Abstract
Synaptic connectivity patterns underlie brain functions. How recognition molecules control where and when neurons form synapses with each other, therefore, is a fundamental question of cellular neuroscience. This chapter delineates adhesion and signaling complexes as well as secreted factors that contribute to synaptic partner recognition in the vertebrate brain. The sections follow a developmental perspective and discuss how recognition molecules (1) guide initial synaptic wiring, (2) provide for the rejection of incorrect partner choices, (3) contribute to synapse specification, and (4) support the removal of inappropriate synapses once formed. These processes involve a rich repertoire of molecular players and key protein families are described, notably the Cadherin and immunoglobulin superfamilies, Semaphorins/Plexins, Leucine-rich repeat containing proteins, and Neurexins and their binding partners. Molecular themes that diversify these recognition systems are defined and highlighted throughout the text, including the neuron-type specific expression and combinatorial action of recognition factors, alternative splicing, and post-translational modifications. Methodological innovations advancing the field such as proteomic approaches and single cell expression studies are additionally described. Further, the chapter highlights the importance of choosing an appropriate brain region to analyze synaptic recognition factors and the advantages offered by laminated structures like the hippocampus or retina. In a concluding section, the profound disease relevance of aberrant synaptic recognition for neurodevelopmental and psychiatric disorders is discussed. Based on the current progress, an outlook is presented on research goals that can further advance insights into how recognition molecules provide for the astounding precision and diversity of synaptic connections.
1. Introduction
The synaptic connectivity patterns in the complex neuropil packed with neurons and glia are being revealed in stunning detail by connectomic reconstruction studies (Kasthuri et al., 2015; Motta et al., 2019). This chapter provides a molecular perspective on how these circuitry patterns are established in the vertebrate brain and reviews adhesion and signaling complexes that contribute to neuronal partner recognition during synapse development and refinement. A series of subcellular events assembles synapses in late prenatal and early postnatal stages, following axon guidance and dendrite differentiation (Biederer, Kaeser, & Blanpied, 2017; Bury & Sabo, 2016; Emperador-Melero & Kaeser, 2020; Südhof, 2018; Yoshihara, De Roo, & Muller, 2009). Filopodia enable axonal contacts with postsynaptic target cells, resulting in the reorganization of the actin cytoskeleton at these sites. In nascent presynaptic terminals, discrete exocytotic areas termed active zones are formed to establish the molecular machinery that couples calcium influx to synaptic vesicle fusion. The active zone precisely aligns with a specialized postsynaptic membrane domain of the target neuron, which is built through the recruitment of scaffold proteins into the postsynaptic density (PSD) and the sorting and stabilization of neurotransmitter receptors. The assembly of pre- and post-synaptic specializations is coordinated and can even be instructed in time and space by adhesion complexes, some of which include secreted molecules. The components of these trans-synaptic complexes often share similar extracellular domains yet engage in diverse cell–cell interactions and exhibit distinct dynamic properties and subcellular localizations (Apóstolo & de Wit, 2019; Benson & Huntley, 2012; Chamma & Thoumine, 2018; Missler, Südhof, & Biederer, 2012). Partner recognition between neurons during synapse development hence involves a rich repertoire of molecular players.
How molecular recognition specifies neuronal connectivity continues to be a question at the forefront of molecular and cellular neuroscience (Sanes & Zipursky, 2020). The cellular expression patterns of recognition molecules provide intriguing leads to ask how they contribute to this diversity (Favuzzi et al., 2019; Foldy et al., 2016; Paul et al., 2017). Moreover, proteomic analyses of synaptic surface proteins have begun to reveal the molecular complexity of these adhesion complexes (Cijsouw et al., 2018; Loh et al., 2016; Takano et al., 2020). Yet, even if each of these recognition factors would execute a different role to specify synapses, they could not individually account for the vast number of connections. Indeed, multiple molecular themes enhance the power of these factors to generate and diversify synaptic recognition patterns as summarized in Box 1 and Fig. 1. These themes are highlighted throughout this chapter.
BOX 1. Molecular concepts at play to generate and diversify synaptic recognition.
Regional expression variation:
The families of synaptic recognition molecules have several members. Restricting their expression to select brain regions, or to subtypes of neurons and synapses, can increase their power to establish connectivity patterns. Case in point: The homophilic protein Cadherin-9 is expressed in the developing hippocampal CA3 and dentate gyrus and facilitates synapse formation between their neuronal populations (Williams et al., 2011).
Combinatorial expression:
The concerted expression and function of recognition molecules can increase their individual ability to specify synaptic connections. Case in point: LRRTM and Neuroligin family members, which both individually engage Neurexins as presynaptic proteins, act together at postsynaptic sites of developing hippocampal neurons to control excitatory synapse number and glutamatergic transmission (Ko, Soler-Llavina, Fuccillo, Malenka, & Südhof, 2011; Siddiqui, Pancaroglu, Kang, Rooyakkers, & Craig, 2010).
Coincidence detection:
Combinatorial assembly of multiple recognition factors into higher order adhesion complexes enables coincidence detection and validate neuronal partner choice. Case in point: Simultaneous binding of Latrophilin 3, FLRT3 and Teneurin-2 in a ternary complex is required for input-specific synapse formation in the hippocampal CA1 area (Sando, Jiang, & Südhof, 2019).
Temporally defined roles:
Recognition molecules act during restricted temporal windows to enable proteins to act in different developmental contexts. Case in point: Pre- and post-synaptic Cadherins are required for synapse assembly in young neurons, but dispensable for synapse assembly in maturing neurons (Bozdagi, Valcin, Poskanzer, Tanaka, & Benson, 2004).
Post-transcriptional modifications:
Alternative splicing profoundly increases the molecular diversity of synaptic recognition molecules. Case in point: Cell-type select utilization of six alternative splice sites in Neurexins gives rise to ~1300 isoforms that can differentially engage with a variety of ligands (Schreiner, Simicevic, Ahrne, Schmidt, & Scheiffele, 2015; Treutlein, Gokce, Quake, & Südhof, 2014).
Post-translational modifications:
The range of recognition interactions can be increased through post-translational modifications that endow molecules with different binding properties. Case in point: Modification of the extracellular domain of presynaptic Neurexin-1 with heparan sulfate glycans promotes binding to postsynaptic LRRTM proteins (Roppongi et al., 2020; Zhang et al., 2018).
Subcellular targeting:
Adhesive recognition molecules can interact with receptors/ligands on distinct target domains of neurites to guide inputs. Case in point: Netrin-G1 and Netrin-G2 are expressed in axons originating from different neuronal populations and restrict their cognate Netrin-G ligands NGL-1 and NGL-2 to subdendritic segments of hippocampal CA1 neurons (Matsukawa et al., 2014; Nishimura-Akiyoshi, Niimi, Nakashiba, & Itohara, 2007).
Restrictive factors:
Along with positive factors enabling synaptic partner recognition, negative factors can lower the rate of synapse formation, restrict inappropriately formed synapses, or tip the balance to remove synapses that only need to be formed transiently in development. Case in point: MDGA Ig superfamily members bind Neuroligins in cis to restrict interactions between Neuroligins and Neurexins in trans (Lee et al., 2013; Pettem, Yokomaku, Takahashi, Ge, & Craig, 2013).
Fig. 1.
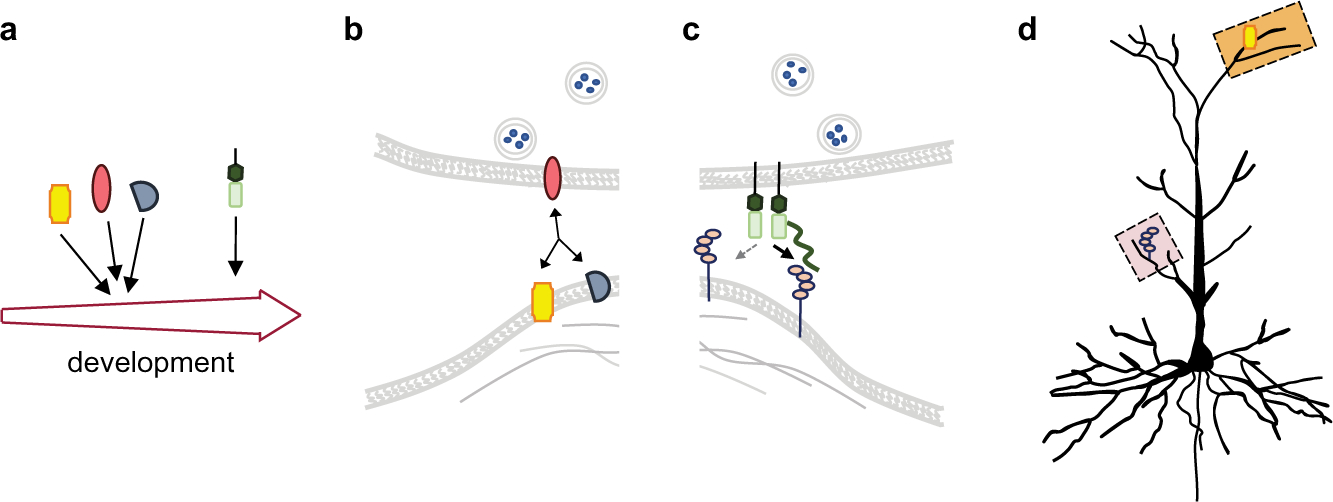
Molecular themes that increase the coding power of synaptic recognition factors described in Box 1. (A) The diagram depicts recognition factors expressed during distinct developmental windows. These patterns include transient co-expression, providing for temporally defined functional cooperation. (B) The combinatorial expression of synaptic recognition factors that form multimeric complexes enables them to cooperate. (C) Post-translational modifications such as glycans (green line) can modulate and even enable recognition. (D) Recognition factors can exhibit subcellular localization to different domains of neurites. This cellular targeting defines their synaptic site of action.
This chapter follows a developmental perspective on synaptic recognition molecules in vertebrate systems. In Section 2, we discuss how these molecules guide the establishment of neuronal connectivity. Synaptic recognition not only helps to find the right partners, it also allows for rejection of incorrect partner choices as described in Section 3. Section 4 presents how synapse organizing adhesion molecules can act beyond the formation of synapses and contribute to the diversification of synapse types. Once synapses are formed, removing or pruning those connections recognized as inappropriate is a key developmental process and described in Section 5. The concluding Section 6 outlines the profound disease relevance of aberrant synaptic recognition, with a focus on neurodevelopmental and psychiatric disorders. The role of glial factors in synapse development is the focus of another chapter (reference chapter “Role of astrocytes in synapse formation and maturation” by Tan et al.). Throughout, we provide examples that highlight that it is crucial to choose an appropriate brain region to analyze synaptic recognition and how laminated structures like the retina and hippocampus provide an anatomical matrix that facilitates these studies. The scope of this chapter is unusually broad, and we would like to apologize to the colleagues whose work we could not reference here.
2. Wiring neuronal partners through adhesive recognition
The specificity of synaptic recognition is perhaps best illustrated by those regions of the central nervous system (CNS) that exhibit a laminar architecture of neuronal connectivity, providing a structural basis for the integration of inputs. The anatomical stratification of these brain regions facilitates studies of synaptic recognition and examples are depicted in Fig. 2.
Fig. 2.
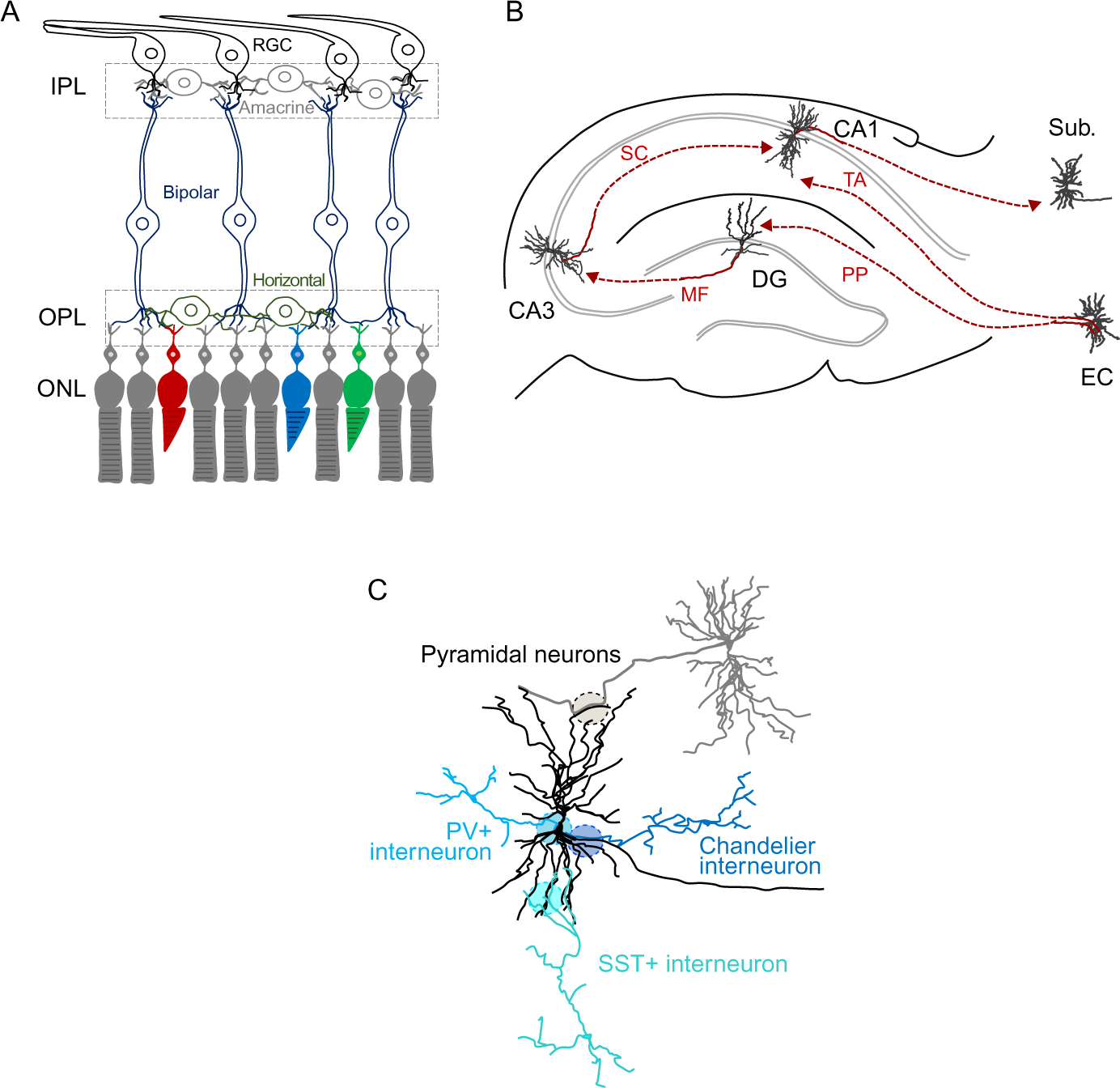
Simplified illustrations of neuronal connectivity in select brain regions. (A) The retina exhibits exemplary laminar organization of cell types and synaptic connections. Rod (grey) and cone (red, green, blue) photoreceptors are located in the outer nuclear layer (ONL). Photoreceptors form synapses with bipolar cells (dark blue) and horizontal cells (dark green) in the outer plexiform layer (OPL). In the inner plexiform layer (IPL), neurites from retinal ganglion cells (RGCs, black), bipolar cells and amacrine cells (light grey) form synapses. (B) In the classic trisynaptic circuit of the hippocampus, dentate gyrus (DG) granule cells receive via the perforant path (PP) inputs from the entorhinal cortex (EC). DG neurons project via mossy fibers (MF) to CA3 pyramidal neurons, which project via Schaffer collaterals (SC) to CA1 pyramidal cells. In one of the additional projections, CA1 pyramidal neurons receive direct inputs from the EC via the temporoammonic (TA) pathway. CA1 pyramidal cells project to the subiculum (Sub) and EC (not shown). (C) Cortical pyramidal neurons (black) receive excitatory inputs from other pyramidal neurons (grey) and in addition subcellularly targeted inhibitory inputs from GABAergic neurons including Parvalbumin-positive (PV) interneurons, Somatostatin (SST)-positive interneurons, and Chandelier cells.
The highly stereotyped laminar architecture of the retina has provided for pioneering studies to identify cell type-specific connectivity (Fig. 2A). It is organized into layers that contain either cell nuclei (the outer and inner nuclear layers and ganglion cell layer) or synaptic connections between projecting axons and dendrites (the outer and inner plexiform layers). In the outer plexiform layer, rod and cone photoreceptor cells form synapses with bipolar and horizontal cells. In the narrow inner plexiform layer of the mouse retina, the dendrites of >40 types of retinal ganglion cells (RGCs) receive inputs from >50 types of bipolar and amacrine cells (Macosko et al., 2015; Yan et al., 2020; Zeng & Sanes, 2017).
Another important brain region organized in a laminated manner is the hippocampus, where granule cells in the dentate gyrus, pyramidal neurons in the CA3 area, and pyramidal neurons in CA1 form three cell layers that are connected into a trisynaptic circuit (Fig. 2B). Additional projections add to these intrinsic connections as illustrated by pyramidal neurons in CA1. While their dendritic segments in the stratum radiatum receive inputs from Schaffer collateral axons of CA3 neurons, the more distal domains of apical dendrites of CA1 neurons in the stratum lacunosum-moleculare are contacted by axons of the temporoammonic branch of the perforant path from the entorhinal cortex. Specific partner recognition is required for all these connections, including subcellular input targeting.
The patterning of synaptic connectivity on a subcellular level is also exemplified by Purkinje cells, the principal output neurons of the cerebellum. The proximal and distal dendritic segments of a Purkinje cell receive glutamatergic innervation originating from the inferior olivary nucleus (one climbing fiber input) and cerebellar granule cells (thousands of parallel fiber inputs), respectively (Hirano, 2018; Kano, Watanabe, Uesaka, & Watanabe, 2018). In addition, Purkinje cells receive GABAergic inputs from local stellate and basket cells that form synapses on the shafts of their dendrites and soma, respectively.
How connectivity patterns arise can not only be understood by analyzing target cells but also by comparing different populations of cells providing inputs to the same target neurons. For example, interneurons in the hippocampus and cortex make their inhibitory inputs to specific subcellular sites of pyramidal neurons, a targeting precision that plays central roles in controlling neuronal activity and network function (Cardin, 2018; Pelkey et al., 2017). Different types of GABAergic neurons synapse onto distinct subcellular compartments like soma and proximal dendrites, distal dendrites, or the axon initial segment, as shown for fast-spiking Parvalbumin (PV)-positive basket cells, Somatostatin-positive interneurons, and Chandelier cells, respectively (Fig. 2C).
In this section, we focus on major classes of recognition factors known to play key roles in establishing connectivity between distinct neuronal populations, including Cadherins, immunoglobulin superfamily molecules and leucine-rich repeat domain containing proteins. Roles of molecules in the recognition events that diversify synapse types, such as Neurexins and their partners, are described in Section 4. Fig. 3 shows proteins discussed below and their domain organization.
Fig. 3.
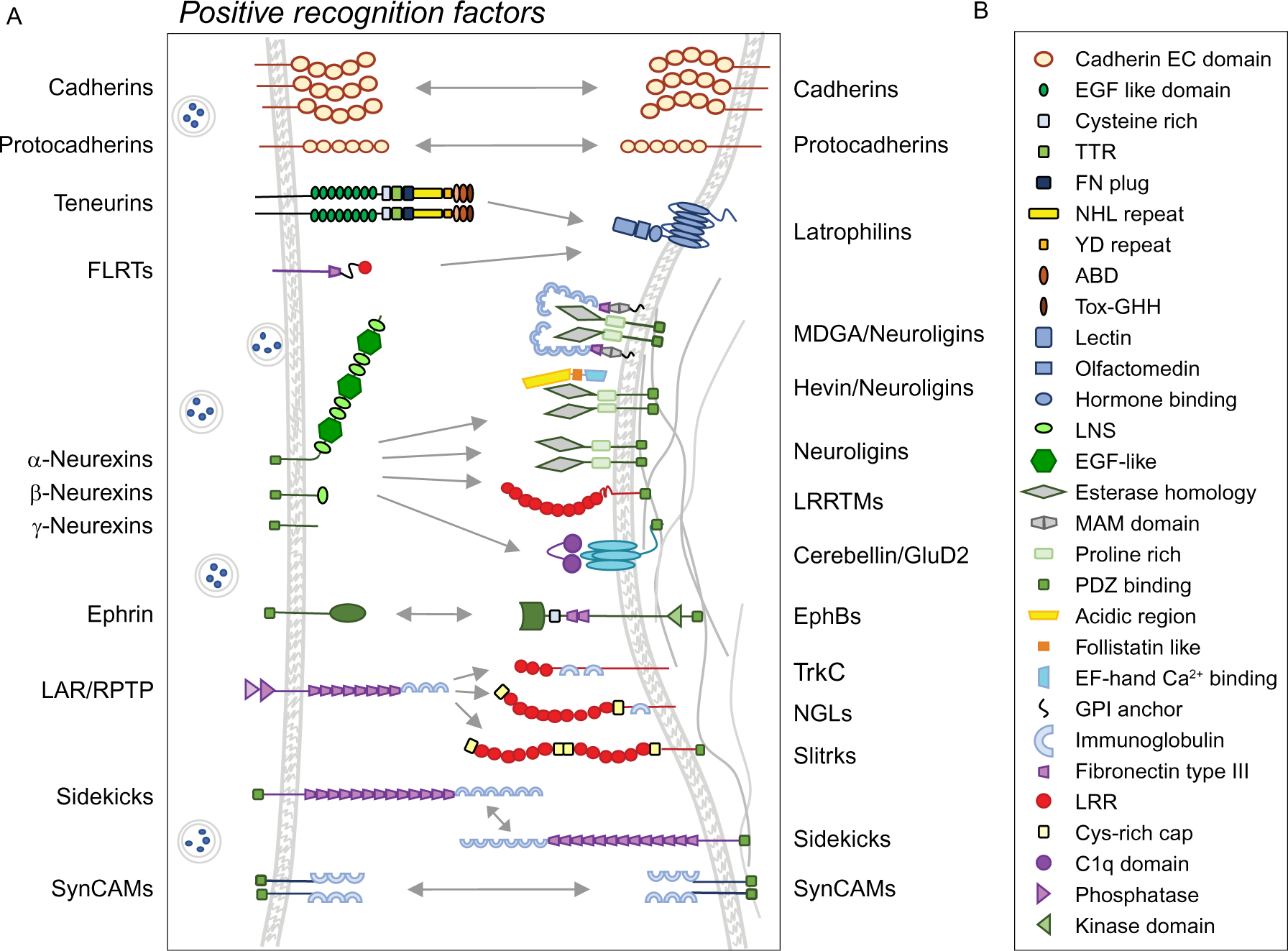
Recognition molecules that promote synaptic connectivity. (A) Factors promoting recognition and synaptic connections. Pre- and postsynaptic membranes are shown on the left and right, respectively. EphB, EphB receptors; FLRTs, fibronectin LRR transmembrane proteins; GluD2, glutamate receptor delta 2; LAR/RPTPs, LAR-type receptor protein tyrosine phosphatases; LRRTMs, leucine-rich repeat transmembrane proteins; MDGA, MAM domain-containing GPI-anchor proteins; NGLs, Netrin-G ligands; Slitrk, Slit- and Trk-like protein; SynCAMs, Synaptic Cell Adhesion Molecules; Trk, neurotrophin receptor-tyrosine kinase. (B) Domains utilized by the recognition molecules shown in (A). ABD, antibiotic-binding domain-like; C1q, complement component 1q domain; EC, extracellular cadherin domain; EGF, epidermal growth factor; FN, fibronectin; LNS, laminin-neurexin-sex hormone binding globulin domains; LRR, leucine-rich repeat; NHL, ncl-1, HT2A and lin-41 domain; TTR, transthyretin-related domain; YD, tyrosine aspartate repeat.
2.1. Classical Cadherins
The Cadherin (Calcium-dependent adherent protein) superfamily of single-pass transmembrane glycoproteins has more than 100 members in mammals and is characterized by two or more Extracellular Cadherin (EC) domains. Most Cadherins engage in homophilic interactions in trans mediated principally by the most membrane-distal EC domain (Brasch, Harrison, Honig, & Shapiro, 2012). While these interactions tend to be weak, Cadherins can be ordered into arrays for strong adhesion (Al-Amoudi, Diez, Betts, & Frangakis, 2007). Binding of Cadherin proteins to partners in cis further extends their interaction repertoire. The ~20 classical Cadherins, comprised of type I and II Cadherins differing in a motif in the most distal EC domain, share a highly similar intracellular sequence with an extended β-catenin-binding motif. Complex intracellular interactions provide means by which the strength of Cadherin-based adhesion can be adjusted (Brigidi & Bamji, 2011).
Classical Cadherins play diverse roles in the development and maintenance of synaptic circuits (Friedman, Benson, & Huntley, 2015). Most type I Cadherins, including the extensively studied N-Cadherin, are broadly expressed in the CNS, whereas type II Cadherins mostly exhibit distinct expression patterns across areas of brain and spinal cord and are concentrated at nascent synaptic sites early in postnatal development. Cadherins provide for synaptic targeting across many brain regions. For example, blocking N-Cadherin function with antibodies in the developing chick optic tectum causes incoming retinal ganglion cell axons to overshoot their targets in a culture system (Inoue & Sanes, 1997). Similarly, an N-Cadherin zebrafish mutant shows defects in retinal lamination among several other neurite projection deficits of retinal cells (Masai et al., 2003). Moreover, N-Cadherin shapes connectivity by acting together with other family members like Cadherin-8, illustrating the importance of temporally correlated expression. This is exemplified by the topographic mapping of converging thalamic input streams to the barrels of the somatosensory cortex in the early postnatal period. While N-Cadherin becomes concentrated at thalamocortical synapses of the stream arising from ventral posterior medial nucleus (Huntley & Benson, 1999), Cadherin-8 is enriched at synapses of the other stream from the medial division of the posterior nucleus (Gil, Needleman, & Huntley, 2002) when these projections develop. Akin to the abovementioned findings from the chick tectum, the application of N-Cadherin function-blocking antibodies to organotypic co-cultures of thalamus and somatosensory cortex results in thalamic axons overshooting their targets in layer IV (Poskanzer, Needleman, Bozdagi, & Huntley, 2003). Another example for the role of classical Cadherins in target recognition is provided by Cadherin-6. Its deletion in mice impairs axon-target matching for a subset of RGCs whose axons fail to stop at their normal targets in the subcortical visual nuclei, and instead innervate inappropriate visual nuclei (Osterhout et al., 2011). In thecerebellum, Cadherin-7 is expressed in mossy fiber neurons of the pontine nucleus and their targets, the cerebellar granule neurons, but not in climbing fiber neurons, during the synaptogenic stage of development (Kuwako, Nishimoto, Kawase, Okano, & Okano, 2014). In agreement with a role in synaptic recognition, homophilic Cadherin-7 signaling induces presynaptic differentiation of pontine nucleus axons in co-culture assays and knockdown of Cadherin-7 in pontine nucleus neurons in vivo severely impairs their connectivity with granule cells in the developing cerebellum.
Apart from guiding long-range connectivity between brain regions, Cadherins also specify synaptic targeting of distinct neuronal populations within the same region. In the hippocampus, Cadherin-9 is specifically expressed in CA3 pyramidal neurons and dentate gyrus (DG) granule neurons (Bekirov, Needleman, Zhang, & Benson, 2002). Cadherin-9 knockdown from either CA3 or DG neurons exclusively reduces DG-CA3 mossy fiber synapse formation without affecting non-DG synapses (Williams et al., 2011). This is reminiscent of the role of Cadherin-8 in the development of synaptic laminae of the direction-selective retinal circuit within the inner plexiform layer, where different subtypes of bipolar cells specifically connect with their respective targets of starburst amacrine cells and retinal ganglion cells in appropriate sublaminae. Cadherin-8 and 9 are selectively expressed in bipolar cells and control the targeting of their axons to sublaminae in the inner plexiform layer by a heterophilic mechanism (Duan, Krishnaswamy, De la Huerta, & Sanes, 2014). Moreover, combinatorial interactions between six Cadherins (Cadherins 6, 7, 8, 9, 10 and 18) generate the appropriate connectivity between distinct bipolar cells, starburst amacrine cells and retinal ganglion cells to establish the complex direction-selective circuit of the mouse retina (Duan et al., 2018). Together, a wealth of data has established Cadherins as synaptic recognition factors throughout the brain and underlines the importance of their regional and temporal expression patterns, including their combinatorial expression, for circuit development.
2.2. Protocadherins
The largest subfamily within the Cadherin superfamily consists of Protocadherins, each containing six extracellular EC domains that are diverse among the isoforms and a short, constant cytoplasmic domain. Based on the genomic organization of the respective genes, Protocadherins are subdivided into gene clusters with 58 large variable exons in mice encoding α-, β-, and γ-Protocadherins, with multiple members within each cluster (Mountoufaris, Canzio, Nwakeze, Chen, & Maniatis, 2018; Wu & Maniatis, 1999). In addition, there are ~10 nonclustered δ-Protocadherins (Hulpiau & van Roy, 2009). Clustered Protocadherins are expressed in a combinatorial and stochastic fashion (Schreiner & Weiner, 2010; Thu et al., 2014). Protocadherin proteins from the same and different clusters promiscuously form isoform-independent cis dimers through membrane proximal repeats with efficient trans binding between the dimers. Further, Protocadherins show cis interactions with classical Cadherins (Weiner & Jontes, 2013). Apart from multifaceted functions in nervous system development including neuronal survival and dendritic and axonal arborization (Lefebvre, 2017), clustered Protocadherins can generate cell-specific recognition codes. One example is in the mouse barrel cortex, where clustered Protocadherins are required for lineage-dependent postnatal reciprocal synaptic connections between excitatory layer IV neurons (Tarusawa et al., 2016). In hippocampal pyramidal neurons, conditional deletion of the atypical Protocadherin Celsr3 after the first postnatal week results in a ~50% decrease in the number of excitatory but not inhibitory synapses (Thakar et al., 2017). Similar to Dscams (see below), Protocadherins can also mediate self-/non-self-discrimination for self-avoidance and restriction of synapse formation as shown for γ-Protocadherins in starburst amacrine cells in the retina (Ing-Esteves et al., 2018). These studies show intriguing roles of this diverse protein family in synaptic recognition.
2.3. Immunoglobulin superfamily members
The first discovered calcium-independent adhesion molecules are the proteins of the immunoglobulin (Ig) superfamily (IgSF). They were identified in parallel to the Cadherin superfamily and are now known to have at least 500 members, with roughly half expressed in neurons. Many members of the IgSF have been implicated in cell-type-specific recognition throughout brain development. Their extracellular domains are comprised of multiple Ig domains that engage in homophilic as well as heterophilic binding (Verschueren et al., 2020; Wojtowicz et al., 2020), often in combination with fibronectin III domains. A prominent group within the Ig superfamily is the L1 family, consisting of L1 (also known as Neuron-glia Cell Adhesion Molecule NgCAM), Close Homolog of L1 (CHL1), Neuron-glia-related Cell Adhesion Molecule (NrCAM), and Neurofascins. The L1 family plays roles in subcellular-specific targeting in several circuits. In the mouse brain, Neurofascin-186 (NF186) is enriched at the axon initial segment (AIS) of several cell types, such as cerebellar Purkinje neurons, hippocampal granule cells and neocortical pyramidal cells. NF186 in Purkinje neurons binds Neuropilin-1 expressed on axons of GABAergic basket neurons to restrict formation of the complex pinceau synapses at the Purkinje AIS, named for their brush-like appearance (Ango et al., 2004; Telley et al., 2016). In the neocortex, instead of AIS-enriched NF186, pan-axonally expressed L1 is required for selective innervation of pyramidal neuron AIS by GABAergic Chandelier cells (Tai, Gallo, Wang, Yu, & Van Aelst, 2019). In both cases, anchoring the L1 family member by the cytoskeletal ankyrin G/spectrin complex is necessary for this localized innervation. The cooperation and coincidence detection by Ig recognition factors is illustrated by NrCAM and CHL1 in the sensory-motor circuit of the mouse spinal cord. They interact on GABApre interneurons with the Ig complex of Contactin 5 and Cntn-associated protein 4 (Caspr4) on proprioceptive sensory neurons to guide inhibitory synapse formation precisely at the axonal termini of sensory afferents (Ashrafi et al., 2014). Synaptic recognition includes interactions with astrocytic processes and a proximity-labeling approach performed in a partner-specific manner has recently mapped the proteome of astrocyte-neuron contacts in the cortex (Takano et al., 2020). NrCAM was one of the astrocyte-expressed proteins identified at these perisynaptic sites. Intriguingly, extracellular Ig interactions of astrocytic NrCAM are required for normal inhibitory synapse number and strength, while intracortical excitatory synapses are unaffected by NrCAM loss in astrocytes. This work advances our understanding of the roles that perisynaptic astrocyte contacts and NrCAM in particular play in synapse organization. It additionally highlights the power of targeted proteomic approaches to define synaptic recognition.
Studies in the retina have implicated other IgSF families in synaptic recognition, including the Down syndrome cell adhesion molecules (Dscams, comprised of Dscam and Dscam-like1), Sidekicks (Sdk1 and 2) and GPI-anchored Contactins (Cntns 1–6), which mostly bind homophilically. In the chick retina, isoforms of these proteins are expressed by largely non-overlapping subsets of bipolar cells, amacrine cells and retinal ganglion cells, with cells expressing the same molecule projecting to the same inner plexiform layer lamina (Yamagata & Sanes, 2008, 2012; Yamagata, Weiner, & Sanes, 2002). The isoform-specific adhesion between these proteins mediates lamina-specific connectivity in the retina, although interesting differences have been observed across molecules and species. One example are Dscams, which are required for synaptic lamination in the chick retina (Yamagata & Sanes, 2008). Their function in synapse specification does not appear to be conserved in the mammalian retina, and Dscam in the mouse retina facilitates neurite self-avoidance by counteracting cell type-specific adhesion by Cadherins and Protocadherins (Fuerst, Koizumi, Masland, & Burgess, 2008; Garrett, Khalil, Walton, & Burgess, 2018). Sidekicks are expressed in subsets of retinal neurons in chick and mice and are critical for laminar restriction of their neurites (Krishnaswamy, Yamagata, Duan, Hong, & Sanes, 2015; Yamagata & Sanes, 2018). Further, dendrites of mouse amacrine cells and retinal ganglion cells expressing Sdk1 but not Sdk2 arborize in the same stratum in the inner plexiform layer and this sublaminar restriction is disrupted in absence of Sdk1 (Yamagata & Sanes, 2018).
The Ig family of Synaptic Cell Adhesion Molecules (SynCAMs) is comprised of four members also known as Cadms (Cell adhesion molecules) or Nectin-like molecules (Biederer & Shrestha, 2015; Frei & Stoeckli, 2014). These vertebrate-specific proteins engage in homo- and heterophilic binding, with select pairwise interaction patterns between distinct members (Fogel et al., 2007; Kakunaga et al., 2005; Shingai et al., 2003; Thomas, Akins, & Biederer, 2008). SynCAM 1 is required and sufficient for excitatory synapse formation as shown in the hippocampal CA1 area (Robbins et al., 2010). SynCAM 1 also contributes to synaptic recognition in the CA3 area, where it organizes mossy fiber inputs to pyramidal neurons and is additionally required for mossy fibers to form synapses onto fast-spiking, PV-positive interneurons (Park et al., 2016). In the visual cortex, SynCAM 1 acts postsynaptically in PV-positive interneurons to promote their innervation by long-range thalamocortical inputs (Ribic, Crair, & Biederer, 2019) with implications for inhibitory network maturation and cortical plasticity (Ribic & Biederer, 2019). SynCAM 1 organizes synapses in the retina as well and contributes to cell-cell recognition in the outer plexiform layer. Here, it is expressed on mouse rod photoreceptor terminals and is required for their interactions with processes of horizontal cells and bipolar cell dendrites and the assembly of triadic rod ribbon synapses (Ribic, Liu, Crair, & Biederer, 2014).
The homophilic protein Kirrel3/Neph2 has provided insight into roles of Ig interactions in target recognition in the hippocampus. It is expressed by DG granule neurons and calbindin-positive GABAergic neurons in CA3 and regulates the development of mossy fiber synapses between these two cell types (Martin et al., 2015). With respect to hippocampal mossy fiber synapses, a proteomic approach that combined biochemical fractionation and FACS sorting has recently identified the Ig protein IgSF8 as a novel component of these large synaptic specializations (Apóstolo et al., 2020). IgSf8 is not only strongly enriched at mossy fiber synapses, it also acts as presynaptic organizer of their ultrastructure and connectivity. Methodologies that map the proteomes of different synapse types as in this study will advance our understanding of how recognition factors contribute to the staggering diversity of synapses across brain regions.
2.4. Leucine-rich repeat family proteins
The leucine-rich repeat (LRR) family of synaptic adhesion molecules is characterized by the presence of multiple consecutive LRR motifs in the extracellular domain which engage in diverse trans-synaptic interactions. LRR proteins have been implicated in all steps of circuit formation from neuronal migration and neurite outgrowth, to the formation and functional assembly of synaptic contacts (Schroeder & de Wit, 2018). The major subfamilies are Netrin-G ligands (NGLs, also called laminets), LRR Transmembrane proteins (LRRTMs), Slit and NTRK-like proteins (Slitrks), Fibronectin LRR Transmembrane proteins (FLRTs) and Synaptic Adhesion-Like Molecules (SALMs, also called LRFNs). LRR proteins provide examples for differential roles of family members in synaptic recognition and the specification of connectivity. Here, NGL-1 and −2 localize to the postsynaptic membrane and form trans-synaptic complexes selectively with GPI-anchored Netrin-G1 and -G2, respectively. In the neocortex and hippocampus of mice, Netrin-G1 and -G2 are distributed on different populations of developing axons (Nishimura-Akiyoshi et al., 2007). Their partners NGL-1 and −2 are concentrated in distinct segments within dendrites of these target areas that correspond to the termination zones of axons expressing Netrin-G1 or -G2 (Matsukawa et al., 2014). In Netrin-G1 and -G2 deficient mice, axonal pathfinding is normal, but the differential distribution of NGL-1 and −2 across dendritic segments is selectively disrupted. Consistent with a role of this subcellular targeting in synaptic recognition, NGL-2 loss selectively reduces the density of spines in the dendritic segment where CA1 pyramidal neurons receive Schaffer collateral inputs, while spine density on their distal dendrites is unaffected (DeNardo, de Wit, Otto-Hitt, & Ghosh, 2012). These studies provide an example for how differential subcellular targeting and adhesive interactions properties can be utilized to generate recognition codes.
The members of the FLRT family, FLRT1–3, were identified as high-affinity postsynaptic ligands of the Latrophilin family of adhesion-type G-protein coupled receptors (O’Sullivan et al., 2012). FLRT2 and FLRT3 show cell-type-specific expression patterns, with complementary and non-overlapping expression in the hippocampus. FLRT3 is highly expressed during the first 2 postnatal weeks in the principal cells of DG and CA3, and its conditional knockdown in the hippocampus of rat pups reduces spine density in DG granule cells and lowers the strength of perforant path inputs onto these cells (O’Sullivan et al., 2012). The FLRT ligands Latrophilin 2 and 3 localize to different dendritic domains of CA1 pyramidal neurons and are essential for synapse formation by entorhinal cortex afferents and Schaffer collateral axons in these strata, respectively (Anderson et al., 2017; Sando et al., 2019). Both pre- vs postsynaptic localizations of Latrophilins have been reported in different regions of the hippocampus, suggesting they may localize to both sides of synapses, perhaps in a synapse-type specific manner (Anderson et al., 2017; O’Sullivan et al., 2012; Sando et al., 2019).
2.5. Teneurins
Teneurins are large transmembrane proteins that play roles in dendrite morphogenesis, axon pathfinding, partner selection and synapse differentiation (Arac & Li, 2019). They form a family of four proteins comprised of a large C-terminal extracellular domain including eight epidermal growth factor (EGF) motifs, a single transmembrane region, and an N-terminal cytoplasmic domain. Teneurins form constitutive cis dimers through the membrane proximal EGF repeats and are involved in homophilic and heterophilic trans interactions that mediate target recognition. Here, trans-synaptic homophilic interactions of Teneurin-3 control targeting of axons from proximal CA1 neurons to their targets in the distal subiculum (Berns, DeNardo, Pederick, & Luo, 2018). Teneurins can also bind Latrophilins, and these heterophilic high-affinity trans interactions provide for synapse-specifying functions of Teneurins and are regulated by alternative splicing (Li et al., 2018; Silva et al., 2011). Intriguingly, simultaneous binding of Teneurin-2 and FLRT3 to Latrophilin 3 promotes Schaffer collateral-CA1 synapse formation (Sando et al., 2019). This coincidence detection via a ternary interaction, akin to a two-factor authentication protocol, highlights the coding power for target-dependent synapse specification provided by multimeric recognition complexes.
3. Restrictive recognition cues shape neuronal connectivity
In concert with the positive, synapse-promoting cell surface interactions described above, cell-surface interactions and molecules secreted from target and non-target cells can also prevent the inappropriate formation of synapses. Such restrictive recognition cues can act locally to refine connection specificity. Alternatively, soluble restrictive factors can act at a distance to the extent they are diffusible within the neuropil. Select restrictive factors discussed below are depicted in Fig. 4A.
Fig. 4.
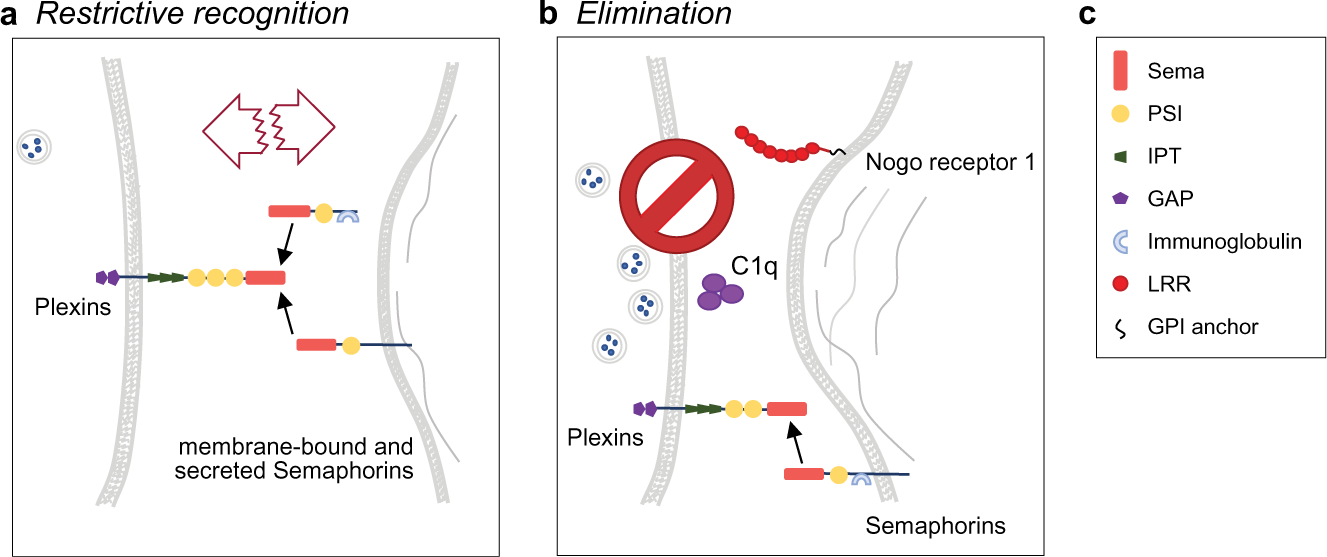
Recognition molecules that restrict synaptic connectivity or participate in synapse elimination. (A) Factors restricting synaptic connections. (B) Factors that eliminate synapses once formed. Pre- and post-synaptic membranes are shown on the left and right, respectively. C1q, complement component 1q. (C) Domains utilized by the molecules in (A, B). GAP, GTPase activating domain; GPI, glycosylphosphatidylinositol; IPT, Immunoglobulin-like fold shared by Plexin and transcription factors, LRR, leucine-rich repeat; PSI, Plexin, Semaphorins and Integrin domain; Sema, Semaphorin domain.
3.1. Semaphorin–Plexin interactions
Some of the best understood restrictive recognition mechanisms are mediated by Semaphorin-Plexin signaling, which prevents mismatches in synaptic connections by inhibiting inappropriate target selection. This has been demonstrated in the hippocampus, retina, olfactory bulb, striatum and spinal cord (Pasterkamp, 2012; Yoshida, 2012). In the mouse hippocampal CA3 area, the transmembrane Semaphorins Sema6A and 6B, expressed on CA3 pyramidal neurons, interact with Plexin A4 on mossy fibers to control their lamina-restricted projection to the stratum lucidum of CA3 (Suto et al., 2007; Tawarayama, Yoshida, Suto, Mitchell, & Fujisawa, 2010). Sema6A and 6B are required for the repulsion of Plexin A4-expressing mossy fibers, preventing them from forming aberrant projections into stratum radiatum and stratum oriens. Semaphorin-Plexin signaling can also restrict connectivity by acting in the same cells as shown in hippocampal DG granule cells. Here, the transmembrane Semaphorin Sema5A is highly expressed in developing granule cells and signals through its receptor Plexin A2 co-expressed by these cells to suppress spinogenesis (Duan et al., 2014).
In the developing mouse retina, Sema6A acts as a repulsive cue to direct laminar termination away from inappropriate sublaminae. Sema6A and its receptor Plexin A4 are expressed in a complementary fashion in a subset of amacrine cells and of retinal ganglion cells in the inner plexiform layer that differ in their response to a luminance change (Matsuoka et al., 2011). Mice lacking Plexin A4 or Sema6A exhibit severe mistargeting of these cell projections to sublaminae of the inner plexiform layer. Similarly, Sema5A and Sema5B, acting through their receptors Plexin A1 and A3, constrain the neurites from multiple retinal neuron subtypes to the inner plexiform layer (Matsuoka et al., 2011). In the absence of these molecules, retinal neurons fail to correctly stratify in the inner plexiform layer and neurites become mistargeted to the outer portions of the developing retina. These results support that Semaphorins specify laminar stratification through restrictive recognition.
Semaphorins also restrict synaptic recognition in other neural circuits. In the mouse spinal cord, repulsive interactions between secreted Sema3E, expressed on the cutaneous maximus motor neurons, and PlexinD1, expressed on sensory afferents, prevents the formation of direct sensory-motor neuron synapses (Pecho-Vrieseling, Sigrist, Yoshida, Jessell, & Arber, 2009). Sema3E-Plexin D1 signaling also negatively regulates thalamo-striatal synapse formation selectively on direct pathway medium spiny neurons (Ding, Oh, Sabatini, & Gu, 2011). While these examples highlight the restrictive activities of Semaphorin–Plexin interactions during circuit assembly, it should be noted that depending on the context, these ligand-receptor systems can promote connectivity as shown, e.g., for Sema4D that enhances inhibitory synapse formation in the hippocampus (Acker, Wong, Kang, & Paradis, 2018). Semaphorins and their receptors additionally play important roles during later stages of circuit refinement that are discussed in Section 5.
3.2. Other restrictive recognition factors
The FLRT family member FLRT2 and Unc-5C, a receptor for Netrin axon guidance cues, are expressed in a strikingly complementary fashion within specific retinal sublaminae of the developing inner plexiform layer. Heterophilic repulsion between FLRT2 and Unc-5c has been proposed to mediate the laminar restriction of presynaptic starburst amacrine cells and a subset of ON-OFF direction-selective ganglion cells (Visser et al., 2015). Synapse-restricting recognition is provided in the hippocampus during early postnatal development by the GPI-anchored Nogo Receptor 1 (NgR1), which acts through its co-receptor TROY to limit excitatory synapse formation and maturation (Lee et al., 2008; Wills et al., 2012).
4. Beyond making connections: Creating synapse diversity
Synapses are structurally and functionally highly diverse. During their assembly, cell-surface adhesion molecules and secreted factors modulate and even instruct pre- and post-synaptic differentiation at nascent contact sites. Many synapse organizing membrane proteins have been identified based on their synaptogenic ability in a mixed co-culture assay of neurons and non-neuronal cells (Biederer & Scheiffele, 2007). The co-culture assay serves to identify the sufficiency of candidate molecules to induce synaptic specializations, however it is unable to differentiate between their activities across the steps of synapse formation, stabilization and maturation. Additional tests are required to dissociate these roles of synapse organizing proteins, including loss-of-function analyses and acute interference with their interactions. Since the structural and functional diversity of synapses is best studied in the complex environment of the brain, we prioritize in this Section in vivo over in vitro studies evaluating roles of these proteins.
As a prerequisite to understand the molecular basis of synapse diversity, much progress has been made to parse out the transcriptional diversity of neurons in the mouse brain (see for examples, Paul et al., 2017; Tasic et al., 2016). Recently, Rico and colleagues made significant progress by demonstrating that cell type-specific expression patterns of recognition factors not only create molecular fingerprints to assign neuronal identities but also underly their connectivity patterns (Favuzzi et al., 2019). Using cell sorting, RNA sequencing and mouse genetics, they identified recognition factors that are differentially expressed between interneuron types in the developing mouse cerebral cortex and that allow these neurons to target distinct subcellular compartments of pyramidal cells. This study revealed presynaptic molecular programs that specify the sites where synaptic inputs are formed (see also Section 4.5). Such innovative analyses of cellular expression patterns promise to advance new insights into the organizing principles of input-specific connectivity and microcircuit wiring. Below, we describe select recognition factors to highlight how they can diversify synapse types.
4.1. Synapse-type specific functions of Neurexin–Neuroligin complexes
Much of our current understanding of the creation of synapse diversity derives from the trans-synaptic interactions of presynaptic Neurexins (Südhof, 2017). Mammalian Neurexins are encoded by three genes giving rise to Neurexin 1–3, each with three promoters that can drive transcription of a longer α-Neurexin and shorter β-Neurexin isoform, and a γ isoform that lacks most of the extracellular domain (Roppongi et al., 2020; Tabuchi & Südhof, 2002; Ushkaryov, Petrenko, Geppert, & Südhof, 1992; Yan et al., 2015). Mice lacking all isoforms of α-Neurexins have ultrastructurally normal synapses and unaltered numbers of excitatory synapses, but Ca2+-triggered neurotransmitter release is severely impaired (Missler et al., 2003; Varoqueaux et al., 2006). Inhibitory synapse number is significantly reduced in Neurexin triple KO mice (Dudanova, Tabuchi, Rohlmann, Südhof, & Missler, 2007). Neuroligins were the first of the postsynaptic ligands identified for Neurexins and form a family of four members. Intriguingly, Neuroligin-1 and Neuroligin-2 localize to excitatory and inhibitory synapses, respectively, pointing to synapse-type specific roles of their complexes with Neurexins (Song, Ichtchenko, Südhof, & Brose, 1999; Varoqueaux, Jamain, & Brose, 2004). Mice lacking the three Neuroligins have impaired synaptic transmission but unaltered synapse number (Varoqueaux et al., 2006). Neurexins engage in multiple extracellular interactions in addition to binding Neuroligins, establishing Neurexins as presynaptic hub proteins (Südhof, 2017).
Neurexin/Neuroligin complexes are involved in the functional specification of synapses. In agreement with their selective localization to excitatory and inhibitory synapses, deletion of Neuroligin-1 or −2 impairs evoked excitatory or inhibitory synaptic transmission, respectively (Chanda, Hale, Zhang, Wernig, & Südhof, 2017; Chubykin et al., 2007). Studies of Neurexins in interneurons reveal an even more diverse picture. Conditional deletion of α and β isoforms of Neurexin 1, 2 and 3 from fast-spiking PV-positive interneurons in the prefrontal cortex results in a loss of the inhibitory synapses they form and a decrease in synaptic strength but no impairment in action potential-triggered Ca2+ influx. In contrast, pan-Neurexin deletion in Somatostatin-positive interneurons causes no synapse loss but a large decrease in action potential-triggered Ca2+ influx that also suppresses synaptic strength (Chen, Jiang, Zhang, Gokce, & Südhof, 2017). Neurexins hence perform distinct roles in specifying synaptic properties that depend on pre- and postsynaptic partner combinations.
4.2. SynCAM cell adhesion molecules
SynCAMs are synaptogenic Ig proteins and induce functional presynaptic specializations (Biederer et al., 2002; Czondor et al., 2013; Fogel et al., 2007). SynCAM 1 is primarily postsynaptic at excitatory hippocampal CA1 synapses, with a smaller fraction present in the presynaptic membrane (Perez de Arce et al., 2015). Loss of SynCAM 1 in mice reduces excitatory synapse number and temporal control of its forebrain expression in mice has demonstrated that it is first sufficient to promote excitatory synapse number in the hippocampal CA1 area and then required to maintain them (Robbins et al., 2010). A postsynaptic role of SynCAM 1 in the specification of synaptic inputs was shown in the visual cortex of mice. Cell-type specific knockdown of SynCAM 1 in PV-positive interneurons revealed that it is required in these cells to receive long-range thalamocortical excitatory inputs while its loss in the same cells does not impact short-range intracortical excitatory synapses (Ribic et al., 2019). SynCAMs hence engage in postsynaptic recognition to promote and specify synaptic connectivity.
4.3. Protein tyrosine phosphatases
The Leukocyte common antigen-receptor protein tyrosine phosphatases (LAR-RPTPs) comprise of the PTPσ, PTPδ and LAR proteins that organize excitatory and inhibitory synapse assembly (Han, Jeon, Um, & Ko, 2016). LAR-RPTPs contribute to the specification of synapses in a target-dependent manner by binding to multiple distinct postsynaptic ligands, such as NGL-3, TrkC, Slitrks, Synaptic Adhesion-Like Molecules (SALMs) and Interleukin 1 receptor accessory protein-like 1 (IL1RAPL1) (Han et al., 2016; Lie, Li, Kim, & Kim, 2018). This target-dependent role in synapse specification is shown by results that PTPσ is required for excitatory synapse induction by Slitrk1 (Han et al., 2018), while PTPδ is required for IL1RAPL1-mediated excitatory presynaptic differentiation (Yoshida et al., 2011) as well as Slitrk3-mediated inhibitory presynaptic differentiation (Yim et al., 2013). Conditional deletion of individual or all LAR-RPTPs either globally or selectively in excitatory cortical and hippocampal neurons does not affect spontaneous or evoked AMPAR-mediated synaptic transmission but does impair NMDA-receptor-mediated responses (Kim et al., 2020; Sclip & Südhof, 2020). This indicates that LAR-RPTPs specify functional postsynaptic properties.
4.4. Ephrin-EphB receptors
An additional class of trans-synaptic organizers with a direct signaling capability are the EphB receptor tyrosine kinases and their ephrinB ligands, which mediate excitatory synapse development in the hippocampus and cortex (Henderson & Dalva, 2018). Here, EphB-ephrinB signaling regulates spinogenesis as well as the clustering of glutamate receptors (Dalva et al., 2000; Henkemeyer, Itkis, Ngo, Hickmott, & Ethell, 2003; Kayser, McClelland, Hughes, & Dalva, 2006; Segura, Essmann, Weinges, & Acker-Palmer, 2007). Postsynaptic EphB2 can simultaneously bind to its ephrinB ligands and NMDA receptors, controlling the mobility of these receptors (Dalva et al., 2000; Washburn, Xia, Zhou, Mao, & Dalva, 2020). Another role in specifying synaptic properties is provided by postsynaptic ephrinB3, which balances the extent to which glutamatergic synapses are formed on dendritic shafts vs spines (Aoto et al., 2007).
4.5. Synapse diversification involves secreted factors
Neurons locally secrete factors that contribute to synapse-type specific recognition. Wingless and Int-1 proteins (Wnts) induce presynaptic assembly (Sahores, Gibb, & Salinas, 2010; Umemori, Linhoff, Ornitz, & Sanes, 2004) and neuronal Pentraxins cluster ionotropic glutamate receptors at excitatory postsynaptic sites (Pelkey et al., 2015). Other secreted neuronal factors are Fibroblast growth factors (FGFs), and FGF-22 and FGF-7 differentially control synapse formation (Dabrowski, Terauchi, Strong, & Umemori, 2015; Terauchi et al., 2015; Umemori et al., 2004). In the hippocampus, they are highly expressed in CA3 pyramidal neurons during synaptogenesis and serve as retrograde presynaptic organizers of excitatory and inhibitory synapses, respectively, on CA3 pyramidal neurons. Another neuronally secreted factor that controls synaptic connectivity is the protein Complement Component 1q Subcomponent-like 3 (C1ql3). C1ql3 contains two globular domains originally identified in the protein C1q that assembles the initiating complex of the complement cascade in the immune system. C1ql family members bind the adhesion G protein-coupled receptor BAI3 (Bolliger, Martinelli, & Südhof, 2011; Sigoillot et al., 2015). Presynaptic deletion of C1ql3 in basolateral amygdala neurons causes a strong loss of their excitatory outputs to the prefrontal cortex (Martinelli et al., 2016).
Cerebellins (Cblns), another class of secreted C1q family members, are critical adaptors for multiple pre- and post-synaptic molecules. Cbln1 is secreted from cerebellar granule neurons and binds simultaneously to its postsynaptic receptor, the Glutamate Receptor Delta-2 (GluD2) on dendritic spines of Purkinje cells and a splice form of presynaptic Neurexin on cerebellar granule cell axons. Neurexin-Cbln1-GluD2 signaling leads to presynaptic vesicle accumulation as well as the postsynaptic accumulation of GluD2, illustrating coordinated pre- and postsynaptic differentiation by trans-synaptic interactions (Ito-Ishida et al., 2012; Matsuda et al., 2010). Other examples for synapse-specifying secreted factors include Cbln4 that is secreted by Somatostatin-positive interneurons to bridge presynaptic Neurexin and GluD1 in layer 2/3 pyramidal neurons in the somatosensory cortex (Fossati et al., 2019). In addition, Cbln4 expressed by cortical Somatostatin-positive interneurons is required for their ability to innervate dendrites of pyramidal neurons, and exogenous expression of Cbln4 in PV-positive interneurons is sufficient to re-direct their inhibitory inputs to pyramidal neuron dendrites (Favuzzi et al., 2019). Cerebellins thereby serve as secreted ‘match-makers’ to spatially specify synapse formation.
Among astrocyte-secreted factors, Hevin/SPARCL1 was reported to bridge interaction-incompatible Neurexin 1α and a splice isoform of Neuroligin-1 at thalamocortical synapses (Singh et al., 2016). While validating the synaptogenic role of Hevin/SPARCL1, another study has used conditional knock-out of Neuroligins and Neurexins to show that Hevin acts independent of them in cultured neurons (Gan & Südhof, 2020). Synapse-type specific roles of Hevin for bridging Neurexin/Neuroligin complexes could resolve this discrepancy but remain to be tested.
4.6. Diversification of synaptic recognition proteins by alternative splicing
Alternative splicing of synaptic recognition molecules enhances their molecular diversity far beyond the limited number of genes, controlling their ectodomain interactions with synaptic ligands. Alternative splicing of cell adhesion molecules regulates synapse development as exemplified by Neurexins. Their alternative splicing at six canonical splice sites (SS1–SS6) in their ectodomains can generate over 1300 detectable isoforms (Schreiner et al., 2014; Treutlein et al., 2014). Single-cell profiling of mRNAs as well as ribosome-associated transcripts complemented by mass-spectrometric profiling of isoforms in the adult mouse brain have revealed hundreds of alternatively spliced Neurexin mRNAs with remarkable cell type-specificity and brain region-select regulation (Fuccillo et al., 2015; Furlanis, Traunmüller, Fucile, & Scheiffele, 2019; Schreiner et al., 2015). For example, in the mouse hippocampus, the SS4+ Neurexin isoform is selectively expressed in GABAergic PV-positive interneurons while the SS4− isoform is the major isoform in glutamatergic pyramidal cells in the CA1 region (Nguyen et al., 2016). This posttranscriptional processing is extensively used to control synaptic recognition by Neurexins. One example is provided by LRRTMs and Latrophilins that bind Neurexins only when they lack an insert in splice site 4 (SS4−) (Boucard, Ko, & Südhof, 2012; Etherton, Blaiss, Powell, & Südhof, 2009; Siddiqui et al., 2010), while Cerebellin 1 only binds to SS4+ Neurexins (Uemura et al., 2010). Alternative Neurexin splicing impacts synaptic composition and transmission as shown by studies in which the SS4 insert in Neurexin-3 was constitutively retained in vivo. This decreased postsynaptic levels of AMPARs in hippocampal neurons, a non-cell-autonomous phenotype shared by Neurexin-3 knock-out neurons (Aoto, Martinelli, Malenka, Tabuchi,& Südhof, 2013). These results highlight the need for profiling the cell-type-specific splicing patterns of other recognition molecules.
4.7. Post-translational modifications modulate and mediate synaptic recognition
Modifications such as phosphorylation, glycosylation, and palmitoylation are abundant in synaptic proteins and regulate their subcellular localization and protein–protein interactions. One example for the regulation of synaptic adhesion molecules with synaptogenic activity by posttranslational modification is provided by SynCAM 1. Site-specific N-glycosylation of its most membrane-distal Ig domain promotes trans-synaptic SynCAM interactions and is required for synapse induction (Fogel et al., 2010). Further, polysialic acid is attached to N-glycosylation sites of a subset of SynCAM 1 proteins in the developing mouse brain and this modification blocks homophilic SynCAM 1 binding (Galuska et al., 2010). Diverse cell-surface recognition codes can be created by glycan modifications as shown for the covalent attachment of long, heterogeneous chains of the glycosaminoglycan heparan sulfate to ectodomains of synapse organizers (Condomitti & de Wit, 2018). These heparan sulfate glycans are bound by heparan sulfate-binding proteins, thereby expanding interactions beyond adhesion of protein domains alone. Here, the glial-derived GPI-anchored Glypican 4 is modified by heparan sulfate and forms a glycan-dependent complex with postsynaptic LRRTM4 to promote excitatory synapse development (de Wit et al., 2013; Siddiqui et al., 2013). The Neurexin-1 extracellular domain is also modified by heparan sulfate and this modification is required for presynaptic differentiation induced by its postsynaptic Neuroligin and LRRTM ligands (Roppongi et al., 2020; Zhang et al., 2018).
4.8. Cooperation of co-expressed recognition molecules
A growing body of evidence demonstrates that multiple synapse organizing proteins are often present at the same synapse, with several of them known to share trans-synaptic partners. This sets the stage for coincidence detection of the presence of recognition factors. At hippocampal DG excitatory synapses, presynaptic Neurexin 1 forms a complex with presynaptic PTPσ that also involves the modification of Neurexin with heparan sulfate, and both proteins cooperate in synaptogenesis mediated by postsynaptic LRRTM4 (Roppongi et al., 2020). This shows that the themes of post-translational diversification and concerted function can be combined to control recognition systems. Combinatorial roles were additionally shown for LRRTMs and Neuroligins in early postnatal development. Whereas individual or combined knockdown of LRRTM1 and LRRTM2 in cultured hippocampal neurons does not affect number of excitatory synapses, the additional loss of Neuroligin-1 and Neuroligin-3 leads to an extensive reduction of synapse number in an activity-dependent manner (Ko et al., 2011). Moreover, N-Cadherin is required for the postsynaptic adhesion molecules Neuroligin-1, SynCAM 1 and LRRTM2 to promote presynaptic differentiation as well as to enable postsynaptic differentiation by Neurexin-1β (Stan et al., 2010; Yamagata, Duan, & Sanes, 2018). Further, development of inhibitory synapses is jointly controlled by the postsynaptic organizers Slitrk3 and Neuroligin-2 (Li et al., 2017). These examples provide intriguing evidence that cooperative action of recognition molecules specifies synapses in a manner that depends on the molecular makeup of their synaptic clefts.
5. Refining neuronal connectivity through eliminating synapses
Once neuronal connections are established, synapses that were inappropriately formed or only need to be present during a select developmental period are removed. This refinement is critical for experience-dependent neuronal network maturation and involves microglial, astrocytic, and neuronal recognition factors. Several molecules involved in synapse elimination are depicted in Fig. 4B.
Tailoring synaptic connectivity includes microglia that migrate to ‘tagged’ synapses and engulf presynaptic terminals (Ji, Akgul, Wollmuth, & Tsirka, 2013; Paolicelli et al., 2011; Schafer et al., 2012; Weinhard et al., 2018). Multiple immune system effector molecules have been implicated in synaptic elimination by microglia in the CNS. The roles of these pruning molecules are best understood in the mouse retinogeniculate system, which is well suited to investigate synaptic pruning due to the anatomical precision with which eye-specific RGC inputs are refined and the fact that pruning in this brain region occurs during a narrow postnatal window. Here, the complement cascade that serves in the innate immune system to tag debris for phagocytosis has been found to mark synapses for removal by microglia (Stevens et al., 2007). The abovementioned complement factor C1q is expressed in developing but not mature RGCs and localizes to the synapses between RGCs and their target neurons in the dorsolateral geniculate nucleus (dLGN). C1q knock-out mice have defects in synaptic refinement in the dLGN by P30, supporting that inappropriate retinal inputs were not properly pruned. Loss of synaptically localized C3, another complement protein, or astrocyte-secreted transforming growth factor-β (TGF-β) also results in decreased synaptic pruning in the retinogeniculate system (Bialas & Stevens, 2013; Schafer et al., 2012; Stevens et al., 2007). Other mechanisms that involve microglia are also in play in the dLGN. Following visual stimulation, microglia upregulate the cytokine TNF-associated weak inducer of apoptosis (TWEAK) and relay neurons increase expression of the TWEAK receptor, the Fibroblast growth factor-inducible protein, 14 kDa (Fn14). Neuronal Fn14 is required for the vision-dependent strengthening of bulbous spines contacted by RGCs when not bound by TWEAK. If Fn14 is bound by TWEAK at synapses proximal to microglia, their signaling decreases the number of bulbous spines via a mechanism distinct from phagocytic engulfment. Microglial TWEAK hence locally balances the refinement of dLGN inputs in a sensory experience-dependent manner (Cheadle, Rivera, Phelps, & Ennis, 2020).
Pruning in the hippocampal CA1 area involves the Triggering Receptor Expressed on Myeloid cells 2 (TREM2), an innate immune receptor that is required by microglia to refine excitatory inputs in CA1 (Filipello et al., 2018). In addition, the fractalkine receptor Cx3cr1, a chemokine receptor expressed by microglia, contributes to pruning in CA1. Mice lacking Cx3cr1 exhibit increased postsynaptic puncta density, CA1 dendritic spine density and mEPSC frequency, in agreement with an excess of excitatory synaptic sites due to a decrease in pruning (Paolicelli et al., 2011). The effect of Cx3cr1 on microglial-mediated pruning appears to be brain region-dependent, as its deletion in the visual cortex results in no change to synapse turnover (Lowery, Tremblay, Hopkins, & Majewska, 2017).
Microglia-dependent synapse elimination accounts for only part of retinogeniculate circuit refinement. Astrocytes and the factors they secrete add to the complexity of synapse removal (Chung et al., 2013; Vainchtein & Molofsky, 2020). Here, astrocyte-expressed phagocytic receptors (MEGF10 and MERTK) and recognition molecules (ephrinB1) contribute to synapse elimination in dLGN and hippocampal CA1, respectively (Chung et al., 2013; Koeppen et al., 2018).
Additionally, neuronally expressed molecules originally identified in the immune system, including the class I major histocompatibility complex (MHC I) and the secreted Pentraxins, homologs of a class of immune proteins recognizing antigens, participate in synapse removal (Bjartmar et al., 2006; Huh et al., 2000). Neuronal Semaphorins that restrict synaptic recognition in earlier development can also act to eliminate synapses once formed in order to refine connectivity (Riccomagno & Kolodkin, 2015). Semaphorin 3F signaling through the Neuropilin-2/Plexin A3 holoreceptor promotes the progressive elimination of synapses transiently formed by infrapyramidal mossy fiber axon collaterals on the basal dendrites of CA3 pyramidal cells in the maturing hippocampus (P25) (Bagri, Cheng, Yaron, Pleasure, & Tessier-Lavigne, 2003; Liu, Low, Jones, & Cheng, 2005). Similar to the elimination of excess synapses in other regions like the retinorecipient superior colliculus (Cheng et al., 2010) or the Plexin A3/A4-dependent stereotypic pruning of inputs by corticospinal tract axons (Low, Liu, Faulkner, Coble, & Cheng, 2008), this synaptic refinement process in the hippocampus precedes retraction of axons. Further, mice lacking the secreted Semaphorin 3F and its receptor Neuropilin-2 have normal spine density in DG granule cells and cortical layer V pyramidal neurons at P14 but higher density at P21 (Tran et al., 2009). This suggests a role for these recognition molecules in restricting synapse number in the maturing hippocampus, in agreement with the increase in the number of dendritic spines after acute deletion of Neuropilin-2 in adult cortical layer V neurons (Assous et al., 2019). Additional support for roles of Semaphorins in the negative control of synapse density comes from studies of the L1 Ig family member NrCAM, an obligate component of the Semaphorin 3F receptor complex Neuropilin-2/Plexin A3. Deletion of NrCAM results in increased spine number on apical dendrites of star pyramidal neurons in layer 4 of the mouse primary visual cortex at both P21 and P60 (Demyanenko et al., 2014). A classical process of synapse elimination occurs in the cerebellum and is also controlled by Semaphorin/Plexin recognition. Here, climbing fibers project from the contralateral inferior olive and synapse onto Purkinje cells (Hashimoto & Kano, 2005; Kano et al., 2018; Sassoe-Pognetto & Patrizi, 2017). During the first week of postnatal development in rodents, Purkinje cells in the cerebellum are innervated by multiple climbing fibers (Crepel, Mariani, & Delhaye-Bouchaud, 1976). However, by postnatal week three, only one of these original climbing fibers innervates a single Purkinje cell and all other climbing fiber inputs are removed (Chedotal & Sotelo, 1993; Crepel, Delhaye-Bouchaud, & Dupont, 1981; Mariani & Changeux, 1981). In this process, Semaphorin 3A acts as a retrograde signal from Purkinje cells to Plexin A4 in climbing fibers to protect one synapse from elimination, whereas Semaphorin 7A facilitates elimination of climbing fiber synapses on Purkinje cells through Plexin C1 and the basement membrane-related protein Integrin β1 (Uesaka et al., 2014).
While it is important for circuit refinement to tag specific subsets of synapses for removal, it is conceivable that molecules present at the retained synapses serve to prevent pruning. Evidence exists that CD47, another immune system molecule, and its receptor SIRPα are among such factors. CD47 is detected at dLGN synapses during the peak period of their pruning and loss of CD47 or its receptor SIRPα results in a decrease in dLGN excitatory synapse number and increased microglial engulfment of presynaptic inputs. This in turn significantly impairs retinal innervation (Lehrman et al., 2018).
6. Aberrant synaptic recognition and brain disorders
Genome wide association studies (GWAS) and the characterization of de novo mutations in neuropsychiatric disorders strongly support that aberrations in synaptic adhesion molecules are associated with increased risk for neurodevelopmental and psychiatric disorders. While functional compensation can occur among synaptic adhesion molecules from the same or different gene families, de novo mutations in neuropsychiatric patients provide evidence that a change as small as a single amino acid substitution in a recognition molecule can impact social behaviors and cognitive functions. The notion that even minor disruptions in synaptic recognition perturb synapses and alter circuits is supported by studies in which disorder-linked mutations were introduced into synaptic adhesion molecules. Among the consequences are synapse-type specific alterations, changes in synaptic transmission, and improper connectivity, all of which can impair brain functions.
We focus here on autism spectrum disorders and schizophrenia that present during early and late brain development, respectively. They were selected because altered synapse number and connectivity patterns are part of the etiology of these disorders. Further, a wealth of human genetic data and results from animal models with disease-linked mutations or deletions in synaptic adhesion molecules are available. While the focus of this chapter lies on developmental aspects, it needs to be considered that phenotypes correlated with mutations in synaptic recognition factors could in part reflect their functions in the maturing and adult brain.
6.1. Autism spectrum disorders
Autism Spectrum Disorders (ASDs) are a group of developmental disorders characterized by deficits in social-emotional reciprocity, impairments in verbal and nonverbal communication, and repetitive patterns of behaviors and interests that start to manifest early in life. Specifically, ASD symptoms can often be diagnosed around the age of 2. These impairments are distinct from intellectual disability or a general developmental delay.
How do synapses come into play? ASD has a high comorbidity with epilepsy and has long been thought to involve altered synaptic connectivity. This agrees with the onset of its symptoms during the period of most intense synaptogenesis, as well as postmortem data showing increased dendritic spine density in prefrontal cortex (PFC) pyramidal neurons of ASD patients (Hutsler & Zhang, 2010). Patients with ASD also present improper excitatory and inhibitory (E/I) synaptic balance resulting in abnormal transmission and oscillatory anomalies on a brain-wide scale (Cornew, Roberts, Blaskey, & Edgar, 2012; Orekhova et al., 2007). A large body of evidence from GWAS and de novo mutation analyses points to malfunctions of synaptic molecules including recognition factors in the etiology of ASD (Geschwind & State, 2015; Sestan & State, 2018). Animal models where synaptic adhesion molecules associated with ASD either carry human disease-linked mutations or are deleted exhibit E/I synaptic imbalance phenotypes as reviewed below. Whether these E/I alterations are cause or consequence of ASD-linked aberrations is being discussed (Antoine, Langberg, Schnepel, & Feldman, 2019), but it can be considered that synaptic adhesion molecules have a role in the homeostatic stabilization of circuits and that disease-linked mutations impair this. Together, the altered expression of ASD-linked synaptic adhesion molecules can impact neuronal transmission and partner recognition as described in this Section. Susceptibility to these mutation effects appears to differ across brain regions and for candidate molecules listed here, the brain area or synapse type that has been characterized is stated.
6.1.1. Neurexins (NRXN genes)
Neurexins have been strongly implicated in ASD, and deletions and rare variants of Neurexin-1α are found in ASD patients (Gauthier et al., 2011; Schaaf et al., 2012; Südhof, 2017; Yan et al., 2008). Neurexin-1α knock-out mice have reduced spontaneous and evoked excitatory synaptic strength in the hippocampus (Etherton et al., 2009). Along with these synaptic changes, Neurexin-1α knock-outs display increased repetitive grooming behaviors, impaired nest-building, and impaired pre-pulse inhibition, behavioral phenotypes that are linked to neurodevelopmental disorders. Non-social cognitive defects were also observed in rats lacking Neurexin-1α (Esclassan, Francois, Phillips, Loomis, & Gilmour, 2015).
6.1.2. Neuroligins (NLGN genes)
Neuroligin family members are genetically associated with ASD, with mutations found in syndromic and non-syndromic ASD patients (Marro et al., 2019; Nakanishi et al., 2017; Südhof, 2017; Xu et al., 2014). One mutation in Neuroligin-3, R451C, is a highly penetrant missense mutation (Jamain et al., 2003). This mutation resides within the extracellular, cholinesterase-like domain and causes altered intracellular protein trafficking, resulting in lower surface expression (De Jaco et al., 2005). While no loss of excitatory or inhibitory synapse density has been found in Neuroligin-3 knock-out or R415C knock-in mouse lines, Neuroligin-3 R415C knock-in mice display increased inhibitory synaptic transmission in cell layer II/III of the somatosensory cortex that is not seen in the Neuroligin-3 knock-out mice, suggesting a pathological dominant negative effect (Tabuchi et al., 2007). In the somatosensory barrel cortex, in vivo spine imaging revealed an increased spine turnover rate in three-week-old Neuroligin-3 R451C knock-in mice (Isshiki et al., 2014). Neuroligin-3 R451C knock-in mice also display social novelty defects with decreased activity in the medial prefrontal cortex (mPFC), an area implicated in ASD. Local field potential recordings in the mPFC of Neuroligin-3 R451C knock-in mice revealed reduced gamma band activity as well as reduced gamma-to-theta amplitude coupling, indicative of inappropriate synaptic connectivity (Cao et al., 2018). In vitro patch-clamp recordings in the mPFC found reduced excitability of PV-positive interneurons, but not pyramidal neurons. Interestingly, oscillation-coupled excitation of mPFC PV-positive interneurons via optogenetics was able to rescue gamma-to-theta coupling in Neuroligin-3 R451C knock-in mice, as well as social novelty defects (Cao et al., 2018). Targeting the connectivity of interneurons could therefore be an entry point for the treatment of ASD. ASD-relevant effects of Neuroligin-3 mutations also manifest in the striatal subregion of the nucleus accumbens, where Neuroligin-3 deletion decreases inhibitory transmission and results in repetitive behaviors (Rothwell et al., 2014).
6.1.3. Contactins (CNTNAP genes)
Contactin Associated Protein-like 2 (CNTNAP2) shares extracellular domains with Neurexins and strong genetic data implicate it in ASD, with genetic variants and microdeletions in the CNTNAP2 gene associated with ASD (Alarcon et al., 2008; Al-Murrani, Ashton, Aftimos, George, & Love, 2012; Arking et al., 2008; Poot et al., 2010). There is also evidence that a common genetic variant affects inter-region connectivity in the human PFC, with carriers of the risk variant having impaired functional connectivity and significant reductions in grey and white matter volume (Scott-Van Zeeland et al., 2010; Tan, Doke, Ashburner, Wood, & Frackowiak, 2010).
Loss of CNTNAP2 reduces dendritic spine density and in vivo imaging in the somatosensory cortex of mice shows that it contributes to an accelerated loss of spines, suggesting CNTNAP2 plays a role in the stabilization of excitatory synaptic connections (Gdalyahu et al., 2015; Varea et al., 2015). In the mPFC, a decrease in both excitatory and inhibitory synaptic inputs was found in cell layer II/III in CNTNAP2 knock-out mice (Lazaro et al., 2019). Further, analyses of local field potentials and unit spiking in awake CNTNAP2 knock-out mice found impairments in oscillations, in agreement with a reduction in coordinated neuronal population activity (Lazaro et al., 2019).
6.1.4. SynCAMs (CADM genes)
Two different missense mutations (H246N and Y251S) in the gene encoding SynCAM 1 have been identified in ASD patients (Zhiling et al., 2008). Both mutations occur in the immunoglobulin domain that is proximal to the cell membrane, which is required for its lateral cis interactions (Fogel, Stagi, Perez de Arce, & Biederer, 2011). These mutations render SynCAM 1 more susceptible to protease cleavage, alter its intracellular trafficking, and shorten dendrite length (Fujita et al., 2010; Zhiling et al., 2008). A biological concept-based analysis of ASD-linked SNPs that was cross-validated with patient gene expression data identified several disease-linked clusters, with a prominent cluster for adhesion that includes the gene encoding SynCAM 1 (Esteban, Tonellato, & Wall, 2020). SynCAM 2, a heterophilic binding partner of SynCAM 1, has also been implicated through GWAS in ASD, and is additionally linked to attention-deficit hyperactive disorder, cognitive processing speed and educational attainment (Albayrak et al., 2013; Casey et al., 2012; Davies et al., 2016; Ibrahim-Verbaas et al., 2016; Okbay et al., 2016). Mice in which SynCAM 1 is deleted exhibit lower dendritic spine density and a reduction in excitatory transmission in the hippocampal CA1 area (Robbins et al., 2010) as well as impaired connectivity and E/I balance in the CA3 region (Park et al., 2016). Loss of SynCAM 1 impacts the cortex, too, and reduces thalamocortical inputs to PV-positive interneurons and impedes inhibitory maturation (Ribic et al., 2019).
6.1.5. Cadherins and protocadherins (CDH and PCDH genes)
Single nucleotide polymorphisms (SNPs) close to the genes encoding Cadherin 8, 9 and 10 (CDH8–10) are strongly associated with ASD, as well as large deletions in Cadherin 13 (CDH13) (Lin, Frei, Kilander, Shen, & Blatt, 2016; Sanders et al., 2011; Wang et al., 2009). The Protocadherin family is also implicated in ASD. Multiple SNPs within the Protocadherin-α gene cluster show significant associations with autism (Anitha et al., 2013). CNVs in Protocadherin 9 (encoded by PCDH9) and homozygous deletions in Protocadherin 10 (PCH10) have been reported in ASD cases, as well as numerous Protocadherin 19 mutations in families with members diagnosed with epilepsy and mental retardation. Five of these mutations result in early stop codons and two are missense mutations that are predicted to affect calcium binding (Dibbens et al., 2008; Marshall et al., 2008; Morrow et al., 2008). Correspondingly, loss of Protocadherins in mice results in autism-relevant phenotypes. Protocadherin 19 knock-out male mice show abnormal sociability as well as increased grooming, while Protocadherin 10 knock-out male mice exhibit social novelty defects and abnormal gamma oscillations in the basolateral amygdala (Lim, Ryu, Kang, Noh, & Kim, 2019; Schoch et al., 2017).
6.2. Schizophrenia
Schizophrenia, a neurodevelopmental disorder also considered to be synaptic in pathology, presents later in adolescence in a time period that corresponds with the final maturation of the PFC. Postmortem studies of schizophrenia patients show decreased dendritic spine density in layer II/III in the PFC, pointing to a significant loss in synapse number (Garey et al., 1998; Glantz & Lewis, 2000) Schizophrenia patients also display altered PFC gamma band oscillations which are driven through PV-positive interneurons and are presumed to synchronize local cortical networks (McNally & McCarley, 2016). These lower gamma band oscillations in schizophrenia patients suggest chronically dysfunctional long-range synaptic transmission in the cortex (Chen et al., 2014; Grent-t-Jong et al., 2018; Grutzner et al., 2013).
While there has been significantly less success in identifying de novo mutations in proteins in schizophrenia patients as compared to ASD due to the challenging genetic heterogeneity of schizophrenia, GWAS studies have provided substantial progress. Genetic risk loci include synapse organizing proteins, suggesting that schizophrenia is a disorder that involves, at least in part, improper synaptic connectivity during development (Schizophrenia Working Group of the Psychiatric Genomics, 2014). We highlight below representative schizophrenia-relevant synapse organizing proteins for which animal studies have been performed.
6.2.1. Neurexins
Neurexin-1α has been implicated in schizophrenia through copy number variant deletions and duplications (Gauthier et al., 2011; Kirov et al., 2009; Rujescu et al., 2009). As outlined for ASD, Neurexin-1α mice have reduced excitatory synapse strength in the hippocampus, as well as altered behaviors. Specifically, Neurexin-1α knock-out mice display impaired pre-pulse inhibition (PPI), a behavioral assay that measures sensory gating and attentive processing, which is also impaired in human schizophrenia patients (Etherton et al., 2009).
6.2.2. Neuroligins
Damaging missense mutations have been found in the NLGN2 gene in a cohort of schizophrenia patients. One disease-linked mutant (R215H), fails to bind presynaptic Neurexin and disrupts GABA transmission in a reconstituted system (Sun et al., 2011). Introducing the R215H mutation into Neuroligin-2 in mice results in reduced miniature and evoked inhibitory post-synaptic currents and abnormal gamma oscillations in the PFC (Chen et al., 2020). Neuroligin-2 R215H knock-in mice also exhibit reduced inhibitory synaptic transmission in the hippocampus, with lower inhibitory synaptic marker density and impaired memory processes (Jiang et al., 2018). In the PPI test, Neuroligin-2 R215H mice have performed differently given the study; in one they display impaired PPI, in another enhanced, but this effect may be due to differences in the genetic makeup of the mice analyzed (Chen, Lee, Liao, & Chang, 2017; Jiang et al., 2018).
6.2.3. Leucine-rich repeat transmembrane proteins (LRRTM genes)
The postsynaptic adhesion molecule and Neurexin partner LRRTM1 is encoded byan imprinted gene (disease risk associatedwith paternal inheritance) and hypomethylation of the promoter significantly increases the risk of developing schizophrenia (Brucato, DeLisi, Fisher, & Francks, 2014; Francks et al., 2007; Ludwig et al., 2009). LRRTM1 is also linked to schizotypy in a non-clinical population (Leach, Prefontaine, Hurd, & Crespi, 2014). Altered excitatory presynaptic protein distribution in the CA1 but not the CA3 region of the hippocampus and a significant decrease in excitatory synapses in the CA1 stratum radiatum have been observed in LRRTM1 knock-out mice, indicating that LRRTM1 supports hippocampal connectivity (Linhoff et al., 2009; Schroeder & de Wit, 2018; Takashima et al., 2011). To what extent these phenotypes involve interactions with Neurexins is currently unknown.
7. Outlook
As reviewed in this chapter, recognition molecules are now known to play critical roles in neuronal partner identification and the formation and specification of synapses. What are the next key questions? On a functional level, the roles of recognition during the sequential steps of partner contact and synapse assembly remain to be elucidated. As reviewed here, multiple examples exist for proteins that are required and sufficient for neuronal partner recognition. This is not the case for synapse formation, where several proteins are sufficient to drive this process, but none is required. Future studies can test whether select factors that control neuronal partner recognition switch roles after contact and work with synapse-organizing proteins to initiate synapse development. These recognition processes may include positive cooperation as well as competition between recognition factors, mechanisms that we are only beginning to grasp. From a molecular perspective, it will be important to delineate the stoichiometry, subsynaptic distribution and dynamic properties of recognition factors at different synapse types, aiming to reach single synapse resolution. These measurements will determine to what extent the relative abundance and dynamics of recognition factors guide the trajectories along which different synapse types emerge.
The design of future studies will benefit from comparing brain regions and neuron types as this provides opportunities to determine contextual functions of synaptic recognition factors. Region- and neuron-specific roles can be of high relevance for understanding disease processes, including why certain brain areas are more vulnerable to synaptic aberrations than others. An additional health-relevant goal will be to analyze the roles of synaptic recognition once development has been completed. Many of the molecules discussed here persist at mature synapses, indicating functions beyond development that may include synapse maintenance, synaptic plasticity, synapse-type specific control of network maturation, or circuit remodeling. A better understanding of how synaptic recognition shapes the mature CNS can generate leads for therapeutic intervention in disorders of the adult and aging brain and for healthy aging.
These new directions are bound to advance our knowledge of how recognition molecules provide for the precise connectivity of the CNS and the astounding structural and functional diversity of synapses.
Acknowledgments
The authors would like to thank Drs. Deanna Benson, George Huntley, Roman Giger, the Reviewer, and members of the Biederer group for their helpful input. Synaptic biology research in the Biederer group is supported by NIH grants R01 DA018928, R01 MH119826 and R21 NS109637 (to T.B.).
References
- Acker DWM, Wong I, Kang M, & Paradis S(2018). Semaphorin 4Dpromotesinhibitory synapse formation and suppresses seizures in vivo. Epilepsia, 59(6), 1257–1268. 10.1111/epi.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amoudi A, Diez DC, Betts MJ, & Frangakis AS (2007). The molecular architecture of cadherins in native epidermal desmosomes. Nature, 450(7171), 832–837. 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. (2008). Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics, 82(1), 150–159. 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayrak O, Putter C, Volckmar AL, Cichon S, Hoffmann P, Nothen MM, et al. (2013). Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 162B(4), 295–305. 10.1002/ajmg.b.32144. [DOI] [PubMed] [Google Scholar]
- Al-Murrani A, Ashton F, Aftimos S, George AM, & Love DR (2012). Amino-terminal microdeletion within the CNTNAP2 gene associated with variable expressivity of speech delay. Case Reports in Genetics, 2012, 172408. 10.1155/2012/172408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Maxeiner S, Sando R, Tsetsenis T, Malenka RC, & Südhof TC (2017). Postsynaptic adhesion GPCR latrophilin-2 mediates target recognition in entorhinal-hippocampal synapse assembly. The Journal of Cell Biology, 216(11), 3831–3846. 10.1083/jcb.201703042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, & Huang ZJ (2004). Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell, 119(2), 257–272. 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Anitha A, Thanseem I, Nakamura K, Yamada K, Iwayama Y, Toyota T, et al. (2013). Protocadherin alpha (PCDHA) as a novel susceptibility gene for autism. Journal of Psychiatry & Neuroscience, 38(3), 192–198. 10.1503/jpn.120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, & Feldman DE (2019). Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron, 101(4), 648–661.e644. 10.1016/j.neuron.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, & Südhof TC (2013). Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell, 154(1), 75–88. 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, & Chen L (2007). Postsynaptic EphrinB3 promotes shaft glutamatergic synapse formation. The Journal of Neuroscience, 27(28), 7508–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apóstolo N, & de Wit J (2019). Compartmentalized distributions of neuronal and glial cell-surface proteins pattern the synaptic network. Current Opinion in Neurobiology, 57, 126–133. 10.1016/j.conb.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Apóstolo N, Smukowski SN, Vanderlinden J, Condomitti G, Rybakin V, Ten Bos J, et al. (2020). Synapse type-specific proteomic dissection identifies IgSF8 as a hippocampal CA3 microcircuit organizer. Nature Communications, 11(1). 10.1038/s41467-020-18956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arac D, & Li J (2019). Teneurins and latrophilins: Two giants meet at the synapse. Current Opinion in Structural Biology, 54, 141–151. 10.1016/j.sbi.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. (2008). A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. American Journal of Human Genetics, 82(1), 160–164. 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi S, Betley JN, Comer JD, Brenner-Morton S, Bar V, Shimoda Y, et al. (2014). Neuronal Ig/Caspr recognition promotes the formation of axoaxonic synapses in mouse spinal cord. Neuron, 81(1), 120–129. 10.1016/j.neuron.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Martinez E, Eisenberg C, Shah F, Kosc A, Varghese K, et al. (2019). Neuropilin 2 signaling mediates corticostriatal transmission, spine maintenance, and goal-directed learning in mice. The Journal of Neuroscience, 39(45), 8845–8859. 10.1523/JNEUROSCI.1006-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, & Tessier-Lavigne M (2003). Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell, 113(3), 285–299. 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Bekirov IH, Needleman LA, Zhang W, & Benson DL (2002). Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience, 115(1), 213–227. 10.1016/s0306-4522(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Benson DL, & Huntley GW (2012). Building and remodeling synapses. Hippocampus, 22(5), 954–968. 10.1002/hipo.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns DS, DeNardo LA, Pederick DT, & Luo L (2018). Teneurin-3 controls topographic circuit assembly in the hippocampus. Nature, 554(7692), 328–333. 10.1038/nature25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, & Stevens B (2013). TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature Neuroscience, 16(12), 1773–1782. 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Biederer T, Kaeser PS, & Blanpied TA (2017). Transcellular nanoalignment of synaptic function. Neuron, 96(3), 680–696. 10.1016/j.neuron.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. (2002). SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science, 297(5586), 1525–1531. 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Biederer T, & Scheiffele P (2007). Mixed-culture assays for analyzing neuronal synapse formation. Nature Protocols, 2(3), 670–676. 10.1038/nprot.2007.92. [DOI] [PubMed] [Google Scholar]
- Biederer T, & Shrestha N (2015). SynCAM proteins. In Caplan M (Ed.), Reference module in biomedical research Elsevier. [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, et al. (2006). Neuronal pentraxins mediate synaptic refinement in the developing visual system. The Journal of Neuroscience, 26(23), 6269–6281. 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger MF, Martinelli DC, & Südhof TC (2011). The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proceedings of the National Academy of Sciences of the United States of America, 108(6), 2534–2539. 10.1073/pnas.1019577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Ko J, & Südhof TC (2012). High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. The Journal of Biological Chemistry, 287(12), 9399–9413. 10.1074/jbc.M111.318659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Valcin M, Poskanzer K, Tanaka H, & Benson DL (2004). Temporally distinct demands for classic cadherins in synapse formation and maturation. Molecular and Cellular Neurosciences, 27(4), 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch J, Harrison OJ, Honig B, & Shapiro L (2012). Thinking outside the cell: How cadherins drive adhesion. Trends in Cell Biology, 22(6), 299–310. 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigidi GS, & Bamji SX (2011). Cadherin-catenin adhesion complexes at the synapse. Current Opinion in Neurobiology, 21(2), 208–214. 10.1016/j.conb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Brucato N, DeLisi LE, Fisher SE, & Francks C (2014). Hypomethylation of the paternally inherited LRRTM1 promoter linked to schizophrenia. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 165B(7), 555–563. 10.1002/ajmg.b.32258. [DOI] [PubMed] [Google Scholar]
- Bury LA, & Sabo SL (2016). Building a terminal: Mechanisms of presynaptic development in the CNS. The Neuroscientist, 22(4), 372–391. 10.1177/1073858415596131. [DOI] [PubMed] [Google Scholar]
- Cao W, Lin S, Xia QQ, Du YL, Yang Q, Zhang MY, et al. (2018). Gamma oscillation dysfunction in mPFC leads to social deficits in Neuroligin 3 R451C knockin mice. Neuron, 97(6), 1253–1260. 10.1016/j.neuron.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Cardin JA (2018). Inhibitory interneurons regulate temporal precision and correlations in cortical circuits. Trends in Neurosciences, 41(10), 689–700. 10.1016/j.tins.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R, et al. (2012). A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Human Genetics, 131(4), 565–579. 10.1007/s00439-011-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamma I, & Thoumine O (2018). Dynamics, nanoscale organization, and function of synaptic adhesion molecules. Molecular and Cellular Neurosciences, 91, 95–107. 10.1016/j.mcn.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Chanda S, Hale WD, Zhang B, Wernig M, & Südhof TC (2017). Unique versus redundant functions of Neuroligin genes in shaping excitatory and inhibitory synapse properties. The Journal of Neuroscience, 37(29), 6816–6836. 10.1523/JNEUROSCI.0125-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle L, Rivera SA, Phelps JS, Ennis KA, et al. (2020). Sensory experience engages microglia to shape neural connectivity through a non-phagocytic mechanism. Neuron, 108(3), 451–468. 10.1016/j.neuron.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A, & Sotelo C (1993). The ‘creeper stage’ in cerebellar climbing fiber synaptogenesis precedes the ‘pericellular nest’- -ultrastructural evidence with parvalbumin immunocytochemistry. Brain Research. Developmental Brain Research, 76(2), 207–220. 10.1016/0165-3806(93)90209-s. [DOI] [PubMed] [Google Scholar]
- Chen J, Dong B, Feng X, Jiang D, Chen G, Long C, et al. (2020). Aberrant mPFC GABAergic synaptic transmission and fear behavior in neuroligin-2 R215H knock-in mice. Brain Research, 1730. 10.1016/j.brainres.2020.146671, 146671. [DOI] [PubMed] [Google Scholar]
- Chen LY, Jiang M, Zhang B, Gokce O, & Südhof TC (2017). Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron, 94(3), 611–625.e614. 10.1016/j.neuron.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen CH, Lee PW, Liao HM, & Chang PK (2017). Neuroligin 2 R215H mutant mice manifest anxiety, increased prepulse inhibition, and impaired spatial learning and memory. Frontiers in Psychiatry, 8, 257. 10.3389/fpsyt.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, et al. (2014). GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage: Clinical, 4, 531–539. 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TW, Liu XB, Faulkner RL, Stephan AH, Barres BA, Huberman AD, et al. (2010). Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. The Journal of Neuroscience, 30(48), 16376–16382. 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. (2007). Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron, 54(6), 919–931. 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature, 504(7480), 394–400. 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cijsouw T, Ramsey AM, Lam TT, Carbone BE, Blanpied TA, & Biederer T (2018). Mapping the proteome of the synaptic cleft through proximity labeling reveals new cleft proteins. Proteomes, 6(4). 10.3390/proteomes6040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condomitti G, & de Wit J (2018). Heparan sulfate proteoglycans as emerging players in synaptic specificity. Frontiers in Molecular Neuroscience, 11. 10.3389/fnmol.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornew L, Roberts TP, Blaskey L, & Edgar JC (2012). Resting-state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(9), 1884–1894. 10.1007/s10803-011-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, & Dupont JL (1981). Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Research, 227(1), 59–71. 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, & Delhaye-Bouchaud N (1976). Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. Journal of Neurobiology, 7(6), 567–578. 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Czondor K, Garcia M, Argento A, Constals A, Breillat C, Tessier B, et al. (2013). Micropatterned substrates coated with neuronal adhesion molecules for high-content study of synapse formation. Nature Communications, 4, 2252. 10.1038/ncomms3252. [DOI] [PubMed] [Google Scholar]
- Dabrowski A, Terauchi A, Strong C, & Umemori H (2015). Distinct sets of FGF receptors sculpt excitatory and inhibitory synaptogenesis. Development, 142(10), 1818–1830. 10.1242/dev.115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. (2000). EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell, 103(6), 945–956. 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, et al. (2016). Genome-wide association study of cognitive functions and educational attainment in UK Biobank. Molecular Psychiatry, 21(6), 758–767. 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaco A, Kovarik Z, Comoletti D, Jennings LL, Gaietta G, Ellisman MH, et al. (2005). A single mutation near the C-terminus in alpha/beta hydrolase fold protein family causes a defect in protein processing. Chemico-Biological Interactions, 157–158, 371–372. 10.1016/j.cbi.2005.10.057. [DOI] [PubMed] [Google Scholar]
- de Wit J, O’Sullivan ML, Savas JN, Condomitti G, Caccese MC, Vennekens KM, et al. (2013). Unbiased discovery of glypican as a receptor for LRRTM4 in regulating excitatory synapse development. Neuron, 79(4), 696–711. 10.1016/j.neuron.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Mohan V, Zhang X, Brennaman LH, Dharbal KE, Tran TS, et al. (2014). Neural cell adhesion molecule NrCAM regulates Semaphorin 3F-induced dendritic spine remodeling. The Journal of Neuroscience, 34(34), 11274–11287. 10.1523/JNEUROSCI.1774-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo LA, de Wit J, Otto-Hitt S, & Ghosh A (2012). NGL-2 regulates input-specific synapse development in CA1 pyramidal neurons. Neuron, 76(4), 762–775. 10.1016/j.neuron.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, et al. (2008). X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nature Genetics, 40(6), 776–781. 10.1038/ng.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Oh WJ, Sabatini BL, & Gu C (2011). Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nature Neuroscience, 15(2), 215–223. 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, & Sanes JR (2014). Type II cadherins guide assembly of a direction-selective retinal circuit. Cell, 158(4), 793–807. 10.1016/j.cell.2014.06.047. [DOI] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, Laboulaye MA, Liu J, Peng YR, Yamagata M, et al. (2018). Cadherin combinations recruit dendrites of distinct retinal neurons to a shared interneuronal scaffold. Neuron, 99(6), 1145–1154. 10.1016/j.neuron.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Wang SH, Song J, Mironova Y, Ming GL, Kolodkin AL, et al. (2014). Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. eLife, 3. 10.7554/eLife.04390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Südhof TC, & Missler M (2007). Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. The Journal of Comparative Neurology, 502(2), 261–274. 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Emperador-Melero J, & Kaeser PS (2020). Assembly of the presynaptic active zone. Current Opinion in Neurobiology, 63, 95–103. 10.1016/j.conb.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclassan F, Francois J, Phillips KG, Loomis S, & Gilmour G (2015). Phenotypic characterization of nonsocial behavioral impairment in neurexin 1α knockout rats. Behavioral Neuroscience, 129(1), 74–85. 10.1037/bne0000024. [DOI] [PubMed] [Google Scholar]
- Esteban FJ, Tonellato PJ, & Wall DP (2020). Enrichment of genomic variation in pathways linked to autism. bioRxiv. 10.1101/2020.10.19.346072. [DOI] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, & Südhof TC (2009). Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proceedings of the National Academy of Sciences of the United States of America, 106(42), 17998–18003. 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Deogracias R, Marques-Smith A, Maeso P, Jezequel J, Exposito-Alonso D, et al. (2019). Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science, 363(6425), 413–417. 10.1126/science.aau8977. [DOI] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, et al. (2018). The microglial innate immune receptor TREM2 Is required for synapse elimination and normal brain connectivity. Immunity, 48(5), 979–991.e978. 10.1016/j.immuni.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, & Biederer T (2007). SynCAMs organize synapses through heterophilic adhesion. The Journal of Neuroscience, 27(46), 12516–12530. 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, et al. (2010). N-glycosylation at the SynCAM (Synaptic Cell Adhesion Molecule) immunoglobulin interface modulates synaptic adhesion. The Journal of Biological Chemistry, 285(45), 34864–34874. 10.1074/jbc.M110.120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel AI, Stagi M, Perez de Arce K, & Biederer T (2011). Lateral assembly of the immunoglobulin protein SynCAM 1 controls its adhesive function and instructs synapse formation. The EMBO Journal, 30(23), 4728–4738. 10.1038/emboj.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Darmanis S, Aoto J, Malenka RC, Quake SR, & Südhof TC (2016). Single-cell RNAseq reveals cell adhesion molecule profiles in electrophysiologically defined neurons. Proceedings of the National Academy of Sciences of the United States of America, 113(35), E5222–E5231. 10.1073/pnas.1610155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati M, Assendorp N, Gemin O, Colasse S, Dingli F, Arras G, et al. (2019). Trans-synaptic signaling through the Glutamate Receptor Delta-1 mediates inhibitory synapse formation in cortical pyramidal neurons. Neuron, 104(6), 1081–1094. 10.1016/j.neuron.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, et al. (2007). LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Molecular Psychiatry, 12(12), 1129–1139. 1057. 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei JA, & Stoeckli ET (2014). SynCAMs extend their functions beyond the synapse. The European Journal of Neuroscience, 39(11), 1752–1760. 10.1111/ejn.12544. [DOI] [PubMed] [Google Scholar]
- Friedman LG, Benson DL, & Huntley GW (2015). Cadherin-based transsynaptic networks in establishing and modifying neural connectivity. Current Topics in Developmental Biology, 112, 415–465. 10.1016/bs.ctdb.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo MV, Foldy C, Gokce O, Rothwell PE, Sun GL, Malenka RC, et al. (2015). Single-cell mRNA profiling reveals cell-type-specific expression of Neurexin isoforms. Neuron, 87(2), 326–340. 10.1016/j.neuron.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, & Burgess RW (2008). Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature, 451(7177), 470–474. 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Dai H, Tanabe Y, Zhiling Y, Yamagata T, Miyakawa T, et al. (2010). Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death & Disease, 1. 10.1038/cddis.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanis E, Traunmüller L, Fucile G, & Scheiffele P (2019). Landscape of ribosome-engaged transcript isoforms reveals extensive neuronal-cell-class-specific alternative splicing programs. Nature Neuroscience, 22(10), 1709–1717. 10.1038/s41593-019-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, et al. (2010). Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10250–10255. 10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan KJ, & Südhof TC (2020). SPARCL1 promotes excitatory but not inhibitory synapse formation and function independent of Neurexins and Neuroligins. The Journal of Neuroscience, 40(42), 8088–8102. 10.1523/JNEUROSCI.0454-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. (1998). Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry, 65(4), 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AM, Khalil A, Walton DO, & Burgess RW (2018). DSCAM promotes self-avoidance in the developing mouse retina by masking the functions of cadherin superfamily members. Proceedings of the National Academy of Sciences of the United States of America, 115(43), E10216–E10224. 10.1073/pnas.1809430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, et al. (2011). Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Human Genetics, 130(4), 563–573. 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalyahu A, Lazaro M, Penagarikano O, Golshani P, Trachtenberg JT, & Geschwind DH (2015). The autism related protein contactin-associated protein-Like 2 (CNTNAP2) stabilizes new spines: An in vivo mouse study. PLoS One, 10(5). 10.1371/journal.pone.0125633, e0125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, & State MW (2015). Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet Neurology, 15, S1474–S4422. 10.1016/S1474-4422(15)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Needleman L, & Huntley GW (2002). Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. The Journal of Comparative Neurology, 453(4), 372–388. 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Glantz LA, & Lewis DA (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry, 57(1), 65–73. 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Grent-t-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, et al. (2018). Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife, 7. 10.7554/eLife.37799, e37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzner C, Wibral M, Sun L, Rivolta D, Singer W, Maurer K, et al. (2013). Deficits in high-(>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Jeon S, Um JW, & Ko J (2016). Emergent synapse organizers: LAR-RPTPs and their companions. International Review of Cell and Molecular Biology, 324, 39–65. 10.1016/bs.ircmb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Han KA, Ko JS, Pramanik G, Kim JY, Tabuchi K, Um JW, et al. (2018). PTPsigma drives excitatory presynaptic assembly via various extracellular and intracellular mechanisms. The Journal of Neuroscience, 38(30), 6700–6721. 10.1523/JNEUROSCI.0672-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Woo D, Kim S, Choii G, Jeon S, Won SY, et al. (2016). Neurotrophin-3 regulates synapse development by modulating TrkC-PTPsigma synaptic adhesion and intracellular signaling pathways. The Journal of Neuroscience, 36(17), 4816–4831. 10.1523/JNEUROSCI.4024-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, & Kano M (2005). Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neuroscience Research, 53(3), 221–228. 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Henderson NT, & Dalva MB (2018). EphBs and ephrin-Bs: Trans-synaptic organizers of synapse development and function. Molecular and Cellular Neurosciences, 91, 108–121. 10.1016/j.mcn.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, & Ethell IM (2003). Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. The Journal of Cell Biology, 163(6), 1313–1326. 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T (2018). Purkinje neurons: Development, morphology, and function. Cerebellum, 17(6), 699–700. 10.1007/s12311-018-0985-7. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, & Shatz CJ (2000). Functional requirement for class I MHC in CNS development and plasticity. Science, 290(5499), 2155–2159. 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulpiau P, & van Roy F (2009). Molecular evolution of the cadherin superfamily. The International Journal of Biochemistry & Cell Biology, 41(2), 349–369. 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Huntley GW, & Benson DL (1999). Neural (N)-cadherin at developing thalamocortical synapses provides an adhesion mechanism for the formation of somatopically organized connections. The Journal of Comparative Neurology, 407(4), 453–471. [PubMed] [Google Scholar]
- Hutsler JJ, & Zhang H (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research, 1309, 83–94. 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Ibrahim-Verbaas CA, Bressler J, Debette S, Schuur M, Smith AV, Bis JC, et al. (2016). GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Molecular Psychiatry, 21(2), 189–197. 10.1038/mp.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing-Esteves S, Kostadinov D, Marocha J, Sing AD, Joseph KS, Laboulaye MA, et al. (2018). Combinatorial effects of alpha- and gamma-protocadherins on neuronal survival and dendritic self-avoidance. The Journal of Neuroscience, 38(11), 2713–2729. 10.1523/JNEUROSCI.3035-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, & Sanes JR (1997). Lamina-specific connectivity in the brain: Regulation by N-cadherin, neurotrophins, and glycoconjugates. Science, 276(5317), 1428–1431. 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Isshiki M, Tanaka S, Kuriu T, Tabuchi K, Takumi T, & Okabe S (2014). Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nature Communications, 5, 4742. 10.1038/ncomms5742. [DOI] [PubMed] [Google Scholar]
- Ito-Ishida A, Miyazaki T, Miura E, Matsuda K, Watanabe M, Yuzaki M, et al. (2012). Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron, 76(3), 549–564. 10.1016/j.neuron.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, et al. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics, 34(1), 27–29. 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, & Tsirka SE (2013). Microglia actively regulate the number of functional synapses. PLoS One, 8(2), e56293. 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang DY, Wu Z, Forsyth CT, Hu Y, Yee SP, & Chen G (2018). GABAergic deficits and schizophrenia-like behaviors in a mouse model carrying patient-derived neuroligin-2 R215H mutation. Molecular Brain, 11(1), 31. 10.1186/s13041-018-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, et al. (2005). Nectin-like molecule-1/TSLL1/SynCAM3: A neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. Journal of Cell Science, 118(Pt. 6), 1267–1277. 10.1242/jcs.01656. [DOI] [PubMed] [Google Scholar]
- Kano M, Watanabe T, Uesaka N, & Watanabe M (2018). Multiple phases of climbing fiber synapse elimination in the developing cerebellum. Cerebellum, 17(6), 722–734. 10.1007/s12311-018-0964-z. [DOI] [PubMed] [Google Scholar]
- Kasthuri N, Hayworth KJ, Berger DR, Schalek RL, Conchello JA, Knowles-Barley S, et al. (2015). Saturated reconstruction of a volume of neocortex. Cell, 162(3), 648–661. 10.1016/j.cell.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, & Dalva MB (2006). Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. The Journal of Neuroscience, 26(47), 12152–12164. 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Shin W, Kang M, Lee S, Kim D, Kang R, et al. (2020). Presynaptic PTPsigma regulates postsynaptic NMDA receptor function through direct adhesion-independent mechanisms. eLife, 9, e54224. 10.7554/eLife.54224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, & Owen MJ (2009). Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophrenia Bulletin, 35(5), 851–854. 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Soler-Llavina GJ, Fuccillo MV, Malenka RC, & Südhof TC (2011). Neuroligins/LRRTMs prevent activity- and Ca2 +/calmodulin-dependent synapse elimination in cultured neurons. The Journal of Cell Biology, 194(2), 323–334. 10.1083/jcb.201101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen J, Nguyen AQ, Nikolakopoulou AM, Garcia M, Hanna S, Woodruff S, et al. (2018). Functional consequences of synapse remodeling following astrocyte-specific regulation of Ephrin-B1 in the adult hippocampus. The Journal of Neuroscience, 38(25), 5710–5726. 10.1523/JNEUROSCI.3618-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy A, Yamagata M, Duan X, Hong YK, & Sanes JR (2015). Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature, 524(7566), 466–470. 10.1038/nature14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwako KI, Nishimoto Y, Kawase S, Okano HJ, & Okano H (2014). Cadherin-7 regulates mossy fiber connectivity in the cerebellum. Cell Reports, 9(1), 311–323. 10.1016/j.celrep.2014.08.063. [DOI] [PubMed] [Google Scholar]
- Lazaro MT, Taxidis J, Shuman T, Bachmutsky I, Ikrar T, Santos R, et al. (2019). Reduced prefrontal synaptic connectivity and disturbed oscillatory population dynamics in the CNTNAP2 model of autism. Cell Reports, 27(9), 2567–2578.e2566. 10.1016/j.celrep.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach EL, Prefontaine G, Hurd PL, & Crespi BJ (2014). The imprinted gene LRRTM1 mediates schizotypy and handedness in a nonclinical population. Journal of Human Genetics, 59(6), 332–336. 10.1038/jhg.2014.30. [DOI] [PubMed] [Google Scholar]
- Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW, et al. (2013). MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proceedings of the National Academy of Sciences of the United States of America, 110(1), 336–341. 10.1073/pnas.1219987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, et al. (2008). Synaptic function for the Nogo-66 receptor NgR1: Regulation of dendritic spine morphology and activity-dependent synaptic strength. The Journal of Neuroscience, 28(11), 2753–2765. 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL (2017). Neuronal territory formation by the atypical cadherins and clustered protocadherins. Seminars in Cell & Developmental Biology, 69, 111–121. 10.1016/j.semcdb.2017.07.040. [DOI] [PubMed] [Google Scholar]
- Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. (2018). CD47 protects synapses from excess microglia-mediated pruning during development. Neuron, 100(1), 120–134. 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han W, Pelkey KA, Duan J, Mao X, Wang YX, et al. (2017). Molecular dissection of Neuroligin 2 and Slitrk3 reveals an essential framework for GABAergic synapse development. Neuron, 96(4), 808–826. 10.1016/j.neuron.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shalev-Benami M, Sando R, Jiang X, Kibrom A, Wang J, et al. (2018). Structural basis for Teneurin function in circuit-wiring: A toxin motif at the synapse. Cell, 173(3), 735–748.e715. 10.1016/j.cell.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie E, Li Y, Kim R, & Kim E (2018). SALM/Lrfn family synaptic adhesion molecules. Frontiers in Molecular Neuroscience, 11. 10.3389/fnmol.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ryu J, Kang S, Noh HJ, & Kim CH (2019). Autism-like behaviors in male mice with a Pcdh19 deletion. Molecular Brain, 12(1). 10.1186/s13041-019-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Frei JA, Kilander MB, Shen W, & Blatt GJ (2016). A subset of autism-associated genes regulate the structural stability of neurons. Frontiers in Cellular Neuroscience, 10. 10.3389/fncel.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, et al. (2009). An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron, 61(5), 734–749. 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, & Cheng HJ (2005). Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. The Journal of Neuroscience, 25(40), 9124–9134. 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KH, Stawski PS, Draycott AS, Udeshi ND, Lehrman EK, Wilton DK, et al. (2016). Proteomic analysis of unbounded cellular compartments: Synaptic clefts. Cell, 166(5), 1295–1307. 10.1016/j.cell.2016.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, & Cheng HJ (2008). Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 105(23), 8136–8141. 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery RL, Tremblay ME, Hopkins BE, & Majewska AK (2017). The microglial fractalkine receptor is not required for activity-dependent plasticity in the mouse visual system. Glia, 65(11), 1744–1761. 10.1002/glia.23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig KU, Mattheisen M, Muhleisen TW, Roeske D, Schmal C, Breuer R, et al. (2009). Supporting evidence for LRRTM1 imprinting effects in schizophrenia. Molecular Psychiatry, 14(8), 743–745. 10.1038/mp.2009.28. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. (2015). Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell, 161(5), 1202–1214. 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, & Changeux JP (1981). Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. The Journal of Neuroscience, 1(7), 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro SG, Chanda S, Yang N, Janas JA, Valperga G, Trotter J, et al. (2019). Neuroligin-4 regulates excitatory synaptic transmission in human neurons. Neuron, 103(4), 617–626. 10.1016/j.neuron.2019.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. (2008). Structural variation of chromosomes in autism spectrum disorder. American Journal of Human Genetics, 82(2), 477–488. 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Muralidhar S, Wang Z, Cervantes DC, Basu R, Taylor MR, et al. (2015). The intellectual disability gene Kirrel3 regulates target-specific mossy fiber synapse development in the hippocampus. eLife, 4, e09395. 10.7554/eLife.09395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli DC, Chew KS, Rohlmann A, Lum MY, Ressl S, Hattar S, et al. (2016). Expression of C1ql3 in discrete neuronal populations controls efferent synapse numbers and diverse behaviors. Neuron, 91(5), 1034–1051. 10.1016/j.neuron.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, et al. (2003). N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development, 130(11), 2479–2494. 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, et al. (2010). Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science, 328(5976), 363–368. 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- Matsukawa H, Akiyoshi-Nishimura S, Zhang Q, Lujan R, Yamaguchi K, Goto H, et al. (2014). Netrin-G/NGL complexes encode functional synaptic diversification. The Journal of Neuroscience, 34(47), 15779–15792. 10.1523/JNEUROSCI.1141-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, et al. (2011). Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron, 71(3), 460–473. 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, & Kolodkin AL (2011). Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature, 470(7333), 259–263. 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, & McCarley RW (2016). Gamma band oscillations: A key to understanding schizophrenia symptoms and neural circuit abnormalities. Current Opinion in Psychiatry, 29(3), 202–210. 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Südhof TC, & Biederer T (2012). Synaptic cell adhesion. Cold Spring Harbor Perspectives in Biology, 4(4). 10.1101/cshperspect.a005694, a005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, et al. (2003). Alpha-neurexins couple Ca2 + channels to synaptic vesicle exocytosis. Nature, 423(6943), 939–948. 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, et al. (2008). Identifying autism loci and genes by tracing recent shared ancestry. Science, 321(5886), 218–223. 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta A, Berning M, Boergens KM, Staffler B, Beining M, Loomba S, et al. (2019). Dense connectomic reconstruction in layer 4 of the somatosensory cortex. Science, 366-(6469). 10.1126/science.aay3134. [DOI] [PubMed] [Google Scholar]
- Mountoufaris G, Canzio D, Nwakeze CL, Chen WV, & Maniatis T (2018). Writing, reading, and translating the clustered Protocadherin cell surface recognition code for neural circuit assembly. Annual Review of Cell and Developmental Biology, 34, 471–493. 10.1146/annurev-cellbio-100616-060701. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Nomura J, Ji X, Tamada K, Arai T, Takahashi E, et al. (2017). Functional significance of rare neuroligin 1 variants found in autism. PLoS Genetics, 13(8). 10.1371/journal.pgen.1006940, e1006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TM, Schreiner D, Xiao L, Traunmuller L, Bornmann C, & Scheiffele P (2016). An alternative splicing switch shapes neurexin repertoires in principal neurons versus interneurons in the mouse hippocampus. eLife, 5, e22757. 10.7554/eLife.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura-Akiyoshi S, Niimi K, Nakashiba T, & Itohara S (2007). Axonal netrin-Gs trans-neuronally determine lamina-specific subdendritic segments. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14801–14806. 10.1073/pnas.0706919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533(7604), 539–542. 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, et al. (2007). Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry, 62(9), 1022–1029. 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Osterhout JA, Josten N, Yamada J, Pan F, Wu SW, Nguyen PL, et al. (2011). Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron, 71(4), 632–639. 10.1016/j.neuron.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR 3rd, et al. (2012). FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron, 73(5), 903–910. 10.1016/j.neuron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science, 333(6048), 1456–1458. 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Park KA, Ribic A, Laage Gaupp FM, Coman D, Huang Y, Dulla CG, et al. (2016). Excitatory synaptic drive and feedforward inhibition in the hippocampal CA3 circuit are regulated by SynCAM 1. The Journal of Neuroscience, 36(28), 7464–7475. 10.1523/JNEUROSCI.0189-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ (2012). Getting neural circuits into shape with semaphorins. Nature Reviews. Neuroscience, 13(9), 605–618. 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Paul A, Crow M, Raudales R, He M, Gillis J, & Huang ZJ (2017). Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell, 171(3), 522–539.e520. 10.1016/j.cell.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, & Arber S (2009). Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature, 459(7248), 842–846. 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, et al. (2015). Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron, 85(6), 1257–1272. 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, & McBain CJ (2017). Hippocampal GABAergic inhibitory interneurons. Physiological Reviews, 97(4), 1619–1747. 10.1152/physrev.00007.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Arce K, Schrod N, Metzbower SWR, Allgeyer E, Kong GK, Tang AH, et al. (2015). Topographic mapping of the synaptic cleft into adhesive nanodomains. Neuron, 88(6), 1165–1172. 10.1016/j.neuron.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Takahashi H, Ge Y, & Craig AM (2013). Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. The Journal of Cell Biology, 200(3), 321–336. 10.1083/jcb.201206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M, Beyer V, Schwaab I, Damatova N, Van’t Slot R, Prothero J, et al. (2010). Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics, 11(1), 81–89. 10.1007/s10048-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, & Huntley GW (2003). N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. The Journal of Neuroscience, 23(6), 2294–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic A, & Biederer T (2019). Emerging roles of synapse organizers in the regulation of critical periods. Neural Plasticity, 2019. 10.1155/2019/1538137, 1538137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic A, Crair MC, & Biederer T (2019). Synapse-selective control of cortical maturation and plasticity by parvalbumin-autonomous action of SynCAM 1. Cell Reports, 26(2), 381–393. 10.1016/j.celrep.2018.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic A, Liu X, Crair MC, & Biederer T (2014). Structural organization and function of mouse photoreceptor ribbon synapses involve the immunoglobulin protein Synaptic Cell Adhesion Molecule 1. The Journal of Comparative Neurology, 522(4), 900–920. 10.1002/cne.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, & Kolodkin AL (2015). Sculpting neural circuits by axon and dendrite pruning. Annual Review of Cell and Developmental Biology, 31, 779–805. 10.1146/annurev-cellbio-100913-013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, et al. (2010). SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron, 68(5), 894–906. 10.1016/j.neuron.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppongi RT, Dhume SH, Padmanabhan N, Silwal P, Zahra N, Karimi B, et al. (2020). LRRTMs organize synapses through differential engagement of Neurexin and PTPsigma. Neuron, 106(1), 108–125.e112. 10.1016/j.neuron.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, et al. (2014). Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell, 158(1), 198–212. 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, et al. (2009). Disruption of the neurexin 1 gene is associated with schizophrenia. Human Molecular Genetics, 18(5), 988–996. 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahores M, Gibb A, & Salinas PC (2010). Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development, 137(13), 2215–2225. 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. (2011). Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron, 70(5), 863–885. 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R, Jiang X, & Südhof TC (2019). Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science, 363(6429). 10.1126/science.aav7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, & Zipursky SL (2020). Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell, 181(6), 1434–1435. 10.1016/j.cell.2020.05.046. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, & Patrizi A (2017). The Purkinje cell as a model of synaptogenesis and synaptic specificity. Brain Research Bulletin, 129, 12–17. 10.1016/j.brainresbull.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Schaaf CP, Boone PM, Sampath S, Williams C, Bader PI, Mueller JM, et al. (2012). Phenotypic spectrum and genotype-phenotype correlations of NRXN1 exon deletions. European Journal of Human Genetics, 20(12), 1240–1247. 10.1038/ejhg.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74(4), 691–705. 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch H, Kreibich AS, Ferri SL, White RS, Bohorquez D, Banerjee A, et al. (2017). Sociability deficits and altered amygdala circuits in mice lacking Pcdh10, an autism associated gene. Biological Psychiatry, 81(3), 193–202. 10.1016/j.biopsych.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, Nguyen TM, Russo G, Heber S, Patrignani A, Ahrne E, et al. (2014). Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron, 84(2), 386–398. 10.1016/j.neuron.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Simicevic J, Ahrne E, Schmidt A, & Scheiffele P (2015). Quantitative isoform-profiling of highly diversified recognition molecules. eLife, 4, e07794. 10.7554/eLife.07794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, & Weiner JA (2010). Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14893–14898. 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, & de Wit J (2018). Leucine-rich repeat-containing synaptic adhesion molecules as organizers of synaptic specificity and diversity. Experimental & Molecular Medicine, 50(4). 10.1038/s12276-017-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclip A, & Südhof TC (2020). LAR receptor phospho-tyrosine phosphatases regulate NMDA-receptor responses. eLife, 9, e53406. 10.7554/eLife.53406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, et al. (2010). Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Science Translational Medicine, 2(56), 56ra80. 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, & Acker-Palmer A (2007). Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nature Neuroscience, 10(3), 301–310. 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Sestan N, & State MW (2018). Lost in translation: Traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron, 100(2), 406–423. 10.1016/j.neuron.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, et al. (2003). Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. The Journal of Biological Chemistry, 278(37), 35421–35427. 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, & Craig AM (2010). LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. The Journal of Neuroscience, 30(22), 7495–7506. 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Tari PK, Connor SA, Zhang P, Dobie FA, She K, et al. (2013). An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron, 79(4), 680–695. 10.1016/j.neuron.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Sigoillot SM, Iyer K, Binda F, Gonzalez-Calvo I, Talleur M, Vodjdani G, et al. (2015). The secreted protein C1QL1 and its receptor BAI3 control the synaptic connectivity of excitatory inputs converging on cerebellar Purkinje cells. Cell Reports, 10(5), 820–832. 10.1016/j.celrep.2015.01.034. [DOI] [PubMed] [Google Scholar]
- Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, et al. (2011). Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proceedings of the National Academy of Sciences of the United States of America, 108(29), 12113–12118. 10.1073/pnas.1019434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, et al. (2016). Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via Hevin. Cell, 164(1–2), 183–196. 10.1016/j.cell.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, & Brose N (1999). Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proceedings of the National Academy of Sciences of the United States of America, 96(3), 1100–1105. 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, et al. (2010). Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11116–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell, 131(6), 1164–1178. [DOI] [PubMed] [Google Scholar]
- Südhof TC (2017). Synaptic neurexin complexes: A molecular code for the logic of neural circuits. Cell, 171(4), 745–769. 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2018). Towards an understanding of synapse formation. Neuron, 100(2), 276–293. 10.1016/j.neuron.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Cheng MC, Qin R, Liao DL, Chen TT, Koong FJ, et al. (2011). Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia. Human Molecular Genetics, 20(15), 3042–3051. 10.1093/hmg/ddr208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, et al. (2007). Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron, 53(4), 535–547. 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. (2007). A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science, 318(5847), 71–76. 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, & Südhof TC (2002). Structure and evolution of neurexin genes: Insight into the mechanism of alternative splicing. Genomics, 79(6), 849–859. 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Tai Y, Gallo NB, Wang M, Yu JR, & Van Aelst L (2019). Axo-axonic innervation of neocortical pyramidal neurons by GABAergic Chandelier cells requires AnkyrinG-associated L1CAM. Neuron, 102(2), 358–372.e359. 10.1016/j.neuron.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Wallace JT, Baldwin KT, Purkey AM, Uezu A, Courtland JL, et al. (2020). Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature. 10.1038/s41586-020-2926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima N, Odaka YS, Sakoori K, Akagi T, Hashikawa T, Morimura N, et al. (2011). Impaired cognitive function and altered hippocampal synapse morphology in mice lacking Lrrtm1, a gene associated with schizophrenia. PLoS One, 6(7), e22716. 10.1371/journal.pone.0022716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GC, Doke TF, Ashburner J, Wood NW, & Frackowiak RS (2010). Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. NeuroImage, 53(3), 1030–1042. 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Sanbo M, Okayama A, Miyashita T, Kitsukawa T, Hirayama T, et al. (2016). Establishment of high reciprocal connectivity between clonal cortical neurons is regulated by the Dnmt3b DNA methyltransferase and clustered protocadherins. BMC Biology, 14(1), 103. 10.1186/s12915-016-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. (2016). Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nature Neuroscience, 19(2), 335–346. 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawarayama H, Yoshida Y, Suto F, Mitchell KJ, & Fujisawa H (2010). Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. The Journal of Neuroscience, 30(20), 7049–7060. 10.1523/JNEUROSCI.0073-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley L, Cadilhac C, Cioni JM, Saywell V, Jahannault-Talignani C, Huettl RE, et al. (2016). Dual function of NRP1 in axon guidance and subcellular target recognition in cerebellum. Neuron, 91(6), 1276–1291. 10.1016/j.neuron.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Timmons KM, Kikuma K, Pechmann Y, Kneussel M, & Umemori H (2015). Selective synaptic targeting of the excitatory and inhibitory presynaptic organizers FGF22 and FGF7. Journal of Cell Science, 128(2), 281–292. 10.1242/jcs.158337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar S, Wang L, Yu T, Ye M, Onishi K, Scott J, et al. (2017). Evidence for opposing roles of Celsr3 and Vangl2 in glutamatergic synapse formation. Proceedings of the National Academy of Sciences of the United States of America, 114(4), E610–E618. 10.1073/pnas.1612062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Akins MR, & Biederer T (2008). Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. The Journal of Comparative Neurology, 510(1), 47–67. 10.1002/cne.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu CA, Chen WV, Rubinstein R, Chevee M, Wolcott HN, Felsovalyi KO, et al. (2014). Single-cell identity generated by combinatorial homophilic interactions between alpha, beta, and gamma protocadherins. Cell, 158(5), 1045–1059. 10.1016/j.cell.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, et al. (2009). Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature, 462(7276), 1065–1069. 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Gokce O, Quake SR, & Südhof TC (2014). Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proceedings of the National Academy of Sciences of the United States of America, 111(13), E1291–E1299. 10.1073/pnas.1403244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, et al. (2010). Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell, 141(6), 1068–1079. 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, et al. (2014). Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science, 344(6187), 1020–1023. 10.1126/science.1252514. [DOI] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, & Sanes JR (2004). FGF22 and Its close relatives are presynaptic organizing molecules in the mammalian brain. Cell, 118(2), 257–270. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, & Südhof TC (1992). Neurexins: Synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science, 257(5066), 50–56. [DOI] [PubMed] [Google Scholar]
- Vainchtein ID, & Molofsky AV (2020). Astrocytes and microglia: In sickness and in health. Trends in Neurosciences, 43(3), 144–154. 10.1016/j.tins.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schurmann B, Fleming HJ, Fawcett-Patel JM, et al. (2015). Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proceedings of the National Academy of Sciences of the United States of America, 112(19), 6176–6181. 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. (2006). Neuroligins determine synapse maturation and function. Neuron, 51(6), 741–754. 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, & Brose N (2004). Neuroligin 2 is exclusively localized to inhibitory synapses. European Journal of Cell Biology, 83(9), 449–456. 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Verschueren E, Husain B, Yuen K, Sun Y, Paduchuri S, Senbabaoglu Y, et al. (2020). The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome. Cell, 182(2), 329–344.e319. 10.1016/j.cell.2020.06.007. [DOI] [PubMed] [Google Scholar]
- Visser JJ, Cheng Y, Perry SC, Chastain AB, Parsa B, Masri SS, et al. (2015). An extracellular biochemical screen reveals that FLRTs and Unc5s mediate neuronal subtype recognition in the retina. eLife, 4. 10.7554/eLife.08149, e08149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. (2009). Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature, 459(7246), 528–533. 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn HR, Xia NL, Zhou W, Mao YT, & Dalva MB (2020). Positive surface charge of GluN1 N-terminus mediates the direct interaction with EphB2 and NMDAR mobility. Nature Communications, 11(1), 570. 10.1038/s41467-020-14345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, & Jontes JD (2013). Protocadherins, not prototypical: A complex tale of their interactions, expression, and functions. Frontiers in Molecular Neuroscience, 6. 10.3389/fnmol.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications, 9(1), 1228. 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, et al. (2011). Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron, 71(4), 640–655. 10.1016/j.neuron.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ZP, Mandel-Brehm C, Mardinly AR, McCord AE, Giger RJ, & Greenberg ME (2012). The nogo receptor family restricts synapse number in the developing hippocampus. Neuron, 73(3), 466–481. 10.1016/j.neuron.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Vielmetter J, Fernandes RA, Siepe DH, Eastman CL, Chisholm GB, et al. (2020). A human IgSF cell-surface interactome reveals a complex network of protein-protein interactions. Cell, 182(4), 1027–1043. 10.1016/j.cell.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, & Maniatis T (1999). A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell, 97(6), 779–790. 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Xu X, Xiong Z, Zhang L, Liu Y, Lu L, Peng Y, et al. (2014). Variations analysis of NLGN3 and NLGN4X gene in Chinese autism patients. Molecular Biology Reports, 41(6), 4133–4140. 10.1007/s11033-014-3284-5. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Duan X, & Sanes JR (2018). Cadherins interact with synaptic organizers to promote synaptic differentiation. Frontiers in Molecular Neuroscience, 11. 10.3389/fnmol.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, & Sanes JR (2008). Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature, 451(7177), 465–469. 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, & Sanes JR (2012). Expanding the Ig superfamily code for laminar specificity in retina: Expression and role of contactins. The Journal of Neuroscience, 32(41), 14402–14414. 10.1523/JNEUROSCI.3193-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, & Sanes JR (2018). Expression and roles of the immunoglobulin superfamily recognition molecule Sidekick1 in mouse retina. Frontiers in Molecular Neuroscience, 11. 10.3389/fnmol.2018.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, & Sanes JR (2002). Sidekicks: Synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell, 110(5), 649–660. 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yan W, Laboulaye MA, Tran NM, Whitney IE, Benhar I, & Sanes JR (2020). Mouse retinal cell atlas: Molecular identification of over sixty amacrine cell types. The Journal of Neuroscience, 40(27), 5177–5195. 10.1523/JNEUROSCI.0471-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C, et al. (2008). Neurexin 1α structural variants associated with autism. Neuroscience Letters, 438(3), 368–370. 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- Yan Q, Weyn-Vanhentenryck SM, Wu J, Sloan SA, Zhang Y, Chen K, et al. (2015). Systematic discovery of regulated and conserved alternative exons in the mammalian brain reveals NMD modulating chromatin regulators. Proceedings of the National Academy of Sciences of the United States of America, 112(11), 3445–3450. 10.1073/pnas.1502849112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, et al. (2013). Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 4057–4062. 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y (2012). Semaphorin signaling in vertebrate neural circuit assembly. Frontiers in Molecular Neuroscience, 5. 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yasumura M, Uemura T, Lee SJ, Ra M, Taguchi R, et al. (2011). IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. The Journal of Neuroscience, 31(38), 13485–13499. 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, & Muller D (2009). Dendritic spine formation and stabilization. Current Opinion in Neurobiology, 19(2), 146–153. 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Zeng H, & Sanes JR (2017). Neuronal cell-type classification: Challenges, opportunities and the path forward. Nature Reviews. Neuroscience, 18(9), 530–546. 10.1038/nrn.2017.85. [DOI] [PubMed] [Google Scholar]
- Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, et al. (2018). Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell, 174(6), 1450–1464. 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiling Y, Fujita E, Tanabe Y, Yamagata T, Momoi T, & Momoi MY (2008). Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. Biochemical and Biophysical Research Communications, 377(3), 926–929. 10.1016/j.bbrc.2008.10.107. [DOI] [PubMed] [Google Scholar]


