Abstract
A multilocus sequence typing (MLST) scheme has been developed for Staphylococcus aureus. The sequences of internal fragments of seven housekeeping genes were obtained for 155 S. aureus isolates from patients with community-acquired and hospital-acquired invasive disease in the Oxford, United Kingdom, area. Fifty-three different allelic profiles were identified, and 17 of these were represented by at least two isolates. The MLST scheme was highly discriminatory and was validated by showing that pairs of isolates with the same allelic profile produced very similar SmaI restriction fragment patterns by pulsed-field gel electrophoresis. All 22 isolates with the most prevalent allelic profile were methicillin-resistant S. aureus (MRSA) isolates and had allelic profiles identical to that of a reference strain of the epidemic MRSA clone 16 (EMRSA-16). Four MRSA isolates that were identical in allelic profile to the other major epidemic MRSA clone prevalent in British hospitals (clone EMRSA-15) were also identified. The majority of isolates (81%) were methicillin-susceptible S. aureus (MSSA) isolates, and seven MSSA clones included five or more isolates. Three of the MSSA clones included at least five isolates from patients with community-acquired invasive disease and may represent virulent clones with an increased ability to cause disease in otherwise healthy individuals. The most prevalent MSSA clone (17 isolates) was very closely related to EMRSA-16, and the success of the latter clone at causing disease in hospitals may be due to its emergence from a virulent MSSA clone that was already a major cause of invasive disease in both the community and hospital settings. MLST provides an unambiguous method for assigning MRSA and MSSA isolates to known clones or assigning them as novel clones via the Internet.
Staphylococcus aureus is a major pathogen that is associated with serious community-acquired and nosocomial disease (8, 29). In the United Kingdom, S. aureus is the second most common isolate from blood cultures after Escherichia coli and is by far the most common hospital-acquired organism (1). Clinical syndromes associated with severe disease include bacteremia, pneumonia, endocarditis, septic arthritis, osteomyelitis, and deep abscess formation.
In the preantibiotic era, the rate of mortality from invasive S. aureus disease was high and the introduction of penicillin had a dramatic impact on treatment. The semisynthetic penicillin methicillin was introduced in 1959 to overcome the problems that arose from the increasing prevalence of penicillinase-producing isolates of S. aureus resistant to penicillin G and penicillin V. Methicillin-resistant S. aureus (MRSA) strains rapidly emerged and became a major clinical problem within hospitals during the 1960s in Europe and the 1970s in the United States and elsewhere (12, 20, 27). Many MRSA strains are resistant to most other classes of antimicrobial agents and are susceptible only to glycopeptides and new investigational drugs. The recent reports of MRSA with decreased susceptibility to glycopeptides (glycopeptide-intermediate S. aureus) [GISA]) threatens to compromise further our ability to treat hospital-acquired S. aureus infections (13, 26).
Although the infection control problems caused by MRSA and GISA and the limited therapeutic options are of major concern for hospitals, the majority of S. aureus infections acquired within hospitals and almost all S. aureus infections acquired within the community are caused by strains that are susceptible to methicillin and most other classes of antibiotics (methicillin-susceptible S. aureus [MSSA]). Few studies have characterized MSSA isolates (19, 33), and it is unclear whether some MSSA clones that are circulating within the community (or in hospitals) have a particular ability to cause serious infections (hypervirulent clones) and have an international distribution. We are also ignorant of the basic features of the population and evolutionary biology of this important pathogen.
Many of the basic questions about the population biology of S. aureus and a better understanding of the origins and spread of MRSA clones and of the relatedness of MRSA and MSSA clones described by different laboratories could be answered if we could characterize isolates of this species unambiguously. The most widely used molecular typing method for the study of the local and global epidemiologies of MRSA is pulsed-field gel electrophoresis (PFGE) (2). This method has proved very successful for the investigation of nosocomial outbreaks (4, 7, 21) and has also been used to identify MRSA clones that have a particular ability to cause major outbreaks and to spread nationally and internationally (epidemic MRSA clones; EMRSA [6, 22]). A major disadvantage of PFGE and all methods that depend on comparisons of DNA fragment patterns on gels is the difficulty of comparing the results from different laboratories. For this reason there is considerable confusion about the genetic relatedness of the clones of EMRSA described by different laboratories and a pressing need for a method that allows EMRSA clones and virulent MSSA clones to be identified unambiguously without the need to exchange reference strains.
Multilocus sequence typing (MLST) is a highly discriminatory method of characterizing bacterial isolates on the basis of the sequences of ∼450-bp internal fragments of seven housekeeping genes (16). For each gene fragment, the different sequences are assigned as distinct alleles, and each isolate is defined by the alleles at each of the seven housekeeping loci (the allelic profile or sequence type [ST]). As there are many alleles at each of seven loci, isolates are highly unlikely to have identical allelic profiles by chance, and isolates with the same allelic profile can be assigned as members of the same clone (16, 28). Sequence data are readily compared between laboratories, and a major advantage of MLST is the ability to compare the results obtained in different studies via the Internet. In addition, the data obtained by MLST can be used to address basic questions about the evolutionary and population biology of bacterial species (11, 28).
MLST has been developed for the identification of the hypervirulent lineages of Neisseria meningitidis (16) and for assigning Streptococcus pneumoniae strains to the major hypervirulent clones (9, 10) and to the major penicillin-resistant and multiple-antibiotic-resistant clones (10, 25). In this report we describe the development and validation of an MLST scheme for S. aureus and demonstrate the utility of the method by identifying the MRSA and MSSA clones within a sample of 155 recent isolates from patients with serious community-acquired and hospital-acquired infections.
MATERIALS AND METHODS
Bacterial isolates.
We selected 155 consecutive bacterial isolates from prospectively selected patients with invasive S. aureus disease identified in the Oxford, United Kingdom, region in 1997 or 1998. Invasive S. aureus disease was defined as the isolation of the organism from a normally sterile site in a patient with clinical signs and symptoms consistent with S. aureus infection. Isolates from patients with deep local infections associated with prosthetic material were excluded, as were isolates from patients with septicemia in which S. aureus was isolated from only one blood culture bottle (of at least two inoculated). Patients were assigned to one of two groups on the basis of clinical details: those with community-acquired disease and those with hospital-acquired disease. Community-acquired disease was defined as an illness consistent with invasive S. aureus disease that required admission to hospital and that resulted in the isolation of S. aureus from a specimen taken from a normally sterile site within 24 h of admission. In addition, patients hospitalized for any reason within the preceding 6 months were excluded from this category. Of the 155 isolates, 61 were from patients with community-acquired disease and 94 were from patients with hospital-acquired disease. One hundred forty isolates were obtained from blood cultures, and the other 15 were obtained from deep pus samples from patients with invasive S. aureus disease. Isolates were confirmed to be S. aureus by positive tube coagulase and DNase tests. Resistance to methicillin was determined by measuring the oxacillin MIC for the organism. This was done by using the oxacillin E-test (AB Biodisk, Solna, Sweden), with resistance defined as an MIC of >2 μg ml−1 (18).
MLST.
The DNA sequences of 14 housekeeping genes were supplied by M. Burnham of SmithKline Beecham. The sequences of their gene products were compared with those in the EMBL/GenBank database by using BlastP (http://www.ncbi.nlm.nih.gov/blast/blast.cgi), and the amino acid sequences of the pneumococcal protein and its homologs were aligned and the most variable regions were identified. Primers were designed by using the sequences of the highly conserved regions that flank the more variable regions. Each primer pair amplified an internal fragment of the housekeeping gene (about 500 bp) and allowed accurate sequencing of ∼450-bp fragments of each gene on both strands.
The following seven housekeeping genes were used in the final MLST scheme and the fragments were amplified by using the primers shown in Table 1: carbamate kinase (arcC), shikimate dehydrogenase (aroE), glycerol kinase (glp), guanylate kinase (gmk), phosphate acetyltransferase (pta), triosephosphate isomerase (tpi), and acetyl coenzyme A acetyltransferase (yqiL).
TABLE 1.
Sequences of primers used in the PCR
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| Carbamate kinase (arcC) | arcC-Up | TTGATTCACCAGCGCGTATTGTC |
| arcC-Dn | AGGTATCTGCTTCAATCAGCG | |
| Shikimate dehydrogenase (aroE) | aroE-Up | ATCGGAAATCCTATTTCACATTC |
| aroE-Dn | GGTGTTGTATTAATAACGATATC | |
| Glycerol kinase (glpF) | glpF-Up | CTAGGAACTGCAATCTTAATCC |
| glpF-Dn | TGGTAAAATCGCATGTCCAATTC | |
| Guanylate kinase (gmk) | gmk-Up | ATCGTTTTATCGGGACCATC |
| gmk-Dn | TCATTAACTACAACGTAATCGTA | |
| Phosphate acetyltransferase (pta) | pta-Up | GTTAAAATCGTATTACCTGAAGG |
| pta-Dn | GACCCTTTTGTTGAAAAGCTTAA | |
| Triosephosphate isomerase (tpi) | tpi-Up | TCGTTCATTCTGAACGTCGTGAA |
| tpi-Dn | TTTGCACCTTCTAACAATTGTAC | |
| Acetyl coenzyme A acetyltransferase (yqiL) | yqiL-Up | CAGCATACAGGACACCTATTGGC |
| yqiL-Dn | CGTTGAGGAATCGATACTGGAAC |
Chromosomal DNA was extracted by the method of Jordens and Pennington (14), but the method was modified for S. aureus by the inclusion of lysostaphin (Sigma) at a final concentration of 30 μg ml−1 at the cell lysis step. PCRs were carried out with 50-μl reaction volumes containing 0.5 μl of chromosomal DNA (approximately 0.5 μg), 0.5 μg of each primer, 1 U of Taq DNA polymerase (Qiagen, Crawley, United Kingdom), 5 μl of 10× buffer (supplied with the Taq polymerase), and 0.2 mM deoxynucleoside triphosphates (Perkin-Elmer Applied Biosystems; Foster City, Calif.). The PCR was performed in a PTC-200 DNA engine (MJ Research, Boston, Mass.) with an initial 5-min denaturation at 95°C, followed by 30 cycles of annealing at 55°C for 1 min, extension at 72°C for 1 min, and denaturation at 95°C for 1 min, followed by a final extension step of 72°C for 5 min. The amplified products were precipitated with 20% polyethylene glycol–2.5 M NaCl (15), resuspended in cold 70% ethanol, and reprecipitated; and the sequences of both strand were determined with an ABI Prism 377 DNA sequencer with BigDye fluorescent terminators and the primers used in the initial PCR amplification.
For each locus, the sequences obtained from all 155 isolates were compared and the different sequences were assigned allele numbers. For each isolate, the alleles at each of the seven loci defined the allelic profile which corresponded to its ST. The clustering of isolates was achieved by the unweighted pair group method with arithmetic averages (UPGMA) from the matrix of the percentage of pairwise differences between the allelic profiles of the isolates by using Statistica (StatSoft, Tulsa, Okla.). The nonrandomness in the distribution of variable sites along the sequence of each gene fragment was examined by the method of Sawyer (23). Polymorphic sites were displayed by using Sequence Output, a Macintosh program available from the MLST website (http://mlst.zoo.ox.ac.uk).
PFGE.
Chromosomal DNA from S. aureus was prepared in agarose blocks and was cleaved with SmaI by the method of Bannerman et al. (2). The samples were run on 1% agarose gel in 0.5% TBE buffer (44.5 mM Tris, 44.5 mM borate, 1 mM EDTA) on a CHEF DR-III PFGE system (Bio-Rad, Hemel Hempstead, Hertsfordshire, United Kingdom) by using an initial switching time of 1 s which was increased to 5 s for 12 h, followed by 12 h with an initial switching time of 15 s which was increased to 30 s, by using a voltage of 6 V cm−1 (3). Concatenated bacteriophage lambda DNA (New England Biolabs, Beverly, Mass.) was used to provide molecular size markers.
Detection of mecA.
The presence of mecA was determined by using the primers MR1 and MR2, which were used in the PCR to amplify a 1,339-bp internal fragment of the gene (32). PCR was carried out for 30 cycles of 1 min at 95°C, 1 min at 55°C, and 2 min at 72°C.
Nucleotide sequence accession numbers.
The DNA sequences of each allele at the seven loci used in this study have been deposited in GenBank under accession nos. AJ252295-2310, AJ271251-89, AJ271387-403, and AJ271482-510, and can be downloaded from the website mentioned above.
RESULTS
Sequences of the seven housekeeping gene fragments.
Fourteen housekeeping gene fragments were sequenced from 10 S. aureus strains, obtained from different geographic locations, and the seven gene fragments that provided the greatest number of alleles were chosen for use in the MLST scheme. These seven housekeeping gene fragments, which were of between 402 and 516 bp (Table 2), were then sequenced from each of 155 recent isolates of S. aureus from patients with invasive disease in the Oxfordshire region. For each isolate, the sequences obtained at each of the seven loci were compared with those of every other isolate, and the alleles were numbered consecutively. Sequences were assigned as distinct alleles even if they differed at a single nucleotide site; no weighting was applied to reflect the number of nucleotide differences between alleles.
TABLE 2.
Sequence variation at the seven loci
| Gene | Sequence length (bp) | No. of alleles | No. of polymorphic sites |
|---|---|---|---|
| arcC | 456 | 17 | 19 |
| aroE | 456 | 17 | 23 |
| glpF | 465 | 11 | 14 |
| gmk | 429 | 11 | 13 |
| pta | 474 | 15 | 18 |
| tpi | 402 | 14 | 18 |
| yqiL | 516 | 16 | 19 |
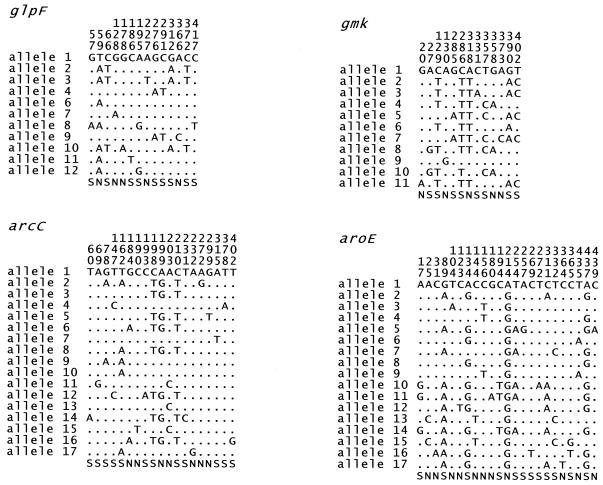
Between 11 alleles (glpF and gmk) and 17 alleles (arcC and aroE) were present at each locus (Table 2), with a mean of 14.4 alleles per locus, which allowed >100 × 106 STs to be distinguished. The S. aureus genes were relatively uniform; the number of polymorphic (variable) nucleotide sites at the seven loci varied between 13 (gmk) and 23 (aroE). The polymorphic sites within the two most uniform and the two most diverse gene fragments are shown in Fig. 1. Visual inspection of the sequences suggested that the distribution of polymorphic sites along each gene fragment was random, and this was confirmed by the test described by Sawyer (23).
FIG. 1.
Polymorphic sites in four of the gene fragments. The nucleotides present at each variable site among the 155 S. aureus isolates are shown for allele 1. For the other alleles, only those sites that differ are shown; sites that are the same as those in allele 1 are shown by periods. Nucleotide sites that are the same in all alleles are not shown. The position of each polymorphic site within the sequenced fragment is shown above, in vertical format. The polymorphisms that are synonymous (S) and nonsynonymous (N) are shown below. Allele 5 of glpF is not shown as this allele was found in S. aureus isolates that are not described in this paper. The glpF and gmk fragments were the most uniform of the seven housekeeping gene fragments used in the MLST scheme, and arcC and aroE were the most variable.
Validation of the S. aureus MLST scheme.
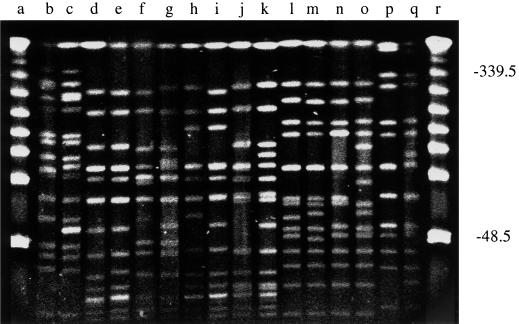
Table 3 shows the 53 different allelic profiles or STs identified among the 155 S. aureus isolates. The MLST scheme was validated by selecting one pair of isolates from each of the 17 STs that contained multiple isolates and examining by PFGE the similarities of the SmaI fragments obtained from the chromosomal DNA of each pair. For STs 12 and 22, which included both MRSA and MSSA isolates, one of the selected pair was MRSA and one was MSSA. The SmaI PFGE fingerprints for eight pairs of isolates are shown in Fig. 2. One of the 17 pairs of isolates (ST34) had five fragment differences, but the other pairs, including the two pairs consisting of both MRSA and MSSA isolates, differed at four fragments or less.
TABLE 3.
Properties of the 53 STs
| ST | No. of isolates (no. methicillin resistant) | No. of isolates community acquired: no. of isolates hospital acquired | Allelic profile (allele no.)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| arcC | aroE | glpF | gmk | pta | tpi | yqiL | |||
| 1 | 7 (0) | 5:2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 (0) | 1:0 | 1 | 1 | 1 | 8 | 1 | 1 | 1 |
| 3 | 1 (0) | 0:1 | 1 | 1 | 1 | 9 | 1 | 1 | 12 |
| 4 | 1 (0) | 0:1 | 3 | 1 | 1 | 8 | 8 | 8 | 1 |
| 5 | 7 (0) | 3:4 | 1 | 4 | 1 | 4 | 12 | 1 | 10 |
| 6 | 2 (0) | 2:0 | 12 | 4 | 1 | 4 | 12 | 1 | 3 |
| 7 | 1 (0) | 1:0 | 5 | 4 | 1 | 4 | 4 | 6 | 3 |
| 8 | 8 (0) | 4:4 | 3 | 3 | 1 | 1 | 4 | 4 | 3 |
| 9 | 2 (0) | 0:2 | 3 | 3 | 1 | 1 | 1 | 1 | 10 |
| 10 | 1 (0) | 0:1 | 3 | 3 | 1 | 2 | 1 | 1 | 10 |
| 11 | 1 (0) | 1:0 | 1 | 6 | 1 | 4 | 13 | 5 | 11 |
| 12 | 5 (1) | 2:3 | 1 | 3 | 1 | 8 | 11 | 5 | 11 |
| 13 | 1 (0) | 0:1 | 1 | 3 | 1 | 10 | 11 | 5 | 11 |
| 14 | 1 (0) | 1:0 | 11 | 13 | 1 | 1 | 12 | 11 | 13 |
| 15 | 5 (0) | 2:3 | 13 | 13 | 1 | 1 | 12 | 11 | 13 |
| 16 | 1 (0) | 0:1 | 15 | 13 | 1 | 1 | 12 | 11 | 13 |
| 17 | 1 (0) | 1:0 | 13 | 6 | 1 | 1 | 12 | 11 | 13 |
| 18 | 2 (0) | 0:2 | 13 | 15 | 1 | 1 | 12 | 11 | 13 |
| 19 | 1 (0) | 0:1 | 4 | 9 | 1 | 1 | 1 | 10 | 8 |
| 20 | 1 (0) | 0:1 | 4 | 9 | 1 | 8 | 1 | 10 | 8 |
| 21 | 1 (0) | 0:1 | 7 | 6 | 1 | 5 | 1 | 1 | 6 |
| 22 | 8 (4) | 4:4 | 7 | 6 | 1 | 5 | 8 | 8 | 6 |
| 23 | 1 (0) | 1:0 | 7 | 6 | 7 | 5 | 8 | 8 | 6 |
| 24 | 1 (0) | 0:1 | 7 | 8 | 1 | 7 | 8 | 8 | 6 |
| 25 | 12 (0) | 8:4 | 4 | 1 | 4 | 1 | 5 | 5 | 4 |
| 26 | 1 (0) | 0:1 | 4 | 1 | 4 | 1 | 5 | 5 | 9 |
| 27 | 1 (0) | 0:1 | 4 | 13 | 4 | 1 | 5 | 5 | 4 |
| 28 | 1 (0) | 0:1 | 4 | 1 | 9 | 1 | 5 | 5 | 4 |
| 29 | 1 (0) | 0:2 | 2 | 2 | 2 | 1 | 6 | 3 | 2 |
| 30 | 17 (0) | 8:9 | 2 | 2 | 2 | 2 | 6 | 3 | 2 |
| 31 | 1 (0) | 1:0 | 2 | 2 | 2 | 3 | 6 | 3 | 2 |
| 32 | 1 (0) | 0:1 | 2 | 12 | 2 | 2 | 6 | 3 | 2 |
| 33 | 1 (0) | 0:1 | 2 | 17 | 2 | 2 | 6 | 3 | 2 |
| 34 | 2 (0) | 2:0 | 8 | 2 | 2 | 2 | 6 | 3 | 2 |
| 35 | 1 (1) | 0:1 | 2 | 1 | 2 | 2 | 3 | 3 | 2 |
| 36 | 22 (22) | 0:22 | 2 | 2 | 2 | 2 | 3 | 3 | 2 |
| 37 | 1 (0) | 1:0 | 2 | 2 | 2 | 2 | 15 | 3 | 2 |
| 38 | 1 (1) | 0:1 | 2 | 2 | 10 | 2 | 3 | 3 | 2 |
| 39 | 8 (0) | 3:5 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 40 | 1 (0) | 1:0 | 2 | 2 | 2 | 2 | 6 | 2 | 2 |
| 41 | 1 (0) | 0:1 | 2 | 2 | 3 | 2 | 2 | 2 | 2 |
| 42 | 1 (0) | 1:0 | 8 | 2 | 2 | 11 | 2 | 2 | 2 |
| 43 | 1 (0) | 1:0 | 9 | 10 | 8 | 6 | 10 | 3 | 2 |
| 44 | 1 (0) | 1:0 | 10 | 14 | 8 | 2 | 10 | 3 | 2 |
| 45 | 6 (0) | 2:4 | 10 | 14 | 8 | 6 | 10 | 3 | 2 |
| 46 | 1 (0) | 0:1 | 10 | 14 | 8 | 6 | 14 | 3 | 2 |
| 47 | 4 (0) | 2:2 | 10 | 11 | 8 | 6 | 10 | 3 | 2 |
| 48 | 1 (0) | 0:1 | 17 | 11 | 8 | 6 | 10 | 3 | 2 |
| 49 | 2 (0) | 1:1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 50 | 1 (0) | 0:1 | 16 | 16 | 12 | 2 | 13 | 13 | 15 |
| 51 | 1 (0) | 1:0 | 6 | 5 | 6 | 2 | 7 | 7 | 5 |
| 52 | 1 (0) | 0:1 | 6 | 5 | 6 | 6 | 7 | 14 | 5 |
| 53 | 1 (0) | 0:1 | 8 | 7 | 6 | 6 | 9 | 9 | 7 |
FIG. 2.
PFGE of pairs of isolates with identical allelic profiles. Chromosomal DNA from pairs of isolates of STs 25 (lanes b and c), 30 (lanes d and e), 34 (lanes f and g), 36 (lanes h and i), 39 (lanes j and k), 45 (lanes l and m), 47 (lanes n and o), and 49 (lanes p and q) were digested with SmaI and were separated by PFGE. Concatenated bacteriophage lambda molecular size markers were run in lanes a and r. Numbers on the right are in kilobase pairs.
Similarities in the patterns of SmaI fragments were also observed between the isolates of STs that appeared to be relatively closely related on the dendrogram constructed from the MLST data (Fig. 3; see below). For example, the isolates of STs 30 and 34 (five or six fragment differences), STs 30 and 36 (two to five fragment differences), and STs 45 and 49 (four to seven fragment differences) showed clear similarities by PFGE (Fig. 2). In contrast, isolates of STs that appeared to be distantly related on the dendrogram showed a large number of fragment differences. For example, by MLST, STs 25 and 30 had different alleles at all seven loci and STs 39 and 45 differed at six of seven loci (Table 3); by PFGE, there were at least 20 fragment differences between the isolates of these two pairs of STs (Fig. 2).
FIG. 3.
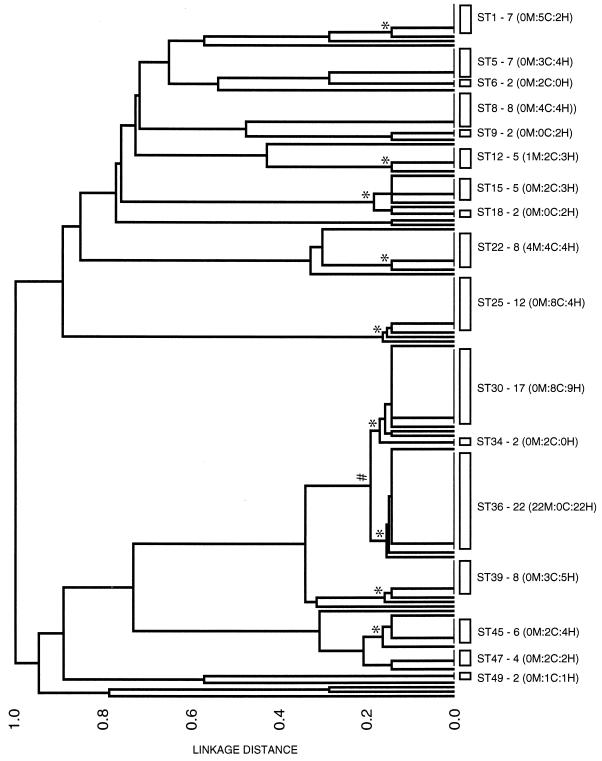
Dendrogram showing the genetic relatedness of the 155 S. aureus isolates. The STs that are represented by multiple isolates are shown by the open rectangles and are labeled on the right, with the number of isolates in the ST and, in parentheses, the number of MRSA isolates in the ST (M), followed by a colon and the number of isolates from patients with community-acquired infections (C), followed by a colon and the number of isolates from patients with hospital-acquired infections (H). For example, ST1 - 7 (0M:5C:2H) contains seven isolates, none of which are methicillin resistant, five isolates from patients with community-acquired infections, and two isolates from patients with hospital-acquired infections. The asterisks show the nodes that define the major clonal complexes, which include a group of isolates with the same allelic profile plus those closely related isolates that differ at only one of the seven loci. The node that defines the large cluster of closely related isolates of STs 29 to 38 is shown by a hash sign.
Clustering of the invasive S. aureus isolates.
Figure 3 shows a dendrogram constructed from the matrix of pairwise differences between the allelic profiles of the 155 isolates. ST36 contained the largest number of isolates, all 22 of which were MRSA. The second largest cluster was ST30. The 17 isolates of ST30 were all MSSA and were closely related in genotype to the MRSA isolates of ST36, as their allelic profiles differed at only one locus (pta). Nine other STs were represented by at least five isolates. All 11 of these prevalent STs except ST36, which included only hospital-acquired MRSA isolates, were represented by isolates recovered both from patients with community-acquired infections and from patients with hospital-acquired infections.
Nine of the 11 prevalent STs were part of clonal complexes, which included the isolates of the prevalent ST and closely related isolates whose allelic profiles differed from the profile of the prevalent ST at only a single locus (Fig. 3; Table 3). In three cases, the clonal complexes included a larger number of STs whose allelic profiles differed from each other at only one two loci (Fig. 3; Table 3). For example, the isolates of STs 29 to 38 (see below) were part of a large cluster which included 48 of the 155 isolates (31%) and which included the two major STs. Similarly, STs 44 to 48 and STs 14 to 18 included 14 and 10 closely related isolates, respectively.
Analysis of MRSA isolates.
Of the 155 isolates, 29 (19%) were methicillin resistant and 126 (81%) were methicillin susceptible. Only one of the MRSA isolates was obtained from a patient with a community-acquired infection. The major clone identified in this study (ST36) included 22 MRSA isolates. Eleven of these MRSA isolates were submitted to the Central Public Health Laboratory (Colindale, London, United Kingdom) and were assigned as members of the EMRSA-16 clone by PFGE. EMRSA-15 (21) and EMRSA-16 (4) are the most common MRSA clones recovered in hospitals in the United Kingdom in recent years (1). The allelic profiles of reference strains of EMRSA-15 (strain 90/10685) and EMRSA-16 (strain 96/32101), kindly provided by B. Cookson of the Central Public Health Laboratory, were determined. The allelic profile of the EMRSA-16 reference strain was identical to those of the 22 MRSA isolates of ST36.
Similarly, the allelic profile of the EMRSA-15 reference strain was identical to those of the isolates of ST22. ST22 included four MRSA isolates, including the only one in the collection that was community acquired, as well as four MSSA, three of which were community acquired.
Only three of the MRSA isolates did not belong to either ST36 (EMRSA-16) or ST22 (EMRSA-15). Two of these isolates (which belonged to ST35 and ST38) were very closely related to EMRSA-16, as they differed at only a single locus, and are considered to be minor variants of EMRSA-16. The other MRSA isolate (which belonged to ST12) was indistinguishable from a cluster of four MSSA isolates and was not similar to any of the reference strains of MRSA clones that we examined.
The presence of mecA was determined for all isolates of the two STs that included both MRSA and MSSA isolates (STs 12 and 22) and for at least two members of the other STs containing MRSA isolates. All MRSA isolates tested contained the mecA gene, whereas this gene was not detected in the MSSA isolates.
Analysis of MSSA isolates.
Methicillin-susceptible isolates comprised the majority of the invasive S. aureus isolates collected in this survey (126 isolates; 81%), and they were responsible for 98% of all community-acquired infections and 70% of all hospital-acquired infections. There were 60 community-acquired MSSA isolates and 66 hospital-acquired MSSA isolates among the 155 isolates in the collection.
The majority of the MSSA isolates (71%) had allelic profiles that were identical to that of at least one other MSSA isolate, with 14 of the 17 STs that included multiple isolates consisting entirely of MSSA isolates. There was no clear difference between hospital-acquired and community-acquired MSSA isolates. All 11 STs that were represented by at least three MSSA isolates contained isolates from both patients with hospital-acquired infections and patients with community-acquired infections (Fig. 3). The MSSA clones with the most isolates contained 17 (ST30), 12 (ST25), 8 (ST8 and ST39), and 7 (ST1 and ST5) isolates, accounting for 47% of the MSSA isolates in this study.
DISCUSSION
Molecular methods have been used to study the epidemiology of MRSA in hospital, national, and global settings, with PFGE proving the most satisfactory method on the basis of its discriminatory ability and reproducibility (24, 30). The major disadvantage of PFGE and other methods that compare DNA fragments on gels is the difficulty of comparing the results obtained in different laboratories, even when reagents and conditions are standardized (3, 5, 34). MLST provides a highly discriminatory method that defines each isolate as a string of seven integers—the allelic profile—and which produces data that can be held in a central database on the World Wide Web, along with associated epidemiological data, and which can be interrogated via the Internet (16, 28). This approach allows any laboratory that uses MLST to submit the sequences of the seven gene fragments of an S. aureus isolate to the MLST website (http://mlst.ox.ac.uk), where an allelic profile can be assigned and compared with those of all of the MRSA and MSSA isolates maintained in the database.
The clustering of isolates obtained by MLST agreed well with that obtained by PFGE. Isolates that were identical by MLST had either identical SmaI fragment patterns or patterns that differed at two to five fragments. PFGE patterns that have less than four fragment differences are considered to be the same strain, and isolates that differ at four to six fragments are considered to be of the same genetic lineage (31). The criterion for assigning isolates as the same strain by PFGE is used in the context of outbreaks of disease to infer epidemiological links. In many cases, strains from outbreaks are likely to be identical or very similar by PFGE, as they are descended from a common ancestor (the isolate from the index case patient who introduced the outbreak strain) that may have existed only a matter of weeks or months earlier. In MLST, the variation within housekeeping genes accumulates relatively slowly (28), and isolates with the same allelic profile (ST) may be descended from a common ancestor that existed many years ago. It is therefore not surprising that, for some pairs of isolates with the same ST, the number of fragment differences obtained by PFGE is slightly greater than the criterion number used to define isolates of the same strain. STs that appeared to be closely related by MLST also had similar PFGE fragment patterns, whereas those that appeared to be distantly related had very few fragments in common. This congruence between MLST and PFGE was expected since MLST is highly discriminatory, and it is therefore very unlikely that totally unrelated isolates will, by chance, have the same allelic profile.
The major clone (ST36) identified in this study corresponded to clone EMRSA-16, one of the two major MRSA clones circulating in British hospitals in recent years (1). Isolates of the other major British MRSA clone (clone EMRSA-15) were also found (ST22), although at a lower frequency. At present the relatedness between the major MRSA clones described by different laboratories in different countries is unclear. MLST will provide a simple means of defining each MRSA clone unambiguously. The advantage of the MLST approach is shown by the ease with which isolates of STs 22 and 36 were assigned as members of the EMRSA-15 and EMRSA-16 clones. The allelic profiles of the major MRSA clones will be available on the S. aureus MLST database (M. C. Enright, unpublished data), and any MRSA clone (or isolate) can be assigned by MLST to a previously identified clone or as a novel clone by using the Internet.
Three STs (STs 12, 15, and 22) contained both MRSA and MSSA isolates. The identification of MSSA and MRSA isolates that were indistinguishable by MLST was slightly unexpected, and the possibility that the MLST scheme lacked resolution had to be considered. The expected frequency of occurrence of any allelic profile by chance can be calculated from the product of the observed frequencies of each of the seven alleles in the population. The expected frequencies of isolates of STs 12, 15, and 22 were between 1.5 × 10−7 and 8 × 10−8, and it is therefore highly unlikely that unrelated MSSA and MRSA isolates would be assigned to the same ST by chance. The close similarity of the MRSA and MSSA isolates within STs 12, 15, and 22 was also confirmed by PFGE.
We therefore believe that the genotypes of MRSA and MSSA isolates with the same allelic profiles are very closely related. The most likely explanation is that the MSSA isolates represent the genotype from which the MRSA isolates recently arose by the acquisition of the methicillin resistance determinant (mec) by horizontal gene transfer. The mec determinant probably entered S. aureus only after the introduction of methicillin into medicine in 1959 (12), and many MRSA isolates will have arisen much more recently than this by the horizontal transfer of mec into new MSSA lineages (17). There has therefore been little time for MRSA isolates to have accumulated sequence variation within their housekeeping genes that would distinguish them from their MSSA ancestors. Some MRSA clones are therefore expected to be identical or very closely related in allelic profile to their MSSA ancestors. An alternative possibility is that some of the MRSA clones contain isolates in which the mec genes have been lost or inactivated. This possibility is difficult to discount, but none of the MSSA isolates that had the same allelic profiles as MRSA isolates possessed the mecA gene.
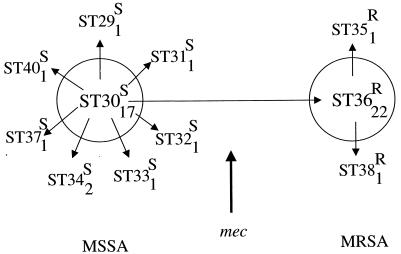
ST36 (clone EMRSA-16) was very closely related to the major MSSA clone associated with invasive disease in the Oxford region (ST30); these STs differed at only one of the seven loci. A dendrogram, as shown in Fig. 3, identifies the clusters of isolates with identical allelic profiles, but it cannot always accurately represent the relationships between isolates with similar allelic profiles. Figure 4 shows the relationships among the isolates that differ from ST30 or ST36 at a single locus. The most parsimonious way of relating the allelic profiles of the isolates in this clonal complex and their resistance or susceptibility to methicillin is to propose that one of the several single-locus variants of ST30 became methicillin resistant to result in EMRSA-16 (ST36), which subsequently expanded to become a prevalent cause of hospital-acquired invasive disease and which itself spawned methicillin-resistant single-locus variants (STs 35 and 38). It is interesting that EMRSA-16 appears to be part of a major cluster of isolates that are associated with invasive disease (STs 29 to 38) and that included 31% of the isolates recovered from patients with invasive disease in the Oxford region. The development of EMRSA-16 from a virulent clone (ST30) that was a major cause of invasive disease in both the hospital and the community settings may partly explain the success of EMRSA-16 in causing invasive disease in the hospital setting.
FIG. 4.
Relatedness among the major cluster of isolates. The STs within the large clonal complex defined by the node indicated with a hash sign in Fig. 3 (STs 29 to 38) are shown, with the number of isolates in each ST indicated by the subscript and the susceptibility (S) or resistance (R) to methicillin indicated as a superscript. The circles indicate the two major STs in the cluster, and the STs that differ from these two major STs at only a single locus are shown by arrows. ST36 (clone EMRSA-16) differs from ST30 at only a single locus but includes exclusively MRSA isolates. ST36 is hypothesized to have arisen as a minor variant of ST30 that acquired the mec determinant by horizontal gene transfer and that has subsequently diversified slightly to give rise to the single-locus variants ST35 and ST38, which are also MRSA. All of the isolates that are not linked by arrows differ at more than one locus. ST37 differs from both ST30 and ST36 at a single locus (all three STs have different pta alleles) but, on parsimony grounds, is considered to be derived from ST30 since it is susceptible to methicillin. ST40 is included since it differs from ST30 at a single locus, although this is not apparent in Fig. 3 as it clustered anomalously when UPGMA was used.
Most of the MSSA clones were recovered from both patients with community-acquired invasive disease and patients with hospital-acquired invasive disease. The isolates that caused community-acquired invasive S. aureus disease were, with one exception, MSSA. These isolates caused disease in previously healthy individuals, and the prevalent STs associated with community-acquired invasive disease (e.g., STs 1, 25, and 30, isolates of which caused 35% of cases of community-acquired disease) may identify particularly virulent MSSA clones.
Much larger studies that have examined isolates from worldwide sources will be required to establish the existence of widely distributed S. aureus clones that have a special ability to cause community-acquired invasive disease. Such studies are under way. However, the major use of the MLST scheme for S. aureus will probably be to allow investigators to unambiguously identify MRSA or GISA isolates and to probe the origins and evolution of these strains.
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust. B.G.S. and N.P.J.D. were in receipt of a Wellcome Trust Principal Research Fellowship and a Wellcome Trust Career Development Fellowship, respectively.
We are grateful to Paul Wilkinson for managing our nucleotide sequencing facility, Man-Suen Chan for developing the MLST website, and Martin Burnham for providing S. aureus sequences.
REFERENCES
- 1.Anonymous. Epidemic methicillin-resistant Staphylococcus aureus. Commun Dis Rep Weekly. 1997;7:1. [PubMed] [Google Scholar]
- 2.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cookson B D, Aparicio P, Deplano A, Streulens M, Goering R, Marples R. Inter-centre comparison of pulsed-field gel electrophoresis for the typing of methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1996;44:179–184. doi: 10.1099/00222615-44-3-179. [DOI] [PubMed] [Google Scholar]
- 4.Cox R A, Conquest C, Mallaghan C, Marples R R. A major outbreak of methicillin-resistant Staphylococcus aureus caused by a new phage type (EMRSA-16) J Hosp Infect. 1995;29:87–106. doi: 10.1016/0195-6701(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 5.de Lencastre H, Severina E P, Roberts R B, Kreiswirth B N, Tomasz A. Testing the efficacy of a molecular surveillance network: methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) genotypes in six hospitals in the metropolitan New York City area. Microb Drug Resist. 1996;2:343–351. doi: 10.1089/mdr.1996.2.343. [DOI] [PubMed] [Google Scholar]
- 6.de Sousa M A, Sanches I S, Ferro M L, Vaz M J, Saraiva Z, Tendeiro T, Serra J, de Lencastre H. Intercontinental spread of a multidrug-resistant methicillin-resistant Staphylococcus aureus clone. J Clin Microbiol. 1998;36:2590–2596. doi: 10.1128/jcm.36.9.2590-2596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez M A, de Lencastre H, Liñares J, Tomasz A. Spread and maintenance of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone during an outbreak of MRSA disease in a Spanish hospital. J Clin Microbiol. 1994;32:2081–2087. doi: 10.1128/jcm.32.9.2081-2087.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emori T G, Gaynes R P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright M, Spratt B G. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 10.Enright M C, Fenoll A, Griffiths D, Spratt B G. The three major Spanish clones of penicillin-resistant Streptococcus pneumoniae are the most common clones recovered in recent cases of meningitis in Spain. J Clin Microbiol. 1999;37:3210–3216. doi: 10.1128/jcm.37.10.3210-3216.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feil E J, Maiden M C J, Achtman M, Spratt B G. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol Biol Evol. 1999;16:1496–1502. doi: 10.1093/oxfordjournals.molbev.a026061. [DOI] [PubMed] [Google Scholar]
- 12.Grubb W B. Genetics of MRSA. Rev Med Microbiol. 1998;9:153–162. [Google Scholar]
- 13.Hiramatsu K, Hanaki H, Ito T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1998;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Jordens J Z, Pennington T H. Characterization of Neisseria meningitidis isolates by ribosomal RNA gene restriction patterns and restriction endonuclease digestion of chromosomal DNA. Epidemiol Infect. 1991;107:253–262. doi: 10.1017/s0950268800048901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lis J T, Schleif R. Size fractionation of double-stranded DNA by precipitation with polyethylene glycol. Nucleic Acids Res. 1975;2:383–389. doi: 10.1093/nar/2.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiden M C J, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser J M, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Ninth informational supplement. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 19.Na'Was T, Hawwari A, Hendrix E, Hebden J, Edelman R, Martin M, Campbell W, Naso R, Schwalbe R, Fattom A I. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J Clin Microbiol. 1998;36:414–420. doi: 10.1128/jcm.36.2.414-420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panlilio A L, Culver D H, Gaynes R P, Bannerjee S, Henderson T S, Tolson J S, Martone W J. Methicillin-resistant Staphylococcus aureus in US hospitals, 1975–1991. Infect Control Hosp Epidemiol. 1992;13:582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 21.Richardson J F, Reith S. Characterization of a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J Hosp Infect. 1993;25:45–52. doi: 10.1016/0195-6701(93)90007-m. [DOI] [PubMed] [Google Scholar]
- 22.Roman R S, Smith J, Walker M, Byrne S, Ramotar K, Dyck B, Kabani A, Nicolle L E. Rapid geographic spread of a methicillin-resistant Staphylococcus aureus strain. Clin Infect Dis. 1996;25:698–705. doi: 10.1086/513758. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer S. Statistical tests for gene conversion. Mol Biol Evol. 1989;6:526–538. doi: 10.1093/oxfordjournals.molbev.a040567. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz F-J, Steirt M, Tichy H-V, Hofmann B, Verhoef J, Heinz H-P, Köhrer K, Jones M E. Typing of methicillin-resistant Staphylococcus aureus isolates from Düsseldorf by six genotypic methods. J Med Microbiol. 1998;47:341–351. doi: 10.1099/00222615-47-4-341. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z-Y, Enright M C, Wilkinson P, Griffiths D, Spratt B G. Identification of three major clones of multiply antibiotic-resistant Streptococcus pneumoniae in Taiwanese hospitals using multilocus sequence typing. J Clin Microbiol. 1998;36:3514–3519. doi: 10.1128/jcm.36.12.3514-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M J, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide intermediate Staphylococcus aureus working group. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 27.Speller D C, Johnson A P, James D, Marples R R, Charlett A, George R C. Resistance to methicillin and other antibiotics in isolates of Staphylococcus aureus from blood and cerebrospinal fluid, England and Wales, 1989–1995. Lancet. 1997;250:323–325. doi: 10.1016/s0140-6736(97)12148-1. [DOI] [PubMed] [Google Scholar]
- 28.Spratt B G. Multilocus sequence typing: molecular typing of bacterial pathogens in an era of rapid DNA sequencing and the Internet. Curr Opin Microbiol. 1999;2:312–316. doi: 10.1016/S1369-5274(99)80054-X. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg J P, Clark C C, Hackman B O. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996;23:255–259. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 30.Streulens, M. J., R. Bax, A. Deplano, W. G. V. Quint, and A. van Belkum. Concordant clonal delineation of methicillin-resistant Staphylococcus aureus by macrorestriction analysis and polymerase chain reaction genome fingerprinting. J. Clin. Microbiol. 31:1964–1970. [DOI] [PMC free article] [PubMed]
- 31.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trzcinski K, Hryniewicz W, van Leeuwen W, Sijmons M, Dulny G, Verbrugh H, van Belkum A. Simultaneous persistence of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus in a neonatal ward of a Warsaw hospital. J Hosp Infect. 1997;36:291–303. doi: 10.1016/s0195-6701(97)90056-6. [DOI] [PubMed] [Google Scholar]
- 34.van Belkum A, van Leewen W, Kaufmann M E, Cookson B, Forey F, Etienne J, Goering R, Tenover F, Steward C, O'Brien F, Grubb W, Tassios P, Legakis N, Morvan A, El Solh N, de Ryck R, Streulens M, Salmenlinna S, Vuopio-Varkila J, Kooistra M, Talens A, Witte W, Verbrugh H. Assessment of resolution and intercenter reproducibility of results of genotyping Staphylococcus aureus by pulsed-field gel electrophoresis of SmaI macrorestriction fragments: a multicenter study. J Clin Microbiol. 1998;36:1653–1659. doi: 10.1128/jcm.36.6.1653-1659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]