Abstract
Drosophila telomeres contain arrays of the retrotransposonlike elements HeT-A and TART. Their transposition to broken chromosomal termini has been implicated in chromosome healing and telomere elongation. The HeT-A element is attached by its 3′ end, which contains the promoter. To monitor the behavior of HeT-A elements, we used the yellow gene with terminal deficiencies consisting of breaks in the yellow promoter region that result in the y-null phenotype. Attachment of the HeT-A element provides the promoterless yellow gene with a promoter that activates yellow expression in bristles. The frequency of HeT-A transpositions to the yellow terminal deficiency depends on the genotype of the line and varies from 2 × 10−3 to less than 2 × 10−5. Loss of the attached HeT-A due to incomplete replication at the telomere leads to inactivation of yellow expression, which is restored by attachment of a new HeT-A element upstream of yellow. New HeT-A additions occur at a frequency of about 1.2 × 10−3. Short DNA attachments are generated by gene conversion using the homologous telomeric sequences as templates. Longer DNA attachments are generated either by conventional transposition of an HeT-A element to the chromosomal terminus or by recombination between the 3′ terminus of telomeric HeT-A elements and the receding end of HeT-A attached to the yellow gene.
Specialized mechanisms have evolved to add DNA to the termini of eukaryotic chromosomes, balancing the loss that occurs as a result of incomplete terminal DNA replication (11, 37). In most eukaryotes a special reverse transcriptase, telomerase, adds telomeric DNA repeats to the chromosomal ends by using an internal RNA template (11, 25, 26, 38). In contrast, Drosophila telomeres consist of multiple copies of HeT-A and TART elements sharing similarities with non-LTR-type retrotransposons (7, 33, 36, 38). In particular, they have an oligo(A) tract at the 3′ end. HeT-A and TART in telomeres have head-to-tail orientation (28, 33, 36, 38). Telomeres are believed to elongate by transposition of these elements to the ends of chromosomes (5, 6, 7, 36, 38, 42). All available data suggest that the HeT-A and TART elements are attached with 3′ oligo(A) tails to their target sites (4, 5, 42). The structures and functions of HeT-A and TART reveal similarities with telomeres: the TART reverse transcriptase is related to the catalytic subunit of telomerase (38). Still, the mechanism and the regulation of the telomere elongation by transposition remain unclear.
The terminal deficiencies that remove the chromosome end and are broken within the yellow gene have been used to study the mechanism of telomere recession and elongation (2, 3, 4, 5, 6, 35). The yellow gene is required for larval and adult cuticle pigmentation and is transcribed in the distal-to-proximal direction. The enhancers that control yellow expression in the wings and body cuticle are located in the 5′ upstream region of the yellow gene, whereas the enhancer controlling yellow expression in bristles resides in the intron (2, 24, 32). Therefore, flies with the terminal DNA breakpoints in the 5′ upstream region removing the wing and body enhancers display a y2-like phenotype: wild-type pigmentation in bristles and lack of pigmentation in the body cuticle and wing blade (2). Terminal deficiencies with breaks located at the yellow promoter or within the yellow transcription unit result in the y1-like phenotype, i.e., complete repression of yellow function (2, 3). Biessmann et al. (4) described the RT394 strain carrying a HeT-A element attached to the 5′ end of the yellow transcription unit. RT394 flies displayed the y2-like phenotype in spite of deletion of the yellow promoter. Danilevskaya et al. (16) showed that HeT-A elements have a promoter element at the 3′ end. As a result, the HeT-A promoter initiates transcription of sequences downstream of the element. One can suggest that the HeT-A promoter restores yellow expression in bristles.
Using these observations, we have developed a genetic method to analyze the frequency of HeT-A transposition to the receding promoterless yellow terminus. Here we have found that transposition depends on the genotype of a line and varies from less than 2 × 10−5 to 2 × 10−3. Thus, the genotype strongly affects the frequency of HeT-A transposition to the broken chromosomal end. Previously, we observed that the ends of the yellow terminal deficiencies could also be elongated by gene conversion if the yellow gene on the homologous chromosome served as a template (35). It was suggested that elongation of the HeT-A array might occur not only by virtue of transposition but also by an alternative mechanism, such as gene conversion.
To monitor the fate of the receding HeT-A element, we exploited the observation that less than 300 bp of the 3′ end of HeT-A could not activate yellow transcription. Addition of a new HeT-A element to the 5′ end of a truncated element renews yellow transcription. Using such a genetic screen we isolated a number of flies with elongated chromosomal termini. Southern blot analysis and sequencing showed that some HeT-A attachments were generated by transposition to the chromosome terminus, while others were generated by gene conversion using as a template a HeT-A element from the homologous chromosome. A significant fraction of HeT-A attachments were characterized by a large size, exceeding 20 kb. Their structure might be explained in terms of recombination between the 3′ terminus of the telomeric HeT-A element and the receding end of HeT-A attached to the yellow gene. As a result, the terminal deficiency carrying a single HeT-A element acquired a large array of telomere sequences. Our results suggest that Drosophila HeT-A arrays can be elongated not only by transposition but also by gene conversion and/or recombination mechanisms.
MATERIALS AND METHODS
Genetic crosses.
All Drosophila stocks were maintained at 25°C on a standard yeast medium. Genetic symbols for the yellow alleles and their origin were described elsewhere (22, 35). Most of genetic markers used were described by Lindsley and Zimm (30). The yac chromosome contains a deletion of the yellow and achaete genes but not of any vital genes and thus provides an opportunity to examine the behavior of the yellow gene on a homologue in the absence of other yellow sequences.
For determination of the yellow phenotype, the levels of pigmentation in different tissues of adult flies were estimated visually in 3- to 5-day-old males and females developing at 25°C as described in reference 22.
Molecular methods.
For Southern blot hybridization, DNA from adult flies was isolated using the protocol described by Ashburner (1). Treatment of DNA with restriction endonucleases, blotting, fixation, and hybridization with radioactive probes prepared by random primer extension were performed as described in the protocols for Hybond-N+ nylon membrane (Amersham, Arlington Heights, Ill.) and in the laboratory manual (41).
The junctions between newly transposed mobile elements and the DNA terminus were cloned by DNA amplification with two oligonucleotide primers. PCR was done by standard techniques (21). The primers used in DNA amplification were from the yellow gene and the HeT-A element. The numbers of nucleotide map positions are given below in parentheses in accordance with the yellow sequences (23) and the HeT-A element (6). The primers for the yellow gene are as follows: y1, CCTGGAACATTGCAC (3053 to 3039); y2, AAGACGGCGTCACCAAGGTGATC (3101 to 3078); and y3, ACTTCCACTTACCATCACGCCAG (3293 to 3271). The primers in the HeT-A element are as follows: h1, ATACTGCAAGTGGCGCGCATCC (455 to 434); and h2, GGTGCTTCCGTACTTCTGGCGG (359 to 338).
The products of amplification were fractionated by electrophoresis in 1.5% agarose gels. The successfully amplified products were cloned in a Bluescript plasmid (Stratagene, La Jolla, Calif.) and were sequenced using the Amersham sequence kit.
RESULTS
Determination of the yellow promoter region which is sufficient for maintaining yellow expression at the chromosome terminus.
In order to study the frequency of HeT-A transposition to a broken chromosomal end, we used the alleles with terminal deficiencies consisting of breaks in the yellow gene, designated yellow terminal deficiencies (yTD). Breaks that place the end of the chromosome at the yellow promoter or within the yellow transcription unit result in the y1-like phenotype (Fig. 1). Transposition of a promoter-containing HeT-A element to the end of a deficient chromosome should activate yellow expression in bristles (y2-like phenotype) if the yellow translation start site has not been deleted. This model system provides a simple genetic screen for monitoring HeT-A additions.
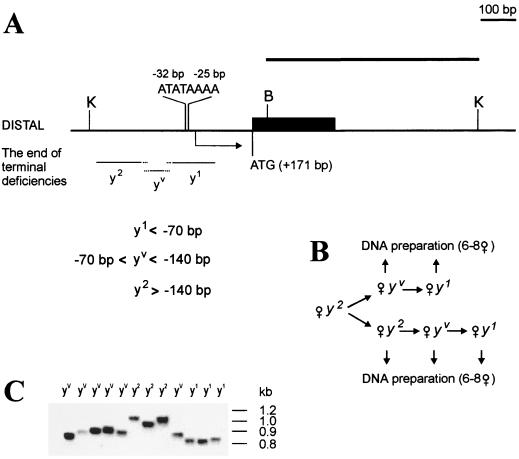
FIG. 1.
Minimal promoter region responsible for yellow activation at the tip of the terminal deficiencies. (A) A schematic presentation of terminal yellow deficiencies associated with different y phenotypes. The localization of regulatory regions, promoter, and start of translation are indicated according to the transcription start site of the yellow gene. The coding yellow region is shown as a black box. The sequence and localization of the yellow promoter is presented. The start of yellow transcription is shown by an arrow. The translation start codon (ATG) is indicated. The thin horizontal lines show the regions of yellow sequence in which the termini of the yTD line that correspond to the same class of y phenotype have been mapped. A BamHI-KpnI genomic fragment used as a probe for Southern blot analysis is indicated by a thick line in the upper part of the figure. Restriction enzyme abbreviations: B, BamHI; K, KpnI. (B) Scheme used to study the correlation between the DNA structure and y phenotype. In subsequent generations two or three yTD/yac sisters displaying either the y2-like or the yv or y1-like phenotype were crossed individually with yac males. Other females in groups of six to eight were combined according to their phenotypes and used for DNA preparation. (C) Southern blot analysis of DNAs prepared from six to eight yTD/yac sisters displaying either y2-like or yv or y1-like phenotype. DNAs were digested with KpnI. The filter was hybridized with the BamHI-KpnI probe.
To establish the model system we determined the minimal regulatory region in the deficient chromosomes that allows yellow activation in bristles (Fig. 1). By Southern blot analysis four lines carrying deficiencies terminating in the region from −400 bp to −300 bp upstream of the yellow transcription start site were selected. Flies of these lines displayed a y2-like phenotype due to yellow activation in bristles by the enhancer located in the yellow intron (2, 24, 32). The yellow terminal deficiencies displaying a y2-like phenotype were designated as y2TD. The y2TD/yac females were crossed individually with yac males. After several generations exceptional flies with variegated bristle pigmentation (yv phenotype) were found among y2-like females (Fig. 1). A few of the y2-like and yv-like females were taken for DNA preparation; other yv females and their y2-like sisters were crossed individually with yac males (Fig. 1B). Among their progeny yv females gave rise to y1-like (designated y1TD) females. In the next generation all progeny exhibited a y1 phenotype. DNA was isolated from groups of six to eight sisters with the same phenotype, as shown in Fig. 1B.
Southern blot analysis (Fig. 1C) showed that the distance between the end of the chromosome and the transcription start site was 140 bp or more in y2-like flies and less than 70 bp in y1-like flies. It varied from 140 to 70 bp in yv-like flies. Thus, deficiency chromosomes that terminate at position −70 bp have slightly less than the minimal sequence necessary to express the yellow gene in any tissue.
The model system also allowed us to identify additions of HeT-A elements into the ends of these deficient chromosomes. Transposition of a HeT-A element onto the end of the deficient chromosome can be detected visually in progeny of y1TD females carrying a terminal deficiency chromosome broken in the interval between −70 bp and +171 bp (the position of the translation start codon of the yellow gene). Such transposition produces y2-like progeny of y1-like parents. If the HeT-A element is attached to the yellow sequence downstream of +171 bp, the bristle pigmentation is not restored. As the deficiency terminus loses about 70 bp per generation on average (2–6, 27, 42), the interval between −70 bp and +171 bp is expected to be lost over a period of three generations.
The frequency of the HeT-A transposition to the broken chromosome terminus in the yellow gene depends on the genotype.
The above system was used to determine the frequency of HeT-A transposition. We obtained nine independent y2TD/yac lines that had a terminally deficient chromosome broken approximately 300 bp upstream of the yellow transcription start site. The terminal yellow deficiencies in these lines originated as described previously (35) from single females after crosses with different laboratory strains, such as those with the genotype y1w or yacw or yacw+ or Oregon-R. As a result, the lines had similar but not identical chromosomal contexts.
y2TD/yac lines were propagated for several generations, and newly arising y1-like females were crossed individually with yac males for three subsequent generations. For any of the nine y1TD/yac lines we examined 6,000 to 14,000 flies (altogether about 65,000 flies were examined). Approximately the same number of flies from each generation was scored.
The appearance of y2-like flies among the y1-like offspring was observed in only one of these lines. Fourteen independent y1 → y2 transitions were found among ca. 6,900 flies scored; i.e., the frequency of y2-phenotype appearance was about 2 × 10−3. No y2-like females were found in the other eight y1TD/yac lines (58,100 flies scored). Thus, the frequency of terminal elongation in these lines was lower than 2 × 10−5. These results suggest that the frequency of the HeT-A transposition strongly depends on the particular chromosomal context of the line.
To determine the molecular nature of y1 → y2 transitions, the DNAs of the y2-like derivatives were studied with the aid of Southern blot analysis (Fig. 2). In all y2-like alleles, the appearance of an additional DNA sequence at the broken end was observed. The sizes of DNA additions were measured by Southern blot hybridization of genomic DNA restricted with NruI, which usually does not cleave HeT-A DNA (5); and the sizes varied from 1.4 to 6.6 kb. These y2 derivatives were referred to as yhTD (HeT-A-healed terminal deletions) and numbered from 1 to 14.
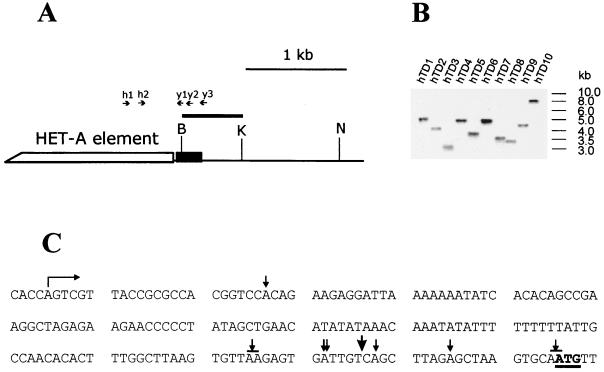
FIG. 2.
HeT-A transpositions to the broken chromosome terminus in the yellow gene. (A) Partial restriction map of the wild-type yellow gene region, indicating the position of the BamHI-KpnI probe that was used for Southern blot analysis. The coding yellow region is shown as a black box. Restriction enzyme abbreviations: B, BamHI; K, KpnI; N, NruI. The primers in the HeT-A element and the yellow gene used for DNA amplification are shown by arrows. (B) HeT-A additions as indicated by Southern blot hybridization of genomic DNA restricted with NruI and probed with the BamHI-KpnI fragment. The NruI site is at position +1835 relative to the transcription start site of the yellow gene. (C) Attachment points of HeT-A elements in the yellow gene. The start of yellow transcription is shown by a bent arrow. The translation start codon ATG is underlined. The points of HeT-A attachment are shown by small arrows. The attachment of HeT-A in the yhTD3 line is indicated by a large arrow. One or two A bases at the junctions may originate either from the receding yellow sequences or from the oligo(A) tail of the 3′ end of the attached HeT-A element.
To directly show the transposition of the HeT-A elements to ends broken at yellow, we amplified by PCR the DNA between primers in the yellow coding region and the 3′ region of the HeT-A element (Fig. 2). The junctions between mobile elements and the yellow gene were sequenced in eight y2-like derivatives (Fig. 2C). In all cases, a string of adenine residues was present between the yellow and HeT-A sequences. The 3′ HeT-A sequences (5′ CCTCCACCAGCAAAGTT 3′) were highly conserved and identical to the previously sequenced HeT-A elements (4, 5, 6). These results suggest that HeT-A addition to broken chromosomal termini occurs through transposition if the homologous sequences allowing gene conversion (35) are absent.
Estimation of a minimal HeT-A region that is sufficient for maintaining yellow expression in bristles.
In order to monitor the fate of the HeT-A element attached previously to the yellow sequences, we first determined the minimal HeT-A region sufficient for yellow expression in bristles (Fig. 3). For this the yhTD3 line, carrying a yellow deficiency terminating with a 1.4-kb HeT-A sequence, was selected (Fig. 2B). Using Southern blot hybridization of DNA from the progeny of individual yhTD3/yac females, two yhTD3 sublines carrying yellow terminal deficiencies with an approximately 500-bp HeT-A sequence attached were isolated. These yhTD3/yac females were crossed individually with yac males; after several generations some females acquired variegated bristle pigmentation (yv phenotype). Sisters displaying either y2-like or yv phenotypes were mated individually with yacw males (Fig. 3B). Six to eight y2-like or yv sisters were collected for DNA preparation and Southern blot analysis. In the next generation, the progeny of yv females displayed the y1-like phenotype, whereas progeny of y2-like sisters acquired variegated bristle pigmentation. Again, six to eight sisters displaying either the y1-like or yv phenotype were collected for DNA preparation.
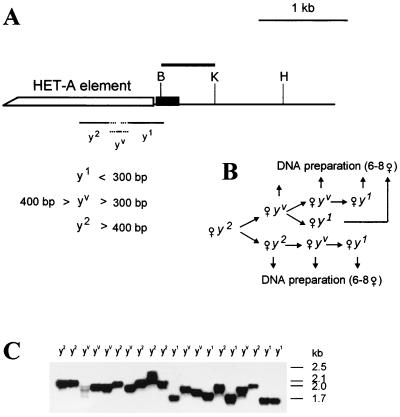
FIG. 3.
Minimal region of the HeT-A element which is sufficient for compensation of the yellow promoter deletion at the tip of the X chromosome. (A) Scheme of terminal deficiencies associated with different y phenotypes. The yellow coding region is shown by a black box. The thin horizontal lines in the lower part of the scheme show the regions of the HeT-A element (numbered from the 3′ HeT-A end) in which termini that correspond to the y phenotype have been mapped. The figures show the distance from the first nucleotide of the 3′ end of the HeT-A element. Restriction enzyme abbreviation: H, SphI. Other designations are as in Fig. 1. (B) The scheme used to study the relationship between the length of the terminal HeT-A element and the y phenotype. (C) Southern blot analysis of yhTD3/yac sisters displaying y2-like, yv, or y1-like phenotypes. DNAs were digested with SphI. The filter was hybridized with the BamHI-KpnI fragment indicated by the thick line in panel A. The SphI site is 1435 bp from the HeT-A attachment in yhTD3.
The results of Southern blot analysis showed that the terminal HeT-A element was longer than 400 bp in y2-like flies and shorter than 300 bp in y1-like flies (Fig. 3C). The size of the HeT-A element varied from 300 to 400 bp in yv flies. Thus, 400 bp of the HeT-A 3′ terminus is sufficient for wild-type levels of yellow expression in bristles.
Detection of new HeT-A attachments to the terminal HeT-A element.
As shown above, the presence of less than 300 bp from the 3′ end of HeT-A does not compensate for the absence of the yellow promoter. It may be expected that the attachment of a new HeT-A element should restore the yellow activation. If this is the case, it is possible to monitor the addition of novel HeT-A elements to a preexisting HeT-A element by visual analysis of bristle pigmentation.
To monitor the behavior of receding HeT-A termini, we obtained four yhTD3 sublines started from a single yhTD3 female. These lines were termed A, B, C, and D. At the beginning of the experiment the X chromosome in all four sublines carried about 400 bp of HeT-A sequence attached at position +152 bp of yellow sequence and as a result displayed a y2-like phenotype. In the offspring of yhTD3/yac females we selected females with y1-like phenotype and individually crossed them with yac males for three successive generations. For any of four yhTD3 lines we examined about 14,000 flies (altogether 53,500 flies were scored). In all sublines, y2-like females were found as single events (31 cases) or in clusters (10 cases). Sixty-four y2-like females were found altogether. This gives an average frequency of y1→y2 phenotype transition of ca. 1.2 × 10−3.
DNA was prepared from the offspring of these selected y2-like females for Southern blot analysis (Fig. 4). Many HeT-A elements have sites for KpnI and EcoRI restriction endonucleases at the 3′ end, have sites for SpeI and EcoRV in the central region, and have no sites for NruI (4–6). On the other hand, all these endonucleases have sites in the yellow transcription unit in the vicinity of the HeT-A attachment (Fig. 4A). Therefore, these enzymes were used for DNA hydrolysis. The BamHI-KpnI fragment subcloned from the yellow gene was used as a probe (Fig. 4).
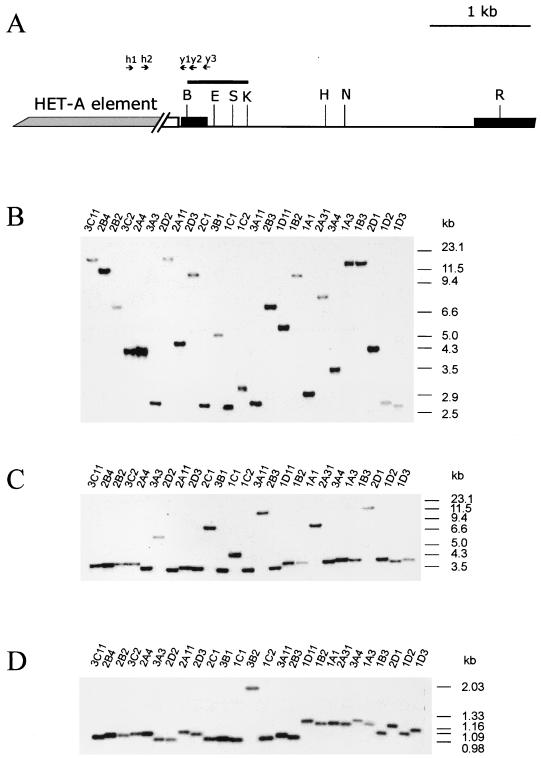
FIG. 4.
Molecular additions to the tip of the X chromosome. (A) Partial restriction map of the wild-type yellow gene region, indicating the position of sites for restriction enzymes used for Southern blot analysis. The HeT-A addition is shown by a shaded box. The coding yellow regions are shown by black boxes. Restriction enzyme abbreviations: B, BamHI; K, KpnI; E, EcoRV; H, SphI; S, SpeI; N, NruI; R, EcoRI. Other designations are as in Fig. 1 and 2. (B through D) Southern blot analysis of DNAs prepared from y2-like lines. DNAs were digested with NruI (B), EcoRI (C), and KpnI (D). The filters were hybridized with the BamHI-KpnI fragment.
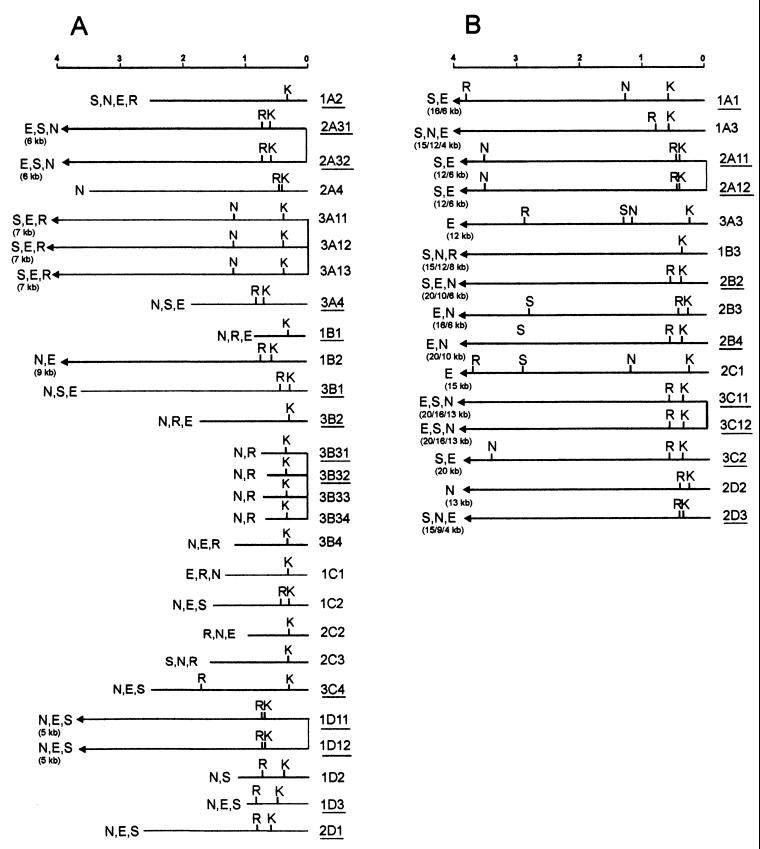
Usually, different individual y2-like lines derived from the same cluster have identical restriction maps of the new HeT-A attachments (Fig. 4 and 5), suggesting that DNA additions happened at the premeiotic stage in the germ line. Most of the DNA attachments had KpnI and EcoRI sites at the 3′ end, as is typical of the 3′ end of the HeT-A element (Fig. 5). The size of new DNA additions varied widely, from less than 1 kb to more than 20 kb. In the case of large DNA additions, we could not precisely estimate their size because of a possible existence of additional restriction sites in the new DNA sequences and also due to the low resolution of large DNA fragments by conventional Southern blot analysis. For convenience, we divided the DNA attachments into two groups according to their size: those with a size between 0.5 and 8 kb (Fig. 5A) and those with a size exceeding 10 kb (Fig. 5B).
FIG. 5.
Restriction map of the DNA additions to the terminal HeT-A element as inferred from genomic Southern blot analysis. The maps of the DNA additions obtained as clusters of identical events are linked by vertical lines. The maps start from the BamHI site located in the yellow gene at 39 bp from the HeT-A attachment site in the yhTD3 line. (A) Restriction maps of the DNA attachments with a determined size; (B) restriction maps of the DNA attachments with undetermined size. The lines described in Fig. 6 and 7 are underlined. Abbreviations of restriction enzymes are as in Fig. 4.
Mechanisms of attachment of short HeT-A sequences: transposition of new HeT-A elements and terminal DNA extension by gene conversion.
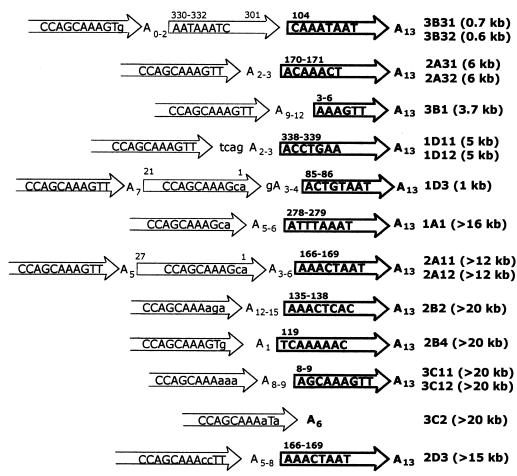
To study the mechanisms of attachment of HeT-A sequences we cloned by PCR and sequenced junctions between terminal HeT-A elements and new DNA attachments (Fig. 6 and 7). Two primers were used for DNA amplification, one located in the yellow gene and the other in the conserved region of the HeT-A element between 330 and 460 bp from the 3′ end (Fig. 4A). The latter was absent from the terminal HeT-A element in y1-like derivatives but present in the newly attached HeT-A elements. The junctions were identified by comparing the relevant sequences from y2-like derivative lines and the original yhTD3 line.
FIG. 6.
Aligned sequences of the original HeT-A element and four y2-like derivatives. All sequences end with the last nucleotide at the 3′ end of the original HeT-A element. The sequences are shown in the 5′-to-3′ orientation. Only the last 350 nucleotides of aligned sequences are shown. The small letters show the substitutions in the sequence. Asterisks indicate missing nucleotides. Arrows represent the 3′ ends of new HeT-A elements. The sequences that may refer to the original HeT-A element in the yhTD3 line are shown by bold letters.
FIG. 7.
Diagram of HeT-A additions to the receding HeT-A element. The numbers in parentheses show the approximate sizes of the attachments. The arrows indicate the directions of the HeT-A elements. The bold arrows correspond to the original HeT-A element. The numbers above the arrows indicate distances of either the 5′ terminus or the 5′ and 3′ termini of the HeT-A element from the 3′ terminus of a standard element. For this, the terminal HeT-A element present in the original yhTD3 line was used (Fig. 6). A base at the junctions may originate either from the terminal HeT-A element or from the 3′ oligo(A) tail of the new HeT-A element. The base pairs at the junction between new and old HeT-A elements are shown. The lowercase letters indicate substitutions in the conserved sequence at the 3′ end of the HeT-A element.
We analyzed a total of 23 y2-like lines. In the cases where two y2-like lines were obtained from the same progenitor fly, the restriction maps and nucleotide sequences were identical between the two lines (Fig. 5 and 7). Further, the structure of only independently obtained y2-like lines will be discussed below.
Sequencing of four y2 lines showed no oligo(A) tracts. Further, the extension on the chromosome end in these lines contained sequences internal to the HeT-A element immediately 5′ of the old element and not at the 3′-most end of the element (Fig. 6). The newly attached DNA of these two y2-like lines (3B2 and 3C4) contained many nucleotide substitutions and small gaps compared to the original HeT-A element attached to the yellow terminal deficiency in the yhTD3 line (Fig. 6). The presence of multiple changes in the DNA sequence suggests the DNA elongation by gene conversion on the template of a homologous, but not identical, HeT-A sequence located somewhere else in other telomeres. In two other lines (1A2 and 1B1), the elongated HeT-A element had the same structure as the original, suggesting the use of a HeT-A element whose structure was identical to that of receding HeT-A as the template (data not shown).
In two lines (2D1 and 3A4) we found an oligo(A) tract and the conservative 3′ end of a new HeT-A element. However, sequences proximal to the HeT-A 3′ terminus contained many nucleotide substitutions compared to the original HeT-A element. This suggests that such attachments are also generated by gene conversion. A short, truncated HeT-A element and the 3′ terminus of an adjacent HeT-A element may serve as the template in this case.
All these attachments were no longer than 2.7 kb. This is consistent with our previous observation that the average length of converted tracts as determined for terminal deficiencies in the yellow gene was an estimated 2.6 kb (34).
Finally, in the 3B31 line the sequence of the original HeT-A element was interrupted at the position of 104 bp (numbered relative to the 3′ end of the HeT-A element). Attached at this point were a short, altered sequence from another HeT-A element (from 301 to 330 bp), two T bases, and the 3′ terminus of the third HeT-A element (Fig. 7). The small size of the HeT-A attachment (0.6 to 0.7 kb) and the presence of an additional DNA tract between the receding HeT-A element and the 3′ end of the new HeT-A addition suggest DNA elongation by gene conversion. Possibly a homologous HeT-A region was used as a conversion template. Thus, in 6 or 7 out of the 18 independent HeT-A attachments tested, gene conversion is implicated.
In two lines (2A31 and 3B1), HeT-A elements appear to have used their oligo(A) tails of different lengths to attach to the target sites (Fig. 7). Sequences of the 3′ HeT-A ends (5′CCAGCAAAGTT 3′) were conserved as in all the cases of HeT-A transposition to the yellow locus at the deficient terminus (see above). These observations suggest DNA elongation by true transposition of the HeT-A elements. The size of attachments (3 to 6 kb) is also consistent with these data.
Two independent lines (1D11 and 1D3) displayed a more complex structure at the junction. In the 1D11 line, the newly attached sequence begins with three A bases representing the oligo(A) tail and an additional sequence, TCAG, inserted between the oligo(A) and the conserved 3′ terminus of the new HeT-A element (Fig. 7). In the 1D3 line, we found a duplication of the HeT-A 3′ tail consisting of two tandem 3′ HeT-A regions (Fig. 7). The sizes of the attachments were 5 and 1 kb, respectively. The structures of the newly attached HeT-A sequences in the 1D11 and 1D3 lines are difficult to explain in terms of either of the two terminal DNA elongation mechanisms discussed here. Still, neither of them can be ruled out.
The large HeT-A attachments may be generated by an alternative mechanism.
Seven independent DNA attachments (1A1, 2A11, 2B2, 2B4, 2D3, 3C11, and 3C2) belong to the second group characterized by extension exceeding 10 kb (Fig. 5B).
Apart from large size, all these DNA attachments (Fig. 7) begin with an oligo(A) tail and a conserved 3′ end typical of HeT-A elements. Sequence comparison revealed that the target HeT-A element contains several A bases at the junction. Thus, some of these A bases may belong to either the oligo(A) of the new HeT-A element or the target HeT-A element. All of these newly attached HeT-A elements bear different base substitutions in the normally conserved 3′ terminal GTT triplet. The generation of such DNA attachments as well as their large size may not be explained by HeT-A transposition or gene conversion. The presence of several A bases at the target sequence, the extremely large size of DNA attachments, and the aberrant 3′ terminal sequence suggest that they were formed as a result of recombination between the receding HeT-A element at the yellow locus and some other telomeric HeT-A element rather than transposition (see Discussion).
DISCUSSION
Transposition of HeT-A elements to terminally deficient X chromosomes.
The HeT-A element has a promoter at the 3′ end (16). Here, we show that the 400-bp sequence at the 3′ end of the HeT-A element is sufficient for activation of yellow expression in bristles. Specific activation of yellow transcription by the HeT-A promoter in bristles only is probably due to the presence of the bristle enhancer located in the yellow intron. The body and wing enhancers are upstream of the yellow promoter and have been removed. A possible role of the HeT-A promoter specificity should also be considered.
The attachment of HeT-A or TART elements to the terminally deficient X chromosome may be considered real transposition events because of the absence of extended homology between the mobile elements and target site within the yellow locus, which is necessary for DNA elongation by gene conversion. In all cases, the oligo(A) track and the conserved 3′ end sequence of HeT-A necessary for transposition were found at the junction, confirming the transposition mechanism for HeT-A attachments (4–6; this study). The joining of the 3′ end of TART or HeT-A to the broken end is explained by a model for terminal transposition in which reverse transcription of the retrotransposon is initiated at its 3′ end by using the chromosome end as a primer (4, 5, 7, 8, 28, 33, 38, 42, 45). This model explains the invariant orientation of the HeT-A and TART copies which were isolated from native telomere.
The genetic system described here selectively visualizes only attachments of HeT-A elements. The TART element seems to contain no promoter at the 3′ terminus (19), and its attachments seem to fail to support yellow expression. We found that the frequency of HeT-A additions to the yellow sequences was relatively low and depended strongly on chromosomal context.
Attachment of a new HeT-A element to the terminal HeT-A may occur by different mechanisms.
Recombination of repetitive telomeric ends has been considered an alternative reserve pathway for telomere elongation (8, 9, 11, 15, 29, 31, 34, 38, 39, 40, 46). Indirect evidence exists that telomeres of Chironomus and Anopheles gambiae are extended by recombination and gene conversion mechanisms involving long complex terminal repeats (8, 15, 31, 39). This pathway has been well documented for yeast, where telomeres are extended by telomerase, but recombination and/or gene conversion serves as an efficient bypass mechanism for chromosome length maintenance when telomerase is inactive (11, 12, 29, 38). Recombination has also been suggested as the mechanism for telomere maintenance in several immortalized human cell lines that harbor no telomerase activity (10, 13, 14, 25, 26, 43, 44). Thus, recombination-based mechanisms are present in most organisms as an alternative mechanism of unregulated telomere elongation (37). Normally, the recombination pathway may be blocked by a special class of telomere-bound proteins and may be tightly regulated during DNA replication (38).
Previous data (4, 5) and our observations showed that the attachment of new HeT-A element(s) to the receding end of a terminal HeT-A element happened with a rather high frequency. However, in contrast to previous observations, we found that DNA elongation did not occur only by transposition of the mobile elements. Short DNA attachments were more frequently generated by DNA extension through conversion using homologous HeT-A sequences located on another telomere as a template. The average size of DNA extensions is approximately the same as that obtained in the experiments with terminal DNA elongation of the yellow sequences by conversion mechanisms (35). Sometimes the 3′ sequence of a new HeT-A element was found in close vicinity to the start of the conversion track. This may reflect the structure of telomeres, which often contain arrays of truncated 3′ ends of HeT-A elements (17, 28, 37).
The mechanism of long DNA attachments.
Unexpectedly, many HeT-A attachments had a large size exceeding severalfold the size expected for the full-length transcript of the HeT-A element (17, 38). Interestingly, the large DNA attachments occur frequently. Previously, among four independent HeT-A attachments to a terminal HeT-A, two exceeded 14 kb (5). In our experiments they comprised 13 of 33 DNA elongations. The second feature of this class of attachments is the presence of substitutions in the 3′ terminal nucleotides of the HeT-A element. These nucleotides were conserved among all 10 studied HeT-A additions to the terminal yellow sequences, suggesting their importance for transposition. These observations argue against the transposition mechanism for the long DNA attachments.
The existence of a small amount of high-molecular-weight RNA homologous to HeT-A (19) still does not allow one to exclude the transposition of long HeT-A arrays via an RNA intermediate. This minor fraction of HeT-A RNA is thought to represent readthrough transcripts of tandem HeT-A elements (19). However, it is difficult to imagine that such long transcripts are much more efficient in transposition than the truncated or full-length HeT-A transcripts.
It is more likely that the large DNA attachments are generated by site-specific recombination using several A bases at the terminal HeT-A and the oligo(A) tail of a HeT-A located at another telomere. As a result, a large fragment of telomere sequence is transferred to the chromosome end.
Analysis of the sequenced HeT-A elements showed that the 3′ noncoding region of HeT-A was rather conserved, and only a few HeT-A subfamilies existed (17, 18). This conservation of sequences within HeT-A subfamilies is difficult to explain from the viewpoint that telomeres are elongated only by transposition of HeT-A elements via an RNA-templated step. Rapid sequence change has been reported for many elements with an RNA-based step in replication (20). The conservation of HeT-A sequence was explained by postulating a limited number of replicatively active HeT-A elements (17). In this case, the majority of elements in the genome would be separated from a transcriptionally active HeT-A element by only one step of reverse transcription. Another explanation may be that the homogeneity of HeT-A sequences has been established by a conversion and/or recombination mechanism. The same mechanism was suggested to explain the gradient homogenization of termini in the yeast (12); the sequences closest to the ends share the highest degree of homology. More likely, both mechanisms are responsible for the HeT-A conservation and for telomere elongation.
ACKNOWLEDGMENTS
We are sincerely grateful to James Mason for critical reading of the manuscript, corrections, and comments. We also are greatly indebted to Tatyana Loukianova for manuscript editing and to the anonymous reviewers for helpful suggestions. We greatly appreciate A. Soldatov's help with DNA sequencing.
This work was supported by the Russian State Program “Frontiers in Genetics,” by the Russian Foundation for Basic Research, and by an International Research Scholar award from the Howard Hughes Medical Institute to P.G.
REFERENCES
- 1.Ashburner M. Drosophila: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 2.Biessmann H, Mason J M. Progressive loss of DNA sequences from terminal chromosome deficiencies in Drosophila melanogaster. EMBO J. 1988;7:1081–1086. doi: 10.1002/j.1460-2075.1988.tb02916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biessmann H, Carter S B, Mason J M. Chromosome ends in Drosophila without telomeric DNA sequences. Proc Natl Acad Sci USA. 1990;87:1758–1761. doi: 10.1073/pnas.87.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biessmann H, Mason J M, Ferry K, d'Hulst M, Valgeirsdottir K, Traverse K L, Pardue M L. Addition of telomere-associated HeT DNA sequences “heals” broken chromosome ends in Drosophila. Cell. 1990;61:663–673. doi: 10.1016/0092-8674(90)90478-w. [DOI] [PubMed] [Google Scholar]
- 5.Biessmann H, Champion L E, O'Hair K, Ikenaga K, Kasravi B, Mason J M. Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. EMBO J. 1992;11:4459–4469. doi: 10.1002/j.1460-2075.1992.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biessmann H, Valgeirsdottir K, Lofsky A, Chin C, Ginther B, Levis R W, Pardue M-L. HeT-A, a transposable element specifically involved in “healing” broken chromosome ends in Drosophila melanogaster. Mol Cell Biol. 1992;12:3910–3918. doi: 10.1128/mcb.12.9.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biessmann H, Walter M F, Mason J M. Drosophila telomere elongation. Ciba Found Symp. 1997;211:53–67. doi: 10.1002/9780470515433.ch5. [DOI] [PubMed] [Google Scholar]
- 8.Biessmann H, Mason J M. Telomere maintenance without telomerase. Chromosoma. 1997;106:63–69. doi: 10.1007/s004120050225. [DOI] [PubMed] [Google Scholar]
- 9.Biessmann H, Kobeski F, Walter M F, Kasravi A, Roth C W. DNA organization and length polymorphism at the 2L telomeric region of Anopheles gambiae. Insect Mol Biol. 1998;7:83–93. doi: 10.1046/j.1365-2583.1998.71054.x. [DOI] [PubMed] [Google Scholar]
- 10.Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 11.Blasco M A, Gasser S M, Lingner J. Telomeres and telomerase. Genes Dev. 1999;13:2353–2359. doi: 10.1101/gad.13.18.2353. [DOI] [PubMed] [Google Scholar]
- 12.Bosco G, Haber J E. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan T M, Marusic L, Bacchetti S, Namba M, Reddel R R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryan T M, Englezou A, Gupta J, Bacchetti S, Reddel R R. The telomere lengthening in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum Mol Genet. 1997;6:921–926. doi: 10.1093/hmg/6.6.921. [DOI] [PubMed] [Google Scholar]
- 15.Cohn M, Edstrom J E. Telomere-associated repeats in Chironomus form discrete subfamilies generated by gene conversion. J Mol Evol. 1992;35:114–122. doi: 10.1007/BF00183222. [DOI] [PubMed] [Google Scholar]
- 16.Danilevskaya O N, Arkhipova I R, Traverse K L, Pardue M L. Promoting in tandem: the promoter for telomere transposon HeT-A and implications for the evolution of retroviral LTRs. Cell. 1997;88:647–655. doi: 10.1016/s0092-8674(00)81907-8. [DOI] [PubMed] [Google Scholar]
- 17.Danilevskaya O N, Lowenhaupt K, Pardue M L. Conserved subfamilies of the Drosophila HeT-A telomere-specific retrotransposon. Genetics. 1998;148:233–242. doi: 10.1093/genetics/148.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danilevskaya O N, Tan C, Wong J, Alibhal M, Pardue M L. Unusual features of the Drosophila melanogaster telomere transposable element HeT-A are conserved in Drosophila yakuba telomere elements. Proc Natl Acad Sci USA. 1998;95:3770–3775. doi: 10.1073/pnas.95.7.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danilevskaya O N, Traverse K L, Hogan N C, DeBaryshe P G, Pardue M L. The two Drosophila telomeric transposable elements have very different patterns of transcription. Mol Cell Biol. 1999;19:873–881. doi: 10.1128/mcb.19.1.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake J W. Rates of spontaneous mutation among RNA viruses. Proc Natl Acad Sci USA. 1993;90:4171–4175. doi: 10.1073/pnas.90.9.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlich H A. PCR technology. New York, N.Y: Stockton Press; 1989. [Google Scholar]
- 22.Georgiev P, Tikhomirova T, Yelagin V, Belenkaya T, Gracheva E, Parshikov A, Evgen'ev M B, Samarina O P, Corces V G. Insertions of hybrid P elements in the yellow gene of Drosophila cause a large variety of mutant phenotypes. Genetics. 1997;146:583–594. doi: 10.1093/genetics/146.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyer P K, Spana C, Corces V G. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986;5:2657–2662. doi: 10.1002/j.1460-2075.1986.tb04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geyer P K, Corces V G. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987;1:996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- 25.Greider C W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 26.Greider C W. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998;95:90–92. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levis R W. Viable deletions of a telomere from a Drosophila chromosome. Cell. 1989;58:791–801. doi: 10.1016/0092-8674(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 28.Levis R W, Ganesan R, Houtchens K, Tolar L A, Sheen F-M. Transposons in place of telomere repeats at a Drosophila telomere. Cell. 1993;75:1083–1093. doi: 10.1016/0092-8674(93)90318-k. [DOI] [PubMed] [Google Scholar]
- 29.Li B, Lustig A J. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10:1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- 30.Lindsley D L, Zimm G G. The genome of Drosophila melanogaster. New York, N.Y: Academic Press; 1992. [Google Scholar]
- 31.López C C, Nielsen L, Edström J-E. Terminal long tandem repeats in chromosomes from Chironomus pallidivittatus. Mol Cell Biol. 1996;16:3285–3290. doi: 10.1128/mcb.16.7.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin M, Meng Y B, Chia W. Regulatory elements involved in the tissue-specific expression of the yellow gene of Drosophila. Mol Gen Genet. 1989;218:118–126. doi: 10.1007/BF00330574. [DOI] [PubMed] [Google Scholar]
- 33.Mason J M, Biessmann H. The unusual telomeres of Drosophila. Trends Genet. 1995;11:58–62. doi: 10.1016/s0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- 34.McEachern M J, Blackburn E H. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 1996;10:1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 35.Mikhailovsky S, Belenkaya T, Georgiev P. Broken chromosome ends can be elongated by conversion in Drosophila melanogaster. Chromosoma. 1999;108:114–120. doi: 10.1007/s004120050358. [DOI] [PubMed] [Google Scholar]
- 36.Pardue M L, Danilevskaya O N, Lowenhaupt K, Slot F, Traverse K L. Drosophila telomeres: new views on chromosome evolution. Trends Genet. 1996;12:48–52. doi: 10.1016/0168-9525(96)81399-0. [DOI] [PubMed] [Google Scholar]
- 37.Pardue M L, Danilevskaya O N, Lowenhaupt K, Wong J, Erby K. The gag coding region of the Drosophila telomeric retrotransposon, HeT-A, has an internal frame shift and a length polymorphic region. J Mol Evol. 1996;43:572–583. doi: 10.1007/BF02202105. [DOI] [PubMed] [Google Scholar]
- 38.Pardue M L, DeBaryshe P G. Telomeres and telomerase: more than the end of the line. Chromosoma. 1999;108:73–82. doi: 10.1007/s004120050354. [DOI] [PubMed] [Google Scholar]
- 39.Pluta A F, Zakian V A. Recombination occurs during telomere formation in yeast. Nature. 1989;337:429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- 40.Roth C W, Kobeski F, Walter M F, Biessmann H. Chromosome end elongation by recombination in the mosquito Anopheles gambiae. Mol Cell Biol. 1997;17:5176–5183. doi: 10.1128/mcb.17.9.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning:a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Sheen F M, Levis R W. Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. Proc Natl Acad Sci USA. 1994;91:12510–12514. doi: 10.1073/pnas.91.26.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoppler H, Hartmann D P, Sherman L, Schlegel R. The human papilloma virus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem. 1997;272:13332–13337. doi: 10.1074/jbc.272.20.13332. [DOI] [PubMed] [Google Scholar]
- 44.Strahl C, Blackburn E H. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol. 1996;16:53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traverse K L, Pardue M L. A spontaneously opened ring chromosome of Drosophila melanogaster has acquired He-T DNA sequences at both new telomeres. Proc Natl Acad Sci USA. 1988;85:8116–8120. doi: 10.1073/pnas.85.21.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S S, Zakian V A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990;345:456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]