient in this scenario, suggests diffuse fibrosis, which could be secondary to cardiomyopathy.
Conclusions
To the best of our knowledge, this is the first reported case of COVID-19 in an adolescent with history of remote dilated cardiomyopathy who presents with the precipitation of thyrotoxicosis crisis. This case report highlights the importance of history and physical examination to ensure appropriate diagnosis and management. Also, we recommend caution in patients with a previous history of thyroid disease, as SARS-CoV-2 could provoke thyroid storms. It is not fully understood about the effects of SARS-CoV-2 on the heart and circulation, especially in pre-existing dilated cardiomyopathy patients. More research is needed about the long-term impact of SARS CoV-2 infection on the thyroid gland and the heart, especially with pre-existing myocardial dysfunction.
Acknowledgements
We are thankful to Pediatric ICU team for their contribution in excellent care and patient management.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
References
- 1.COVID-19 Map. John Hopkins University Coronavirus Resource Center. Retrieved April 26, 2021, fromhttps://coronavirus.jhus.edu/map
- 2. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. CMMID COVID-19 working group. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med 2020; 26: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. United States COVID-19 Cases and Deaths by State. Updated August 19, 2020. Retrieved April 13, 2021, from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 4. Alsaied T, Tremouulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021; 143: 78–88. [DOI] [PubMed] [Google Scholar]
- 5. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009; 53: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pastor S, Molina Sr. A, Celis De E. Thyrotoxicocrisis and COVID-19 infection: an extraordinary case and literature review. Cureus 2020; 12: e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endocrinol Metab 2020; 105: 2367–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Retrieved March 30, 2021, from https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/children/mis-c.html
- 9. The European Society of Cardiology.ESC Guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Retrieved March 30, 2021, from https://www.escardio.org/Education/VOVID-19-and-Cardiology/ESC-COVID-19-Guidance
- 10. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020; 22: 2128–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kristoffersen AW, Knudsen PK, Moller T. SARS-CoV-2 in an infant with severe dilated cardiomyopathy. Cardiol Young 2021; 31: 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020; 5: 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]