Abstract
Background
Disease recurrence and progression remain major challenges in the treatment of non‐muscle invasive bladder cancer (NMIBC). Blue light‐enhanced transurethral resection of bladder cancer (TURBT) is an approach to improve staging and achieve a complete resection of NMIBC.
Objectives
To assess the effects of blue light‐enhanced TURBT compared to white light‐based TURBT in the treatment of NMIBC.
Search methods
We searched several medical literature databases, including the Cochrane Library, MEDLINE, and Embase, as well as trial registers, including ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform. We performed a comprehensive search with no restrictions on language of publication or publication status until March 2021.
Selection criteria
We included randomized controlled trials using blue light versus white light TURBT. Included participants had a high level of suspicion based on imaging or ‘visible diagnosis’ for primary urothelial carcinoma of the bladder or recurrent urothelial carcinoma of the bladder upon cytoscopy. We excluded studies in which blue light was used in a surveillance setting.
Data collection and analysis
Two review authors independently performed data extraction and risk of bias assessment. Our primary outcomes were time to disease recurrence, time to disease progression, and serious surgical complications. Secondary outcomes were time to death from bladder cancer, any adverse events, and non‐serious complications. We rated the certainty of evidence using the GRADE approach.
Main results
We included 16 randomized controlled trials involving a total of 4325 participants in the review. The studies compared blue light versus white light TURBT for treatment of NMIBC.
Primary outcomes
Blue light TURBT may reduce the risk of disease recurrence over time (hazard ratio (HR) 0.66, 95% confidence interval (CI) 0.54 to 0.81; low‐certainty evidence) depending on baseline risk. For participants with low‐, intermediate‐, and high‐risk NMIBC, this corresponded to 48 (66 fewer to 27 fewer), 109 (152 fewer to 59 fewer), and 147 (211 fewer to 76 fewer) fewer recurrences per 1000 participants when compared to white light TURBT, respectively.
Blue light TURBT may also reduce the risk of disease progression over time (HR 0.65, 95% CI 0.50 to 0.84; low‐certainty evidence) depending on baseline risk. For participants with low‐, intermediate‐, and high‐risk NMIBC, this corresponded to 1 (1 fewer to 0 fewer), 17 (25 fewer to 8 fewer), and 56 (81 fewer to 25 fewer) fewer progressions per 1000 participants when compared to white light TURBT, respectively.
Blue light TURBT may have little or no effect on serious surgical complications (risk ratio (RR) 0.54, 95% CI 0.14 to 2.14; low‐certainty evidence). This corresponded to 10 fewer (19 fewer to 25 more) surgical complications per 1000 participants with blue light TURBT.
Secondary outcomes
Blue light TURBT may have little or no effect on the risk of death from bladder cancer over time (HR 0.55, 95% CI 0.19 to 1.61; low‐certainty evidence). This corresponded to 22 deaths per 1000 participants with white light TURBT and 10 fewer (17 fewer to 13 more) deaths per 1000 participants with blue light TURBT.
We are very uncertain how blue light TURBT affects the outcome adverse events of any grade (RR 1.09, 95% CI 0.88 to 1.33; low‐certainty evidence).
No analysis was possible for the outcome non‐serious surgical complications, as it was not reported by any of the included studies.
Authors' conclusions
Blue light‐enhanced TURBT for the treatment of non‐muscle invasive bladder cancer compared to white light‐based TURBT may reduce the risk of disease recurrence and disease progression over time depending on baseline risk. There may be little or no effect on serious surgical complications. The certainty of evidence for our findings was low, meaning that future studies are likely change to the reported estimates of effect. Frequent issues that led to downgrading of the certainty of the evidence were study limitations, inconsistency, and imprecision.
Plain language summary
Blue light‐enhanced versus white light resection in the treatment of non‐muscle invasive bladder cancer
Review question
How does a resection (surgical removal) of bladder cancer supported with a special visualization method (blue light) compare to a standard resection with white light in people in whom a tumor of the inner bladder wall is suspected?
Background
In people suspected of having bladder cancer, suspicious tissue is cut from the inner bladder wall using a special instrument inserted through the urethra into the bladder. However, it is sometimes difficult to tell what is normal bladder versus what is cancer. In order to see the tumor better and remove it completely, a substance, or 'contrast agent,' is put into the bladder through a catheter. During surgery, a special light is used that is meant to make the cancerous area light up blue.
Study characteristics
We only included randomized controlled trials (a type of study where participants are randomly assigned to one of two or more treatment groups) for inclusion in the review, as this type of clinical study is considered to be of the highest quality producing the most reliable results. We included people who were very likely to have had bladder cancer because if had been seen on an imaging study (like a computed tomography (CT) scan) or when looking into the bladder. We included studies of people with newly suspected tumors and those who had been treated for bladder cancer before and there was concern it had come back.
Key results
We included 16 studies addressing our review question. Overall, blue light‐enhanced resection of bladder cancer may reduce the risk of disease recurrence over time compared to white light resection (low‐certainty evidence) and may reduce the risk of disease progression over time (low‐certainty evidence). However, whether this effect is big enough to be meaningful to people with bladder cancer depends on whether they belong to the low, intermediate and high risk group for disease recurrence or progression.
We also found that blue light may have little or no effect on the occurrence of serious surgical complications (low‐certainty evidence) or the risk of death from bladder cancer over time (low‐certainty evidence). We are very uncertain as to whether blue light TURBT reduces the incidence of unwanted side effects, as the certainty of the evidence was assessed as low. We do not know how non‐serious surgical complications are affected as no data were reported for this outcome.
Quality of the evidence
The certainty of the evidence was low, meaning that future research would likely change our results.
Summary of findings
Summary of findings 1. Blue light compared to white light for transurethral resection of NMIBC .
| Blue versus white light for transurethral resection of non‐muscle invasive bladder cancer | ||||||
|
Population: people with non‐muscle invasive bladder cancer Setting: inpatient or outpatient Intervention: blue light transurethral resection Comparison of interest: white light transurethral resection | ||||||
| Outcome |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) | Anticipated absolute effects | What happens | |
| Assumed risk1 | ||||||
| White light |
Risk difference with blue light |
|||||
|
Time to disease recurrence (absolute event rates based on 12 months follow‐up; MCID 5%) |
2994 (15 RCTs) | ⨁⨁◯◯ LOW a,b | HR 0.66 (0.54 to 0.81) | Low2 | Blue light TURBT may have little or no effect on the risk of recurrence in people at low risk, but may reduce the risk of recurrence in those at intermediate and high risk. | |
| 150 per 1000 |
48 fewer per 1000 (66 fewer to 27 fewer) |
|||||
| Intermediate2 | ||||||
| 380 per 1000 |
109 fewer per 1000 (152 fewer to 59 fewer) |
|||||
| High2 | ||||||
| 610 per 1000 |
147 fewer per 1000 (211 fewer to 76 fewer) |
|||||
|
Time to disease progression (absolute event rates based on 12 months follow‐up; MCID 2%) |
2200 (9 RCTs) | ⨁⨁◯◯ LOW a,c | HR 0.65 (0.50 to 0.84) | Low2 | Blue light TURBT may have little or no effect on the risk of progression in people at low and intermediate risk, but may reduce the risk of progression in those at high risk. | |
| 2 per 1000 |
1 fewer per 1000 (1 fewer to 0 fewer) |
|||||
| Intermediate2 | ||||||
| 50 per 1000 |
17 fewer per 1000 (25 fewer to 8 fewer) |
|||||
| High2 | ||||||
| 170 per 1000 |
56 fewer per 1000 (81 fewer to 25 fewer) |
|||||
|
Surgical complications, serious (up to 90 days; MCID 2%) |
525 (1 RCT) | ⨁⨁◯◯ LOW a,c | RR 0.54 (0.14 to 2.14) | 22 per 1000 |
10 fewer per 1000 (19 fewer to 25 more) |
Blue light TURBT may have little to no effect on serious surgical complications. |
|
Time to death from bladder cancer (absolute event rates based on 60 months follow‐up; MCID 2%) |
407 (1 RCT) |
⨁⨁◯◯ LOW a,c |
HR 0.55 (0.19 to 1.61) |
22 per 1000 |
10 fewer per 1000 (17 fewer to 13 more) |
Blue light TURBT may have little to no effect on the time to death from bladder cancer. |
|
Any adverse events (up to 90 days; MCID 5%) |
1375 (3 RCTs) | ⨁⨁◯◯ LOW d,e | RR 1.09 (0.88 to 1.33) | 397 per 1000 |
36 more per 1000 (48 fewer to 131 more) |
We are very uncertain how blue light may affect adverse events. |
|
Surgical complications, non‐serious (up to 90 days; MCID 5%) |
‐ | ‐ | Not estimable | ‐ | ‐ | We do not know how non‐serious surgical complications are affected as no data were reported for this outcome. |
1We provide absolute effect size estimates for time to recurrence and time to progression to reflect risk stratification in clinical practice. Corresponding data were not found for time to death from bladder cancer. Baseline risk for other outcomes is assumed to be similar. 2Baseline risk at 12 months taken from Sylvester 2006.
aDowngraded by one level for study limitations due to concerns about performance, attrition, and reporting bias. bDowngraded by one level for clinically relevant inconsistency (I2 > 60%). cDowngraded by one level for imprecision given that 95% CI is consistent with both no effect and clinically important reduction. dDowngraded by one level for study limitations due to concerns about performance and reporting bias. eDowngraded by one level for imprecision given wide 95% CI consistent with both large increase and large reduction of adverse events.
MCID: minimal clinically important difference
NMIBC: non‐muscle invasive bladder cancer
Background
Description of the condition
Bladder cancer is the second most common malignancy in urologic cancer patients (Bray 2018), with a rising incidence in recent decades. In 2018, 549,393 new cases of bladder cancer were reported globally, leading to an estimated 200,000 cancer‐related deaths per year, making bladder cancer the 10th most common malignancy worldwide (Bray 2018).
The most common presenting symptom is hematuria, either macroscopic or microscopic. Other, even less specific, symptoms include recurrent urinary tract infections or irritative voiding symptoms. Some bladder tumors are found incidentally on cross‐sectional imaging either in the form of a bladder mass or secondary ureteral obstruction with hydronephrosis, or both.
The diagnostic workup for suspected bladder cancer typically includes a urine analysis and urine culture (to rule out infection), an upper tract study such as a computed tomogram with intravenous pyelogram, urine cytology, and a white light office cystoscopy. For both diagnostic and therapeutic purposes, patients then undergo transurethral bladder tumor resection (TURBT), as well as additional bladder biopsies of suspicious‐appearing areas as indicated.
At initial diagnosis, about 70% to 75% of patients present with non‐muscle invasive tumors (Burger 2013; Schned 2012). Non‐muscle invasive bladder cancer (NMIBC) is defined as tumors that are limited to the mucosa (pTa, carcinoma in situ) or submucosa (pT1) and do not infiltrate the underlying deeper muscle layers (Humphrey 2016). Based on their appearance, NMIBC can either present as papillary or non‐papillary tumor. Involvement of the deep muscle layer of muscularis propria constitutes muscle invasive (pT2) disease or muscle invasive bladder cancer and mandates different, more aggressive management, ideally in the form of radical cystectomy.
NMIBC, especially carcinoma in situ (CIS), is known to have a high risk of tumor recurrence and progression after TURBT. Tumor recurrence at 3 and 12 months after TURBT as detected by cystoscopy is reported in up to 30% and 50% of patients, respectively (Palou 2015). The five‐year recurrence rate is as high as 80%. Progression to muscle invasive bladder cancer may occur in up to 45% of patients within five years (Sylvester 2006). Additional interventions that have been demonstrated to improve the risk of recurrence and potentially progression are various forms of adjuvant intravesical therapy (Jones 2012; Schmidt 2020).
Description of the intervention
Effective treatment of NMIBC relies on both accurate staging, including the identification of CIS when present, as well as the complete resection of all visible tumor. White light cystoscopy of the bladder is the current gold standard procedure for the detection of bladder cancer. However, its sensitivity (6% to 84%) and specificity (43% to 98%) is limited, so not all tumors are always visualized (Jocham 2008). The diagnosis of small papillary tumors as well as CIS can be especially difficult, which may result in missed tumors, failure to provide adequate treatment, and cancer progression (Jocham 2008).
Different optical imaging techniques in combination with white light cystoscopy have been investigated to improve the visualization of tumors. Photodynamic diagnosis (PDD), or blue light (synonymous terms), is performed using fluorescent light after intravesical instillation of 5‐aminolevulinic acid (5‐ALA) or hexaminolevulinic acid (HAL, Hexvix), both for detection only at the time of diagnostic cystoscopy and therapeutically at the time of TURBT. HAL, an ester derivative of 5‐ALA with improved pharmacokinetic characteristics, is the only approved drug for blue light in the United States (FDA 2010); however, both agents appear comparable in terms of sensitivity and specificity (Mowatt 2011).
Blue light is applied by intravesical instillation of the photoactive agents approximately one hour prior to cystoscopy either in the clinic or the operating room. Visualization and TURBT is then performed after drainage of the instilled fluid and activation of a fluorescent light.
How the intervention might work
Blue light uses photoactive compounds, and their interaction with fluorescent light is used to increase the optical difference between normal and malignant tissue (Krieg 2002; Witjes 2018). In malignant tissue, a dysregulation in the activity of transport proteins leads to the accumulation of protoporphyrin IX (PPIX) up to 20‐fold. PPIX is a precursor of hemoglobin produced during the biosynthesis of 5‐ALA, a natural amino acid. Since PPIX is photoactive, a red fluorescence is emitted by excitation at certain wavelengths of light, in particular visible blue light (375 to 445 nm) (Inoue 2017; Witjes 2018). A limiting factor in the use of 5‐ALA is its low depth of penetration into the tissue due to its lipophilic characteristics (Krieg 2002). In contrast, with HAL, a synthesized ester derivative of 5‐ALA, the uptake in cells is increased by passive diffusion of the cell membrane (Zaak 2007). HAL undergoes local conversion to porphyrins that preferentially accumulate in malignant cells, and the use of blue light with selective filters can highlight these areas within the bladder mucosa (typically with a red appearance before a dark‐blue background).
Consequently, with the contrast enhancement of blue light from healthy to malignant tissue, more tumors can be made visible, which can then be resected more completely.
Adverse effects of the intervention
The accumulation of HAL in inflammatory tissue reduces its specificity and may lead to the resection of non‐cancerous areas of the bladder, thereby resulting in unnecessary overtreatment of patients (Ray 2010). This may potentially result in a higher risk of bleeding, clot retention, and the need for secondary procedures. Other grave complications of more extensive resection include bladder perforation with the need for open surgical repair or tumor spillage into the abdomen, which may result in incurable spread.
In addition, the use of blue light cystoscopy requires changes in workflow, specialized training of staff, and oftentimes capital investment for suitable equipment, which all represent additional costs. Due to this operative time may be longer.
Why it is important to do this review
Several systematic reviews have investigated the role of blue light in aiding TURBT, and their findings suggest improved cancer outcomes over white light TURBT. Based on these findings, several widely used evidence‐based, AUA 2016; EAU 2021; NICE 2015, and consensus‐based, NCCN 2020, guidelines support the use of blue light. However, the existing reviews have since become outdated (Chou 2017; Mowatt 2011), as additional trials have become available, and existing trials have provided longer follow‐up. In addition, none of the existing systematic reviews has applied the same methodological rigor that is the current standard for a Cochrane Review, which includes the comprehensiveness of the search, completion of study screening and data abstraction in duplicate, risk of bias assessment on per‐outcome basis, and the use of the GRADE approach to rate the certainty of the evidence. The results of this review may therefore provide important new insights to inform guideline recommendations that will add to the existing suite of Cochrane Reviews on non‐muscle invasive bladder cancer (Han 2021; Hwang 2019; Jung 2017; Schmidt 2020; Shepherd 2017). A closely related review on narrow band imaging is currently ongoing (Lai 2021).
Objectives
To assess the effects of blue light‐enhanced TURBT compared to white light‐based TURBT in the treatment of non‐muscle invasive bladder cancer.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomized controlled trials (RCTs) to address the comparisons of interest. We planned to include pseudo‐randomized studies given their greater risk of selection bias, but our search did not identify any. We did not consider cluster‐randomized or cross‐over trials, as they are not applicable to our review question.
Types of participants
We included participants older than 18 years with a high level of suspicion or ‘visible diagnosis’ for primary urothelial carcinoma of the bladder or recurrent urothelial carcinoma of the bladder. This high level of suspicion was based on one or more of the following:
bladder mass or abnormal bladder mucosa findings based on white light office cystoscopy;
findings suggestive of a bladder mass (bladder filling defect or hydronephrosis, or both) based on cross‐sectional imaging;
positive or atypical urinary cytology (and/or other markers such as positive fluorescence in situ hybridization (FISH) test).
We only considered studies of participants without any evidence of distant metastatic disease. We did not consider studies of participants in which blue light was used in a surveillance setting (cystoscopy in a diagnostic setting only).
Types of interventions
We planned to investigate the following comparisons of experimental intervention versus comparator intervention. Concomitant interventions had to be the same in the experimental and comparator groups to establish fair comparisons.
Experimental intervention
Blue light cystoscopy with 5‐ALA or HAL in combination with TURBT.
Comparator intervention
White light cystoscopy in combination with TURBT.
Comparison
Blue light cystoscopy via 5‐ALA or HAL versus white light cystoscopy.
Types of outcome measures
The measurement of outcomes assessed in this review was not a study eligibility criterion.
Primary outcomes
Time to disease recurrence (time‐to‐event outcome).
Time to disease progression (time‐to‐event outcome).
Surgical complications, serious (grade III, IV, and V according to Clavien‐Dindo) (dichotomous outcome) (Clavien 2009).
Secondary outcomes
Time to death from bladder cancer (time‐to‐event outcome).
Any adverse events (assessed using the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE)) (dichotomous outcome).
Surgical complications, non‐serious (grade I and II according to Clavien‐Dindo) (dichotomous outcome).
Method and timing of outcome measurement
Time to disease recurrence: measured from the time of random sequence generation to the time of any recurrence of bladder cancer (based on TURBT; irrespective of tumor stage or grade).
Time to disease progression: measured from the time of random sequence generation to the time of progression of bladder cancer as documented by histopathology at the time of TURBT (prompted by abnormal cystoscopy). We defined progression as an increase in tumor stage (defined as lamina propria invasion, e.g. increase from Ta to T1, or CIS to T1; development of muscle invasive disease (stage ≥ T2) and development of new lymph node involvement or metastatic disease) or grade (increase in grade from low to high (including CIS) following the definition of the International Bladder Cancer Group) (Lamm 2014).
Surgical complications, serious: measured within 90 days of initial TURBT according to the Clavien‐Dindo classification.
Time to death from bladder cancer: measured from the time of random sequence generation to the time of death from bladder cancer.
Adverse events: adverse events of any grade measured by CTCAE and within 90 days of initial TURBT.
Surgical complications, non‐serious: measured within 90 days of initial TURBT according to the Clavien‐Dindo classification.
Had we been unable to obtain the necessary information to analyze time‐to‐event outcomes for time to disease recurrence, time to disease progression, and time to death from bladder cancer, we would instead have analyzed these outcomes as dichotomous outcomes up to 12 months (short term) or 13 to 24 months (longer term) after randomization.
We have presented a summary of findings table reporting the following outcomes listed according to priority.
Time to disease recurrence
Time to disease progression
Surgical complications, serious
Time to death from bladder cancer
Any adverse events
Surgical complications, non‐serious
Thresholds for clinical relevance of outcomes
Time to disease recurrence: we assumed the effect to be of clinical relevance if the observed absolute difference was 5% or greater at 12 months follow‐up.
Time to disease progression: we assumed the effect to be of clinical relevance if the observed absolute difference was 2% or greater at 12 months follow‐up.
Surgical complications, serious: we assumed the effect to be of clinical relevance if the observed absolute difference was 2% or greater at initial TURBT or re‐resection.
Time to death from bladder cancer: we assumed the effect to be of clinical relevance if the observed absolute difference was 2% or greater at 12 months follow‐up.
Adverse events of any grade: we assumed the effect to be of clinical relevance if the observed difference was 5% or greater at 12 months follow‐up.
Surgical complications, non‐serious: we assumed the effect to be of clinical relevance if the observed difference was 5% or greater at initial TURBT or re‐resection.
These thresholds were established based on the input of the clinical experts on the author team considering the relative importance of a given outcome and the expected control event rate.
Search methods for identification of studies
We performed a comprehensive search with no restrictions on language of publication or publication status. We re‐ran searches approximately every three to six months, most recently on March 17, 2021.
Electronic searches
We searched the following databases.
-
Cochrane Library (via Wiley; 1970 to 17 March 2021; Appendix 1)
Cochrane Database of Systematic Reviews (CDSR)
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3 of 12, March 2021)
MEDLINE (via Ovid; 1946 to 17 March 2021; Appendix 2)
Embase (1947 to 17 March 2021; Appendix 3)
Web of Science Core Collection (1900 to 17 March 2021; Appendix 4)
Scopus (2004 to 17 March 2021; Appendix 5)
LILACS (Latin American and Caribbean Health Sciences Information database; 1982 to 17 March 2021; Appendix 6)
OpenGrey (1997 to 17 March 2021; Appendix 7)
We searched the following trial registers.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; 2000 to 17 March 2021; Appendix 8)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/; 2007 to 17 March 2021; Appendix 9)
Our electronic searches also included abstract proceedings of the European Association of Urology (EAU), American Urological Association (AUA), American Society of Clinical Oncology (ASCO), and American Society of Clinical Oncology Genitourinary (ASCO‐GU) meetings that are included in the above databases.
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We also contacted study authors of the included trials to identify any further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished trials.
Data collection and analysis
Selection of studies
We first used the reference management software EndNote to identify and remove duplicate records (EndNote 2019). We used the reference management software Covidence for the study selection process (Covidence). Two of three review authors (PM, AK or JV) independently scanned the titles and abstracts of the remaining records to determine which studies should be assessed further. We investigated all articles deemed potentially relevant as full text. Using Covidence, we categorized the full‐text studies as ‘included studies’ or ‘excluded studies.’ Any discrepancies were resolved through consensus or through recourse to a third review author (PD). We have presented an adapted PRISMA flowchart documenting the process of study selection and the total number of identified, included, and excluded studies (Liberati 2009). We listed all articles excluded after full‐text screening along with the reasons for their exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
For each included study, two of three review authors (PM, AK or JV) independently extracted key participant and intervention data using a data extraction form based on guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). A second review author (AK and JV) checked these data. Any disagreements were resolved by consensus or by consulting a third review author (PD) if required.
We extracted the following data.
Study information: author, title, source, publication date, publication type, language, duplicate publications, source of funding, authors’ conflicts of interest.
Study characteristics: study design, randomization method, number of study centers, country of study centers, inclusion and exclusion criteria, subgroup analysis, statistical methods, period of enrollment, follow‐up period.
Participant characteristics: number of participants, number of participants per study arm, age, gender, ethnicity, clinical stage of disease (presentation, focality, tumor size), number of participants recruited/allocated/evaluated.
Intervention/comparator information: name, dosage, frequency, duration of treatment, adjuvant therapy, re‐intervention, follow‐up.
Outcomes: according to our predefined primary and secondary outcomes (including tumor stage and grade), events of intervention/comparator, timing of outcome measurement, number of re‐resections.
We extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain the numbers of events and totals for population of a 2 x 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information. For time‐to‐event outcomes, we attempted to obtain hazard ratios (HRs) with corresponding measures of variance or data necessary to calculate this information.
Information about potentially relevant ongoing studies including trial identifier is presented in the 'Characteristics of ongoing studies’ table.
We contacted authors of the included studies if relevant data were missing.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (PM and AK) independently assessed the risk of bias of each included study. Any disagreements were resolved by consensus or by consultation with a third review author (PD).
We assessed risk of bias using Cochrane's risk of bias assessment tool (Higgins 2011b; Higgins 2021), which includes the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
For each study, we judged the risk of bias for each domain as being low, high, or unclear, according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b; Higgins 2021). We have presented a risk of bias summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and grouped outcomes according to whether they were measured subjectively or objectively in the risk of bias tables.
Performance bias
We considered all outcomes similarly susceptible to performance bias.
Detection bias
We considered all outcomes similarly susceptible to detection bias.
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and presented the judgement for each outcome separately when reporting our findings in the risk of bias tables. We collapsed reporting for outcomes with identical judgments. We considered a < 10% rate of attrition as low; 10% to 20% as unclear; and ≥ 20% in at least one trial arm as high risk of bias.
We assessed reporting bias on a per‐study basis. We classified the risk of bias for this domain as low only if we are able to identify an a priori protocol, and the reporting of outcomes and their analyses matched what the investigators had prespecified.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). We used the risk of bias assessment on a per‐study basis to inform the preplanned sensitivity analyses.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. Should we identify trials for inclusion with more than two intervention groups in further updates of this review, we will address these in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). For studies with more than two intervention groups (multi‐arm studies), we will only consider interventions that address the review objective for a pairwise comparison in our analysis (Higgins 2019b). In the event of repeated reporting of outcome measurements (e.g. at different time points), we used the data representing the longest follow‐up.
Dealing with missing data
Where feasible, we attempted to obtain missing data from study investigators. If this information was available, we performed an intention‐to‐treat (ITT) analysis. If it was not, we performed a modified intention‐to‐treat (mITT) analysis, adhering to ITT principles with the exception that participants with missing outcome data were excluded. If mITT analysis was not possible, we performed the analysis as‐treated and per‐protocol population (Higgins 2019c). This was rated as a potential source of bias. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals) and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)) if used by the study authors. We did not impute missing data for this review.
Assessment of heterogeneity
In the event of substantial clinical, methodological, or statistical heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis, instead providing a narrative description of the results of each study.
We identified heterogeneity (inconsistency) by visual inspection of the forest plots to assess the amount of overlap of CIs, and by using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted the I2 statistic as follows (Deeks 2019):
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
In the case of heterogeneity, we attempted to determine the potential causes by examining individual study and subgroup characteristics.
Assessment of reporting biases
In order to identify missing trial data, we searched for completed but not reported trials in the above‐mentioned trial registers.
We attempted to obtain study protocols to assess for selective outcome reporting.
If we include 10 or more studies investigating a given outcome in further updates of the review, we will use funnel plots to assess small‐study effects. There are several possible explanations for asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias. We will therefore use care in our interpretation of the results.
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we summarized data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). We used the Mantel‐Haenszel method for dichotomous outcomes and the generic inverse‐variance method for time‐to‐event outcomes. We used Review Manager 5 software to perform the analyses (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and therefore planned to carry out subgroup analyses with investigation of interactions for each comparison group by:
setting: primary versus recurrent bladder cancer;
multifocality: solitary versus multiple lesions of bladder cancer;
tumor size: tumor size 3 cm or less versus greater than 3 cm;
stage: positive cytology and/or history of CIS (in the case of recurrent disease).
Our rationale for these subgroup analyses was as follows.
Setting: according to the European Organisation for Research and Treatment of Cancer (EORTC) criteria (Sylvester 2006), the setting of primary versus recurrent (primary versus ≤ 1 recurrence versus > 1 recurrence) bladder cancer predicts recurrence and progression; it may represent an effect modifier.
Multifocality: according to the EORTC criteria (Sylvester 2006), the number of tumors (1 versus 2 to 7 versus ≥ 8) predicts recurrence and progression; it may represent an effect modifier.
Tumor size: according to the EORTC criteria (Sylvester 2006), tumor size (< 3 cm versus ≥ 3 cm) predicts recurrence and progression; it may represent an effect modifier.
Stage: compared to other histological types, the detection of CIS is particularly difficult due to its flat growth within the cell level; the effect of blue light versus white light may vary based on participant risk of harboring CIS.
At the request of several external peer referees, we performed one additional post hoc subgroup analysis:
use of 5‐ALA versus HAL as photodynamic agent in combination with TURBT.
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses if the number of studies was sufficient (Review Manager 2020).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (where applicable) on effect sizes.
Restricting the analyses by taking into account risk of bias, excluding studies at 'high risk' overall.
Restricting the analyses by taking into account the procedure for re‐resection, excluding studies in which all participants underwent re‐resection on a routine basis (rather than selectively based on high‐risk criteria concordant with current guidelines such as visible tumor left behind, pT1 high‐grade tumors). Routine re‐resection may potentially mitigate any benefits of blue light (Bogdan 2021; Doisy 2019; Tadrist 2021).
Summary of findings and assessment of the certainty of the evidence
We have presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (PM; AK or JV) independently rated the certainty of evidence for each outcome as 'high,' 'moderate,' 'low,' or 'very low' using GRADEpro GDT (GRADEpro GDT). Any discrepancies were resolved through consensus or by arbitration from a third review author (PD) if necessary. For each comparison, we have presented a summary of the evidence for the main outcomes in a summary of findings table, which provides key information regarding the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and a rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2019). If meta‐analysis was not possible, we presented the results in a narrative summary of findings table.
We have presented a summary of findings table reporting the following outcomes listed according to a priority rating established by the clinicians on our team with input from external experts.
Time to disease recurrence
Time to disease progression
Surgical complications, serious
Time to death from bladder cancer
Adverse events
Surgical complications, non‐serious
We used a minimally contextualized approach that focused on absolute effect size estimates for the interpretation of the results, Hultcrantz 2017, which we have reported using proposed language for GRADE narratives (Santesso 2020).
Results
Description of studies
We initially identified 1285 references following our database search, of which 144 were excluded as duplicates. After title and abstract screening, we retrieved the full texts for 112 studies which we assessed for inclusion in the review.
Results of the search
Sixteen studies ultimately met the inclusion criteria for assessment of the study question. The process of study selection is presented in a PRISMA flow diagram (see Figure 1).
1.
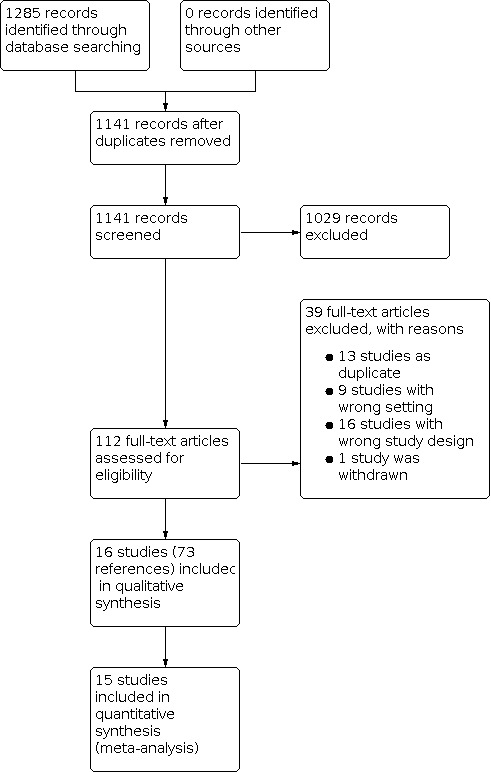
Included studies
We identified 16 trials that were eligible for inclusion (Babjuk 2005; Drăgoescu 2017; Filbeck 2002; Geavlete 2010; Geavlete 2012; Gkritsios 2014; Hermann 2011; Karaolides 2012; Kriegmaier 2002; Neuzillet 2014; O’Brien 2013; Riedl 2001; Rolevich 2017; Schumacher 2010; Stenzl 2010).
Full‐text publications in the English language were available for all of the included trials. All studies were RCTs; nine were single center, and seven were multicenter. The studies were conducted between 2001 and 2017. In 5 studies participants with primary NMIBC were included, and in 11 studies participants with primary or recurrent NMIBC were included. Inclusion of participants with suspicion of bladder cancer was based on positive urinary cytology, sonography, cystoscopy, or computed tomography. The trials compared white light TURBT to blue light TURBT using 5‐ALA and HAL as photoactive compound in seven and nine studies, respectively. Only one study used 50 mL solvent as a comparator in a placebo‐controlled setting. Predefined outcomes of the studies were broad and included time to disease recurrence, time to disease progression, time to death from bladder cancer, cancer‐specific survival, overall survival, recurrence rate, progression rate, detection rate, residual tumor rate, false‐positive rate, surgical complications, and adverse events of any grade. Nine trials reported no funding; four trials reported receiving funding from pharmaceutical companies; and three trials received funding through national government institutions. Nine studies reported conflict of interest statements, of which five declared to have none.
For a detailed description of included studies see Characteristics of included studies, Table 2, and Table 3.
1. Baseline characteristics of included studies.
| Study name | Trial period (year to year) | Setting/country | Description of participants | Intervention(s) and comparator(s) | Adjuvant instillation*/re‐resection |
Duration of follow‐up (median) |
Age (years (mean), range) | Gender (male/female) |
| Babjuk 2005 | 2001 to 2005 | Single center/Czech Republic | Primary and recurrent NMIBC, only Ta/T1 | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/NA | 24 months | 67.9 ± NA | 43/17 |
| Comparator: WL‐TURBT |
67.9 ± NA | 39/23 | ||||||
| Drăgoescu 2017 | 2009 to 2011 | Single center/Romania | Primary NMIBC | Intervention: 0.85 g HAL + BL‐TURBT |
Mitomycin C, doxorubicin, farmorubicin/NA | 60 months | 59.4 ± 9.9 | 45/12 |
| Comparator: WL‐TURBT |
60.3 ± 10.2 | 43/13 | ||||||
| Filbeck 2002 | 1997 to 2000 | Single center/Germany | Primary and recurrent NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/WL‐TURBT after 6 weeks | 24 months | 68.0 (31 to 88) | NA |
| Comparator: WL‐TURBT |
70.0 (32 to 89) | NA | ||||||
| Geavlete 2010 | 2007 to 2009 | Single center/Romania | Primary NMIBC | Intervention: HAL + BL‐TURBT |
Mitomycin C/WL‐TURBT after 6 weeks | 6 weeks | 64.0 (32 to 86) | 327/119 |
| Comparator: WL‐TURBT | ||||||||
| Geavlete 2012 | NA | Single center/Romania | Primary and recurrent NMIBC | Intervention: HAL + BL‐TURBT |
Mitomycin C/NA | 24 months | 66.8 (31 to 85) | 267/95 |
| Comparator: WL‐TURBT | ||||||||
| Gkritsios 2014 | NA | Single center/Greece | Primary and recurrent NMIBC | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
Epirubicin/NA | 40 months | 66.0 ± NA | 43/11 |
| Comparator: WL‐TURBT |
68.2 ± NA | 44/6 | ||||||
| Hermann 2011 | NA | Multicenter/Denmark | Primary and recurrent NMIBC, only Ta/T1 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
NA/NA | 12 months | 71.0 (35 to 96) | NA |
| Comparator: WL‐TURBT |
69.0 (41 to 92) | NA | ||||||
| Karaolides 2012 | 2008 to 2010 | Single center/Greece | Primary and recurrent NMIBC | Intervention: HAL + BL‐TURBT |
Epirubicin/NA | 18 months | 66.3 (37 to 82) | 33/8 |
| Comparator: WL‐TURBT |
63.8 (39 to 88) | 40/5 | ||||||
| Kriegmaier 2002 | 1997 to 1998 | Multicenter/Germany, Austria | Primary and recurrent NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/WL‐TURBT after 10 to 14 days | 2 weeks | 69.3 (38 to 88) | 53/12 |
| Comparator: WL‐TURBT |
69.6 (34 to 94) | 45/19 | ||||||
| Neuzillet 2014 | 2009 to 2012 | Multicenter/France | Primary NMIBC | Intervention: 0.85 g HAL + BL‐TURBT |
NA/PDD‐TURBT after 6 weeks | 6 weeks | 74.0 ± 10.3 | 64/8 |
| Comparator: WL‐TURBT |
74.0 ± 10.4 | 69/10 | ||||||
| O’Brien 2013 | 2005 to 2010 | Single center/United Kingdom | Primary NMIBC | Intervention: HAL + BL‐TURBT |
Mitomycin C/NA | 12 months | 68.0 (31 to 95) | 95/34 |
| Comparator: WL‐TURBT |
68.0 (29 to 90) | 88/32 | ||||||
| Riedl 2001 | 1998 to 2000 | Multicenter/Germany, Austria | Primary NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/PDD‐TURBT after 6 weeks | 60 months | 67.0 (19 to 86) | 36/15 |
| Comparator: WL‐TURBT |
37/14 | |||||||
| Rolevich 2017 | 2008 to 2012 | Single center/Republic of Belarus | Primary and recurrent NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
Doxorubicin/NA | 60 months | NA | 134/40 |
| Comparator: WL‐TURBT |
NA | 156/47 | ||||||
| Schumacher 2010 | 2002 to 2005 | Multicenter/Sweden | Primary and recurrent NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/WL‐TURBT after 5 to 7 weeks in pts with pT1 G2‐3 or T2 | 24 months | 70.1 ± 10.1 | 103/38 |
| Comparator: WL‐TURBT |
68.9 ± 10.8 | 104/34 | ||||||
| Stenzl 2010 | NR | Multicenter/USA, Canada, Europe | Primary and recurrent NMIBC, only Ta/T1 | Intervention: 0.85 g HAL + BL‐TURBT |
NA/NA | 9 months | 68.0 ± 10.8 | 212/59 |
| Comparator: WL‐TURBT |
69.6 ± 10.7 | 223/57 | ||||||
| Stenzl 2011 | NR | Multicenter/Germany, Austria | Primary NMIBC | Intervention: 1 g 5‐ALA + BL‐TURBT |
NA/WL‐TURBT after 2 to 4 weeks in pts with pT1 G2‐3 or T2 | 12 months | 66.0 ± 12.0 | 259/100 |
| Comparator: WL‐TURBT |
*Only immediate postoperative instillations, no Bacille Calmette‐Guerin schedule.
5‐ALA: 5‐aminolevulinic acid
BL‐TURBT: blue light transurethral resection of bladder tumor
HAL: hexaminolevulinic acid
NA: not available
NMIBC: non‐muscle invasive bladder cancer
NR: not reported
PDD‐TURBT: photodynamic diagnosis‐assisted transurethral resection of bladder tumor
pts: participants
WL‐TURBT: white light transurethral resection of bladder tumor
2. Participants in the included studies.
| Study name | Intervention(s) and comparator(s) | Screened/eligible (N) | Randomized (N) | Analyzed (N): efficacy | Analyzed (N): safety | Finishing trial (N) |
| Babjuk 2005 | Intervention: 1 g 5‐ALA + BL‐TURBT |
128/122 | 64 | 60 | NA | 60 |
| Comparator: WL‐TURBT | 64 | 62 | NA | 62 | ||
| Drăgoescu 2017 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
113/113 | 57 | 57 | NA | 57 |
| Comparator: WL‐TURBT | 56 | 56 | NA | 56 | ||
| Filbeck 2002 | Intervention: 1 g 5‐ALA + BL‐TURBT |
301/191 | 151 | 88 | NA | 88 |
| Comparator: WL‐TURBT | 150 | 103 | NA | 103 | ||
| Geavlete 2010 | Intervention: HAL + BL‐TURBT |
NA/446 | 223 | 176 | NA | NA |
| Comparator: WL‐TURBT | 233 | 159 | NA | NA | ||
| Geavlete 2012 | Intervention: HAL + BL‐TURBT |
362/269 | 181 | 125 | NA | 48 |
| Comparator: WL‐TURBT | 181 | 114 | NA | 37 | ||
| Gkritsios 2014 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
130/104 | 66 | 48 | NA | 48 |
| Comparator: WL‐TURBT | 64 | 37 | NA | 37 | ||
| Hermann 2011 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
223/223 | 115 | 68 | NA | 68 |
| Comparator: WL‐TURBT | 118 | 77 | NA | 77 | ||
| Karaolides 2012 | Intervention: HAL + BL‐TURBT |
102/102 | 49 | 41 | NA | 41 |
| Comparator: WL‐TURBT | 53 | 45 | NA | 45 | ||
| Kriegmaier 2002 | Intervention: 1 g 5‐ALA + BL‐TURBT |
165/129 | 83 | 65 | NA | 65 |
| Comparator: WL‐TURBT | 82 | 64 | NA | 64 | ||
| Neuzillet 2014 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
151/151 | 72 | 72 | NA | 43 |
| Comparator: WL‐TURBT | 79 | 79 | NA | 50 | ||
| O’Brien 2013 | Intervention: HAL + BL‐TURBT |
249/185 | 129 | 86 | NA | 63 |
| Comparator: WL‐TURBT | 120 | 82 | NA | 67 | ||
| Riedl 2001 | Intervention: 1 g 5‐ALA + BL‐TURBT |
115/102 | NA | 51 | 51 | NA |
| Comparator: WL‐TURBT | NA | 51 | 51 | NA | ||
| Rolevich 2017 | Intervention: 1 g 5‐ALA + BL‐TURBT |
525/377 | 252 | 174 | NA | NA |
| Comparator: WL‐TURBT | 273 | 203 | NA | NA | ||
| Schumacher 2010 | Intervention: 1 g 5‐ALA + BL‐TURBT |
300/279 | 153 | 141 | NA | 136 |
| Comparator: WL‐TURBT | 147 | 138 | NA | 134 | ||
| Stenzl 2010 | Intervention: 0.85 g 5‐ALA + BL‐TURBT |
814/766 | 382 | 200 | 421 | 200 |
| Comparator: WL‐TURBT | 384 | 202 | 391 | 202 | ||
| Stenzl 2011 | Intervention: 1 g 5‐ALA + BL‐TURBT |
381/370 | 192 | 183 | 187 | NA |
| Comparator: WL‐TURBT | 189 | 176 | 183 | NA | ||
| Intervention total | 2169 | 1635 | 659 | 994 | ||
| Comparator total | 2183 | 1648 | 625 | 1011 | ||
| Grand total | 4352 | 3283 | 1284 | 2005 | ||
5‐ALA: 5‐aminolevulinic acid
BL‐TURBT: blue light transurethral resection of bladder tumor
HAL: hexaminolevulinic acid
NA: not available
WL‐TURBT: white light transurethral resection of bladder tumor
Ongoing studies
We identified two ongoing trials addressing our objective (Boström 2018; Tandogdu 2019). At the time of publication of this review, the study of Boström 2018 was recruiting. Regarding the study of Tandogdu 2019, the manuscript was prepared, but no published data were available yet. We reached out to the authors of both studies.
For a detailed description of ongoing studies see Characteristics of ongoing studies.
Excluded studies
During title and abstract screening we excluded 1029 records that did not meet our inclusion criteria. We excluded 39 studies after full‐text screening. Reasons for exclusion included wrong study setting (e.g. surveillance after TURBT; 9 publications); duplicate records (13 publications); wrong study design (e.g. non‐randomized; 16 studies); and study withdrawn before accrual of any participants (1 study; NCT00785694). For a detailed description of excluded studies see Characteristics of excluded studies.
Risk of bias in included studies
For details see the risk of bias sections in Characteristics of included studies, Figure 2, and Figure 3.
2.

3.

Allocation
Random sequence generation
We assessed the majority of studies (10 of 16) as at unclear risk of bias for sequence generation (Babjuk 2005; Drăgoescu 2017; Filbeck 2002; Hermann 2011; Karaolides 2012; Kriegmaier 2002; Riedl 2001; Schumacher 2010; Stenzl 2010; Stenzl 2011). We rated the remaining six studies as low risk of bias (Geavlete 2010; Geavlete 2012; Gkritsios 2014; Neuzillet 2014; O’Brien 2013; Rolevich 2017)
Allocation concealment
Similar to above, we judged the majority of studies (10 of 16) as at unclear risk of bias for allocation concealment (Babjuk 2005; Drăgoescu 2017; Filbeck 2002; Gkritsios 2014; Hermann 2011; Karaolides 2012; Neuzillet 2014; Riedl 2001; Stenzl 2010). We rated the remaining six studies as low risk of bias. (Geavlete 2010; Geavlete 2012; O’Brien 2013; Rolevich 2017; Schumacher 2010; Stenzl 2010).
Blinding
Performance bias: blinding of surgeon
Due to the nature of the intervention, in none of the included studies was the surgeon blinded, therefore all studies were rated as high risk of bias for this domain.
Performance bias: blinding of participants and other study personnel
Only one study reported blinding of participants and other study personnel; we judged this study to be at low risk of bias (Stenzl 2011). We assessed all other studies as at unclear risk of bias.
Detection bias: surgical complications
Subjective outcomes (all other outcomes)
One study reported blinding of outcome assessors adequately and was assessed as at low risk of bias (Stenzl 2011).
Objective outcomes (surgical complications)
We judged all studies to be at low risk of bias for the objective outcome of surgical complications.
Incomplete outcome data
We rated the risk of attrition bias on a per‐outcome basis but grouped outcomes with identical ratings together.
Oncological outcomes
We identified six studies where randomized participants were included adequately in the analysis for oncological outcomes (Babjuk 2005; Filbeck 2002; Karaolides 2012; Neuzillet 2014; Schumacher 2010; Stenzl 2011). We judged risk of bias to be unclear for five studies due to attrition rates of 10% to 20% (Drăgoescu 2017; Geavlete 2010; Geavlete 2012; O’Brien 2013; Riedl 2001). We judged four studies to be at high risk of bias for this domain (Gkritsios 2014; Hermann 2011; Rolevich 2017; Stenzl 2010).
Surgical complications
One study reported the outcome surgical complications (Rolevich 2017). All participants were included in the analysis, therefore we rated the study as at low risk of bias.
Any adverse events
Only three studies reported this outcome (Schumacher 2010; Stenzl 2010; Stenzl 2011). In all of these studies the vast majority of randomized participants (> 90% per arm) were included in the analysis, therefore we judged the risk of attrition bias to be low.
Selective reporting
We failed to identified protocols for the majority of studies (11 of 16); we rated these studies as at unclear risk of reporting bias (Babjuk 2005; Drăgoescu 2017; Filbeck 2002; Geavlete 2010; Geavlete 2012; Karaolides 2012; Kriegmaier 2002; Neuzillet 2014; Riedl 2001; Rolevich 2017; Schumacher 2010). We identified study protocols for four of the included studies (Gkritsios 2014; Hermann 2011; O’Brien 2013; Stenzl 2010). One additional study protocol was provided by the trial sponsor (Stenzl 2011). The reported primary and secondary outcomes corresponded to how they were planned, therefore we rated these five studies as at low risk of reporting bias.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
See also Table 1.
Primary outcomes
Time to disease recurrence
Blue light TURBT may reduce the risk of disease recurrence over time compared to white light TURBT (hazard ratio (HR) 0.66, 95% confidence interval (CI) 0.54 to 0.81; 15 studies; 2994 participants; low‐certainty evidence; Analysis 1.1; Figure 4), depending on baseline risk. For participants with low‐risk NMIBC with a baseline risk of 15.0% according to EORTC risk categories (Sylvester 2006), this corresponds to 48 fewer (66 fewer to 27 fewer) recurrences per 1000, which falls below our predefined threshold for minimal clinically important difference (MCID) of 50 per 1000. For participants with intermediate‐ and high‐risk NMIBC with baseline risks of 38.0% and 61.0% according to EORTC risk categories (Sylvester 2006), this corresponds to 109 (152 fewer to 59 fewer) and 147 (211 fewer to 76 fewer) fewer recurrences per 1000 participants, respectively, when compared to white light TURBT. We downgraded the certainty of the evidence by one level each for study limitations as well as clinically relevant inconsistency.
4.

For two studies, all the information for the analysis was contained in the publications (Drăgoescu 2017; Rolevich 2017); for nine studies the HR and P value (if not provided) were calculated with the Parmar method (Geavlete 2010; Geavlete 2012; Gkritsios 2014; Hermann 2011; Karaolides 2012; Neuzillet 2014; O’Brien 2013; Riedl 2001; Stenzl 2011); and for the remaining four studies we performed data reconstruction due to digitalization of Kaplan‐Meier curves and HRs and CIs were calculated by the Tierney method (Babjuk 2005; Filbeck 2002; Schumacher 2010; Stenzl 2010).
Time to disease progression
Blue light TURBT may reduce the risk of disease progression over time compared to white light TURBT (HR 0.65, 95% CI 0.50 to 0.84; 9 studies; 2200 participants; low‐certainty evidence; Analysis 1.2; Figure 5), depending on the baseline risk. For people with low‐ and intermediate‐risk NMIBC with assumed baseline risks of 0.2% and 5% based on EORTC risk categories (Sylvester 2006), this corresponds to 2 (1 fewer to 0 fewer) and 50 fewer (25 fewer to 8 fewer) progressions per 1000 people at 12 months, respectively, when compared to white light TURBT, which falls below our predefined threshold for MCID of 20 per 1000. For people with high‐risk NMIBC with an assumed baseline risk of 17.0% per EORTC risk categories (Sylvester 2006), this corresponds to 170 fewer (81 fewer to 25 fewer) progressions per 1000 participants at 12 months. We downgraded the certainty of the evidence by one level each for risk of bias (the majority of studies had an unclear method of randomization, unclear allocation concealment, and lacked an a prior protocol) and imprecision.
1.2. Analysis.

Comparison 1: Blue light versus white light, Outcome 2: Time to disease progression
5.

For one study, all the information for the analysis was contained in the publication (Rolevich 2017); for six studies the HR and P value (if not provided) were calculated with the Parmar method (Babjuk 2005; Drăgoescu 2017; Geavlete 2010; O’Brien 2013; Riedl 2001; Stenzl 2010); and for two studies we performed data reconstruction due to digitalization of Kaplan‐Meier curves and HRs and CIs were calculated by the Tierney method (Schumacher 2010; Stenzl 2011).
Surgical complications, serious
Blue light TURBT may have little or no effect on serious surgical complications (risk ratio (RR) 0.54, 95% CI 0.14 to 2.14; 1 study; 525 participants; low‐certainty evidence; Analysis 1.3; Figure 6). Assuming a risk of serious complications of 2.2% in the white light group, this corresponds to 10 fewer (19 fewer to 25 more) surgical complications per 1000 participants with blue light TURBT, which falls below our predefined threshold for MCID of 20 per 1000. We judged the certainty of evidence to be low (downgraded one level for risk of bias and one level for imprecision).
1.3. Analysis.

Comparison 1: Blue light versus white light, Outcome 3: Surgical complications, serious
6.

Secondary outcomes
Time to death from bladder cancer
Blue light TURBT may have little or no effect on the risk of death from bladder cancer over time (HR 0.55, 95% CI 0.19 to 1.61; 1 study; 407 participants; low‐certainty evidence; Analysis 1.4). Assuming a risk of bladder cancer of 2.2% in the white light group, this corresponds to 22 deaths from bladder cancer per 1000 participants with white light TURBT and 10 fewer (17 fewer to 13 more) deaths from bladder cancer per 1000 participants with blue light TURBT, which falls below our predefined threshold for MCID of 20 per 1000. We judged the certainty of evidence to be low (downgraded one level for risk of bias and one level for imprecision).
1.4. Analysis.

Comparison 1: Blue light versus white light, Outcome 4: Time to death from bladder cancer
Any adverse events
We are very uncertain as to whether blue light TURBT reduces the incidence of adverse events of any grade as we assessed the certainty of the evidence as low (RR 1.09, 95% CI 0.88 to 1.33; 3 studies; 1375 participants; low‐certainty evidence; Analysis 1.5). Assuming a risk of any adverse event of 39.7% in the white light group, this corresponds to 397 adverse events of any grade per 1000 participants with white light TURBT and 36 more (48 fewer to 131 more) any adverse events per 1000 participants with blue light TURBT, which falls below our predefined threshold for MCID of 50 per 1000. We judged the certainty of evidence to be low (downgraded one level for risk of bias and one level for imprecision).
1.5. Analysis.

Comparison 1: Blue light versus white light, Outcome 5: Adverse events
Surgical complications, non‐serious
No analysis was possible as this outcome was not reported by any study.
Subgroup analyses
I. Primary versus recurrent bladder cancer
Time to disease recurrence
The pooled effect size in participants with primary bladder tumor (HR 0.79, 95% CI 0.60 to 1.03; 2 studies; 368 participants) was similar to that of participants with recurrent disease (HR 0.80, 95% CI 0.67 to 0.95; 2 studies; 422 participants; Analysis 2.1). All comparisons were across studies and did not suggest a potential subgroup effect (P = 0.95).
2.1. Analysis.

Comparison 2: Blue light versus white light—subgroup analysis: primary versus recurrent bladder cancer, Outcome 1: Time to disease recurrence
Time to disease progression
We were unable to perform this analysis due to lack of reported data.
Surgical complications, serious
We were unable to perform this analysis due to lack of reported data.
II. Solitary versus multiple lesions of bladder cancer
Time to disease recurrence
The pooled effect size in participants with a solitary bladder tumor (HR 0.60, 95% CI 0.38 to 0.95; 3 studies; 230 participants) was similar to that of participants with multiple tumors (HR 0.53, 95% CI 0.31 to 0.90; 3 studies; 241 participants; Analysis 3.1). All comparisons were across studies, and we did not find any suggestion of a potential subgroup effect (P = 0.74).
3.1. Analysis.

Comparison 3: Blue light versus white light—subgroup analysis: solitary versus multiple lesions of bladder cancer, Outcome 1: Time to disease recurrence
Time to disease progression
We were unable to perform this analysis due to lack of reported data.
Surgical complications, serious
No additional analysis was possible as the primary analysis (Analysis 1.3) included only one study (Rolevich 2017).
III. Tumor size 3 cm or less versus greater than 3 cm
No analysis was possible as no data were reported by any included study.
IV. Positive cytology and/or history of CIS (in the case of recurrent disease)
No analysis was possible as no data were reported by any included study.
V. 5‐ALA versus HAL (post hoc subgroup analysis)
Time to disease recurrence
The pooled effect size of studies using 5‐ALA (HR 0.76, 95% CI 0.57 to 1.00; 6 studies; 1430 participants) was similar to that of studies using HAL (HR 0.60, 95% CI 0.45 to 0.78; 9 studies; 1564 participants; Analysis 4.1). All comparisons were across studies, and we did not find any suggestion of a potential subgroup effect (P = 0.23).
4.1. Analysis.

Comparison 4: Blue light versus white light—subgroup analysis of 5‐ALA versus HAL (post hoc), Outcome 1: Time to disease recurrence
Time to disease progression
The pooled effect size of studies using 5‐ALA (HR 0.72, 95% CI 0.47 to 1.11; 5 studies; 1239 participants) was similar to that of studies using HAL (HR 0.69, 95% CI 0.48 to 0.98; 4 studies; 961 participants; Analysis 4.2). All comparisons were across studies, and we did not find any suggestion of a potential subgroup effect (P = 0.87).
4.2. Analysis.

Comparison 4: Blue light versus white light—subgroup analysis of 5‐ALA versus HAL (post hoc), Outcome 2: Time to disease progression
Surgical complications, serious
No subgroup analysis was possible as the analysis (Analysis 1.3) included only one study (Rolevich 2017).
Sensitivity analyses
I. Sensitivity analysis by studies at high risk of bias overall
No analysis was possible as no study was judged to be at low risk of bias.
II. Sensitivity analysis by re‐resection
We were able to perform sensitivity analyses by excluding studies in which all participants underwent re‐resection on a routine basis for the three primary outcomes of this review.
Time to disease recurrence
The effect size (HR 0.64, 95% CI 0.56 to 0.74; 9 studies; 1776 participants; Analysis 5.1) was similar to that of the primary analysis (HR 0.66, 95% CI 0.54 to 0.81; 15 studies; 2994 participants).
5.1. Analysis.

Comparison 5: Blue light versus white light—sensitivity analysis by re‐resection, Outcome 1: Time to disease recurrence
Time to disease progression
The effect size (HR 0.64, 95% CI 0.46 to 0.90; 6 studies; 1460 participants; Analysis 5.2) as similar to that of the primary analysis (HR 0.65, 95% CI 0.50 to 0.84; 9 studies; 2200 participants).
5.2. Analysis.

Comparison 5: Blue light versus white light—sensitivity analysis by re‐resection, Outcome 2: Time to disease progression
Surgical complications, serious
No additional analysis was possible as the primary analysis (Analysis 1.3) included only one study (Rolevich 2017).
Discussion
Summary of main results
We identified 16 RCTs including a total of 4325 participants that compared blue light‐enhanced TURBT with white light‐based TURBT in the treatment of primary or recurrent non‐muscle invasive bladder cancer. Of 4352 randomized participants, 3283 could be included in the analysis. Of the randomized participants, 2169 received a blue light TURBT and 2183 participants received a white light TURBT.
Blue light may reduce the risk of disease recurrence over time (low‐certainty evidence) and may also reduce the risk of disease progression over time (low‐certainty evidence). For both outcomes, the magnitude of this treatment effect in absolute terms depends greatly on the baseline risk as reflected by the EORTC prognostic group. Meanwhile, blue light may have little to no effect on the incidence of serious surgical complications (low‐certainty evidence).
We also found that blue light may have little to no effect on the risk of death from bladder cancer over time (low‐certainty evidence), and we are very uncertain of the effect on adverse events of any grade (low‐certainty evidence). We do not know the effect of blue light on non‐serious surgical complications as none of the included trials reported this outcome.
None of our predefined subgroup analysis suggested evidence for a subgroup effect. We also did not find any evidence of a subgroup effect when comparing 5‐ALA versus HAL.
A sensitivity analysis based on risk of bias was not possible. Our analytic results appeared robust to a sensitivity analysis that excluded studies in which all participants underwent a re‐resection on a routine basis.
Overall completeness and applicability of evidence
This Cochrane Review is based on 16 RCTs including participants with primary or recurrent non‐muscle invasive bladder cancer (stage Ta, T1, and carcinoma in situ), which represents a population of patients routinely managed in clinical practice.
The studies included in this review were published between 2001 and 2017. During this period, technological progress has led to improvements in the instruments used for visualization and resection. Unfortunately, not all of the included studies described the equipment used in detail. Accordingly, the influence of the equipment used on the results of the included studies remains unclear. Examples of this include the recently US Food and Drug Administration‐approved use of flexible cystoscopes for blue light visualization (used for surveillance) as well as the type of white light cystoscopy equipment used (i.e. high‐definition versus standard‐definition camera systems).
Aside from the studies' comparable intervention of blue light to white light TURBT, there were differences in the implementation of the individual studies. Both 5‐ALA and HAL were used as the fluorescent agent, and furthermore only one study used a solvent as a placebo instillation. There were also differences in the postoperative instillation regimen. Administrations of mitomycin C as well as epirubicin or doxorubicin were used. Due to the heterogeneity of the treatments used, we could not consider these data in our analysis, which may have affected the results. Another difference was whether a re‐TURBT was performed, and if so, at what point in time. In most of the studies, the time between five and seven weeks after the initial TURBT was chosen, but re‐TURBT was rarely performed again under blue light. Overall, all of these differences reflect clinical heterogeneity that may have impacted our study results.
We were unable to address whether blue light TURBT may obviate the need for routine re‐resection (Doisy 2019; Tadrist 2021). In addition, we did not address the issue of cost consequences or effectiveness, which is relevant given the required capital investment and personnel‐intensive workflow alterations (Klaassen 2017; Witjes 2014a).
A major source of heterogeneity in the included studies was the postoperative use of intravesical chemotherapy (e.g. postoperative mitomycin installation) as well as the initiation of intravesical induction therapy. These agents are known to impact outcomes (Han 2021; Hwang 2019; Shepherd 2017), and were applied differently in the included studies, therefore representing a potential source of bias.
Lastly, this review indicates a paucity of published, direct trial‐derived evidence on adverse events related to the installation of the photodynamic agent used for blue light cytoscopy. A sponsor‐supported study on the issue of safety specific to HLA has been published using pooled trial data (Witjes 2014). It reported no serious adverse events related to the agent, but did not permit trial‐specific data attribution and verification that would have permitted inclusion in the review.
Quality of the evidence
We rated the certainty of evidence as low throughout all endpoints. Reasons for downgrading of the evidence included concerns over study limitations for risk of bias, inconsistency, and imprecision.
Study limitations: a majority of studies did not provide assurance of allocation concealment (thereby raising concerns over selection bias); very few studies explicitly reported blinding of study participants and personnel or outcome assessors; and more than half of the included studies had substantial attrition of participants. This was in part due to purposeful exclusion of participants at various time points in the course of each study due to a diagnosis of muscle invasive bladder cancer or histopathological findings without malignancy. Different ways of dealing with excluded participants may have distorted the study results. For example, participants with a diagnosis of muscle invasive bladder cancer who subsequently underwent cystectomy may have experienced surgical complications or adverse events that were not accounted for. In addition, few studies had an a priori protocol that was retrievable, which made it difficult to judge the risk of reporting bias. It is notable that much fewer studies reported on serious surgical complications and adverse events than on time to recurrence or progression. All of these issues prompted us to consistently rate down the certainty of evidence by one level.
Inconsistency: we downgraded the certainty of evidence by one level for the outcome time to disease recurrence in accordance with GRADE guidance considering not only the I2 value, but also the clinical implications of using a minimally contextualized approach (Hultcrantz 2017).
Imprecision: we downgraded most of the outcomes by one level for imprecision, as CIs for the pooled effect sizes were consistent with both no effect and clinically important reduction.
Potential biases in the review process
In order to minimize potential bias in the review process, we performed a sensitive search strategy in multiple databases. Despite a comprehensive and unrestricted search, we may have missed studies (unpublished, non‐English). Two review authors independently performed literature screening, data extraction, and risk of bias assessment.
Some studies did not provide hazard ratios for the outcomes time to disease recurrence and time to disease progression. We therefore calculated hazard ratios either with Parmar method (Parmar 1998), or conducted data reconstruction via digitalization of Kaplan‐Meier curves and by Tierney method (Tierney 2007). Both methods can only reconstruct hazard ratios according to the information available, which may have resulted in bias.
The subgroup analysis comparing 5‐ALA versus HAL was conducted post hoc (as described in Differences between protocol and review section) and was motivated by the comments of several peer referees suggesting that HAL may have superior oncological outcomes (Gakis 2015). Interpreting the results in accordance with Cochrane guidance, we did not find evidence to support a subgroup effect.
Agreements and disagreements with other studies or reviews
Relatively few higher‐quality systematic reviews exist on this topic, which are described as follows.
Mowatt 2011 published a systematic review and meta‐analysis that addressed both the diagnostic accuracy of photodynamic cytoscopy as well as its clinical effectiveness compared to white light. Based on a literature search up to 2008, the review identified four RCTs with 709 participants (Babjuk 2005; Filbeck 2002; Kriegmaier 2002; Riedl 2001), and reported a substantially reduced risk of recurrence (RR 0.37, 95% CI 0.20 to 0.69) and greater progression‐free survival (RR 1.37, 95% CI 1.18 to 1.59) compared to white light. The authors did not rate the certainty of evidence. The risk of bias assessment also reported unclear allocation concealment and failure to blind participants, personnel, or outcome assessors.
Rink 2013 reported a similar systematic review that assessed both diagnostic accuracy and comparative effectiveness and identified a total of 13 randomized trials (date of last search October 2012), but double‐counted several studies that had presented both initial results as well as longer‐term follow‐up. The authors also provided no meta‐analysis of their own.
Tran 2017 reported an evidence report by the Canadian Agency for Drugs and Technologies with a search date of up to 2016. Their findings suggested both short‐term (based on 10 RCTs) and long‐term (based on 12 RCTs) reduced risk of recurrence, but no reduced risk of progression. The results of subgroup analyses for both outcomes favored HAL over 5‐ALA, but the tests of interaction were not significant, thereby indicating chance as a potential explanation for these findings. The strength of evidence was described as low and moderate, respectively.
Chou 2017 reported the most recent high‐quality systematic review on this topic to date; however, the latest search date is reported as September 2015, and the review only included studies published as full text, thereby raising concerns about publication bias. Since publication of Chou 2017, one study has published extended follow‐up data (Drăgoescu 2017), and results of one additional study have become available (Rolevich 2017). In addition, our review adds an analysis based on time‐to‐event outcomes using HR both for time to recurrence and progression, whereas Chou 2017 used RR that were analyzed as short‐ (less than three months), intermediate‐ (three months to less than one year), and long‐term (one year or more). Whereas the interpretation of Chou 2017 focused on the presence or absence of statistical significance, we applied a minimally contextualized approach to GRADE (Hultcrantz 2017), with predefined thresholds of what we considered the MCID for each outcome. Lastly, we were able to provide a more nuanced interpretation of our results by applying the relative effect size measures to different baseline risk groups. As a result, our findings provide more support for the notion that blue light cytoscopy‐guided TURBT extends time to progression, but that any clinically important effect may be limited to individuals stratified as high risk.
Sari 2021, a recent systematic review and network meta‐analysis, sought to also assess whether a single intravesical chemotherapy installation added to the therapeutic effectiveness of blue light and narrow band imaging‐assisted TURBT compared to white light. The findings were consistent with ours in that blue light cystoscopy‐assisted TURBT outperformed white light, and the positive effect was accentuated by intravesical chemotherapy, as one might have expected. Missing from this review, however, was any attempt to rate the certainty of evidence. Also, narrow band imaging‐assisted TURBT appeared to outperform blue light. While this is outside the scope of our review, it is the topic of an ongoing companion review, Lai 2021, of this study.
Authors' conclusions
Implications for practice.
The findings of this review suggest a favorable impact of blue light transurethral bladder tumor resection (TURBT) on the risk of disease recurrence and progression; however, whether this risk reduction is clinically relevant greatly depends on the baseline risk of patients. Time to death from bladder cancer may not be impacted. We did not find an increase in severe surgical complications with blue light cystoscopy, and we did not find any trial evidence on other, non‐surgical adverse events.
We expect findings of the review to help further inform evidence‐based guidelines by major professional organizations such as the American Urological Association (AUA) and the European Association of Urology (EAU). Current EAU guidelines, EAU 2021, make a conditional recommendation for the use of "methods to improve tumor visualization" referring to both blue light cytoscopy and narrow band imaging, but further qualify this by "if available." The latest AUA guidelines, AUA 2016, (amended in 2020) state that clinicians "should offer" blue light cytoscopy (moderate recommendation) without further qualifiers to "increase detection and decrease recurrence." Baseline risk as defined by risk category may be an important criterion to help guide the use of blue light technology in clinical practice.
Implications for research.
Although we identified 16 randomized controlled trials addressing the objective of the review, the certainty of the evidence is low, therefore future high‐quality trials are likely to change our results. Future studies should be conducted with greater methodological rigor with regard to safeguards against selection, performance, and detection bias; have a published a priori protocol; and ensure greater completeness of follow‐up. Authors should extend follow‐up and plan ahead to address questions with regard to participant subgroups.
Future studies should also take into account recent technological developments such as digital imaging technology or LED (light‐emitting diode) illumination. It remains unclear how such innovations in white light technology may alter the assessment of the comparative effectiveness of blue light technology. To identify the most effective treatment option for individuals with non‐muscle invasive bladder cancer, head‐to‐head trials comparing white light, blue light, and other therapeutic options, such as narrow band imaging, should be conducted. Furthermore, surveillance strategies combining blue light and flexible cystoscopy should be examined.
History
Protocol first published: Issue 11, 2020
Acknowledgements
We would like to acknowledge the support of all members of the Cochrane Urology Group.
We thank the Cancer Network for their support of this review.
We thank Rodney Breau, Stefanie Schmidt, Eugene Pietzak, and Ambui Kumar for reviewing the protocol.
We thank Gautier Marcq, Hamed Ahmadi, Rodney Breau, and Georgios Gakis for reviewing the full review.
Appendices
Appendix 1. Cochrane Library search strategy
#1 MeSH descriptor: [undefined] explode all trees
#2 (bladder* near/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)):ti,ab,kw (Word variations have been searched)
#3 (NMIBC):ti,ab,kw (Word variations have been searched)
#4 (TURBT):ti,ab,kw (Word variations have been searched)
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Photosensitizing Agents] explode all trees
#7 (photodynamic and diagnosis):ti,ab,kw (Word variations have been searched)
#8 (PDD):ti,ab,kw (Word variations have been searched)
#9 MeSH descriptor: [Fluorescence] explode all trees
#10 (fluorescence):ti,ab,kw (Word variations have been searched)
#11 MeSH descriptor: [Aminolevulinic Acid] explode all trees
#12 (Aminolaevulinate):ti,ab,kw (Word variations have been searched)
#13 (hexaminolevulinate):ti,ab,kw (Word variations have been searched)
#14 (ALA or HAL):ti,ab,kw (Word variations have been searched)
#15 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #5 and #15
Appendix 2. MEDLINE via Ovid search strategy
1 exp urinary bladder neoplasms/
2 (bladder$ adj3 (cancer$ or carcinoma$ or neoplas$ or tumo?r$)).tw.
3 NMIBC.tw.
4 TURBT.tw.
5 1 or 2 or 3 or 4
6 exp Photosensitizing Agents/
7 photodynamic.tw. and (diagnosis.fs. or diagnosis.tw.)
8 PDD.tw.
9 exp Fluorescence/
10 fluorescence.tw.
11 exp Aminolevulinic Acid/
12 Aminolaevulinate.tw.
13 hexaminolevulinate.tw.
14 (ALA or HAL).tw.
15 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 5 and 15
17 randomized controlled trial.pt.
18 controlled clinical trial.pt.
19 randomized.ab.
20 placebo.ab.
21 drug therapy.fs.
22 randomly.ab.
23 trial.ab.
24 groups.ab.
25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26 exp animals/ not humans.sh.
27 25 not 26
28 16 and 27
Appendix 3. Embase search strategy
#1 'bladder tumor'/exp
#2 (bladder* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)):ab,ti
#3 nmibc:ab,ti
#4 turbt:ab,ti
#5 #1 OR #2 OR #3 OR #4
#6 'photosensitizing agent'/exp
#7 photodynamic:ab,ti AND (diagnosis:lnk OR diagnosis:ab,ti)
#8 pdd:ab,ti
#9 'fluorescence'/exp
#10 'fluorescence':ab,ti
#11 'aminolevulinic acid'/exp
#12 aminolaevulinate:ab,ti
#13 hexaminolevulinate:ab,ti
#14 ala:ab,ti OR hal:ab,ti
#15 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#16 #5 AND #15
#17 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti
#18 'animals'/exp NOT ('humans'/exp AND 'animals'/exp)
#19 #17 NOT #18
#20 #16 AND #19
Appendix 4. Web of Science search strategy
#1 TS=((bladder* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)) OR NMIBC OR TURBT)
#2 TS=((("Photosensitizing Agents" OR photodynamic) AND diagnosis) fluorescence OR "Aminolevulinic Acid" OR Aminolaevulinate OR hexaminolevulinate)
#3 TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#4 #1 AND #2 AND #3
Appendix 5. Scopus search strategy
#1 TITLE‐ABS‐KEY((bladder* W/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour*)) OR NMIBC OR TURBT) AND
#2 TITLE‐ABS‐KEY((("Photosensitizing Agents" OR photodynamic) AND diagnosis) fluorescence OR "Aminolevulinic Acid" OR Aminolaevulinate OR hexaminolevulinate)
#3 ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomization" OR "placebo" ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical trial* OR trial* OR rct* OR random* OR blind* ) )
#4 #1 AND #2 AND #3
Appendix 6. LILACS search strategy
((mh:("Urinary Bladder Neoplasms")) OR (tw:("bladder cancer" OR "bladder carcinoma" OR "bladder neoplasm" OR "bladder tumor" OR "bladder tumour" OR "NMIBC" OR "TURBT"))) AND ((mh:("Photosensitizing Agents" OR "Fluorescence" OR "Aminolevulinic Acid")) OR (tw:((photodynamic AND diagnosis) OR "PDD" OR "fluorescence" OR "Aminolaevulinate" OR "hexaminolevulinate" OR "ALA" OR "HAL"))) AND ((PT:"randomized controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomized controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter studies as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro")
Appendix 7. OpenGrey literature search strategy
"Bladder Cancer" AND (photodyanmic OR fluorescence)
Appendix 8. ClinicalTrials.gov search strategy
#1 Bladder Cancer
#2 photodyanmic OR fluorescence
#3 1 AND 2
Appendix 9. WHO ICTRP search strategy
1 bladder cancer AND photodynamic
2 bladder cancer AND fluorescence
3 1 OR 2
Data and analyses
Comparison 1. Blue light versus white light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to disease recurrence | 15 | 2994 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.54, 0.81] |
| 1.2 Time to disease progression | 9 | 2200 | Hazard Ratio (IV, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 1.3 Surgical complications, serious | 1 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.14, 2.14] |
| 1.4 Time to death from bladder cancer | 1 | 407 | Hazard Ratio (IV, Random, 95% CI) | 0.55 [0.19, 1.61] |
| 1.5 Adverse events | 3 | 1375 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.88, 1.33] |
1.1. Analysis.

Comparison 1: Blue light versus white light, Outcome 1: Time to disease recurrence
Comparison 2. Blue light versus white light—subgroup analysis: primary versus recurrent bladder cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Time to disease recurrence | 2 | 790 | Hazard Ratio (IV, Random, 95% CI) | 0.79 [0.69, 0.92] |
| 2.1.1 Primary bladder cancer | 2 | 368 | Hazard Ratio (IV, Random, 95% CI) | 0.79 [0.60, 1.03] |
| 2.1.2 Recurrent bladder cancer | 2 | 422 | Hazard Ratio (IV, Random, 95% CI) | 0.80 [0.67, 0.95] |
Comparison 3. Blue light versus white light—subgroup analysis: solitary versus multiple lesions of bladder cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Time to disease recurrence | 3 | 471 | Hazard Ratio (IV, Random, 95% CI) | 0.58 [0.45, 0.76] |
| 3.1.1 solitary bladder cancer | 3 | 230 | Hazard Ratio (IV, Random, 95% CI) | 0.60 [0.38, 0.95] |
| 3.1.2 multiple bladder cancer | 3 | 241 | Hazard Ratio (IV, Random, 95% CI) | 0.53 [0.31, 0.90] |
Comparison 4. Blue light versus white light—subgroup analysis of 5‐ALA versus HAL (post hoc).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Time to disease recurrence | 15 | 2994 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.54, 0.81] |
| 4.1.1 5‐ALA | 6 | 1430 | Hazard Ratio (IV, Random, 95% CI) | 0.76 [0.57, 1.00] |
| 4.1.2 HAL | 9 | 1564 | Hazard Ratio (IV, Random, 95% CI) | 0.60 [0.45, 0.78] |
| 4.2 Time to disease progression | 9 | 2200 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.63, 0.96] |
| 4.2.1 5‐ALA | 5 | 1239 | Hazard Ratio (IV, Random, 95% CI) | 0.72 [0.47, 1.11] |
| 4.2.2 HAL | 4 | 961 | Hazard Ratio (IV, Random, 95% CI) | 0.69 [0.48, 0.98] |
Comparison 5. Blue light versus white light—sensitivity analysis by re‐resection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Time to disease recurrence | 9 | 1776 | Hazard Ratio (IV, Random, 95% CI) | 0.64 [0.56, 0.74] |
| 5.2 Time to disease progression | 6 | 1460 | Hazard Ratio (IV, Random, 95% CI) | 0.64 [0.46, 0.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Babjuk 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | Grant of Czech Health Ministry | |
| Declarations of interest | NA | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author; original study data no longer available |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to two groups." Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to two groups." Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: "six [participants] (two in group A and four in group B) were excluded after histological examination, as in two there was no histological evidence of TCC, in three there was muscle invasion, and in one there was a multiple T1G3 tumour with concomitant carcinoma in situ (CIS) treated with immediate cystectomy." Comment: loss to follow‐up was < 10%, and variation in loss to follow‐up between groups was low |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Drăgoescu 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: 30 to 50 mg mitomycin C, doxorubicin, farmorubicin within 6 hours after surgery |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | National Scientific Research Council (CNCSIS) within the National Exploratory Research Project Program (PCE–2) | |
| Declarations of interest | None | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the study designed as a prospective randomized clinical trial." Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "distribution of patients in both groups was conducted in a randomized single blind manner." Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Unclear risk | Quote: "between 2009 and 2011, 113 patients with primary NMIBC were enrolled in our prospective study and randomized in two parallel groups: 57 patients in the study group (PDD) and 56 patients in the control group (WLC)." Comment: loss to follow‐up was < 10% |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Filbeck 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: BL‐TURBT Comparator: WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients in whom bladder carcinoma was suspected on preoperative endoscopy were randomized to 2 treatment arms" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients in whom bladder carcinoma was suspected on preoperative endoscopy were randomized to 2 treatment arms" Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: "In the white light arm 103 patients were evaluable and 47 were excluded from further analysis because no tumor was identified (22), a muscle invasive urothelial tumor was diagnosed or cystectomy was indicated (23) or followup was refused after initial resection (2 with a stage pTa grade 1 and stage pT1 grade 2 tumor, respectively). Of the 151 patients randomized to the fluorescence diagnosis arm 88 were evaluable, while 63 were excluded from study due to no positive tumor finding (38), muscle invasive disease and/or an indication for cystectomy (23) and loss to followup (2 with stage pTa grade 2 disease)." Comment: most of excluded patients did not meet initial eligibility criteria; those that did and were excluded were few and were similar between groups |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA. Comment: no other bias identified |
Geavlete 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: mitomycin C (no further information on dosage) |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by means of sealed envelopes containing consecutive numbers" Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized by means of sealed envelopes containing consecutive numbers" Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: "to ensure the complete objectivity of the evaluation, the urologists performing the Re‐TURBTs were unaware of the diagnostic and treatment modality initially applied in each case." Comment: blinding of study personnel reported only at the time point of re‐resection |
| Incomplete outcome data (attrition bias) Oncological outcomes | Unclear risk | Quote: "In the blue‐light group, 78.9% of the patients were diagnosed with NMIBC, 14.3% presented muscle‐invasive bladder tumors, and no tumor was found in 6.7% of the cases. In the white‐light arm, these categories of patients represented 71.3%, 14.8%, and 13.9% of the series, respectively." Comment: 78.9% and 71.3% correspond to 176 and 159 participants in the blue and white light groups, respectively. However, only percentages and not raw numbers are provided for follow‐up outcomes, so it was not possible to discern whether percentages reflect number of initially randomized participants or number of participants remaining at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Geavlete 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: mitomycin C (no further information on dosage) |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | NR | |
| Declarations of interest | 1 author: received honoraria from GE Healthcare when spoke at a company‐sponsored symposia | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by means of sealed envelopes, thus ensuring allocation concealment by the ‘sequentially‐numbered, opaque, sealed envelopes’ method" Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized by means of sealed envelopes, thus ensuring allocation concealment by the ‘sequentially‐numbered, opaque, sealed envelopes’ method" Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: "in each case, the urologist performing the procedure was informed on whether HAL‐BLC would be available only after finishing the WLC to ensure maximum attention to the standard investigation" Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: "to ensure the complete objectivity of the evaluation, the urologists performing the follow‐up of WLCs were unaware of the diagnostic and treatment modality initially applied in each case" Comment: blinding of study personnel reported only at the time point of WLCs |
| Incomplete outcome data (attrition bias) Oncological outcomes | Unclear risk | Quote: "during the follow‐up period, 17 patients in the HAL‐BLC arm and 13 in the WLC arm did not complete the 2 years protocol and were consequently excluded from the trial." Comment: unclear rate (10% to 20%) of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Gkritsios 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: 50 mg epirubicin |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization plan was produced with the use of an electronic randomization plan generator (randomization.com), with the method of randomly permuted blocks" Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization plan was produced with the use of an electronic randomization plan generator (randomization.com), with the method of randomly permuted blocks" Comment: method of sequence generation clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | High risk | Quote: "excluded from follow‐up n=6 and n=13, respectively (see figure 1)" Comment: high rate (≥ 20%) of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Quote: NA Comment: reporting according to the protocol (UMIN000008176) of primary and secondary outcome measures |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Hermann 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + WL‐TURBT followed by BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | Photocure ASA; financial support by the Juchum and the Boemske Foundations | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author; provided full study report in co‐operation with the sponsor |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were randomized just before surgery to one of two treatment groups" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "eligible patients were randomized just before surgery to one of two treatment groups" Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | High risk | Quote: "thirteen patients were excluded from the HAL group and one from the white light group (Fig. 2). Fourteen patients were lost to follow‐up." Comment: high rate (≥ 20%) of participants lost to follow‐up, which was also substantially different between groups |
| Selective reporting (reporting bias) | Low risk | Quote: NA Comment: reporting according to the protocol (NCT00412971) of primary outcome measures (tumor recurrence rates, recurrence‐free survival within 1 year) and secondary outcome measures (additional lesion found by blue light cystoscopy, false‐positive detection rate) |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Karaolides 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WL‐TURBT Adjuvant instillation therapy: 50 mg epirubicin |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients with suspected bladder cancer were randomized" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients with suspected bladder cancer were randomized" Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: excluded from follow‐up n = 1 and n = 1, respectively (see Figure 1) Comment: low rate of participants (< 10%) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Kriegmaier 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: PDD‐TURBT Comparator: WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the randomization procedure was stratified according to participating centers and further by the potentially prognostically relevant risk score, defined according to the results of European Organization for the Research and Treatment of Cancer studies 30831, 30790 and 30782 as 1—recurrence, 2—early recurrence at less than 12 months, 3—bacillus Calmette‐Guerin therapy less than 12 months in duration and 4—a history of carcinoma in situ" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the randomization procedure was stratified according to participating centers and further by the potentially prognostically relevant risk score, defined according to the results of European Organization for the Research and Treatment of Cancer studies 30831, 30790 and 30782 as 1—recurrence, 2—early recurrence at less than 12 months, 3—bacillus Calmette‐Guerin therapy less than 12 months in duration and 4—a history of carcinoma in situ" Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | High risk | Quote: NA Comment: loss to follow‐up was > 20% |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Neuzillet 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + WL‐TURBT followed by BLC + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | NA | |
| Declarations of interest | NA | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "based on a computer‐generated random sequence (1:1), sequentially numbered individual sealed envelopes were prepared by an independent statistician with the group the relevant patient was to be assigned, i.e., a first TURB with WLC and PDD (hexaminolevulinate [HAL]arm) or WLC alone (control arm). Randomization was done the day before the first TURB." Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "based on a computer‐generated random sequence (1:1), sequentially numbered individual sealed envelopes were prepared by an independent statistician with the group the relevant patient was to be assigned, i.e., a first TURB with WLC and PDD (hexaminolevulinate [HAL]arm) or WLC alone (control arm). Randomization was done the day before the first TURB" Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: "excluded from follow‐up n=2 and n=2, respectively (see figure)" Comment: low rate of participants (< 10%) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
O’Brien 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: 40 mg mitomycin C in 40 mL saline |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | NR | |
| Declarations of interest | 2 authors: speaker for GE Healthcare; 1 author: speaker for Photocure; 1 author: speaker for Ipsen | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed in the urology department of Guy’s Hospital by means of sealed envelopes in blocks of 20" Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was performed in the urology department of Guy’s Hospital by means of sealed envelopes in blocks of 20" Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Unclear risk | Quote: "excluded from follow‐up see figure 3" Comment: unclear rate (10% to 20%) of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Quote: NA Comment: reporting according to the protocol (ISRCTN14275387) of primary outcome measure (recurrence rate of bladder tumor at 3 months and 12 months postsurgery) and secondary outcome measures (analysis of histology) |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Riedl 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study Setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: BL‐TURBT Comparator: WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author; no original study data available any more |
|
| Notes | none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each center had an individual randomization code formulated before initiation of the study." Comment: method of sequence generation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "each center had an individual randomization code formulated before initiation of the study". Comment: allocation concealment unclear. |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded. |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded. |
| Incomplete outcome data (attrition bias) Oncological outcomes | Unclear risk | Quote: "Of the 115 patients (56 from 1 center and 59 from the other center) initially randomized for the study 13 were excluded after primary transurethral resection because of muscle invasive bladder cancer on microscopic examination." Comment: unclear rate (10‐20%) of randomized participants were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available. |
| Other bias | Low risk | Quote: NA Comment: no other bias identified. |
Rolevich 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by BL‐TURBT + doxorubicin or BL‐TURBT only Comparator: WLC followed by WL‐TURBT + doxorubicin or WL‐TURBT only Adjuvant instillation therapy: 50 mg doxorubicin |
|
| Outcomes | Primary
Secondary
Results
|
|
| Funding sources | Belarusian Ministry of Health | |
| Declarations of interest | None | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author: 19 January 2021 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by the computer software generating random numbers with equal allocation ratio" Comment: method of sequence generation clearly described |
| Allocation concealment (selection bias) | Low risk | Quote: "The procedure was done in the central randomization office via telephone or local network interface, which allowed concealment of generated random sequence." Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: "The follow‐up was arranged mostly by a local healthcare provider outside the study center, which resulted in blinding to the patients’ treatment arm allocation." Comment: blinding of study personnel reported only at the time point of follow‐up |
| Incomplete outcome data (attrition bias) Oncological outcomes | High risk | Quote: "a total of 525 bladder cancer patients entered the study, of these, 377 patients (72 %) were eligible for efficacy analysis (Fig. 1)" Comment: high rate (≥ 20%) of participants lost to follow‐up |
| Incomplete outcome data (attrition bias) Surgical complications | Low risk | Quote: NA Comment: all randomized participants taken into account in analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Schumacher 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by WL‐TURBT + BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | Medac GmbH, Germany | |
| Declarations of interest | None | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: no reply to date |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the urologist telephoned the randomisation office and stated the person‐number, age, gender, and risk group. These data made block randomisation possible, and the urologist was informed whether FL should be used or not" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Quote: "the urologist telephoned the randomisation office and stated the person‐number, age, gender, and risk group. These data made block randomisation possible, and the urologist was informed whether FL should be used or not." Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: "all pathologists were unaware of whether or not FL was used" Comment: unclear whether all study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: "of the 300 patients, 21 (7%) were excluded from the full‐analysis set. Reasons for exclusion were radical cystectomy based on the results of the primary TUR (4.7%) and no first cystoscopy (2.3%)." Comment: low rate (< 10%) of participants lost to follow‐up |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "adverse events were reported in 22.9% of patients and occurred more often in the FL group (28%) than in the WL group (17.5%)" Comment: all randomized participants taken into account in analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: NA Comment: no protocol available |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Stenzl 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: 1) WLC + mapping ‐> 2nd randomization ‐> 2) BLC + mapping followed by BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)*
Results
|
|
| Funding sources | Photocure ASA, Norway | |
| Declarations of interest | 3 authors: financial interest and/or other relationship with Photocure ASA and others | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author: 1 December 2020, reply by sponsor: 22 December 2020 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was performed centrally, and was stratified for patients presenting with initial and recurrent bladder cancer" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was performed centrally, and was stratified for patients presenting with initial and recurrent bladder cancer" Comment: allocation concealment clearly described |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: NA Comment: surgeon unblinded |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Unclear risk | Quote: NA Comment: unclear whether study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Unclear risk | Quote: NA Comment: unclear whether study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | High risk | Quote: excluded from follow‐up see figure 2 Comment: high rate (≥ 20%) of participants lost to follow‐up |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "a similar level of AEs was experienced by patients in both groups (table 5)" Comment: all participants taken into account in intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Quote: NA Comment: reporting according to the protocol (NCT00233402) of primary outcome measures (detection rate, recurrence‐free survival within 9 months) and secondary outcome measures (false‐positive lesions of blue light, CIS lesion detected only by blue light) |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
Stenzl 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants | Study setting
Eligibility criteria
Non‐eligibility criteria
Intervention cohort
Comparator cohort
|
|
| Interventions | Intervention: WLC + BLC followed by BL‐TURBT Comparator: WLC followed by WL‐TURBT Adjuvant instillation therapy: NA |
|
| Outcomes | Outcome(s)
Results
|
|
| Funding sources | medac Gesellschaft fur klinische Spezialpräparate mbH, Hamburg, Germany | |
| Declarations of interest | NR | |
| Contact of study author | Date of contact attempt to first study author: 30 November 2020 Contact status: reply by author: 15 January 2021 |
|
| Notes | Study protocol was provided by the sponsor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified according to 2 categories of the potentially prognostic and relevant European Organization for Research and Treatment of Cancer risk score describing the probability of tumor recurrence and/or tumor progression" Comment: method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "this randomized, double‐blind, placebo‐controlled study comprising 381 patients was conducted at 8 urology centers in Austria and Germany over a period of 27 months" Comment: method of allocation concealment unclear |
| Blinding of participants and personnel (performance bias) Surgeon | High risk | Quote: "the study was designed as a double‐blind trial. During the whole trial, neither the patient, the physicians involved in performing transurethral resections, nor the sponsor staff were aware of the study arm administered. This included anyone determining subject eligibility, evaluating endpoints, or assessing compliance with the protocol. For this trial, 2 independent teams of investigators were required. The first team was responsible for patient registration as well as for the first and second transurethral resections. The second team was responsible for evaluating the primary study endpoint within the control cystoscopies and did not know whether the original procedure had been carried out under white light or fluorescent light." Comment: surgeon unblinded; blinding of surgeon within follow‐up control cystoscopies |
| Blinding of participants and personnel (performance bias) Participants/study personnel | Low risk | Quote: "the study was designed as a double‐blind trial. During the whole trial, neither the patient, the physicians involved in performing transurethral resections, nor the sponsor staff were aware of the study arm administered. This included anyone determining subject eligibility, evaluating endpoints, or assessing compliance with the protocol. For this trial, 2 independent teams of investigators were required. The first team was responsible for patient registration as well as for the first and second transurethral resections. The second team was responsible for evaluating the primary study endpoint within the control cystoscopies and did not know whether the original procedure had been carried out under white light or fluorescent light." Comment: study participants and other study personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all outcomes) | Low risk | Quote: "the study was designed as a double‐blind trial. During the whole trial, neither the patient, the physicians involved in performing transurethral resections, nor the sponsor staff were aware of the study arm administered. This included anyone determining subject eligibility, evaluating endpoints, or assessing compliance with the protocol. For this trial, 2 independent teams of investigators were required. The first team was responsible for patient registration as well as for the first and second transurethral resections. The second team was responsible for evaluating the primary study endpoint within the control cystoscopies and did not know whether the original procedure had been carried out under white light or fluorescent light." Comment: study personnel were blinded |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | Quote: participants excluded from follow‐up see figure 1. Comment: low rate (< 10%) of participants lost to follow‐up |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "Of the 370 patients who underwent cystoscopy with 5‐ALA or the corresponding placebo (safety population), 123 (33.2%) reported adverse events (white light, 33.9%; fluorescent light, 32.6%) (Table 5)." Comment: all randomized participants taken into account in analysis |
| Selective reporting (reporting bias) | Low risk | Quote: NA Comment: reporting of primary and secondary outcome measures according to protocol |
| Other bias | Low risk | Quote: NA Comment: no other bias identified |
*No definition of primary and secondary endpoints.
5‐ALA: 5‐aminolevulinic acid
BCa: bladder cancer
BCG: Bacillus Calmette‐Guerin
BLC: blue light cystoscopy
BL‐TURBT: blue light transurethral resection of bladder tumor
CI: confidence interval
CIS: carcinoma in situ
CT: computed tomography
HAL: hexaminolevulinic acid
HR: hazard ratio
MIBC: muscle invasive bladder cancer
NA: not available
NMIBC: non‐muscle invasive bladder cancer
NR: not reported
PDD‐TURBT: photodynamic diagnosis‐assisted transurethral resection of bladder tumor
PFS: progression‐free survival
RFS: recurrence‐free survival
TURBT: transurethral resection of bladder tumor
WHO: World Health Organization
WLC: white light cystoscopy
WL‐TURBT: white light transurethral resection of bladder tumor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Daneshmand 2018 | Wrong setting (surveillance) |
| Daniltchenko 2004 | Duplicate |
| Doehn 2014 | Wrong setting (diagnostic test accuracy) |
| Doisy 2019 | Wrong study design (cross‐over trial) |
| Drejer 2020 | Wrong setting (surveillance) |
| DRKS00000142 | Wrong setting (wrong comparator) |
| EUCTR2004‐002259‐15‐AT | Duplicate |
| EUCTR2012‐000559‐15‐FI | Duplicate |
| EUCTR2013‐003898‐98‐IT | Duplicate |
| EUCTR2015‐000436‐15‐DK | Wrong setting (surveillance) |
| Filbeck 2003 | Duplicate |
| Fradet 2007 | Wrong setting (surveillance) |
| Gallagher 2017 | Wrong study design (non‐randomized trial) |
| Geavlete 2009 | Wrong study design (non‐randomized trial) |
| ISRCTN14275387 | Duplicate |
| JPRN‐UMIN000001337 | Wrong study design (non‐randomized trial) |
| JPRN‐UMIN000008176 | Duplicate |
| JPRN‐UMIN000009093 | Wrong study design (non‐randomized trial) |
| JPRN‐UMIN000010798 | Wrong study design (non‐randomized trial) |
| JPRN‐UMIN000031471 | Wrong study design (non‐randomized trial) |
| JPRN‐UMIN000035712 | Wrong study design (non‐randomized trial) |
| Lipiński 2008 | Wrong study design (non‐randomized trial) |
| Lykke 2015 | Wrong study design (non‐randomized trial) |
| Madej 2009 | Wrong study design (non‐randomized trial) |
| NCT00052637 | Wrong study design (non‐randomized trial) |
| NCT00412971 | Duplicate |
| NCT00785694 | Withdrawn study (rejected ethics approval in UK and Netherlands) |
| NCT01166230 | Duplicate |
| Otto 2009 | Duplicate |
| Rolevich 2019 | Wrong study design (post hoc analysis) |
Characteristics of ongoing studies [ordered by study ID]
Boström 2018.
| Study name | Treatment of Ta bladder cancer in high risk of recurrence—fluorescence cystoscopy with optimized adjuvant mitomycin‐C (FinnBladder 9) |
| Methods |
|
| Participants | Estimated enrollment
Study setting
Eligibility criteria
Non‐eligibility criteria
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | August 2012 |
| Contact information | Date of contact attempt to first study author: 28 June 2021 Contact status: reply by author 8 July 2021; study still recruiting; date of anticipated publication 2022 to 2023 |
| Notes | clinicaltrials.gov/ct2/show/NCT01675219 |
Tandogdu 2019.
| Study name | PHOTOdynamic versus white light‐guided treatment of non‐muscle invasive bladder cancer: randomised trial of clinical and cost‐effectiveness |
| Methods |
|
| Participants | Estimated enrollment
Study setting
Eligibility criteria
Non‐eligibility criteria
|
| Interventions | Intervention: blue light TURBT Control: white light TURBT |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | September 2014 |
| Contact information | Date of contact attempt to first study author: 28 July 2021 Contact status: reply by author 28 July 2021; manuscript is currently being prepared |
| Notes | www.isrctn.com/ISRCTN84013636 |
CIS: carcinoma in situ
CT: computerized tomography
MIBC: muscle invasive bladder cancer
NMIBC: non‐muscle invasive bladder cancer
PUNLMP: papillary urothelial neoplasm of low malignant potential
TURBT: transurethral resection of bladder tumor
WHO: World Health Organization
Differences between protocol and review
This review is based on a published protocol (Maisch 2020). In response to comments arising during the external review, we made the following changes.
We revised our original definition of progression to correspond to that of the International Bladder Cancer Group (Lamm 2014).
We reconsidered our risk of bias assessment and now consider all outcomes potentially susceptible to detection bias, as their determination includes judgement on the part of the investigators.
We added a subgroup analysis for the use of 5‐aminolevulinic acid (5‐ALA) versus hexaminolevulinic acid (HAL). These have been clearly labeled as post hoc.
Contributions of authors
Draft the protocol: PM, PD, VN, AK, JV, MHK
Study selection: PM, AK, JV, PD
Data extraction: PM, AK, JV
Enter data into Review Manager 5: PM
Carry out the analysis: PM, PD
Interpret the analysis: PM, PD, VN
Disagreement resolution: PD
Sources of support
Internal sources
-
New Source of support, USA
Philipp Dahm received salary support from the Minneapolis VA Healthcare system
External sources
-
New Source of support, Germany
Philipp Maisch received salary support as a Cochrane Urology Fellow through the Deutsche Forschungs Gemeinschaft (DFG)
Declarations of interest
PM, AK, JV, VN, MHK, and PD report no conflicts of interest.
New
References
References to studies included in this review
Babjuk 2005 {published data only}
- Babjuk M, Soukup V, Petrík R, Jirsa M, Dvorácek J. 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU International 2005;96(6):798-802. [DOI: 10.1111/j.1464-410X.2004.05715] [DOI] [PubMed] [Google Scholar]
- Babjuk M, Soukup V, Petrík R, Pavlík I, Jirsa M, Dvorácek J, et al. Fluorescence cystoscopy in the diagnostics and treatment of superficial urinary bladder tumors [Fluorescencní cystoskopie a její místo v diagnostice a lécbe povrchových nádorů mocového mechýre]. Casopis Lékaru Ceských 2005;11(Suppl 2):15-8. [PubMed] [Google Scholar]
Drăgoescu 2017 {published data only}
- Drăgoescu PO, Tudorache Ş, Drocaş AI, Mitroi G, Pănuş A, Drăgoescu NAM, et al. Improved diagnosis and long-term recurrence rate reduction for non-muscle-invasive bladder cancer patients undergoing fluorescent hexylaminolevulinate photodynamic diagnosis. Romanian Journal of Morphology and Embryology 2017;58(4):1279-83. [PubMed] [Google Scholar]
- Dragoescu O, Tomescu P, Panus A, Enache M, Maria C, Stoica L, et al. Photodynamic diagnosis of non-muscle invasive bladder cancer using hexaminolevulinic acid. Romanian Journal of Morphology and Embryology 2011;52(1):123-7. [PubMed] [Google Scholar]
- Dragoescu PO, Enache M, Tomescu PI, Panus A, Maria C, Dragoescu NA, et al. Use of hexaminolevulinate improves diagnosis sensitivity and treat ment efficiency of nonmuscle invasive bladder cancer. European Urology, Supplements 2011;10(9):633. [Google Scholar]
- Panus A, Dragoescu O, Maria C, Tomescu P. Photodynamic diagnosis and treatment of non-muscle invasive bladder cancer using hexaminolevulinic acid. Urology 2013;82(3):S211. [PubMed] [Google Scholar]
- Tomescu PI, Dragoescu PO, Enache MA, Maria C, Panus A, Dragoescu NA, et al. Value of hexaminolevulinate fluorescent cystoscopy and resection in the management of non-muscle invasive bladder cancer. European Urology, Supplements 2012;11(1):e958-e958a. [Google Scholar]
Filbeck 2002 {published data only}
- Denzinger S, Burger M, Walter B, Knuechel R, Roessler W, Wieland WF, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007;69(4):675-9. [DOI: 10.1016/j.urology.2006.12.023] [DOI] [PubMed] [Google Scholar]
- Denzinger S, Wieland WF, Otto W, Filbeck T, Knuechel R, Burger M. Does photodynamic transurethral resection of bladder tumour improve the outcome of initial T1 high-grade bladder cancer? A long-term follow-up of a randomized study. BJU International 2008;101(5):566-9. [DOI: 10.1111/j.1464-410X.2007.07314.x.] [DOI] [PubMed] [Google Scholar]
- Denzinger S, Wieland WF, Otto W, Filbeck T, Knuechel R, Burger M. Does photodynamic transurethral resection of bladder tumour improve the outcome of initial T1 high-grade bladder cancer? A long-term follow-up of a randomized study. BJU International 2008;101(5):566-9. [DOI: 10.1111/j.1464-410X.2007.07314.x] [DOI] [PubMed] [Google Scholar]
- EUCTR2009-012275-98. Trial to compare the gain of photodynamic diagnostic during transuretral resektion of bladder cancer followed by intravesical chemotherapy against white-light-TURB followed by intravesical standard maintenance chemotherapy. www.clinicaltrialsregister.eu/ctr-search/trial/2009-012275-98/DE (first received 11 August 2010).
- Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Roessler W. Clinically relevant improvement of recurrence-free survival with 5-aminolevulinic acid induced fluorescence diagnosis in patients with superficial bladder tumors. Journal of Urology 2002;168(1):67-71. [PubMed] [Google Scholar]
- Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Roessler W. Clinically relevant reduction of recurrence of superficial bladder tumor with 5-aminolevulinic acid-induced fluorescence diagnosis. Results of a 5 year study [Senkung des Rezidivrisikos oberflächlicher Harnblasenkarzinome mittels 5-Aminolävulinsäure-induzierter Fluoreszenzdiagnostik]. Urologe A 2003;42(10):1366-73. [DOI] [PubMed] [Google Scholar]
- Otto W, Burger M, Fritsche HM, Blana A, Roessler W, Knuechel R, et al. Photodynamic diagnosis for superficial bladder cancer: do all risk-groups profit equally from oncological and economic long-term results? Clinical Medicine Insights. Oncology 2009;3:53-8. [DOI: 10.4137/cmo.s1012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geavlete 2010 {published data only}
- Geavlete B, Jecu M, Multescu R, Georgescu D, Geavlete P. HAL blue light cystoscopy in high-risk non-muscle invasive bladder cancer—re-turbt recurrence rates in a prospective, randomized study. Journal of Endourology 2010;24:A125. [DOI] [PubMed] [Google Scholar]
- Geavlete P, Geavlete B, Jecu M, Multescu R, Georgescu D, Dragutescu M. High-risk non-muscle invasive bladder cancer—“the real deal” for blue light cystoscopy and TURBT? BJU International 2010;106:6. [Google Scholar]
- Geavlete B, Jecu M, Multescu R, Georgescu D, Geavlete P. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer—re-TURBT recurrence rates in a prospective, randomized study. Urology 2010;76(3):664-69. [DOI: 10.1016/j.urology.2010.02.067] [DOI] [PubMed] [Google Scholar]
Geavlete 2012 {published data only}
- Geavlete B, Jecu M, Stanescu F, Moldoveanu C, Multescu R, Georgescu D, et al. Hexaminolevulinate fluorescence versus standard white light cystoscopy—does blue light really make a difference in the long run? European Urology, Supplements 2012;11(4):88-9. [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Jecu M, Stanescu F, Moldoveanu C, et al. Four-year non-muscle invasive bladder cancer recurrence rates—a prospective, randomized comparison between hexaminolevulinate blue light and standard white light cystoscopy. Journal of Endourology 2013;27:A147. [Google Scholar]
- Geavlete B, Multescu R, Jecu J, Georgescu D, Stanescu F, Geavlete P. Treatment changes and long-term recurrence rates after hal fluorescence cystoscopy: does blue light really make a difference in the long run? Urology 2011;78(3):S16-7. [Google Scholar]
- Geavlete B, Multescu R, Jecu J, Georgescu D, Stanescu F, Geavlete P. Treatment changes and long-term recurrence rates after HAL fluorescence cystoscopy—does blue light really make a difference in the long run? European Urology, Supplements 2011;10(2):180. [Google Scholar]
- Geavlete B, Multescu R, Jecu M, Georgescu D, Stanescu F, Geavlete P. Treatment changes and long-term recurrence rates after HAL fluorescence cystoscopy—does blue light really make a difference in the long run? Journal of Urology 2011;185(4):e661-2. [Google Scholar]
- Geavlete B, Multescu R, Jecu M, Georgescu D, Stanescu F, Moldoveanu C, et al. Treatment changes and long-term recurrence rates after hal fluorescence cystoscopy: a prospective, randomized, long term comparison to the standard approach. Journal of Endourology 2011;25:A288-9. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Moldoveanu C, et al. A prospective, randomized comparison between the hexaminolevulinate blue light and the standard white light cystoscopy concerning the long term recurrence rates in nonmuscle invasive bladder cancer cases. European Urology, Supplements 2012;11(1):e955-e955a. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Georgescu D, Jecu M, Moldoveanu C, et al. A prospective, randomized comparison between the hexaminolevulinate blue light and the standard white light cystoscopy concerning the long term recurrence rates in non-muscle invasive bladder cancer cases. Journal of Urology 2012;187(4):e511-2. [Google Scholar]
- Geavlete B, Multescu R, Stanescu F, Moldoveanu C, Jecu M, Georgescu D, et al. Hexaminolevulinate fluorescence versus standard white light cystoscopy: does blue light really make a difference in the long run? Journal of Endourology 2012;26:A92. [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Jecu M, Stanescu F, Geavlete P. Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU International 2012;109(4):549-56. [DOI: 10.1111/j.1464-410X.2011.10374.x.] [DOI] [PubMed] [Google Scholar]
- Geavlete B, Multescu R, Georgescu D, Jecu M, Stanescu F, Moldoveanu C, et al. Four-year non-muscle invasive bladder cancer recurrence rates—a prospective, randomized comparison between hexaminolevulinate blue light and standard white light cystoscopy. Journal of Urology 2013;189(4):e523. [DOI: 10.1016/j.juro.2013.02.2633] [DOI] [Google Scholar]
Gkritsios 2014 {published data only}
- Gkritsios P, Hatzimouratidis K, Kazantzidis S, Dimitriadis G, Ioannidis E, Katsikas V. Hexaminolevulinate-guided transurethral resection of non-muscle-invasive bladder cancer does not reduce the recurrence rates after a 2-year follow-up: a prospective randomized trial. International Urology and Nephrology 2014;46(5):927-33. [DOI: 10.1007/s11255-013-0603-z] [DOI] [PubMed] [Google Scholar]
- UMIN000008176. Placebo controlled, randomized trial, comparing the efficacy of white light cystoscopy with the fluorescence cystoscopy with the use of hexaminolevulinate, regarding the diagnosis and treatment of bladder cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000002279 (first received 15 June 2012).
Hermann 2011 {published data only}
- Hermann GG, Mogensen K, Carlsson S, Marcussen N, Duun S. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients—a randomized two-centre study. BJU International 2011;108(8 Pt 2):E297-303. [DOI: 10.1111/j.1464-410X.2011.10090.x.] [DOI] [PubMed] [Google Scholar]
- NCT00412971. A randomized, comparative study of Hexvix fluorescence cystoscopy and standard cystoscopy in patients with non-invasive bladder cancer. clinicaltrials.gov/ct2/show/NCT00412971 (first received 19 December 2006).
Karaolides 2012 {published data only}
- Karaolides T, Skolarikos A, Bourdoumis A, Konandreas A, Mygdalis V, Thanos A, et al. Hexaminolevulinate-induced fluorescence versus white light during transurethral resection of noninvasive bladder tumor: does it reduce recurrences? Urology 2012;80(2):354-9. [DOI: 10.1016/j.urology.2012.03.067] [DOI] [PubMed] [Google Scholar]
- Skolarikos A, Karaoloides T, Bourdoumis A, Logothetis A, Mourmouris P, Konandreas A, et al. Hexaminolevulinate induced fluorescence versus white light during transurethral resection of non-invasive bladder tumor. Does it reduce recurrences? Journal of Endourology 2012;25:A88. [DOI] [PubMed] [Google Scholar]
Kriegmaier 2002 {published data only}
- Kriegmair M, Zaak D, Rothenberger KH, Rassweiler J, Jocham D, Eisenberger F, et al. Transurethral resection for bladder cancer using 5-aminolevulinic acid induced fluorescence endoscopy versus white light endoscopy. Journal of Urology 2002;168(2):475. [PubMed] [Google Scholar]
Neuzillet 2014 {published data only}
- Neuzillet Y, Methorst C, Schneider M, Lebret T, Rouanne M, Radulescu C, et al. Assessment of diagnostic gain with hexaminolevulinate (HAL) in the setting of newly diagnosed non-muscle-invasive bladder cancer with positive results on urine cytology. Urologic Oncology 2014;32(8):1135-40. [DOI: 10.1016/j.urolonc.2014.04.005] [DOI] [PubMed] [Google Scholar]
O’Brien 2013 {published data only}
- EUCTR2004-002760-16. A randomised study of “bluelight” hexyl amino levulinic acid (HAL) photodynamic assisted resection of bladder tumours versus conventional “white light” transurethral resection of newly diagnosed bladder tumours comparing rates of tumour recurrence over on. www.clinicaltrialsregister.eu/ctr-search/trial/2004-002760-16/results (first received 10 June 2011).
- Gan C, Chatterton K, Amery S, Ray E, Khan MS, Thomas K, et al. Long term follow up of a prospective randomised trial of Hexylaminolevulinate (HEXVIX®) photodynamic diagnosis (PDD) assisted versus conventional white-light transurethral resection (TURBT) in newly presenting non-muscle invasive bladder cancer (NMIBC). BJU International 2014;113:8-9. [DOI] [PubMed] [Google Scholar]
- Gan C, Chatterton K, Amery S, Ray E, Khan MS, Thomas K, et al. Long term follow up of a prospective randomised trial of hexylaminolevulinate (Hexvix®) photodynamic diagnosis (PDD) assisted versus conventional white-light transurethral resection (TURBT) in newly presenting non-muscle invasive bladder cancer (NMIBC). Journal of Urology 2014;191(4):e237. [Google Scholar]
- Gan C, Chatterton K, Amery S, Ray E, Khan MS, Thomas K, et al. Long term follow up of a prospective randomised trial of Hexylaminolevulinate (HEXVIX ®) photodynamic diagnosis (PDD) assisted versus conventional white-light transurethral resection (TURBT) in newly presenting non-muscle invasive bladder cancer (NMIBC). Journal of Urology 2014;191(4S):e237. [Google Scholar]
- ISRCTN14275387. A randomised study of 'bluelight' hexyl aminolevulinic acid (HAL) photodynamic assisted resection of bladder tumours versus conventional 'whitelight' transurethral resection of newly diagnosed bladder tumours comparing rates of tumour recurrence over one. www.isrctn.com/ISRCTN14275387 (first received 25 July 2005).
- O'Brien T, Chatterton K, Ray E, Chandra A, Khan S, Thomas K. A prospective randomised trial of hexylaminolevulinate (HAL) assisted transurethral resection (TURBT) plus single shot intravesical mitomycinc (MMC) versus white light TURBT plus single shot MMC in newly presenting bladder cancer. Journal of Urology 2011;185(4):e661. [DOI] [PubMed] [Google Scholar]
- O'Brien T, Ray E, Chatterton K, Khan MS, Chandra A, Thomas K. Prospective randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs conventional white-light TURBT plus mitomycin C in newly presenting non-muscle-invasive bladder cancer. BJU International 2013;112(8):1096-104. [DOI: 10.1111/bju.12355] [DOI] [PubMed] [Google Scholar]
- O'Brien TS, Chatterton K, Ray ER, Wilby D, Chandra A, Dickinson F, et al. A prospective randomised trial of Hexylaminolevulinate (Hexvix) assisted transurethral resection (TURBT) plus single shot intravesical mitomycin (MMC) versus conventional white light TURBT plus single shot MMC in newly presenting bladder cancer. European Urology, Supplements 2011;10(2):150. [Google Scholar]
- Ray E, Chatterton K, Wilby D, Khan MS, Thomas K, O'Brien TS. A prospective randomised trial of Hexylaminolevulinate (HAL) assisted transurethral resection (TURBT) plus single shot intravesical MitomycinC (MMC) versus white light TURBT plus single shot MMC in newly presenting bladder cancer. BJU International 2011;108:8. [DOI] [PubMed] [Google Scholar]
Riedl 2001 {published data only}
- Danilchenko DI, Koenig F, Riedl K, Schnorr D, Waldman A, Al-Shukri S, et al. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU International 2011;108(8 Pt 2):E297-303. [DOI] [PubMed] [Google Scholar]
- Danilchenko DI, Koenig F, Riedl K, Schnorr D, Waldman A, Al-Shukri S, et al. Clinical significance of macroscopic 5-aminolevulinic acid (ALA)-inducer fluorescence in transurethral resection of non-invasive bladder cancer [Klinicheskoe znachenie vizual'noĭ fliuorestsentsii indutsirovannoĭ 5-aminolevulinovoĭ kislotoĭ (ALA) pri transuretral'noĭ rezektsii neinvasivnogo raka mochevogo puzyria]. Voprosy Onkologii 2003;49(6):734-7. [PubMed] [Google Scholar]
- Daniltchenko D, Riedl C, Koenig F, Sachs M, Daha K, Schnorr D, et al. Reducing the risk of cancer recurrence and progression in patients with superficial bladder tumour with 5-aminolevulinic acid-induced fluore scence diagnosis. Results of a prospective randomized 5-year study. European Urology, Supplements 2005;4(3):221. [DOI: 10.1016/S1569-9056(05)80879-6] [DOI] [Google Scholar]
- Daniltchenko D, Riedl C, Koenig F, Daha LK, Sachs M, Schnorr D. The impact of ALA (5-aminolevulinic acid)-fluorescence detection on the prognosis of superficial bladder cancer. A 1-year survival analysis. Aktuelle Urologie 2004;35(6):497-501. [DOI] [PubMed] [Google Scholar]
- Riedl CR, Daniltchenko D, Koenig F, Simak R, Loening SA, Pflueger H. Fluorescence endoscopy with 5-aminolevulinic acid reduces early recurrence rate in superficial bladder cancer. Journal of Urology 2001;165(4):1121-3. [PubMed] [Google Scholar]
- Sachs MD, Daniltchenko DI, Riedl CR, Koenig F, Daha K, Schnorr D, et al. Fluorescence detection with 5-aminolevulinic acid (ALA) reduces the risk of tumor recurrence and progression in patients with superficial bladder cancer: 5 year results of a prospective randomized trial. Journal of Urology 2005;173(4):246. [Google Scholar]
- Sachs MD, Daniltchenko DI, Riedl CR, Koenig F, Daha K, Schnorr D, et al. Fluorescence detection with 5-aminolevulinic acid (ALA) reduces the risk of tumor recurrence and progression in patients with superficial bladder cancer—5 year results of a prospective randomized trial. Journal of Urology 2005;173(4 Suppl):246. [Google Scholar]
Rolevich 2017 {published data only}
- Rolevich A, Minich A, Zhegalik A, Mokhort A, Vasilevich V, Nabebina T, et al. Long-term benefit of fluorescent cystoscopy-assisted transurethral resection in patients with non-muscle invasive bladder cancer is dependent on quality of surgery. European Urology, Supplements 2017;16(5):e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolevich A, Minich A, Vasilevich V, Zhegalik A, Mokhort A, Nabebina T, et al. Efficacy of fluorescent cystoscopy-assisted transurethral resection in patients with non-muscle invasive bladder cancer and quality of surgery: post-hoc analysis of а prospective randomized study. Central European Journal of Urology 2019;72(4):351-6. [DOI: 10.5173/ceju.2019.0003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolevich AI, Sukonko OG, Krasny SA, Polyakov SL, Nabebina TI, Minich AA. Recurrence prevention by combination of photodynamic diagnosis-assisted transurethral resection of the bladder and single postoperative intravesical chemotherapy instillation in patients with nonmuscle-invasive bladder cancer. European Urology, Supplements 2014;13(1):e1003. [Google Scholar]
- Rolevich AI, Zhegalik AG, Minich AA, Nabebina TI, Polyakov SA, Krasny SA, et al. Transurethral resection guided by photodynamic diagnosis can prevent progression in nonmuscle invasive bladder cancer patients. European Urology, Supplements 2016;15(3):e208. [Google Scholar]
- Rolevich AI, Zhegalik AG, Mokhort AA, Minich AA, Vasilevich VY, Polyakov SL, et al. Results of a prospective randomized study assessing the efficacy of fluorescent cystoscopy-assisted transurethral resection and single instillation of doxorubicin in patients with non-muscle-invasive bladder cancer. World Journal of Urology 2017;35(5):745-52. [DOI: 10.1007/s00345-016-1927-y] [DOI] [PubMed] [Google Scholar]
Schumacher 2010 {published data only}
- Schumacher MC, Holmäng S, Davidsson T, Friedrich B, Pedersen J, Wiklund NP. Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-ALA under visible and fluorescent light-multicenter phase III clinical trial. Journal of Urology 2009;181(4):688. [DOI] [PubMed] [Google Scholar]
- Schumacher MC, Holmäng S, Davidsson T, Friedrich B, Pedersen J, Wiklund NP. Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-aminolevulinic acid under visible and fluorescent light: results of a prospective, randomised, multicentre study. European Urology 2010;57(2):293-9. [DOI: 10.1016/j.eururo.2009.10.030] [DOI] [PubMed] [Google Scholar]
Stenzl 2010 {published data only}
- Burger M, Stenzl A, Mynderse L, Kriegmair M, Witjes JA, Soloway M, et al. Long-term reduction in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. European Urology, Supplements 2012;11(1):e957-e957a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRKS00004103. A randomized, comparative, controlled phase III, multicenter study of Hexvix fluorescence cystoscopy and white light cystoscopy in the detection of papillary bladder cancer and the early recurrence rate in patients with bladder cancer. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00004103 (first received 4 October 2005).
- EUCTR2004-002259-15. A randomized, comparative, controlled phase iii, multicenter study of hexvix fluorescence cystoscopy and white light cystoscopy in the detection of papillary bladder cancer and the early recurrence rate in patients with bladder cancer. www.clinicaltrialsregister.eu/ctr-search/search?query=2004-002259-15 (first received 20 December 2004).
- Grossman HB, Mynderse L, Stenzl A, Burger M, Fradet Y, Soloway M, et al. Hexaminolevulinate new data: results from the recurrence study. Urology 2009;74(4):S20-1. [Google Scholar]
- Grossman HB, Stenzl A, Fradet Y, Mynderse LA, Kriegmair M, Witjes JA, et al. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. Journal of Urology 2012;188(1):58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Stenzl A, Mynderse L, Kriegmaier M, Witjes JA, Soloway M, et al. Long-term clinical benefit of hexaminolevulinate enabled fluorescence cystoscopy. Journal of Urology 2012;187(4):e673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Stenzl A, Fradet Y, Mynderse LA, Kriegmair M, Witjes JA, et al. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. Journal of Urology 2012;188(1):58-62. [DOI: 10.1016/j.juro.2012.03.00] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat AM, Cookson M, Witjes JA, Stenzl A, Grossman HB. The impact of blue light cystoscopy with hexaminolevulinate (HAL) on progression of bladder cancer—a new analysis. Bladder Cancer 2016;27(2):273-8. [DOI: 10.3233/BLC-160048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynderse L, Stenzl A, Denzinger S, Fradet Y, Soloway MS, Karl A, et al. Hexaminolevulinate fluorescence cystoscopy improves detection and resection of papillary bladder cancer lesions and reduces early recurrences. Journal of Urology 2009;181(4):689. [Google Scholar]
- NCT00233402. Study to improve detection and early recurrence rate in bladder cancer patients using Hexvix fluorescence cystoscopy. clinicaltrials.gov/ct2/show/NCT00233402 (first received 5 October 2005).
- Stenzl AS, Roessler WR, Fradet YF, Mynderse LM, Soloway MS, Kriegmair MK, et al. Hexvix® fluorescence cystoscopy improves detection and resection of papillary bladder cancer and reduces early recurrence: a multicentre, prospective, randomized study. European Urology, Supplements 2009;8(4):373. [Google Scholar]
- Stenzl A, Burger M, Fradet Y, Mynderse LA, Soloway MS, Witjes JA, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. Journal of Urology 2010;184(5):1907-13. [DOI: 10.1016/j.juro.2010.06.148] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzl A. Photodynamic diagnosis (PDD) and photodynamic treatment (PRT) in bladder cancer. International Journal of Urology 2010;17:A106. [Google Scholar]
Stenzl 2011 {published data only}
- Stenzl A, Penkoff H, Dajc-Sommerer E, Zumbraegel A, Hoeltl L, Scholz M, et al. Detection and clinical outcome of urinary bladder cancer with 5-aminolevulinic acid-induced fluorescence cystoscopy: a multicenter randomized, double-blind, placebo-controlled trial. Cancer 2011;117(5):938-47. [DOI: 10.1002/cncr.25523] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Daneshmand 2018 {published data only}
- Daneshmand S, Patel S, Lotan Y, Pohar K, Trabulsi E, Woods M, et al. Blue light flexible cystoscopy (BLFC) with hexaminolevulinate (HAL) and white light flexible cystoscopy: a prospective, comparative, within-patient controlled multicenter phase 3 study in the detection of bladder cancer during surveillance. Journal of Urology 2017;197(4):e608. [Google Scholar]
- Daneshmand S, Patel S, Lotan Y, Pohar K, Trabulsi E, Woods M, et al. Efficacy and safety of blue light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: a phase III, comparative, multicenter study. Journal of Urology 2018;199(5):1158-65. [DOI: 10.1016/j.juro.2017.11.096] [DOI] [PubMed] [Google Scholar]
- NCT02560584. A Study of Blue Light Flexible Cystoscopy With Cysview in the Detection of Bladder Cancer in the Surveillance Setting. clinicaltrials.gov/show/NCT02560584 March 17, 2021.
- Smith AB, Daneshmand S, Patel S, Pohar K, Trabulsi E, Woods M, et al. Flexible blue light study group collaborators. Patient-reported outcomes of blue-light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: results from a prospective multicentre study. BJU International 2019;123(1):35-41. [DOI: 10.1111/bju.14481] [DOI] [PubMed] [Google Scholar]
Daniltchenko 2004 {published data only}
- Daniltchenko D, Koenig F, Riedl C, Valdman A, Al-Shukri S, Loening SA. Clinical significance of macroscopic 5-aminolevulinic acid (ALA)-inducer florescence at transurethral resections of superficial bladder cancer. Voprosy Onkologii 2003;49(6):734-7. [PubMed] [Google Scholar]
- Daniltchenko D, Riedl C, Koenig F, Daha LK, Sachs M, Schnorr D. The impact of ALA (5-aminolevulinic acid)-fluorescence detection on the prognosis of superficial bladder cancer [Der Stellenwert der ALA-(5-Amino-Lävulinsäure)-Fluoreszenzdetektion für die Prognose des oberflächlichen Blasenkarzinoms. Eine 1-Jahres-Uberlebensanalyse]. Aktuelle Urologie 2004;35(6):497-501. [DOI: 10.1055/s-2004-818544. PMID: 15526230] [DOI] [PubMed] [Google Scholar]
- Daniltchenko DI, Riedl CR, Sachs MD, Koenig F, Daha KL, Pflueger H, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. Journal of Urology 2005;174(6):2129-33. [DOI: 10.1097/01.ju.0000181814] [DOI] [PubMed] [Google Scholar]
Doehn 2014 {published data only}
- Doehn C, Krockenberger K, Zumbe J, Rassler J, Sommerauer M, Feller AC, et al. Comparison of white-light, photodynamic diagnosis and narrow-band imaging for the detection of non-muscle invasive bladder cancer: results from a randomized multicenter diagnostic phase-III study. European Urology, Supplements 2014;13(1):e23. [Google Scholar]
Doisy 2019 {published data only}
- Doisy L, Walz J, Fakhfakh S, Rybikowski S, Koskas Y, Gravis G, et al. Is a routine second transurethral resection of the bladder still necessary after hexaminolévulinate photodynamic diagnosis-assisted TURBT? [La résection transurétrale de vessie de réévaluation est-elle toujours nécessaire en cas de primo-résection sous luminofluorescence par hexaminolévulinate?]. Progrès en Urologie 2019;29(6):332-9. [DOI: 10.1016/j.purol.2019.04.001] [DOI] [PubMed] [Google Scholar]
Drejer 2020 {published data only}
- Drejer D, Moltke AL, Munk Nielsen A, Lam GW, Bjerggaard Jensen J. DaBlaCa-11: Photodynamic diagnosis in flexible cystoscopy—initial findings in a randomized controlled trial. European Urology, Supplements 2018;17(2):e1608. [Google Scholar]
- Drejer D, Béji S, Oezeke R, Nielsen AM, Høyer S, Bjerklund Johansen TE, et al. Comparison of white light, photodynamic diagnosis, and narrow-band Imaging in detection of carcinoma in situ or flat dysplasia at transurethral resection of the bladder: the DaBlaCa-8 study. Urology 2017;102:138-42. [DOI: 10.1016/j.urology.2016.11.032] [DOI] [PubMed] [Google Scholar]
- Drejer D, Moltke AL, Nielsen AM, Lam GW, Jensen JB. DaBlaCa-11: Photodynamic diagnosis in flexible cystoscopy—a randomized study with focus on recurrence. Urology 2020;137:91-6. [DOI: 10.1016/j.urology.2019.12.002] [DOI] [PubMed] [Google Scholar]
DRKS00000142 {published data only}
- DRKS00000142. Prospective, open, tricenter phase-III diagnostic trial to evaluate cystoscopic diagnosis of non-muscle invasive bladder cancer through narrow band imaging (NBI) in comparison to white light and photodynamic diagnosis (PDD). www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000142 March 17, 2021.
EUCTR2004‐002259‐15‐AT {published data only}
- EUCTR2004-002259-15-AT. A randomized, comparative, controlled phase III, multicenter study of hexvix fluorescence cystoscopy and white light cystoscopy in the detection of papillary bladder cancer and the early recurrence rate in patients with bladder cancer. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2004-002259-15-AT March 17, 2021.
EUCTR2012‐000559‐15‐FI {published data only}
- EUCTR2012-000559-15-FI. Treatment of non-invasive bladder cancer with high risk of recurrence—blue light cystoscopy with chemotherapy. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-000559-15-FI March 17, 2021.
EUCTR2013‐003898‐98‐IT {published data only}
- EUCTR2013-003898-98-IT. Comparison of white light transurethral resection (TUR) vs photodynamic diagnosis (PDD)-guided TUR as assessed by second look TUR for the treatment of high risk non muscle invasive bladder cancer (NMIBC). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-003898-98-IT March 17, 2021.
EUCTR2015‐000436‐15‐DK {published data only}
- EUCTR2015-000436-15-DK. Photodynamic diagnosis (PDD) in flexible cystoscopy—a randomized study with focus on significant recurrence [Photodynamisk Diagnostik (PDD) ved flexible cystoskopier—et randomiseret studie med fokus på recidiv]. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-000436-15-DK March 17, 2021.
Filbeck 2003 {published data only}
- Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Rössler W. Reducing the risk of superficial bladder cancer recurrence with 5-aminolevulinic acid-induced fluorescence diagnosis. Results of a 5-year study [Senkung des Rezidivrisikos oberflächlicher Harnblasenkarzinome mittels 5-Aminolävulinsäure-induzierter Fluoreszenzdiagnostik. Resultate einer 5-Jahres-Studie]. Urologe A 2003;42(10):1366-73. [DOI: 10.1007/s00120-003-0355-y] [DOI] [PubMed] [Google Scholar]
Fradet 2007 {published data only}
- Fradet Y, Grossman HB, Gomella L, Lerner S, Cookson M, Albala D, et al. PC B302/01 Study Group. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. Journal of Urology 2007;178(1):68-73. [DOI: 10.1016/j.juro.2007.03.028] [DOI] [PubMed] [Google Scholar]
Gallagher 2017 {published data only}
- Gallagher KM, Gray K, Anderson CH, Lee H, Stewart S, Donat R, et al. 'Real-life experience': recurrence rate at 3 years with Hexvix® photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC-a prospective controlled study. World Journal of Urology 2017;35(12):1871-7. [DOI: 10.1007/s00345-017-2077-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geavlete 2009 {published data only}
- Geavlete B, Multescu R, Georgescu D, Geavlete P. Hexaminolevulinate fluorescence cystoscopy and transurethral resection of the bladder in noninvasive bladder tumors. Journal of Endourology 2009;23(6):977-81. [DOI: 10.1089/end.2008.0574] [DOI] [PubMed] [Google Scholar]
ISRCTN14275387 {published data only}
- ISRCTN14275387. A randomised study of 'bluelight' hexyl aminolevulinic acid (HAL) photodynamic assisted resection of bladder tumours versus conventional 'whitelight' transurethral resection of newly diagnosed bladder tumours comparing rates of tumour recurrence over one. www.isrctn.com/ISRCTN14275387 March 17, 2021.
JPRN‐UMIN000001337 {published data only}
- JPRN-UMIN000001337. Photodynamic diagnosis of bladder cancer using fluorescence cystoscopy with 5-aminolevulinic acid (5-ALA). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001625 March 17, 2021.
JPRN‐UMIN000008176 {published data only}
- JPRN-UMIN000008176. Placebo controlled, randomized trial, comparing the efficacy of white light cystoscopy with the fluorescence cystoscopy with the use of hexaminolevulinate, regarding the diagnosis and treatment of bladder cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000002279 March 17, 2021.
JPRN‐UMIN000009093 {published data only}
- JPRN-UMIN000009093. The impact of PDD for urological cancer. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000009093 March 17, 2021.
JPRN‐UMIN000010798 {published data only}
- JPRN-UMIN000010798. Photodynamic diagnosis of non muscle invasive bladder cancer using fluorescence cystoscopy with oral administration of 5-aminolevulinic acid (5-ALA). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000012641 March 17, 2021.
JPRN‐UMIN000031471 {published data only}
- JPRN-UMIN000031471. Effectiveness of ALA-induced photodynamic diagnosis of bladder cancer on TURBT. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000035923 March 17, 2021.
JPRN‐UMIN000035712 {published data only}
- JPRN-UMIN000035712. Bladder cancer prospective cohort study on high-risk non-muscle invasive cancer after photodynamic diagnosis-assisted transurethral resection of the bladder tumor (BRIGHT study). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000040650 March 17, 2021.
Lipiński 2008 {published data only}
- Lipiński M, Markowski M, Rózański W. The value of photodynamic diagnosis (PDD) in identifying carcinoma in situ (Cis) of urinary bladder. Clinical and Experimental Medical Letters 2008;49(1):55-7. [Google Scholar]
Lykke 2015 {published data only}
- Lykke MR, Nielsen TK, Ebbensgaard NA, Zieger K. Reducing recurrence in non-muscle-invasive bladder cancer using photodynamic diagnosis and immediate post-transurethral resection of the bladder chemoprophylaxis. Scandinavian Journal of Urology 2015;49(3):230-6. [DOI: 10.3109/21681805.2015.1019562] [DOI] [PubMed] [Google Scholar]
Madej 2009 {published data only}
- Madej A, Markowski M, Rózański W, Lipiński M. The comparison of recurrence rate of non-muscle invasive bladder cancer treated with fluorescence-guided and conventional transurethral resection. Onkologia Polska 2009;12(2):47-50. [Google Scholar]
NCT00052637 {published data only}
- Cystoscopy and hexyl 5-aminolevulinate in detecting carcinoma in situ in patients with bladder cancer. www.clinicaltrials.gov March 17, 2021.
- NCT00052637. Cystoscopy and hexyl 5-aminolevulinate in detecting carcinoma in situ in patients with bladder cancer. clinicaltrials.gov/ct2/show/NCT00052637 March 17, 2021.
NCT00412971 {published data only}
- NCT00412971. A randomized, comparative study of hexvix fluorescence cystoscopy and standard cystoscopy in patients with non-invasive bladder cancer. clinicaltrials.gov/show/NCT00412971 March 17, 2021.
NCT00785694 {published data only}
- NCT00785694. Recurrence of bladder cancer after transurethral resection with hexvix. clinicaltrials.gov/ct2/show/NCT00785694 March 17, 2021.
NCT01166230 {published data only}
- NCT01166230. Longer-term recurrence rates in patients with bladder cancer after hexvix (Cysview) fluorescence cystoscopy/TURB. clinicaltrials.gov/show/NCT01166230 March 17, 2021.
Otto 2009 {published data only}
- Otto W, Burger M, Fritsche HM, Blana A, Roessler W, Knuechel R, et al. Photodynamic diagnosis for superficial bladder cancer: do all risk-groups profit equally from oncological and economic long-term results? Clinical Medicine Insights: Oncology 2009;3:53-8. [DOI: 10.4137/cmo.s1012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolevich 2019 {published data only}
- Rolevich A, Minich A, Vasilevich V, Zhegalik A, Mokhort A, Nabebina T, et al. Efficacy of fluorescent cystoscopy-assisted transurethral resection in patients with non-muscle invasive bladder cancer and quality of surgery: post-hoc analysis of а prospective randomized study. Central European Journal of Urology 2019;72(4):351-6. [DOI: 10.5173/ceju.2019.0003] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Boström 2018 {published data only}
- EUCTR2006-000296-15. Treatment of non-invasive bladder cancer with high risk of recurrence—blue light cystoscopy with chemotherapy. www.clinicaltrialsregister.eu/ctr-search/trial/2006-000296-15/HU March 17, 2021.
- NCT01675219. Fluorescence cystoscopy and optimized MMC in recurrent bladder cancer (FinnBladder 9). clinicaltrials.gov/ct2/show/NCT01675219 March 17, 2021.
Tandogdu 2019 {published data only}
- ISRCTN84013636. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer. http://www.isrctn.com/ISRCTN84013636.
- Tandogdu Z, Lewis R, Duncan A, Penegar S, McDonald A, Vale L et al. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer: a study protocol for a randomised trial of clinical and cost-effectiveness. BMJ Open 2019;9(9):e022268. [DOI: 10.1136/bmjopen-2018-022268] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AUA 2016
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO joint guideline (2016). Available from www.auanet.org/guidelines/bladder-cancer-non-muscle-invasive-guideline (accessed prior to 20 October 2020). [DOI] [PubMed]
Bogdan 2021
- Bogdan G. Editorial comment on: 'Primary complete transurethral resection of bladder tumor using photodynamic diagnosis for high-risk nonmuscle invasive bladder cancer: is a restaging photodynamic transurethral resection really necessary?' by Tadrist et al. Journal of Endourology 2021;35(7):1047-8. [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394‐424. [DOI: 10.3322/caac.21492] [DOI] [PubMed] [Google Scholar]
Burger 2013
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. European Urology 2013;63(2):234-41. [DOI: 10.1016/j.eururo.2012.07.033] [DOI] [PubMed] [Google Scholar]
Chou 2017
- Chou R, Selph S, Buckley DI, Fu R, Griffin JC, Grusing S, et al. Comparative effectiveness of fluorescent versus white light cystoscopy for initial diagnosis or surveillance of bladder cancer on clinical outcomes: systematic review and meta-analysis. Journal of Urology 2017;197(3 Pt 1):548-58. [DOI: 10.1016/j.juro.2016.10.061] [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of Surgery 2009;250(2):187-96. [DOI: 10.1097/SLA.0b013e3181b13ca2] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 5 July 2021. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Doisy 2019
- Doisy L, Walz J, Fakhfakh S, Rybikowski S, Koskas Y, Gravis G, Pignot G. [Is a routine second transurethral resection of the bladder still necessary after hexaminolevulinate photodynamic diagnosis-assisted TURBT?]. Prog Urol 2019;29(6):332-9. [DOI] [PubMed] [Google Scholar]
EAU 2021
- Babjuk M, Burger M, Compérat E, Gontero P, Liedberg F, Masson-Lecomte A, et al. EAU Guideline on non-muscle-invasive bladder cancer. uroweb.org/guideline/non-muscle-invasive-bladder-cancer/ (accessed prior to 8 October 2021).
EndNote 2019 [Computer program]
- EndNote. Version X9. Thomson Reuters, 2019. Available from www.endnote.com.
FDA 2010
- FDA. Cysview (hexaminolevulinate hydrochloride), for intravesical solution for bladder instillation only. www.accessdata.fda.gov/drugsatfda_docs/label/2010/022555s000lbl.pdf (accessed prior to 8 October 2021).
Gakis 2015
- Gakis G, Ngamsri T, Rausch S, Mischinger J, Todenhofer T, Schwentner C, et al. Fluorescence-guided bladder tumour resection: impact on survival after radical cystectomy. World Journal of Urology 2015;33(10):1429-37. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 5 July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available from gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Han 2021
- Han MA, Maisch P, Jung JH, Hwang JE, Narayan V, Cleves A, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD009294. [DOI: 10.1002/14651858.CD009294.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2019b
- Higgins JPT, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2019c
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 2016
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: prostate and bladder tumours. European Urology 2016;70(1):106-19. [DOI: 10.1016/j.eururo.2016.02.028] [DOI] [PubMed] [Google Scholar]
Hwang 2019
- Hwang EC, Sathianathen NJ, Jung JH, Kim MH, Dahm P, Risk MC. Single‐dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013160. [DOI: 10.1002/14651858.CD013160.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 2017
- Inoue K. 5-aminolevulinic acid-mediated photodynamic therapy for bladder cancer. International Journal of Urology 2017;24(2):97-101. [DOI: 10.1111/iju.13291] [DOI] [PubMed] [Google Scholar]
Jocham 2008
- Jocham D, Stepp H, Waidelich R. Photodynamic diagnosis in urology: state-of-the-art. European Urology 2008;53(6):1138-48. [DOI: 10.1016/j.eururo.2007.11.048] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD009294. [DOI: 10.1002/14651858.CD009294.pub2] [DOI] [PubMed] [Google Scholar]
Jung 2017
- Jung JH, Gudeloglu A, Kiziloz H, Kuntz GM, Miller A, Konety BR, et al. Intravesical electromotive drug administration for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD011864. [DOI: 10.1002/14651858.CD011864.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klaassen 2017
- Klaassen Z, Li K, Kassouf W, Black PC, Dragomir A, Kulkarni GS. Contemporary cost-consequence analysis of blue light cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. Canadian Urological Association Journal 2017;11(6):173-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krieg 2002
- Krieg RC, Messmann H, Rauch J, Seeger S, Knuechel R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochemistry and Photobiology 2002;76(5):518-25. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lai 2021
- Lai LY, Tafuri SM, Ginier EC, Herrel LA, Dahm P, Maisch P, et al. Narrow band imaging versus white light cystoscopy alone for transurethral resection of non‐muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014887. [DOI: 10.1002/14651858.CD014887] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamm 2014
- Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. Journal of Urology 2014;191(1):20-7. [DOI: 10.1016/j.juro.2013.07.102] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maisch 2020
- Maisch P, Koziarz A, Vajgrt J, Narayan V, Kim MH, Dahm P. Blue versus white light for transurethral resection of non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013776. [DOI: 10.1002/14651858.CD013776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mowatt 2011
- Mowatt G, N'Dow J, Vale L, Nabi G, Boachie C, Cook JA, et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta-analysis. International Journal of Technology Assessment in Health Care 2011;27(1):3-10. [DOI: 10.1017/S0266462310001364] [DOI] [PubMed] [Google Scholar]
NCCN 2020
- Flaig TW, Spiess PE, Argawal N, Bangs R, Boorjian SA, Buyyounousi MK, et al. National Comprehensive Cancer Network Guidelines Version 5.2020 Bladder Cancer, 2020. Available from www.nccn.org/professionals/physician_gls/PDF/bladder.pdf (accessed prior to 21 October 2020).
NICE 2015
- NICE. Bladder cancer: diagnosis and management, NICE guideline [NG2], 2015. Available from www.nice.org.uk/guidance/ng2 (accessed prior to 21 October 2020).
Palou 2015
- Palou J, Hernández C, Solsona E, Abascal R, Burgués JP, Rioja C, et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle-invasive bladder cancer in daily clinical practice: a spanish multicentre observational study. BJU International 2015;116(1):37-43. [DOI: 10.1111/bju.13020] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: 10.1002/(sici)1097-0258(19981230)] [DOI] [PubMed] [Google Scholar]
Ray 2010
- Ray ER, Chatterton K, Khan MS, Chandra A, Thomas K, Dasgupta P, et al. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guérin. BJU International 2010;105(6):789-94. [DOI: 10.1111/j.1464-410X.2009.08839.x] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rink 2013
- Rink M, Babjuk M, Catto JW, Jichlinski P, Shariat SF, Stenzl A, et al. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. European Urology 2013;64(4):624-38. [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al, GRADE Working Group. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Sari 2021
- Sari Motlagh R, Mori K, Laukhtina E, Aydh A, Katayama S, Grossmann NC, et al. Impact of enhanced optical techniques at time of transurethral resection of bladder tumour, with or without single immediate intravesical chemotherapy, on recurrence rate of non-muscle-invasive bladder cancer: a systematic review and network meta-analysis of randomized trials. BJU International 2021;128(3):280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2020
- Schmidt S, Kunath F, Coles B, Draeger DL, Krabbe LM, Dersch R, et al. Intravesical bacillus calmette-guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD011935. [DOI: 10.1002/14651858.CD011935.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schned 2012
- Schned AR, Lenz P, Moore LE, Johnson A, Jones M, Kida M, et al. Analysis of the distribution and temporal trends of grade and stage in urothelial bladder cancer in northern new england from 1994 to 2004. ISRN Pathology 2012;2012:283670. [DOI: 10.5402/2012/283670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Shepherd 2017
- Shepherd ARH, Shepherd E, Brook NR. Intravesical Bacillus Calmette‐Guérin with interferon‐alpha versus intravesical Bacillus Calmette‐Guérin for treating non‐muscle‐invasive bladder cancer. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012112. [DOI: 10.1002/14651858.CD012112.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sylvester 2006
- Sylvester RJ, Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49(3):466-77. [DOI: 10.1016/j.eururo.2005.12.031] [DOI] [PubMed] [Google Scholar]
Tadrist 2021
- Tadrist A, Gondran-Tellier B, McManus R, Al Balushi K, Akiki A, Gaillet S, et al. Primary complete transurethral resection of bladder tumor using photodynamic diagnosis for high-risk nonmuscle invasive bladder cancer: is a restaging photodynamic transurethral resection really necessary? Journal of Endourology 2021;35(7):1042-6. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16):1-16. [DOI: 10.1186/1745-6215-8-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2017
- Tran K, Severn M. Blue light cystoscopy in patients with suspected non-muscle invasive bladder carcinoma: a review of clinical utility. In: www.cadth.ca/sites/default/files/pdf/htis/2017/RC0848_Blue%20Light%20Cystoscopy_Final.pdf. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2017. [PubMed] [Google Scholar]
Witjes 2014
- Witjes JA, Gomella LG, Stenzl A, Chang SS, Zaak D, Grossman HB. Safety of hexaminolevulinate for blue light cystoscopy in bladder cancer. A combined analysis of the trials used for registration and postmarketing data. Urology 2014;84(1):122-6. [DOI] [PubMed] [Google Scholar]
Witjes 2014a
- Witjes JA, Babjuk M, Gontero P, Jacqmin D, Karl A, Kruck S, et al. Clinical and cost effectiveness of hexaminolevulinate-guided blue-light cystoscopy: evidence review and updated expert recommendations. European Urology 2014;66(5):863-71. [DOI] [PubMed] [Google Scholar]
Witjes 2018
- Witjes JA. Cystoscopy in bladder cancer: the case pro. European Urology Supplements 2018;7(5):426-9. [DOI: 10.1016/j.eursup.2007.12.007] [DOI] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI: 10.1136/bmj.39465.451748.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaak 2007
- Zaak D, Karl A, Stepp H, Tritschler S, Tilki D, Burger M, et al. Fluorescence cystoscopy at bladder cancer: present trials [Die fluoreszenzzystoskopie beim harnblasenkarzinom: aktueller stellenwert]. Urologe A 2007;46(11):1519-27. [DOI: 10.1007/s00120-007-1563-7] [DOI] [PubMed] [Google Scholar]


