Abstract
Human kinases comprise a large family of 500+ proteins that catalyze ATP-dependent phosphorylation of protein and metabolite substrates to regulate diverse facets of cell biology. Dysregulation and mutations of protein kinases are linked to human disease, providing opportunities for developing pharmacological agents as potential therapy. Assessing selectivity of pharmacological compounds targeting this enzyme class is critical given that off-target activity of kinase drugs can result in potential toxicity. This protocol outlines the use of ATP acyl phosphate activity-based probes to evaluate the potency and selectivity of kinase inhibitors by fluorescent gel- and mass spectrometry-based detection methods. This competitive chemical proteomic assay can evaluate target engagement of >200 native kinases directly in complex proteomes.
Keywords: Chemical proteomics, kinase, phosphorylation, chemoproteomics, activity-based protein profiling, mass spectrometry, KiNativ, kinome
INTRODUCTION:
ATP and ADP acyl phosphate probes have emerged as chemical probes for interrogation of substrate (e.g. ATP) and inhibitor binding sites of kinases directly in complex proteomes (Patricelli et al., 2011; Patricelli et al., 2007). The probes bind to the kinase active site, where the acyl phosphate reactive group is placed in proximity to the ε-amino group of conserved lysines involved in ATP binding. Nucleophilic attack and ATP release result in covalent attachment of a desthiobiotin tag to kinases (Fig. 1). Probe-labeled kinases can be visualized by 1) SDS-PAGE with a streptavidin fluorophore (Franks et al., 2017; McCloud et al., 2018) or, 2) probe-modified active site peptides can be enriched by avidin affinity chromatography and identified by tandem liquid chromatography-mass spectrometry (LC-MS/MS) analyses (Patricelli et al., 2011; Patricelli et al., 2007; Shin et al., 2018). ATP acyl phosphates can be employed for global profiling of kinase inhibitor potency and selectivity in vitro and in situ (Patricelli et al., 2011). For competitive studies, samples are pre-treated with inhibitor followed by probe labeling with ATP acyl phosphate. Kinase targets of inhibitors are identified by decreased probe labeling that is measured as either decreased fluorescent labeling or reduced active site peptide abundances using gel- or LC-MS-based data, respectively (Fig. 2). When coupled with stable isotope labeling by amino acids in culture (SILAC (Ong et al., 2002)), this platform provides a quantitative readout of native kinase activity and site-specific inhibition directly in proteomes (Campbell et al., 2018; Franks et al., 2017; McCloud et al., 2018; Shin et al., 2018). This platform has facilitated the discovery of novel kinase inhibitors (Ferguson et al., 2019; Olson et al., 2019) and annotation of novel ligand-binding sites of lipid kinases (Franks et al., 2017).
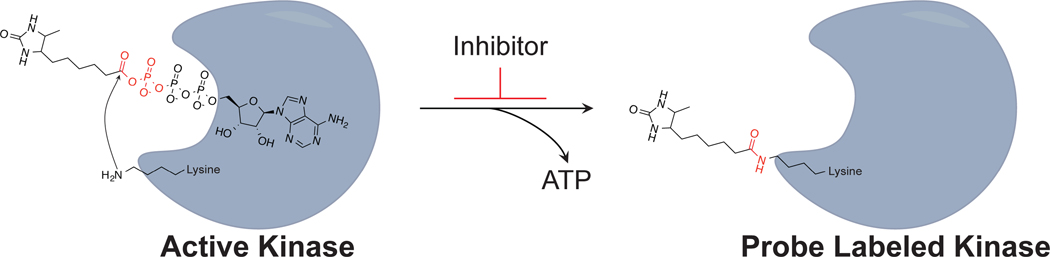
Figure 1. Mechanism of ATP acyl phosphate probe binding.
The probe ATP moiety enters the active kinase ATP-binding domain and where the acyl phosphate group is positioned in proximity to a conserved lysine residue. The lysine residue reacts with the probe to release ATP, which results in covalent modification of the kinase with a desthiobiotin reporter-tag to permit further subsequent analyses. Pre-treatment of cell lysates with inhibitor to inactivate proteins results in blockade of probe labeling.
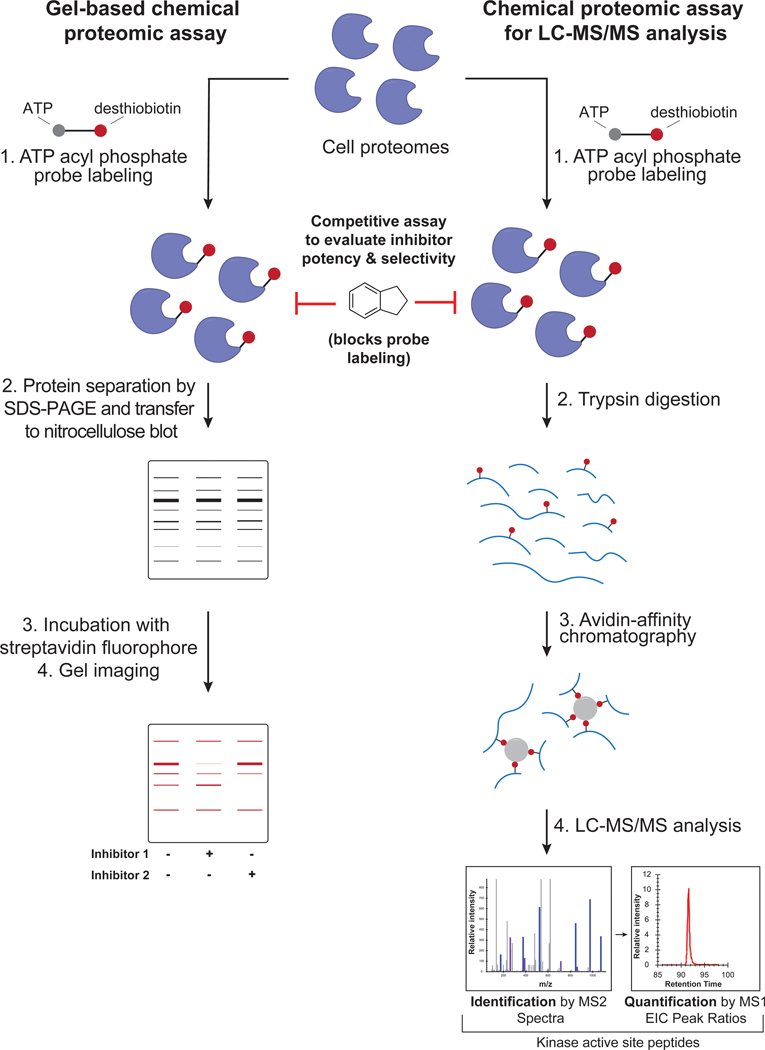
Figure 2. Workflow of gel- and LC-MS/MS-based chemical proteomic assay.
Left: schematic of gel-based chemical proteomic assay. Cell lysates are incubated with ATP acyl phosphate probe, and the probe-labeled proteins separated by SDS-PAGE and then transferred to a nitrocellulose blot. The blot is incubated with a streptavidin fluorophore and then imaged to reveal probe-labeled protein bands. Right: schematic of chemical proteomic assay for LC-MS/MS analysis. Cell lysates are incubated with ATP acyl phosphate probe, proteins digested into peptides with trypsin, and probe-modified peptides enriched using avidin resin. The resulting peptides are analyzed by LC-MS/MS.
The first protocol describes rapid evaluation of inhibitor potency and selectivity kinases and other ATP-binding proteins in proteomes by fluorescent gel-based analyses. The second protocol deploys LC-MS platforms to gain protein target identity and ligand-binding site specificity of inhibitors in competitive activity-based profiling experiments using ATP acyl phosphates.
STRATEGIC PLANNING
Protein concentration
Consideration should be made as to the type of proteome chosen for chemical proteomic analysis. The gel-based assay requires at least 60 μg of protein per sample, while the LC-MS/MS analysis requires at least 500 μg of protein per sample preparation. The latter method may require additional planning depending on use of immortalized or primary cell cultures. Consider increasing the scale of cell cultures for cells that produce lower protein amounts.
Expression of recombinant protein
The production of recombinant protein is an efficient way to investigate inhibitor activity against a protein of interest that may otherwise be expressed at low endogenous levels. One option is to perform transient expression where plasmid DNA is transfected in cell cultures, resulting in immediate or inducible overexpression of recombinant protein (Yang et al., 2017). Consider that transiently transfected cells typically cannot be passaged further without diluting transfected cells. Stable transfection is an alternative option that stably integrates a transgene into the transfected cell’s genome. The ability to select and passage cells that have stably integrated plasmid DNA allows for more homogenous expression of recombinant protein for long-term studies (Chaudhary et al., 2012).
Quantitative LC-MS/MS methodology
Quantitation of inhibitor target engagement in kinase active sites can be achieved by combining SILAC with LC-MS/MS chemical proteomics (Fig. 2). Quantitation by SILAC is achieved by culturing “light” or “heavy” amino acids in media containing 12C, 14N-labeled or 13C, 15N-labeled lysine and arginine, respectively (Franks et al., 2017). Light and heavy amino acids are fully incorporated into proteomes by passaging cells for at least 5 passages with respective lysine and arginine-supplemented media. ATP and ADP acyl phosphate probes can be used in LC-MS/MS studies without SILAC (Patricelli et al., 2011; Patricelli et al., 2007).
BASIC PROTOCOL 1
Gel-based chemical proteomic assay
Introductory paragraph:
This method permits the quantitation of potency and selectivity of kinase inhibitors against the kinome in a rapid gel-based activity-profiling platform. ATP acyl phosphate probe is added to lysates, and conserved lysines found in available ATP binding sites are covalently modified to append a desthiobiotin-reporter tag in kinase (or other ATP binding protein) active sites (Fig. 1). If an inhibitor binds to the ATP pocket of kinases, the binding site will become unavailable for covalent reaction with ATP acyl phosphate probe, which is detected as decreased fluorescence band intensity after SDS-PAGE, transfer to nitrocellulose membrane and in-gel fluorescence analysis using a streptavidin-fluorophore (Fig. 2). Both concentration- and time-dependence can be evaluated to determine potency (e.g. to calculate IC50 values) and mode of inhibition (e.g. reversible versus covalent inhibition), respectively. Selectivity is assessed by determining inhibitor activity against other probe-modified fluorescent bands detected in proteomes, which represent additional active kinases and ATP-binding proteins. Multiple gel analyses can be performed simultaneously to increase throughput and permit small molecule library screens. Quantitation of enzyme activity is achieved by in-gel analysis software to obtain integrated band intensities, which can be used to calculate the magnitude of inhibition (e.g. percent inhibition) by comparing fluorescence protein band signals between vehicle- and compound-treated samples (Fig. 3).
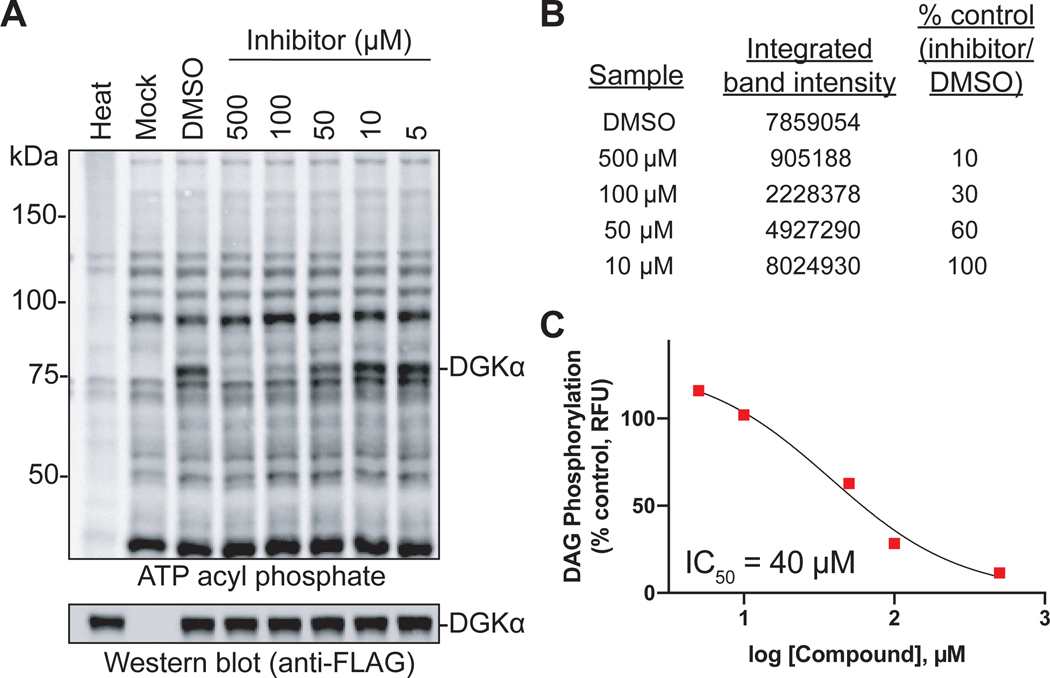
Figure 3. Example of data generated from gel-based chemical proteomic assay.
(A) In-gel fluorescent image of HEK293T cell lysates transiently expressing DGKα protein (~80 kDa; refer to Western blot for total expression level). Cell lysates were pre-treated for 30 minutes with vehicle (DMSO) or inhibitor (5 – 500 μM). Heat denatured control included to account for protein labeling that is not activity dependent. (B) DGKα band intensities were quantified using Bio-Rad Image Lab software, normalized to total protein, and the percent of control (DMSO) calculate(C) Nonlinear regression analysis was used to generate a dose-response curve for the inhibitor from percent control values using GraphPad Prism. IC50 value was quantified from dose-response curve.
Materials:
HEK293T cells (ATCC)
Kinase buffer (see recipe)
Ultracentrifuge tubes
DC protein assay kit II (Bio-Rad #5000112)
Desthiobiotin-ATP acyl phosphate nucleotide probe (Thermo Scientific #88311)
4X SDS-PAGE loading buffer (see recipe)
SDS-PAGE gel
Molecular weight ladder (Precision Plus Protein™ All Blue; Bio-Rad #1610373)
1X SDS-PAGE running buffer (see recipe)
1X Western blot transfer buffer (see recipe)
Nitrocellulose membrane
1X Tris-buffered saline with Tween-20 (TBS-T; see recipe)
Bovine serum albumin (BSA; Reagent Grade; VWR #10842-770)
Streptavidin DyLight 550 (Thermo Fisher Scientific #84542)
Tissue culture hood (biosafety cabinet)
Tissue culture plates
CO2 Incubator (37°C and 5% CO2)
Inverted microscope
Probe-tip sonicator
Ultracentrifuge
Vortex mixer
Gel apparatus
Gel electrophoresis system with transfer insert (Bio-Rad Trans-Blot® Turbo™ Transfer System #1704150)
Rocker
Gel imaging system with fluorescence detection
Harvest cells:
-
1
Aspirate complete DMEM media from HEK293T cells.
-
2Wash cells 2X with warm PBS.
- For adherent cells: Scrape or wash off cells in the first PBS wash, then centrifuge at 400 x g for 5 minutes to pellet cells. Remove supernatant, re-suspend pellet in PBS, and centrifuge again to form a cell pellet for 2X total washes.
- For suspension cells: Use a serological pipette to pull up media with cells and wash the flask bottom, then transfer cells and media to a conical tube. Centrifuge at 400 x g for 5 minutes to pellet, remove supernatant, and re-suspend in PBS. Repeat for a total of 2X washes.
-
3
Snap freeze cell pellet in liquid nitrogen for long-term storage at −80°C or proceed immediately to next step.
Preparation of cell lysates:
-
4Re-suspend cell pellets in kinase buffer for lysis (minimal volumes shown below):
- 1 × 6 cm plate: re-suspend cell pellet in 200 μL
- 1 × 10 cm plate: re-suspend cell pellet in 500 μL
- 1 × 15 cm plate: re-suspend cell pellet in 700 μL
-
5
Lyse cells by sonication at 20% amplitude using a small tip (1/8 in.) sonicator probe. Pulse probe tip in cell mixture 3X for 1 second each.
-
6
To separate membrane and soluble fractions, transfer lysates to ultracentrifuge tubes and centrifuge at 100,000 x g for 45 minutes at 4°C.
-
7
Carefully remove supernatant (soluble fraction) and transfer to a new tube. Gently add kinase buffer to the membrane pellet without disrupting the pellet and discard the wash. Re-suspend membrane pellet using an equivalent volume of kinase buffer, transfer to a new tube and sonicate at 20% amplitude (2–3X 1 second pulses) to re-suspend.
-
8Quantify protein concentration of samples using the DC protein assay kit.
- Notes: HEK293T cells yield ~2–5mg of protein per 10 million cells.
-
9
Samples are ready for chemical proteomic analysis or can be snap frozen as aliquots in liquid nitrogen for long-term storage at −80°Avoid freeze-thaw of proteomes to minimize loss of protein activity.
Chemical proteomic assay:
-
10Dilute HEK293T proteomes (from step 8) to 2 mg/mL in kinase buffer and aliquot each sample (30 μL; see notes below) into 1.5 mL microfuge tubes. Please label tubes carefully with sample conditions.
- The final reaction volume is 30 μL. Leave volume for compound treatment (optional; 0.6 μL of 50X stock) and ATP probe (0.6 μL of 50X stock).
- Example 1: Aliquot 28.8 μL of cell proteome if you plan to treat with compound then analyze with ATP probe.
- Example 2: Aliquot 29.4 μL of cell proteome if you plan to analyze with ATP probe without initial compound treatment.
-
11
(Optional): Pre-treat samples with compound or vehicle (0.6 μL of 50X stock), gently flick tube to mix and incubate for 30 minutes at room temperature.
-
12Add ATP acyl phosphate nucleotide probe to mixture (0.6 μL; 10 μM final concentration), gently flick tube to mix and incubate for 30 minutes at room temperature.
- Preparation of ATP acyl phosphate nucleotide probe: Re-suspend 12.6 μg (one vial) of probe in 20 μL of ddH2O (0.5 mM final concentration).
- Notes: Remove ATP acyl phosphate nucleotide probe from −80°C freezer and prepare immediately before use. Probe is hygroscopic and degrades quickly.
-
13
Quench reaction with 10 μL of 4X SDS-PAGE loading buffer and vortex to mix.
-
14
Samples are now ready for SDS-PAGE analysis and should be run immediately to avoid any excess reaction of ATP acyl phosphate nucleotide probe.
Gel loading and SDS-PAGE:
-
15
Load gel(s) into gel tank and fill gel cassette(s) and tank with 1X SDS-PAGE running buffer. Flush out each well with running buffer.
-
16Load the molecular weight ladder and load 15 μL of each sample.
- The gel percentage and size should be optimized based on your samples. For example, Stain-Free Bio-Rad Midi size 4–20% gels are an option. You may need to load more or less sample depending on the gel capacity.
-
17Run gel at 150V for 60 minutes or until running front reaches the bottom of the cassette.
- These conditions have been optimized for Stain-Free Bio-Rad Midi size 4–20% gels. This may need to be changed depending on your gel size/percentage and samples.
-
18
Remove gel cassette from tank. Pull the two plates of the cassette carefully apart to expose the gel. Cut off the running front dye and loading wells using a razor, and place gel into a container with running buffer.
-
19Optional: Image gel to verify run.
- If you are using a Stain-Free gel to image total protein, the gel must be imaged at this step to cross-link proteins with UV radiation.
Western transfer to nitrocellulose membrane:
-
20
Prepare 1L of 1X Western blot transfer buffer.
-
21
Place 2 stacks and 1 nitrocellulose membrane (per gel) in 1X Western blot transfer buffer and let soak for at least five minutes.
-
22
Place one stack on base of transfer cassette and place one membrane on top. Then, place the gel carefully on top of the nitrocellulose membrane, ensuring all of gel will be captured. Roll out any bubbles using a roller. Place last stack on top of gel and roll out air.
-
23
Place lid on the cassette base, lock it, and insert cassette into transfer base. Run the transfer at 25 V for 10 minutes.
-
24
When transfer is complete, disassemble the blotting sandwich and place the blot in ddH2O.
-
25
Optional: Image blot to verify transfer.
Antibody incubation:
-
26
Block membrane at room temperature for at least one hour with gentle shaking (3% BSA in 1X TBS-T).
-
27
Prepare streptavidin antibody (5% BSA, 0.1% Tween20, and 1:3000 Streptavidin DyLight 550).
-
28
Remove membrane from blocking solution and transfer to streptavidin antibody solution. Incubate for 2 hours at room temperature with gentle shaking.
-
29
Rinse blot 5X with 1X TBS-T for 5 minutes each, then place in ddH2O.
-
30Image gel with the following filters:
- DyLight 650 (MW marker)
- DyLight 550 (streptavidin fluorophore)
- Optional: Stain-Free (total protein)
Notes: An example gel-based chemical proteomic assay is shown in Figure 3.
BASIC PROTOCOL 2
Elucidation of inhibitor site of binding and off-target protein identity can be achieved by analysis of samples by quantitative LC-MS/MS (Figure 2). Inhibitor pretreatments and ATP acyl phosphate probe labeling procedures are similar to gel-based assays. Next, probe-labeled proteomes are proteolytically digested into peptides, including probe-modified peptides that correspond to active site peptides, which are enriched by affinity chromatography using avidin-agarose followed by LC-MS/MS analysis. Inhibitor competition at kinase active sites results in reduced abundances of precursor MS1 probe-modified peptides from target kinases (Fig. 4A). MS2 fragment ion spectra are used to identify probe-modified peptide sequences using statistical database matching of measured and predicted fragment ions (Fig. 4B). Akin to gel-based experiments, concentration- and time-dependence can be evaluated using LC-MS/MS methods with the added benefit of determination of protein identity and site specificity. Proteome-wide selectivity can be assessed by comparing activity and specificity of inhibitors against native kinase active site peptides detected (Fig. 4C).
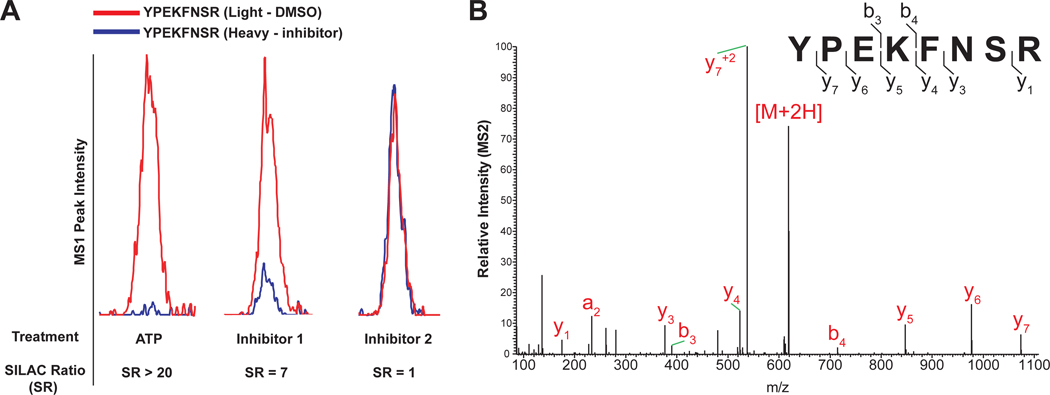
Figure 4. Example of quantitative SILAC data generated from LC-MS/MS chemical proteomic analysis.
(A) MS1-extracted ion chromatograms of probe-modified human DGKα peptides. Heavy cell lysates were treated with free ATP (substrate), Inhibitor 1 or Inhibitor 2, and light cell lysates with vehicle (DMSO) prior to treatment with ATP acyl phosphate prob. SILAC ratios (SR; light/heavy peptide abundances) quantify inhibition of probe labeling (ATP: SR > 20, near-complete inhibition; Inhibitor 1: SR = 7, ~86% inhibition; Inhibitor 2: SR = 1, no inhibition). (B) MS2 spectra of probe-modified human DGKα peptide YPEKFNSR. Major b- and y-type fragment ions that resulted from fragmentation of the precursor ion (M) are indicated on the spectrum in red and annotated on the peptide sequence in black.
Two method options are described below. The difference is use of SILAC or an alternative means for quantitation. A qualitative readout of peptides may be achieved using the non-SILAC protocol. Quantitation of peptide inhibition may be achieved by following the described SILAC protocol.
Chemical proteomic assay for LC-MS/MS analysis
Introductory paragraph:
Materials:
Non-SILAC or SILAC (for optional quantitative analysis) HEK293T cells (ATCC)
Complete SILAC media (optional; see recipe)
Kinase buffer (see recipe)
DC protein assay kit II (Bio-Rad #5000112)
Desthiobiotin-ATP acyl phosphate nucleotide probe (Thermo Scientific #88311)
Two-dram vial
Methanol (Optima™; Fisher Scientific #A454SK-4)
Chloroform (HPLC; Fisher Scientific #C607-4)
Water (HPLC; Fisher Scientific #W5-4)
Pasteur pipette
Screw-top tube (Fisher Scientific #50-809-238)
25 mM AmBic (see recipe)
10 M Urea in 25 mM AmBic (see recipe)
1 M Dithiothreitol (DTT; Make fresh each time; Fisher Scientific #BP172-5)
1 M Iodoacetamide (IAA; Make fresh each time; Light sensitive; store in the dark; Fisher Scientific #AC122270250)
Trypsin/Lys-C mix (Promega #V5073)
Avidin agarose resin (Fisher Scientific #PI-20219)
Dulbecco’s PBS (DPBS; GE Healthcare #SH30028.FS)
Protein LoBind tubes (Eppendorf #022431102)
Gel-loading pipette tips
Elution buffer (see recipe)
18G needle
Mobile phase A (HPLC grade water with 0.1% formic acid; Fisher Scientific #HB5234)
Mobile phase B (see recipe)
Angiotensin I human acetate hydrate (Angtiotensin peptide; Sigma-Aldrich A9650-1MG)
Vasoactive intestinal peptide fragment 1–12 (Vasoactive peptide; Sigma-Aldrich V0131- 0.1MG)
Tissue culture hood (biosafety cabinet)
Tissue culture plates
CO2 Incubator (37°C and 5% CO2)
Inverted microscope
Probe-tip sonicator
Ultracentrifuge
Vortex mixer
Centrifuge
Heat block
Tube revolver/rotator
Vacuum concentrator
Integrated autosampler-LC (Ultimate 3000 RSLC nanoSystem; Dionex)
Trap column (Nano-Trap; Thermo Scientific; 2 cm, 5 μm C18)
Homemade microcapillary analytical column (20 cm, 5 μm C18 packed in 360 μm o.d. x 75 μm i.d. fused silica with an integrated electrospray tip)
Mass spectrometer (Orbitrap Q Exactive Plus; Thermo Scientific)
IP2 software package or similar (ProLuCID search algorithm)
RawConverter software
Skyline-daily software
Harvest cells:
-
1
Aspirate complete DMEM media from HEK293T cells.
-
2Wash cells 2X with warm PBS.
- For adherent cells: Scrape or wash off cells in the first PBS wash, then centrifuge at 400 x g for 5 minutes to pellet cells. Remove supernatant, re-suspend pellet in PBS, and centrifuge again to form a cell pellet for 2X total washes.
- For suspension cells: Use a serological pipette to pull up media with cells and wash the flask bottom, then transfer cells and media to a conical tube. Centrifuge at 400 x g for 5 minutes to pellet, remove supernatant, and re-suspend in PBS. Repeat for 2X total washes.
-
3
Snap freeze in liquid nitrogen for long-term storage at −80°C or proceed immediately to next step.
Preparation of cell lysates:
-
4Re-suspend cell pellets in kinase buffer for lysis (minimal volumes shown below):
- 1 × 6 cm plate: re-suspend cell pellet in 200 μL
- 1 × 10 cm plate: re-suspend cell pellet in 500 μL
- 1 × 15 cm plate: re-suspend cell pellet in 700 μL
-
5Lyse cells by sonication at 20% amplitude using a small tip (1/8 in.) sonicator probe. Pulse probe tip in cell mixture 3X for 1 second each.
- If possible, use a dedicated probe tip for preparations of LC-MS/MS samples. The probe tip sonicator may become contaminated with detergents, etif shared for all lab experiments.
-
6
To separate membrane and soluble fractions, transfer lysates to ultracentrifuge tubes and centrifuge at 100,000 x g for 45 minutes at 4°C.
-
7
Carefully remove supernatant (soluble fraction) and transfer to a new tube. Gently add kinase buffer to the membrane pellet without disrupting the pellet and discard the wash. Re-suspend membrane pellet using an equivalent volume of kinase buffer, transfer to a new tube and sonicate at 20% amplitude (2–3X 1 second pulses) to re-suspend.
-
8
Quantify protein concentration of samples using the DC protein assay kit.
-
9
Samples are ready for chemical proteomic analysis or can be snap frozen as aliquots in liquid nitrogen for long-term storage at −80°Avoid freeze-thaw of proteomes to minimize loss of protein activity.
-
10
Select whether you are proceeding with the Non-SILAC protocol (steps 10–12) or the SILAC protocol (steps 13–15). Then proceed to step 16.
Chemical proteomic assay (A [Non-SILAC] or B [SILAC]):
A. Non-SILAC:
-
11Dilute proteomes to 2 mg/mL in kinase buffer and aliquot each sample (500 μL; see below notes) into 1.5 mL microfuge tubes. Please label tubes carefully with sample conditions.
- The final reaction volume is 500 μL. Leave volume for compound pre-treatment (optional; 10 μL of 50X stock) and ATP probe (10 μL of 50X stock).
- Example 1: Aliquot 480 μL of cell proteome if you plan to pretreat with compound then analyze with ATP probe.
- Example 2: Aliquot 490 μL of cell proteome if you plan to analyze with ATP probe without compound pre- treatment.
-
12
(Optional): Pre-treat samples with compound or vehicle (10 μL of 50X stock), gently flick tube to mix and incubate for 30 minutes at room temperature.
-
13Add ATP acyl phosphate nucleotide probe to mixture (10 μL; 10 μM final concentration), gently flick tube to mix and incubate for 30 minutes at room temperature.
- Preparation of ATP acyl phosphate nucleotide probe: Re-suspend 12.6 μg (one vial) of probe in 20 μL of ddH2O (0.5 mM final concentration).
- Notes: Remove ATP acyl phosphate nucleotide probe from −80°C freezer and prepare immediately before use. Probe is hygroscopic and degrades quickly.
B. SILAC:
-
14Dilute light and heavy proteomes to 2 mg/mL in kinase buffer and aliquot each sample (250 μL; see below notes) into 1.5 mL microfuge tubes. Please label tubes carefully with sample conditions.
- The final reaction volume is 250 μL. Leave volume for compound pre-treatment (optional; 5 μL of 50X stock) and ATP probe (5 μL of 50X stock).
- Example 1: Aliquot 240 μL of cell proteome if you plan to pretreat with compound then analyze with ATP probe.
- Example 2: Aliquot 245 μL of cell proteome if you plan to analyze with ATP probe without compound pre- treatment.
-
15
(Optional): Pre-treat samples with compound or vehicle (5 μL of 50X stock), gently flick tube to mix and incubate for 30 minutes at room temperature.
-
16Add ATP acyl phosphate nucleotide probe to mixture (5 μL; 10 μM final concentration), gently flick tube to mix and incubate for 30 minutes at room temperature.
- Preparation of ATP acyl phosphate nucleotide probe: Re-suspend 12.6 μg (one vial) of probe in 20 μL of ddH2O (0.5 mM final concentration).
- Notes: Remove ATP acyl phosphate nucleotide probe from −80°C freezer and prepare immediately before use. Probe is hygroscopic and degrades quickly.
All reagents from this point forward must be HPLC grade.
MeOH/CHCl3 extraction to remove excess probe and desalt:
-
17Prepare one 8 mL/2 dram glass vial per sample as follows:
- Add 2 mL (4 parts) MeOH
- Add 0.5 mL (1 part) CHCl3
- Add 1.5 mL (3 parts) H2O
- Use one dedicated glass syringe for each solvent. Rinse syringe 3X with solvent before experiment prior to use. Store these separately and only use for mass spectrometry sample preparation.
-
18Transfer each sample (treated proteome from either step 12 or step 15) to an 8 mL/2 dram glass vial with solvents and vortex to mix.
- If preparing SILAC samples, mix light and heavy paired samples at this step and immediately vortex. Steps for Non-SILAC and SILAC preparations are the same from this point forward.
-
19
Centrifuge vials at 1,400 x g for 3 minutes at 25°C.
-
20
Using a Pasteur pipette, carefully remove the top and bottom solvent layers, leaving the protein interface intact.
-
21Add 600 μL MeOH to each vial, and carefully transfer the protein layer to a screw-top microfuge tube using the Pasteur pipette.
- Protein layer may remain as one solid disk or break into many small pieces. Try to transfer as much as possible to the microfuge tube to limit sample loss.
-
22
To each microfuge tube, add 150 μL CHCl3 and 600 μL H2O.
-
23
Vortex to mix and centrifuge at 1,400 x g for 3 minutes at 25°C.
-
24
Using a pipette, remove the top and bottom layers, leaving the protein interface.
-
25
Add 600 μL MeOH to each sample and sonicate (1/8 in. probe tip; 3X 1 second pulse).
-
26
Centrifuge at 14,000 x rpm for 5 minutes at 25°C to pellet protein, then remove MeOH.
Reduction and Alkylation:
-
27
Add 500 μL 10M Urea in 25mM AmBic to each pellet and re-suspend to solubilize. Then, add 500 μL 25 mM AmBic to each sample and mix for a total volume of 1 mL.
-
28
Add 10 μL of 1 M DTT to each sample (10 mM final concentration).
-
29
Incubate samples at 65°C for 15 minutes, then let cool to room temperature or cool briefly on ice.
-
30
Add 40 μL of 1 M IAA to each sample (40 mM final concentration) and incubate at room temperature for 30 minutes in the dark.
MeOH/CHCl3 extraction to desalt:
-
31Prepare one 8 mL/2 dram glass vial per sample as follows:
- Add 2 mL (4 parts) MeOH
- Add 0.5 mL (1 part) CHCl3
- Add 1.5 mL (3 parts) H2O
- Use one dedicated glass syringe for each solvent.
-
32
Transfer each sample to an 8 mL/2 dram glass vial with solvents and vortex to mix.
-
33
Centrifuge vials at 1,400 x g for 3 minutes at 25°C.
-
34
Using a Pasteur pipette, carefully remove the top and bottom solvent layers, leaving the protein interface intact.
-
35Add 600 μL MeOH to each vial and carefully transfer the protein layer to a screw-top microfuge tube using the Pasteur pipette.
- Protein layer may remain as one solid disk or break into many small pieces. Try to transfer as much as possible to the microfuge tube to limit sample loss.
-
36
To each microfuge tube, add 150 μL CHCl3 and 600 μL H2O.
-
37
Vortex to mix and centrifuge at 1,400 x g for 3 minutes at 25°C.
-
38
Using a pipette, remove the top and bottom layers, leaving the protein interface.
-
39
Add 600 μL MeOH to each sample and sonicate (1/8 in. probe tip; 3X 1 second pulse).
-
40
Centrifuge at 14,000 x g for 5 minutes at 25°C to pellet protein, then remove MeOH.
Trypsin digestion:
-
41
Re-suspend protein pellet in 500 μL 25 mM AmBic.
-
42Reconstitute 20 μg of Trypsin/Lys-C (1 vial) in 40 μL of 25 mM AmBic.
- Equilibrate to room temperature before opening and prepare immediately before use.
-
43
Add 7.5 μg (15 μL) Trypsin/Lys-C to each sample and incubate at 37°C for 3 hours.
Labeled protein enrichment:
-
44Remove a 100 μL aliquot of avidin agarose resin per sample and place in a 15 mL conical tube. Wash the resin 3X by adding 10 mL of DPBS to conical tube, centrifuging at 1,400 x g for 1 minute at 25°C, and decanting one time.
- Important note: When decanting, do not invert more than one time. A second inversion will cause the resin to fall out of the conical tube and result in permanent loss of sample. Beads may be pooled together for the wash step into one 15 mL conical tube and re-aliquoted in equivalent volumes for each sample after wash.
-
45
Add one digested sample to each 15 mL conical containing washed avidin resin. Add DPBS to each 15 mL conical for a total volume of 5.5 mL per sample.
-
46
Incubate for 1 hour at room temperature with rotation.
-
47Spin 15 mL conical tubes at 1,400 x g for 1 minute at 25°C to pellet beads, then remove supernatant by decanting one time.
- Important note: When decanting, do not invert more than one time. A second inversion will disrupt the resin and result in potential sample loss.
Wash beads and elute enriched peptides:
-
48
Wash the beads by adding 10 mL of 25 mM AmBic to each tube, centrifuging at 1,400 x g for 3 minutes at 25°C, and decanting one time. Repeat 2 additional times for a total of 3 washes.
-
49
Wash the beads by adding 10 mL of HPLC H2O to each tube, centrifuging at 1,400 x g for 3 minutes at 25°C, and decanting one time. Repeat 2 additional times for a total of 3 washes.
-
50
Re-suspend the beads in the remaining volume of water and transfer each sample to a LoBind microfuge tube.
-
51Spin the LoBind tubes containing beads at 1,400 x g for 3 minutes at 25°C to pellet, let rest 1 minute and remove supernatant by carefully pipetting with a gel-loading pipette tip.
- Important: This is the most technically difficult part of the protocol. You want to remove as much supernatant as possible without disturbing the layer of beads. Try to remove the supernatant from the top and move the pipette tip down as you go. Do not pipette up and down as you will disturb the beads and have to repeat the spin. The 1-minute rest step is extremely important as it allows the beads to fully settle.
-
52
Elute peptides by adding 100 μL of elution buffer to each sample and incubate at room temperature for 3 minutes. Then, spin at 1,400 x g for 3 minutes at 25°C and let rest for 1 minute.
-
53Transfer 75 μL (of the original 100 μL) of eluate to a new LoBind tube using a gel loading pipette tip.
- Again, be very careful at this step. Do not transfer any beads into the new tube.
-
54
Repeat the elution step 2 additional times, adding 75 μL of elution buffer initially to each sample instead of 100 μL. Collect all eluate from each sample into a single LoBind tube and spin down briefly on a bench-top centrifuge to bring down any sample that may have accumulated on the walls of the LoBind tube (~225 μL total volume).
-
55Optional: Spike in synthetic peptide standards for normalization and QC purposes.
- Notes: Human angiotensin and vasoactive peptides may be used as an internal standard. These peptides are spiked at a final concentration of 5 fmol/μL for analysis on an Orbitrap Q Exactive Plus (e.g. 10 fmol from a 2 μL injection of sample).
- Example: Add 2.5 μL of 100 fmol/μL angiotensin and vasoactive peptide to each sample (from step 50). This yields a final concentration of 5 fmol/μL when sample is resuspended in 50 μL mobile phase A (step 54).
-
56Using the 18G needle, poke holes into the inside of the LoBind sample caps outwards to prepare for vacuum concentration.
- Poking holes from inside of tube out minimizes plastic debris that may contaminate sample.
-
57
Freeze peptides on dry ice and dry (remove solvent)on the vacuum concentrator.
-
58
Re-suspend peptides in 50 μL of mobile phase A.
LC-MS/MS analysis:
-
59
Using the integrated autosampler-LC, load the peptides onto the trap column and wash for 2 minutes with 1% mobile phase B.
-
60Elute peptides from the trap column and through the nanocapillary analytical column using a 180 minute 1–95% B reverse-phase LC gradient with the following parameters:
- 0–2 minutes: 1% B, 400 nL/min
- 2–144 minutes: increase 1 to 95% B, 300 nL/min
- 44.1–180 minutes: decrease 95 to 1% B, 400 nL/min
-
61
Peptides are introduced into mass spectrometer by electrospray ionization and analyzed with a top 10 data-dependent acquisition method (1 MS1 followed by MS2 scans of the top 10 most abundant peptides in the MS1) or a data-independent parallel reaction monitoring method (targeted method for low abundance peptides).
Data analysis:
-
62
Generate searchable MS1 and MS2 data from the .raw files using RawConverter.
-
63Upload MS1, MS2, and .raw files for ProLuCID (Xu et al., 2015) (or other suitable peptide identification algorithm) and search the data against an appropriate protein database (e.g. UniProt human protein database) using the ProLuCID algorithm with the following parameters:
- static carbamidomethyl modification of cysteine (+57.0142 Da)
- differential modification of oxidized methionine (+15.9949 Da)
- differential modification of desthiobiotin-labeled lysine residues (+196.1212 Da)
- Optional: added masses of the SILAC “heavy”-labeled amino acids (+10.0083 Da for arginine, +8.0142 Da for lysine)
- trypsin enzyme specificity with 2 missed cleavages
- DTASelect 2.0 filter with --mass, --modstat, and --trypstat options
- Peptide FDR (false discovery rate) = 1%
- 10 ppm precursor mass tolerance, 20 ppm fragment mass tolerance
-
64
Create an .mzXML file from the .raw file using RawConverter and download the .mzIdent files that result from the IP2 searches.
-
65
Upload the .mzXML and .mzIdent files into Skyline-daily.
REAGENTS AND SOLUTIONS:
Use deionized, distilled water in all recipes listed below unless otherwise stated.
25 mM AmBic
Ammonium bicarbonate (Fisher Scientific BP2413-500)
Water (HPLC; Fisher Scientific #W5-4)
Add 19.8mg ammonium bicarbonate to 10mL of water to make 25mM AmBiPrepare fresh.
10 M Urea in 25 mM AmBic
25mM AmBic
Urea (Fisher Scientific
Add 0.9g urea to 1.5mL 25mM AmBic to make 10M urea in 25mM AmBiPrepare fresh.
Complete SILAC media
SILAC media
Dialyzed fetal bovine serum (FBS; Omega Scientific #FB-03)
L-glutamine (Thermo Fisher Scientific #25030-081)
Light lysine (L-lysine dihydrochloride; Acros Organics #657-26-1)
Light arginine (L-arginine hydrochloride; Acros Organics #1119-34-2)
Heavy lysine (L-lysine-3C6, 15N2 hydrochloride; Sigma-Aldrich #608041)
Heavy arginine (L-arginine-3C6, 15N4 hydrochloride; Sigma-Aldrich #608033)
For light media: Combine SILAC media, dialyzed FBS (10% final volume) and L-glutamine (1% final volume). Add light lysine and light arginine to a final concentration of 100 μg/mL.
For heavy media: Combine SILAC media, dialyzed FBS (10% final volume) and L-glutamine (1% final volume). Add heavy lysine and heavy arginine to a final concentration of 100 μg/mL. Store at 4°C for up to 1 month.
Elution buffer
Acetonitrile (Optima™; Fisher Scientific #A955-4)
Water (HPLC; Fisher Scientific #W5-4)
Formic acid (Optima™; Thermo Fisher Scientific #A117-50)
Combine for a final concentration of 50% acetonitrile and 0.1% formic aciStore at room temperature for up to 3 months.
Kinase buffer
10 mL Dulbecco’s PBS (GE Healthcare #SH30028.FS)
20 mM magnesium chloride hexahydrate (40.7 mg in 10 mL; Fisher Scientific #BP214-500)
1 protease inhibitor mini tablet (Thermo Fisher Scientific #A32955)
Combine in a 15 mL conical tubVortex for ~1 minute until all components are dissolveStore at 4°C for up to 1 month.
Mobile phase B
Water with 0.1% formic acid (HPLC Grade; Fisher Scientific #HB5234)
Acetonitrile with 0.1% formic acid (HPLC Grade; Fisher Scientific #HB98234)
Combine 4 parts acetonitrile with 0.1% formic acid to 1-part water with 0.1% formic acid (80% acetonitrile/20% water final concentration). Store at room temperature according to manufacturer’s recommendation.
SDS-PAGE loading buffer, 4X
3.02 g TRIS base (Tocris #3163)
40 mL glycerol (Thomas Scientific #5092-02)
Concentrated HCl
8 g sodium dodecyl sulfate (SDS; Thermo Scientific #BP166-100)
10 mL β-Mercaptoethanol (BME; Fisher Scientific #O3446I-100)
Bromophenol Blue (Fisher Scientific #B392-5)
Add 3.02 g TRIS base to 40 mL water, and then slowly add 40 mL glycerol. Using concentrated HCl bring the pH to 6.7Add 8 g SDS. Under a fume hood, add 10 mL BME, 10 mL ddH2O, and then add a pinch of bromophenol bluAliquot and store at −20°C for up to 1 year.
SDS-PAGE running buffer, 1X
100 mL 10X TRIS/Glycine/SDS (Bio-Rad #1610772)
900 mL water
Measure and combine in a 1 L graduate cylinder. Store at room temperature for up to 1 year.
Tris-buffered saline, 10X
87.6 g Sodium chloride
2.1 g TRIS base (Tocris #3163)
Concentrated HCl
Combine in a 1 L bottle and add 900 mL water. Mix gently until dissolveAdjust pH to 7.4 with concentrated HCl and then fill to 1 L with water. Store at room temperature for up to 1 year.
Tris-buffered saline with Tween20 (TBS-T), 1X
100 mL 10X TBS
900 mL water
1 mL Tween20
Combine in a 1 L bottle and invert to mix. Prepare fresh.
Western blot transfer buffer, 1X
200 mL Trans-Blot® Turbo™ 5X Transfer Buffer (Bio-Rad #10026938)
600 mL Water
200 mL Ethanol
Combine in a 1 L bottle and invert to mix. Store at 4°C for up to 1 year.
COMMENTARY BACKGROUND INFORMATION:
Kinases are key regulators of cell biology by catalyzing transfer of the γ-phosphate of ATP onto hydroxyl groups of protein and metabolite substrates (Manning et al., 2002). Kinases account for ~5% of all protein-coding genes, and their activities regulate ubiquitous cellular activities including signal transduction (Lightfoot et al., 2019). Despite their importance, only a small fraction of kinases have been functionally annotated and even a smaller fraction explored for therapeutic purposes (Cohen et al., 2013). Development of selective inhibitors for individual kinases has been challenging because kinases share similar structures (principally in ATP binding domains) despite diversity in primary sequences, substrates, and functions. Consequently, the high potential for off-target activity has precluded development of specific chemical probes for preclinical studies and drug candidates with reduced toxicity (Klaeger et al., 2017). New assays for evaluating target engagement and proteome-wide specificity of kinase inhibitors are needed to expand the number and type of kinase targets that are pharmacologically accessible for basic and translational research efforts.
Traditional in vitro assays have been implemented to study kinase activity including e.radiochemical ATP assays (Hastie et al., 2006; Karra et al., 2017) and coupled bioluminescent assays (ADP-Glo) (Franks et al., 2017; Tai et al., 2011). Advantages of these assays include the potential for medium- to high-throughput analyses. Limitations for these kinase assays, however, include the requirement for recombinant expression and/or purification of the enzyme, preemptive identification of the enzyme substrate, and non-physiological buffer conditions. Considering that kinases are regulated by post-translational activation, protein-protein and protein-lipid interactions, and subcellular localization, the reliance on purified protein assays may not accurately reflect kinase function in complex environments that approximate the cellular milieu. Methods are needed to measure kinase activity in proteomes or live cells, which enables global evaluation of inhibitor selectivity.
Chemical proteomics has emerged as an effective means for profiling kinase inhibitors and drug candidates against the kinomExamples of such methodologies include kinobeads, which utilize pan-kinase inhibitor-bound beads to enrich kinases from complex cell lysates (Bantscheff et al., 2007) and newer activity-based probes such as XO44, which utilizes a reporter-tagged sulfonyl fluoride to covalently modify the catalytic lysine of kinases in lysates and live cells (Zhao et al., 2017). The chemical proteomic assay described in this protocol, now commercialized as the KiNativ platform, was first reported by Patricelli et. al. in 2007 and uses biotinylated acyl phosphates of ATP or ADP to irreversibly modify lysine residues in the conserved ATP binding pocket of kinases and other ATP-binding proteins (Patricelli et al., 2011; Patricelli et al., 2007). Advantages of this platform include the capabilities to profile the kinome in proteomes, to assess compounds in cell and tissue lysates and live cells, and the commercial availability of ATP and ADP acyl phosphate probes. This platform has been used to identify a clinical candidate targeting interleukin-1 receptor associated kinase 4 (IRAK4) (Lee et al., 2017) and biomarkers of LRRK2 inhibition (Thirstrup et al., 2017), as well as validate engagement of a heterobifunctional multi-kinase degrader molecule (Huang et al., 2018), demonstrating the extensive capabilities of this probe technology.
CRITICAL PARAMETERS:
Storage and usage of ATP acyl phosphate nucleotide probe
The ATP acyl phosphate nucleotide probe is extremely hygroscopic and prone to degradation if not stored properly. Aliquot probe vial aliquots into vacuum-sealed bags containing desiccant and store at −80°When removing aliquots from the freezer, be sure to re-seal bags containing probe stocks before placement back into freezer. Let ATP acyl phosphate nucleotide probe vials equilibrate to room temperature prior to use.
Reagent grade
HPLC grade reagents and solvents are required for Basic Protocol 2 once you have reached the first extraction protocol. Use of materials that do not meet these requirements may result in contaminants in your samples that can confound LC-MS/MS analyses.
Choice of sample tubes
The composition of sample tubes is an important factor to consider when preparing samples for LC-MS/MS analysis. Many manufacturers now produce sample tubes designed to minimize protein adherence to the inner walls, resulting in reduced protein loss and improved sample analyses. The tubes described in Basic Protocol 2 were chosen to minimize sample loss and should be used in order to ensure proper sample preparation. Use of LoBind tubes at the final step is critical.
Long-term storage of LC-MS/MS samples
Peptides should not be stored in LoBind tubes because peptides will adsorb to the plastic and reduce LC-MS/MS signals. After reconstituting peptides with mobile phase A, transfer aliquots of peptides into amber-colored vials with spring-loaded glass inserts for storage at −80°C.
TROUBLESHOOTING:
Common problems encountered with each protocol are described in Table 1 and 2 below. Both tables include possible causes, as well as tips to resolve and/or avoid the problem.
Table 1.
Troubleshooting the gel-based chemical proteomic assay
| Issue | Possible Cause | Solution |
|---|---|---|
|
| ||
| No or missing bands on gel image | Streptavidin antibody stock is old Streptavidin antibody solution not prepared fresh Transfer unsuccessful Gel did not run properly Proteome freeze-thawed Gel is old or past the use-by date |
Check the use by date on the Streptavidin DyLight 550 and store the antibody at 4°C with parafilm wrap Prepare antibody solution fresh for each experiment Image blot after transfer to ensure transfer occurred Increase transfer time Ensure transfer buffer has been prepared correctly Watch gel during run to ensure running front is moving in the correct direction Minimize freeze-thaw cycles of samples prior to analysis Check use-by date on gel package or ensure homemade gel has been poured recently |
|
| ||
| Lanes are smeared or streaked | Overloaded sample Sample has high lipid content Loaded poorly Overheating Using old SDS-PAGE buffer Waiting too long after loading to run the samples |
Make sure to use the correct volume of sample for the gel being used Sonicate membrane samples with a small tip (1/8 in.) probe sonicator prior to running the sample to homogenize Use gel-loading pipet tips to load your samples uniformly Ensure the correct voltage/current is being used Prepare fresh SDS-PAGE buffer each time you run a gel Run the gel immediately after loading samples |
Table 2.
Troubleshooting the chemical proteomic LC-MS/MS analysis
| Issue | Possible Cause | Solution |
|---|---|---|
|
| ||
| No protein interface after extraction | Loss of sample after initial extraction Not enough protein used initially Calculated sample concentration incorrectly |
Re-use methanol to try to transfer as much protein as possible Prepare larger quantities of cells to ensure you have 1 mg for experiment Double check protein concentration calculated with DC protein assay |
|
| ||
| Beads in final concentrated sample | Pipetted beads in final elution step | Practice removing eluate from bead solution to verify that you are not removing beads with pipette tip Ensure samples are resting for at least 1 minute after spinning Keep sample tube at the same angle when removing from the centrifuge so that beads do not move around |
|
| ||
| Extended length peptides | Use of Trypsin instead of Trypsin/Lys-C Trypsin digest was not long enough Trypsin digest was not at the correct temperature |
Use Trypsin/Lys-C higher cleavage efficiency lysine residues; use a freshly-aliquoted high-quality enzyme product Digest samples for the full three hours Use a heat block to ensure samples reach correct temperature (37°C) quickly and uniformly |
STATISTICAL ANALYSIS:
Basic Protocol 1: To determine remaining enzyme activity, compare integrated band intensities of inhibitor- versus vehicle-treated samples using Bio-Rad Image Lab or similar softwarUse nonlinear regression analysis to determine IC0 values from a dose-response curve using GraphPad Prism or similar software.
Basic Protocol 2: Assess quality of peptides in Skyline using the following criteria: Isotope dot-product (iDOTP) ≥ 0.8, ratio dot-product (rDOTP) ≥ 0.8 (for SILAC samples), and mass error ≤ 5 ppm. Inspect peptides that meet these criteria manually and verify peak integration. When reporting SILAC (light:heavy peptide abundance) ratios, normalize peptide ratios to DMSO/DMSO peptide ratios to account for potential variations in mixing and sample preparations. Manually inspect raw MS2 spectra to verify peptide identity.
UNDERSTANDING RESULTS:
Expected results for Basic Protocol 1 are shown in Figure 3. Treatment of cell lysates for 30 minutes with desthiobiotin ATP acyl phosphate probe results in covalent modification of active proteins with a desthiobiotin moiety, which is then detected by in-gel fluorescence imaging with a streptavidin fluorophorAnalysis of HEK293T cell lysates transiently expressing recombinant DGKα results in the appearance of a ~80 kDa band corresponding to the molecular weight of DGKα (Fig. 3A). Pre-treatment of cell lysates with inhibitor (5 – 500 μM) results in a concentration dependent decrease in fluorescence signal of the DGKα banSelectivity of the compound is analyzed by assessing decrease in fluorescence intensity of other detectable fluorescent protein bands in each treatment lanPotency of the compound is quantified using BioRad ImageLab or similar software to analyze fluorescence intensity of the bands followed by Excel to calculate percent of vehicle control (Fig. 3B). Nonlinear regression analysis is then used to calculate an IC0 value using GraphPad Prism or similar analysis software (Fig. 3C).
Expected results for Basic Protocol 2 (SILAC version) are shown in Figure 4. Inhibition of desthiobiotin ATP acyl phosphate probe labeling in cell lysates results in decreased MS1 intensity of inhibitor- (heavy) versus control- (light) treated probe-modified peptides. Quantitation of ligand engagement at specific binding sites is achieved by calculating a SILAC ratio (SR) of the peak area under the curve (AUC) for the precursor MS1 probe-modified peptides: SR = light MS1 peak AUC / heavy MS1 peak AUC (Fig. 4A). A SILAC ratio of ~1 indicates equivalent labeling and negligible blockade from a tested inhibitor (Inhibitor 2, Fig. 4A). A SILAC ratio > 1 indicates blockade of probe labeling, which can be potentially interpreted as kinase inhibitory activity at the respective probe-binding site by the tested inhibitor (Inhibitor 1, Fig. 4A); follow-up biochemical assays are recommended to confirm blockade of authentic catalytic activity. The identities of probe-modified peptides are validated by manual evaluation of MS/MS (MS2) spectra using the major b- and y- fragment ions that result from fragmentation of the precursor peptide ion that was detected in the MS1 spectrum (Fig. 4B). Comparison of SILAC ratios for active site peptides across the kinome can provide a measure of selectivity and identify off-targets for kinase inhibitors of interest. Competition with free ATP (1 mM) should result in complete blockade of probe labeling (SR > 20) and is a control that ATP acyl phosphate reaction is occurring in an active site-dependent manner (ATP, Fig. 4A).
TIME CONSIDERATIONS:
Basic Protocol 1: Preparations of samples for the gel-based chemical proteomic assay can be completed within 1 day. Time to prepare lysates and quantify protein concentration is dependent on the number of samples to be analyzeLysates may be stored at −80°C and analyzed on a separate day, however, it is recommended to perform the chemical proteomic assay using fresh lysatTime to complete the chemical proteomic assay, SDS-PAGE and nitrocellulose transfer, streptavidin incubation, and in-gel fluorescence analysis is approximately 6 hours. Optimal results have been obtained with streptavidin incubation for 2 hours; however, incubation times as short as 1 hour may suffice if there are time limitations.
Basic Protocol 2: Preparation of samples for LC-MS/MS analysis requires approximately 16 hours to complete, depending on the number of samples to be analyzeSimilar to Basic Protocol 1, lysates may be stored at −80°C and analyzed on a separate day, however, it is recommended to perform the chemical proteomic assay using fresh lysatAll other protocol steps must be completed on the same day. All incubation times have been minimized so the procedure can be completed within one day. Do not decrease any incubation times or sample quality may be negatively impacted.
ACKNOWLEDGEMENTS:
This work was supported by the National Institutes of Health Grants DA035864 and DA043571 to K.-L. H., T32 GM007055 to F., U.S. Department of Defense (W81XWH-17-1-0487 to K.-L. H.), and the Robbins Family-MRA Young Investigator Award from the Melanoma Research Alliance (K.-L.H).
Contributor Information
Caroline E. Franks, Department of Chemistry, University of Virginia, Charlottesville, VA 22903
Ku-Lung Hsu, P.O. Box 400319, Department of Chemistry, University of Virginia, Charlottesville, VA 22903.
References:
- Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, & Drewes G. (2007). Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol, 25(9), 035–044. [DOI] [PubMed] [Google Scholar]
- Campbell ST, Franks E, Borne L, Shin M, Zhang L, & Hsu KL (2018). Chemoproteomic Discovery of a Ritanserin-Targeted Kinase Network Mediating Apoptotic Cell Death of Lung Tumor Cells. Mol Pharmacol, 94(5), 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S, Pak JE, Gruswitz F, Sharma V, & Stroud RM (2012). Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat Protoc, 7(3), 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, & Alessi R. (2013). Kinase drug discovery--what’s next in the field? ACS Chem Biol, 8(1), 96–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Doctor ZM, Ficarro SB, Browne M, Marto JA, Johnson JL, Yaron TM, Cantley LC, Kim ND, Sim T, Berberich MJ, Kalocsay M, Sorger PK, & Gray NS (2019). Discovery of Covalent CDK14 Inhibitors with Pan-TAIRE Family Specificity. Cell Chem Biol. [DOI] [PMC free article] [PubMed]
- Franks E, Campbell ST, Purow W, Harris TE, & Hsu KL (2017). The Ligand Binding Landscape of Diacylglycerol Kinases. Cell Chem Biol, 24(7), 870–880 e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie J, McLauchlan J, & Cohen P. (2006). Assay of protein kinases using radiolabeled ATP: a protocol. Nat Protoc, 1(2), 968–971. [DOI] [PubMed] [Google Scholar]
- Huang T, Dobrovolsky D, Paulk J, Yang G, Weisberg L, Doctor ZM, Buckley L, Cho JH, Ko E, Jang J, Shi K, Choi G, Griffin JD, Li Y, Treon SP, Fischer S, Bradner JE, Tan L, & Gray NS (2018). A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem Biol, 25(1), 88–99 e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra S, Stippec S, & Cobb MH (2017). Assaying Protein Kinase Activity with Radiolabeled ATP. J Vis Exp(23). [DOI] [PMC free article] [PubMed]
- Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C, Zecha J, Reiter K, Qiao H, Helm D, Koch H, Schoof M, Canevari G, Casale E, Depaolini SR, Feuchtinger A, Wu Z, Schmidt T, Rueckert L, Becker W, Huenges J, Garz K, Gohlke O, Zolg P, Kayser G, Vooder T, Preissner R, Hahne H, Tonisson N, Kramer K, Gotze K, Bassermann F, Schlegl J, Ehrlich C, Aiche S, Walch A, Greif PA, Schneider S, Felder R, Ruland J, Medard G, Jeremias I, Spiekermann K, & Kuster B. (2017). The target landscape of clinical kinase drugs. Science, 358(6367). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Ambler M, Anderson R, Boscoe P, Bree G, Brodfuehrer JI, Chang JS, Choi C, Chung S, Curran KJ, Day JE, Dehnhardt M, Dower K, Drozda SE, Frisbie RK, Gavrin LK, Goldberg JA, Han S, Hegen M, Hepworth D, Hope R, Kamtekar S, Kilty IC, Lee A, Lin LL, Lovering E, Lowe MD, Mathias JP, Morgan M, Murphy A, Papaioannou N, Patny A, Pierce S, Rao VR, Saiah E, Samardjiev IJ, Samas M, Shen MWH, Shin JH, Soutter H, Strohbach JW, Symanowicz PT, Thomason JR, Trzupek JD, Vargas R, Vincent F, Yan J, Zapf W, & Wright SW (2017). Discovery of Clinical Candidate 1-{[(2S,3S,4S)-3-Ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}−7-methoxyisoquinoli ne-6-carboxamide (PF-06650833), a Potent, Selective Inhibitor of Interleukin-1 Receptor Associated Kinase 4 (IRAK4), by Fragment-Based Drug Design. J Med Chem, 60(3), 521–542. [DOI] [PubMed] [Google Scholar]
- Lightfoot L, Goldberg W, & Sedelmeier J. (2019). Evolution of Small Molecule Kinase Drugs. ACS Med Chem Lett, 0(2), 3–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte B, Martinez R, Hunter T, & Sudarsanam S. (2002). The protein kinase complement of the human genome. Science, 298(600), 912–934. [DOI] [PubMed] [Google Scholar]
- McCloud RL, Franks E, Campbell ST, Purow W, Harris TE, & Hsu KL (2018). Deconstructing Lipid Kinase Inhibitors by Chemical Proteomics. Biochemistry, 7(2), 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M, Liang Y, Leggett A, Park WD, Li L, Mills E, Elsarrag SZ, Ficarro SB, Zhang T, Duster R, Geyer M, Sim T, Marto JA, Sorger PK, Westover KD, Lin Y, Kwiatkowski N, & Gray NS (2019). Development of a Selective CDK7 Covalent Inhibitor Reveals Predominant Cell-Cycle Phenotype. Cell Chem Biol. [DOI] [PMC free article] [PubMed]
- Ong SE, Blagoev B, Kratchmarova I, Kristensen B, Steen H, Pandey A, & Mann M. (2002). Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics, 1(5), 376–386. [DOI] [PubMed] [Google Scholar]
- Patricelli MP, Nomanbhoy TK, Wu J, Brown H, Zhou D, Zhang J, Jagannathan S, Aban A, Okerberg E, Herring C, Nordin B, Weissig H, Yang Q, Lee JD, Gray NS, & Kozarich JW (2011). In situ kinase profiling reveals functionally relevant properties of native kinases. Chem Biol, 8(6), 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli MP, Szardenings K, Liyanage M, Nomanbhoy TK, Wu M, Weissig H, Aban A, Chun D, Tanner S, & Kozarich JW (2007). Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry, 46(2), 350–358. [DOI] [PubMed] [Google Scholar]
- Shin M, Franks E, & Hsu K-L (2018). Isoform-selective activity-based profiling of ERK signaling. Chemical Science, 9(9), 2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W, Bojjireddy N, & Balla T. (2011). A homogeneous and nonisotopic assay for phosphatidylinositol 4-kinases. Anal Biochem, 417(1), 97–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirstrup K, Dachsel JC, Oppermann S, Williamson S, Smith P, Fog K, & Christensen KV (2017). Selective LRRK2 kinase inhibition reduces phosphorylation of endogenous Rab10 and Rab12 in human peripheral mononuclear blood cells. Sci Rep, 7(1), 0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X, Wong C, Fonslow B, Delahunty C, Gao Y, Shah H, & Yates JR 3rd. (2015). ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J Proteomics, 29, 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhou X, Li R, Fu X, & Sun P. (2017). Optimized PEI-based Transfection Method for Transient Transfection and Lentiviral Production. Curr Protoc Chem Biol, 9(3), 47–7. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Ouyang X, Wan X, Gajiwala KS, Kath JC, Jones LH, Burlingame L, & Taunton J. (2017). Broad-Spectrum Kinase Profiling in Live Cells with Lysine-Targeted Sulfonyl Fluoride Probes. J Am Chem Soc, 39(2), 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]