Abstract
Green vegetables, fruits, cereals, and pulses are all rich sources of antioxidants. Retinoic acid, ascorbate, proanthocyanidins, tannins, saponins, melatonin, curcumin, allicin, and alpha-lipoic acid stand documented in plants as bioactive compounds. The international dietary committee advocates a specific quantum of these natural antioxidants through diet. Interestingly, environmental pollution has indeed affected most of these farm products. The use of chemical fertilizers, pesticides and heavy metals in soil has a cumulative effect on human health. Enough evidence is available for the presence of phytoestrogen, xenoestrogen, and a host of other endocrine disruptors in the food. These plant-based nutrients can mimic or enhance the natural hormone's health effects. While endocrine disruptors are found in many everyday products, this review aims to address endocrine disruptors from food in the Asian subcontinent. 'Food for thought' justifies the paradigm shift towards good endocrine health by swaying away from the conventional daily dietary recommendations.
Keyword: Endocrine disruptors, Bioactive compounds, Food, Antioxidant
Endocrine Disruptors
Endocrine-disrupting chemicals (EDCs) are the substances present in the environment, food, and consumer products that interfere with hormone biosynthesis, metabolism, or action altering the normal homeostatic balance of an organism. Various validation studies have contributed to the growing knowledge of different EDCs and their mechanism of action (Diamanti-Kandarakis et al. 2009). The previous known mechanism was that they exert their actions through nuclear hormone receptors, including Estrogen receptors (ERs), Androgen receptors (ARs), progesterone receptors, Thyroid receptors (TRs), and retinoid receptors, either acting as an agonist or as an antagonist (Diamanti-Kandarakis et al. 2009). However, present scientific research has elucidated the mechanisms that are broader compared to the previously established. Therefore, endocrine disruptors act via nuclear receptors, nonnuclear steroid hormone receptors (e.g., membrane ERs), nonsteroid receptors (e.g., neurotransmitter receptors such as the serotonin receptor, dopamine receptor, norepinephrine receptor), orphan receptors [e.g., aryl hydrocarbon receptor (AhR)—an orphan receptor], enzymatic pathways involved in steroid biosynthesis and/or metabolism, and numerous other mechanisms that converge upon endocrine and reproductive systems (Diamanti-Kandarakis et al. 2009).
Many chemicals are classified as EDCs as they are heterogeneous and include the synthesized molecules like solvents and lubricants used in industries, food, vegetables and their by-products [polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), dioxins], plastics [bisphenol A (BPA)], plasticizers (phthalates), pesticides [methoxychlor, chlorpyrifos, dichlorodiphenyltrichloroethane (DDT)], fungicides (vinclozolin), and pharmaceutical agents [diethylstilbestrol (DES)]. The synthetic chemicals and even the natural ones used in formulated diets for infants also act as EDCs. Previously, it was thought they possess a low-affinity binding to the estrogen receptors. In this context, a study carried out by Cao Y et al. (2009) suggested that urinary concentrations of genistein and daidzein belonging to phytoestrogens were 500-fold higher in infants fed soy formula compared to those fed cow's milk formula.
According to the jurisdictions, not every chemical is defined as EDC. Therefore, an essential critical step in determining the exposures to chemicals having EDC properties is identifying their intrinsic hazard. Regulatory agencies use various tools to validate the available evidence for its identification (US EPA 2014; Andersson et al. 2018; OECD guidelines 2018). To understand the exact effect of EDCs, researchers have enlisted the key characteristics like Age at exposure, Latency from exposure, Importance of mixtures, Non-traditional dose–response dynamics, Transgenerational and epigenetic effects caused by the toxicant. Additionally, endocrinologists have suggested that these EDCs possess low water solubility and extremely high lipid solubility, ultimately leading to bioaccumulation in adipose tissue (Thorton 2001).
To date, it is challenging to predict the exact sources of EDC exposure due to their diversity and vary widely around the world. Nevertheless, the condition in different countries is constantly evolving because some EDCs were banned decades ago and others more recently. Thus, subjects like migrating people provide a model to study cessation and/or onset of exposure depending on the original and new milieus (Diamanti-Kandarakis et al. 2009). Several past examples of EDCs acting as toxicants through toxic spills or contamination from PCBs and their metabolites show a direct causal relationship between a toxicant and acting as an indicator of endocrine or reproductive dysfunction in wildlife in general and humans in particular. However, these typical illustrations represent single exposures and do not indicate indoor and outdoor toxicants' widespread, persistent exposure. So, household exposure can be viewed by the contamination of soil and groundwater by the agrochemical and industrial effluents possessing a high concentration of toxicants. These highly complex concoctions enter into the food chain and are bio-accumulated in animals leading to an increase in the concentration in the top consumer.
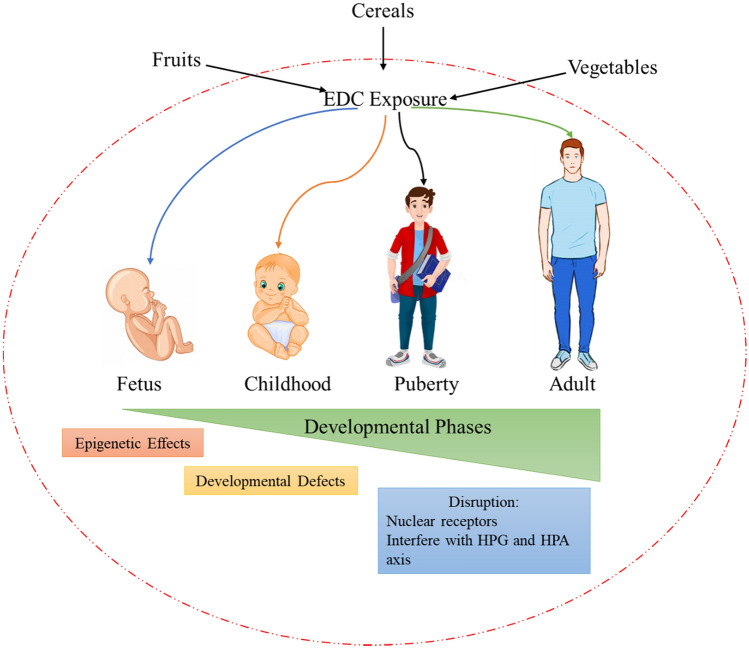
Additionally, instances from an occupation where People work with pesticides, fungicides, and industrial chemicals possess a high risk for exposure and thus ultimately leading to the disruption of normal homeostasis (Fig. 1). Table 1 shows the increased risk posed by EDCs to human health by different chemicals and studies carried across the globe. As the present review focus on the endocrine disruptors from our day-to-day source, the individual sections of EDCs are being discussed in detail.
Fig. 1.
EDCs from different sources like Fruits, Cereals, and Vegetables showing differing effects during the developmental phase
Table 1.
Different EDCs identified from the source point and their experimentally validated effects on Humans
| Name of EDCs | Source/Present in | Effect on humans | Reference |
|---|---|---|---|
| Diethylstilbestrol (DES) | Drugs |
•Higher Chances of Breast Cancer •Genital birth defects in infant males such as hypospadias and cryptorchidism |
Okada et al., (2001); IARC, (2012) |
| Dichlorodiphenyltrichloroethane (DDT) | Insecticide |
•Skin Lesions •Male offspring such as •Hypospadias and undescended testes •Pancreatic cancer •CNS toxicity |
Longnecker et al., (2002); John Beard (2006) |
| Chlorpyrifos, | Insecticide |
•Defects of the gonads, •Reduced activity of the luteinizing hormone (LH), and follicle-stimulating hormone (FSH) •Necrotic changes in the seminiferous Tubules |
Alaa-Eldin et al. (2017) |
| Atrazine, | Herbicide | •Hepatic steatosis | Foulds et al., (2017); Harper AP (2020) |
| 2, 4-D, Glyphosate | Herbicide |
•Oxidative stress, damages in liver and kidneys •Reproduction toxicity •Breast cancer •Endocrine disruption |
Mink et al., (2012); Swanson et al., (2014); Mesnage et al., (2015b); Fluegge and Fluegge, (2016); Fortes et al., (2016); Myers et al., (2016) |
| Bisphenol A (BPA), | Plastics |
•Obesity, •Diabetes mellitus, •Female infertility, •Male sexual dysfunction, •Reduced birth weight and atypical •Neurobehaviours in children |
Rochester (2013); Okugbe and Songhe (2019) |
| Phthalates | Food Storage Materials | •Reduced maternal levels of thyroid hormone | Horton et al., (2015); Steinmaus et al., (2016); Rubin et al., (2017); Knight et al., (2018) |
| Perfluorochemicals and PFAS | Non-Stick Food Wrappers, Microwave Popcorn Bags |
•Alter cholesterol levels •Disrupt thyroid function •Harm liver and kidney function •Alter immune response •Raise the risk of ulcerative colitis •Harm reproductive health •Increase the risk of birth defects •Decrease infant birth weights •Cause tumours and cancer |
Blake et al. (2020) |
Vegetables and Fruits
Vegetables and fruits are essential components of daily diet and provide most minerals, vitamins, folate and dietary fibre. The salad consists of raw vegetables such as carrot, cucumber, beetroot and lettuce. Experimental evidence suggests frequent consumption of salads lowered the risk of diabetes mellitus (Williams et al. 1999). In another study on south Asian subjects, low consumption of vegetables stood associated with greater susceptibility to non-communicable diseases (Jayawardena et al. 2020). In the editorial, del Río-Celestino and Font (2020) suggested that regular consumption of fruits and vegetables is associated with lower life-threatening diseases, such as cardiovascular ailments, metabolic syndromes, neoplastic conditions and neurodegenerative disorders. However, these health benefits of vegetables take a drastic U-turn when one consumes the same vegetables containing a trace quantity of Endocrine-disrupting chemicals (EDCs). Studies by Sandoval-Insausti et al. (2021) documented an association between dietary intake of conventional fruits and vegetables containing pesticide residues with cancer development. Salads are incomplete without carrots (Daucus carota) since this vegetable is rich in antioxidants such as alpha- and beta-carotene, lutein and lycopene (Boadi et al. 2021). Yet, when grown in a polluted environment laden with Polycyclic aromatic hydrocarbon (PAH), the same carrot is likely to cause potent endocrine disruption in adulthood (Zhu et al. 2021). Endocrine-disrupting chemicals (EDCs) are also found in commonly grown fruits and vegetables in Hyderabad, India, prompting us to assess benefits Vs risk of vegetable consumption (Sinha et al., 2009). In yet another innovative study, more than 24 novel Endocrine disrupting (ED) chemicals were assessed in commonly grown fruits and vegetables by employing techniques such as continuous Solid-phase extraction (SPE) and Gas chromatography-mass spectrometry (GC–MS) technique (Hejji et al. 2021). This study made it possible to detect major endocrine-disrupting chemicals such as organophosphorus pesticides, phenyl phenol, alkylphenols, parabens, triclosan, and bisphenol A in vegetable and fruit samples. Heavy metals such as cadmium, hexavalent chromium, tin, arsenic, mercury and lead are common pollutants and potent endocrine disruptors commonly found in the environment (Jia et al. 2021). In food, especially in vegetables, when the level of heavy metals exceeds recommended permitted level of exposure, either singly or in combination, it increases the risk of developing into gestational diabetes, supporting the contention of heavy metals as potential diabetogenic agents (Rezaei et al. 2017; Jia et al. 2021). Studies from our laboratory have documented high levels of endocrine-disrupting heavy metals in vegetables grown along the Baroda effluent channel, impacting the local subjects dwelling around the effluent channel (Ramachandran 2003; Mehrotra et al., 2021). Simulating the exact dosage of cadmium, based on the empirical field value of metals found in cereals and vegetables, we also documented cardiovascular abnormalities in rats (Mukherjee et al. 2011). Thus, it is logically clear to assume that EDCs in vegetables and fruits can mask the benefits of antioxidants and instead create endocrine perturbations.
Poultry
One of the best sources of inexpensive, high-quality protein is eggs, and daily consumption of eggs not only improves cognitive health, but available evidence has documented that regular consumption of table eggs can prevent age-related macular degeneration (Gopinath et al. 2020). Contrary to the popular notion that egg cholesterol content predisposes patients to cardiovascular disease and stroke, a significant cause of concern stands ascribed to the consumption of eggs contaminated with hazardous chemicals (Domingo 2014). Several endocrine disruptors such as hexachlorobenzene (HCB), polychlorinated naphthalenes (PCNs), polychlorinated-dibenzodioxins, polycyclic hydrocarbons (PAHs) have all been found in eggs from polluted sites (Domingo 2014; Pajurek et al. 2019). Home-grown eggs are more nutritious than commercial eggs across the entire Asian subcontinent, especially in the Indian subcontinent. Based on this notion, most villagers in tribal belts rear hen for a supply of eggs and meat. However, abuse of pesticides in the home garden has led to a substantial load of endocrine-disrupting pesticide residues such as hexachlorocyclohexane, aldrin, and malathion in locally laid eggs (Alaboudi et al., 2019). Sometimes the presence of EDCs in food products comes as a surprise. In several parts of the world, DDT is still in use for controlling mosquitoes. Bouwman et al. (2015) reported a high concentration of DDT in eggs in the entire village that used DDT for vector control, supporting the contention that consumption of endocrine disruptors through diet affects human health most unconventionally. Meat is an excellent dietary source of proteins, and it also contains some micronutrients such as vitamin B12, iron and zinc. In general, the global consumption of meat is on the rise due to altered dietary habits and societal influence (Godfray et al., 2018). Toxic agents such as Polybrominated diphenyl ethers (PBDEs), conventionally used as flame retardants, heavy metals and estrogenic endocrine-disrupting chemicals in supranormal concentration have been found in meat products warranting possible side effects on long term consumption (Pietron et al., 2019; Li et al., 2021; Boudray et al., 2021; Wang et al., 2019; Aendo et al., 2019). Both eggs and meat from contaminated areas, despite being protein-rich food, can also lead to endocrine disorders.
Fishes
The inclusion of fish in the diet has several advantages, and one such advantage is regular consumption of fish prevents cardiovascular ailments. A powerful antioxidant, Omega-3 fatty acid present in fishes augments low-density lipoprotein clearance by enhancing the activity of the enzyme lipoprotein lipase leading to diminished plasma triglycerides (Jo et al., 2021). Although the hazard index was below one, endocrine disruptors like dexamethasone, progesterone, and caffeine stand detected in fishes from Malaysian water bodies (Ismail et al., 2021). Under the Indian scenario, 1,1-Dichloro-2,2-bis(p-chlorophenyl) ethylene (p,p’-DDE) was the most abundant endocrine disruptor in fishes. At the same time, Polychlorinated biphenyls (PCBs) and Polybrominated diphenyl ethers (PBDEs) are the other EDCs found in edible fishes of the Indian market (Sharma et al. 2021). Nonylphenol exposure in freshwater fish Labeo rohita elicited oxidative stress and haematological alterations as the toxic response (Karmakar et al. 2021). Since humans consume fish, human exposure to EDCs through aquatic food in general, especially fishes, needs serious regulation.
Beverages
One famous quote on tea states, "Where there's tea, there's hope." Yet hope turns into despair when one comes across Ly et al. (2020) research data. The authors reported a whopping 400 plus pesticide residues in samples of green tea, which is otherwise considered a potent antioxidant. Several investigators have reported pesticide residues in tea and coffee (Cho et al. 2014; Hou et al. 2016; Siraj 2021). In Ethiopia, drinking tea, rich in organochlorine pesticides residues, has been associated with increased susceptibility to diseases (Trevisan et al. 2017). A potent endocrine disruptor, phthalate, has been found in trace quantities in commercial tea packages (Troici et al., 2019). Most average subjects drink a minimum of two cups of tea, a trend that continues for the entire life span. Hence, when present in beverages, Endocrine-disrupting chemicals (EDCs) pose a greater risk to human health when compared with conventional cereals, vegetables, and fruits.
Nutraceuticals
Medicinal plants help cure a wide variety of diseases and form the backbone of the herbal nutraceutical industry. Translocation of heavy metals such as cadmium, arsenic and lead, which are known to exhibit endocrine-disrupting properties, antagonizes these plants' medicinal properties (Tripathi et al. 2021). The innovative fluorescent technique enabled the detection of carbamate residues in Chinese medicinal plants (Wei et al. 2018). Carbamate and the organophosphate group of pesticides are potent endocrine disruptors and exert action by binding to the Androgen receptor (AR) and act as antagonists (Kitamura et al. 2006). Owing to easy availability and minimum side effects, herbal medicines find usage worldwide. In recent times, there is a considerable demand for herbal medicines in both underdeveloped and developed countries. Shaban et al. (2016) have reviewed the impact of heavy metals and pesticide residues in herbal products. According to the authors, the primary source of heavy metals for these plants is the soil and the quality of water used for irrigation. The presence of EDCs and other toxicants in herbal medicines and formulations can have severe implications in the sense that rather than getting cured, a subject would be rendered sick following consumption of herbal formulations contaminated with EDCs.
Gut Biome
In recent times, the gut microbiota has taken centre stage in the development of diseases (Mansour et al. 2021). Daily diet, besides altering microbial diversity, also regulates human health. Diabetes, one of the significant endocrine disorders, is linked to alterations in gut microbiota (Wu et al. 2020). The incidence of diabetes is on the rise, and as of now, diabetes mellitus is considered both a local and public health emergency (Al-Lawati 2017). Fertilizers, plastics, electronic goods, and pesticides release Endocrine-disrupting chemicals (EDCs) into the environment. There is a strong association between exposure to EDCs and predisposition to diabetes since gut microbiota metabolizes these EDCs leading to alterations in their toxicodynamics (Velmurugan, 2017).
Additionally, environmental endocrine disruptors such as pesticides, polychlorinated bisphenyls, heavy metals, bisphenol A, phthalates, and dioxins are linked with diabetes. Discussing the role of EDCs and their association with diet, Gálvez-Ontiveros (2020) highlighted the interrelationships between the complex triad consisting of EDCs, diet and metabolic diseases. The EDCs lead to microbial dysbiosis turning on the xenobiotics pathways wherein the microbial metabolites impact the development of the metabolic syndromes. From the above-stated examples, it is pertinent that gut microbiota and its association with EDCs in the disease set-up needs further research to get additional insights into the intricate mechanism involved in these interactions.
Packaging Industry
Not just food, even the food packaging industry contributes to endocrine disruption. Various materials such as paper used in tetra packs, plastic, ceramics, and metal cans are called Food contact materials (FCMs). While packaging increases the shelf life of food and prevents microbial contamination, the same can be hazardous to endocrine health as packaging materials transfer particles into the food. Bisphenol A, a potential endocrine disruptor affecting human reproduction, has been documented in high concentrations from Food contact materials (FCMs), especially from polycarbonate plastic containers used for hotel parcel service (Cavazza et al. 2021; Park et al. 2018). Sometimes heavy metals can potentiate the toxic effects of bisphenol A. In one such study, Chen et al. (2016) showed the influence of cadmium in enhancing genotoxicity of Bisphenol-A in fibroblast cell lines. FCMs, despite being high in EDCs, may not have any significant health implications, but lack of awareness leading to their improper use can be dangerous. The best example in this regard is microwave reheating of plastics used in the food industry, which leaches phthalate, an endocrine-disrupting chemical in food (Fang et al. 2017). We postulate that the global COVID-19 outbreak and associated changes in food behaviour are considerably related to endocrine health.
Even after the lockdown period, public perception prevented people from going to restaurants for dine-in (Tuzovic et al. 2021). Consumers instead preferred the 'Take away' mode of delivery from restaurants. This has led to the rampant use of plastic containers to cater parcel service by the food industry. Phthalates and bisphenol A (BPA), which leach out of plastic containers, are potent endocrine disruptors and cause obesity and obesity-related metabolic disorders (Biemann et al., 2021). Although 'Take Away' food practice avoided venturing into crowded restaurants and prevented the spread of novel coronavirus, alternations in eating habits pushed us more towards endocrine dysregulation. Diet and dietary patterns both play a crucial role in chronic diseases of the current century. It is becoming more and more evident from studies conducted on the role of nutrition in various conditions that diet plays an inevitable role not only in the pathophysiology of diseases but also in the prevention and protection of disease onset (Bibiloni et al. 2013; Silveira et al. 2018). The world over, there are several varied kinds of diet patterns adopted. The same is undergoing a sea change with the change in modern man's lifestyle with increasing advancements in technology. It is interesting to note that dietary patterns are changing. There is also an equally challenging concern of food toxicity increasing with exposure to unhealthy environments and accumulation of harmful toxicants. All in all, these together are factors that make a strong base for the disease pathogenesis of the current times. With advancements in nutrigenomics, the crosstalk between nutrition and diseases is only becoming clearer further than what is already known and understood (Irimie AI et al., 2019).
Antioxidant Status in Food
Plant-based antioxidants are richly present in fruits and vegetables. These antioxidants are reported to have several promising sound effects on health (Jideani et al., 2021). Citrus fruits, carrots, green leafy vegetables, bananas and several others have compounds with antioxidant properties. Antioxidants are generally known to have several effects on metabolism that regulate the progression and onset of several chronic diseases. Lower dietary intake of antioxidants seems to be associated with an increased risk of cardiovascular disorders and stroke. WHO has recommended a minimum nutritional intake of 400–500 g per day of food rich in antioxidants to prevent diseases like hypertension, stroke, cardiovascular disorder and several other lifestyle disorders. Important antioxidant constituents found in fruits and vegetables and their sources have are in the table below. The most common and well-documented antioxidant components are ascorbic acid, tocopherols, carotenes, alkaloids, sulfur-containing compounds, phenols, and polyphenolics (Table 2).
Table 2.
Type of antioxidant found in different fruits and vegetables which can be of potential use for maintaining healthy life
| Common food: fruits and vegetables (Indian context) | Type of antioxidant present | Reference |
|---|---|---|
| Apple |
Triterpenoids Flavonoids Phenolic acids |
Breda et al., 2018 |
| Apricot |
Tetrapenoids Flavonoids |
Breda et al., 2018 |
| Banana |
Phenolic acids Flavonoids Lignans Triterpenoids |
Breda et al., 2018; Singh et al., 2016 |
| Mango | Tetrapenoids | Breda et al., 2018; Septembre et al., 2016 |
| Orange |
Monoterpenoids Tetrapenoids Flavonoids Organic acids |
Breda et al., 2018; Septembre et al., 2016; Martinez et al., 2021 |
| Papaya |
Tetrapenoids Organic acids |
Septembre et al., 2016 |
| Pomegranate |
Phenolic acid Triterpenoids |
Breda et al., 2018; Moreira et al., 2017 |
| Carrot |
Carotenoids Stilbenoids Flavonoids Lignans Monoterpenoids Tetrapenoids |
Xavier et al., 2016; Breda et al., 2018 |
| Cabbage |
Glucosinolates Phenolic acids Lignans Tetrapenoids |
Breda et al., 2018 |
| Eggplant |
Phenolic acids Flavonoids Glycoalkaloids |
Bouhajeb et al., 2020, Makrogianni et al., 2017 |
| Onion |
Triterpenoids Sulfur compounds Flavonoids Lignans |
Breda et al., 2018 |
| Spinach |
Triterpenoids Tetrapenoids |
Breda et al., 2018 |
| Broccoli |
Tetrapenoids Triterpenoids Flavonoids Tannins |
Casajus et al., 2019 |
Apart from fruits and vegetables, recently, we have also published information on antioxidant constituents in several medicinal plants that have been known in traditional literature for their prevention and cure of several chronic diseases, as also listed in the table below. Such plants are natural sources with powerful antioxidants and have led to several drug molecule discoveries for diseases like cancer. Recently, there has also been an increased interest in identifying bioactive molecules from seaweeds and marine algae, which also have antioxidant properties. These plant sources have been promising new areas of scientific investigation, revealing a potential for drug targeting as well. It will be ironic if such plants also get contaminated with endocrine-disrupting chemicals, compromising their nutraceutical and medicinal values. It would be a paradoxical situation of 'health keepers turning health breakers' in such a scenario.
Dietary Patterns and Health and Disease
In the last decade, there is increasing literature evidence published to discuss the role of healthy versus unhealthy dietary patterns and the risk of diseases such as Alzheimer's, Cancer, Diabetes, etc. (Fénichel and Chevalier 2017; Samandi et al., 2019). There is a growing indication that all these diseases are commonly rooted in unhealthy diet patterns that lack sufficient dietary antioxidants. Oxidative stress and inflammation are the two root causes of these diseases, and unhealthy eating patterns are significant contributors. It has been observed that dietary patterns high in intake of fruits and vegetables than processed meat, sweetened food, and beverages are associated with a significantly increased risk of type 2 diabetes in Chinese men and women in Singapore (Liu et al., 2015). There are reports that dietary patterns and macronutrient profiles may also be responsible for the expression and secretion of inflammatory biomarkers that are associated with chronic disease biology. Some studies have also indicated that greater intake of the western diet is associated with more inferior semen quality in a general Asian population.
Conclusion and Future Prospects
Organisms in general and humans, in particular, are getting exposed to contaminated food, increasing day by day, ultimately leading to deteriorating health. This indicates that necessary action and precaution need to be taken to protect human health, which can be driven by developing regulatory policies for the use of EDCs in our day-to-day life. This policy should be made compulsory to be followed by the industries to achieve the aim of safeguarding human health. More awareness-driven knowledge regarding the safe use of EDCs in fruits, vegetables, etc., should be disseminated to everyday users. Furthermore, studies should be carried out involving revolutionized molecular techniques to understand the chronic studies on transgenerational approach, using Next-generation sequencing, and microarray studies on the routinely used toxicants on different populations to have a complete idea of the effects of these EDCs.
Declarations
Conflict of interest
The authors disclose there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Raktim Mukherjee, Parth Pandya, Darshee Baxi, A. V. Ramachandran have contributed equally to this work.
Contributor Information
Raktim Mukherjee, Email: raktimmukherjee2003@gmail.com.
Parth Pandya, Email: pkpandya.13@gmail.com.
Darshee Baxi, Email: darsheeb@nuv.ac.in.
A. V. Ramachandran, Email: avramachandran@nuv.ac.in
References
- Aendo P, Thongyuan S, Songserm T, Tulayakul P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Science of the Total Environment. 2019;689:215–222. doi: 10.1016/j.scitotenv.2019.06.414. [DOI] [PubMed] [Google Scholar]
- Alaa-Eldin, E.A. et al. 2017. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. 10.1007/s11356-016-7912-6. [DOI] [PubMed]
- Al-Lawati JA. Diabetes mellitus: A local and global public health emergency! Oman Medical Journal. 2017;32(3):177. doi: 10.5001/omj.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, N. et al. 2018. Guidance for the identification of endocrine disruptors in the Context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 16: e05311. [DOI] [PMC free article] [PubMed]
- Baudry J, Rebouillat P, Allès B, Cravedi JP, Touvier M, Hercberg S, Kesse-Guyot E. Estimated dietary exposure to pesticide residues based on organic and conventional data in omnivores, pesco-vegetarians, vegetarians and vegans. Food and Chemical Toxicology. 2021;153:112179. doi: 10.1016/j.fct.2021.112179. [DOI] [PubMed] [Google Scholar]
- Bibiloni MM, Maffeis C, Llompart I, Pons A, Tur JA. Dietary factors associated with subclinical inflammationamong girls. European Journal of Clinical Nutrition. 2013;67(12):1264–1270. doi: 10.1038/ejcn.2013.196. [DOI] [PubMed] [Google Scholar]
- Blake BE, Fenton SE. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology. 2020;443:152565. doi: 10.1016/j.tox.2020.152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boadi, N. O., Badu, M., Kortei, N. K., Saah, S. A., Annor, B., Mensah, M. B., & Fiebor, A. 2021. Nutritional composition and antioxidant properties of three varieties of carrot (Daucus carota). Scientific African, e00801.
- Bouhajeb R, Selmi S, Nakbi A, Jlassi I, Montevecchi G, Flamini G, Zarrad I, Dabbou S. Chemical Composition Analysis, Antioxidant, and Antibacterial Activities of Eggplant Leaves. Chemistry & Biodiversity. 2020;17(12):e2000405. doi: 10.1002/cbdv.202000405. [DOI] [PubMed] [Google Scholar]
- Bouwman H, Bornman R, Van Dyk C, Barnhoorn I. First report of the concentrations and implications of DDT residues in chicken eggs from a malaria-controlled area. Chemosphere. 2015;137:174–177. doi: 10.1016/j.chemosphere.2015.06.097. [DOI] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva and blood of infants—data from a pilot study on the estrogenic activity of soy formula. Journal of Exposure Science & Environmental Epidemiology. 2009;19:223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casajús V, Reyes Jara A, Gergoff G, Gómez Lobato M, Civello P, Martínez G. The time of the day to harvest affects the degreening, antioxidant compounds, and protein content during postharvest storage of broccoli. Journal of Food Biochemistry. 2019;43(7):e12904. doi: 10.1111/jfbc.12904. [DOI] [PubMed] [Google Scholar]
- Cavazza A, Bignardi C, Grimaldi M, Salvadeo P, Corradini C. Oligomers: Hidden sources of bisphenol A from reusable food contact materials. Food Research International. 2021;139:109959. doi: 10.1016/j.foodres.2020.109959. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Liu C, Lu YH, Yang LL, Li M, He MD, Zhou Z. Cadmium exposure enhances bisphenol A-induced genotoxicity through 8-oxoguanine-DNA glycosylase-1 OGG1 inhibition in NIH3T3 fibroblast cells. Cellular Physiology and Biochemistry. 2016;39(3):961–974. doi: 10.1159/000447804. [DOI] [PubMed] [Google Scholar]
- Cho SK, Abd El-Aty AM, Rahman MM, Choi JH, Shim JH. Simultaneous multi-determination and transfer of eight pesticide residues from green tea leaves to infusion using gas chromatography. Food Chemistry. 2014;165:532–539. doi: 10.1016/j.foodchem.2014.05.145. [DOI] [PubMed] [Google Scholar]
- del Río-Celestino Mercedes, Font Rafael. The Health Benefits of Fruits and Vegetables. Foods. 2020;9(3):369. doi: 10.3390/foods9030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL. Health risks of human exposure to chemical contaminants through egg consumption: A review. Food Research International. 2014;56:159–165. [Google Scholar]
- Fang H, Wang J, Lynch RA. Migration of di (2-ethylhexyl) phthalate (DEHP) and di-n-butylphthalate (DBP) from polypropylene food containers. Food Control. 2017;73:1298–1302. [Google Scholar]
- Fénichel P, Chevalier N. Environmental endocrine disruptors: New diabetogens? Comptes Rendus Biologies. 2017;340(9–10):446–452. doi: 10.1016/j.crvi.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Fluegge K, Fluegge K. Glyphosate use predicts healthcare utilization for ADHD in the healthcare cost and utilization project net (HCUPnet): A two-way fixed-effects analysis. Polish Journal of Environmental Studies. 2016;25:1489–1503. [Google Scholar]
- Fortes C, Mastroeni S, Segatto MM, Hohmann C, Miligi L, Bakos L, Bonamigo R. Occupational exposure to pesticides with occupational sun exposure increases the risk for cutaneous melanoma. Journal of Occupational and Environmental Medicine. 2016;58:370–375. doi: 10.1097/JOM.0000000000000665. [DOI] [PubMed] [Google Scholar]
- Foulds CE, Trevino LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nature Reviews Endocrinology. 2017;3:445–57. doi: 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez-Ontiveros Y, Páez S, Monteagudo C, Rivas A. Endocrine disruptors in food: Impact on gut microbiota and metabolic diseases. Nutrients. 2020;12(4):1158. doi: 10.3390/nu12041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray H, Charles J, Aveyard Paul, Garnett Tara, Hall Jim W, Key Timothy J, Lorimer Jamie, Pierrehumbert Ray T, Scarborough Peter, Springmann Marco, Jebb Susan A. Meat consumption, health, and the environment. Science. 2018;361:6399. doi: 10.1126/science.aam5324. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Liew G, Tang D, Burlutsky G, Flood VM, Mitchell P. Consumption of eggs and the 15-year incidence of age-related macular degeneration. Clinical Nutrition. 2020;39(2):580–584. doi: 10.1016/j.clnu.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Harper AP, Finger BJ, Green MP. Chronic Atrazine Exposure Beginning Prenatally Impacts Liver Function and Sperm Concentration With Multi-Generational Consequences in Mice. Front. Endocrinol. 2020;11:580124. doi: 10.3389/fendo.2020.580124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejji L, Azzouz A, Colón LP, Souhail B, Ballesteros E. A multi-residue method for determining twenty-four endocrine disrupting chemicals in vegetables and fruits using ultrasound-assisted solid–liquid extraction and continuous solid-phase extraction. Chemosphere. 2021;263:128158. doi: 10.1016/j.chemosphere.2020.128158. [DOI] [PubMed] [Google Scholar]
- Horton MK, et al. Co-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environment Research. 2015;143:1–9. doi: 10.1016/j.envres.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Lei S, Guo L, Qiu S. Optimization of a multi-residue method for 101 pesticides in green tea leaves using gas chromatography–tandem mass spectrometry. Revista Brasileira De Farmacognosia. 2016;26:401–407. [Google Scholar]
- Irimie AI, Braicu C, Pasca S, Magdo L, Gulei D, Cojocneanu R, Ciocan C, Olariu A, Coza O, Berindan-Neagoe I. Role of Key Micronutrients from Nutrigenetic and Nutrigenomic Perspectives in Cancer Prevention. Medicina (Kaunas). 2019;55(6):283. doi: 10.3390/medicina55060283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail NAH, Wee SY, Aris AZ. Multi-class of endocrine disrupting compounds in aquaculture ecosystems and health impacts in exposed biota. Chemosphere. 2017;188:375–388. doi: 10.1016/j.chemosphere.2017.08.150. [DOI] [PubMed] [Google Scholar]
- Jayawardena R, Jeyakumar DT, Gamage M, Sooriaarachchi P, Hills AP. Fruit and vegetable consumption among South Asians: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(6):1791–1800. doi: 10.1016/j.dsx.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Jia X, Zhang L, Zhao J, Ren M, Li Z, Wang J, Ye R. Associations between endocrine-disrupting heavy metals in maternal hair and gestational diabetes mellitus: A nested case-control study in China. Environment International. 2021;157:106770. doi: 10.1016/j.envint.2021.106770. [DOI] [PubMed] [Google Scholar]
- Jideani Afam I. O, Silungwe Henry, Takalani Thakhani, Omolola Adewale O, Udeh Henry O, Anyasi Tonna A. Antioxidant-rich natural fruit and vegetable products and human health. International Journal of Food Properties. 2021;24(1):41–67. doi: 10.1080/10942912.2020.1866597. [DOI] [Google Scholar]
- Karmakar S, Karmakar S, Jana P, Chhaba B, Das SA, Rout SK. Nonylphenol exposure in Labeo rohita (Ham.): Evaluation of behavioural response, histological, haematological and enzymatic alterations. Comparative Biochemistry and Physiology. 2021;247:109058. doi: 10.1016/j.cbpc.2021.109058. [DOI] [PubMed] [Google Scholar]
- Kitamura, S., Sugihara, K., & Fujimoto, N. 2006. Endocrine disruption by organophosphate and carbamate pesticides. In Toxicology of Organophosphate & Carbamate Compounds (481–494). Academic Press.
- Knight BA, et al. Effect of perchlorate and thiocyanate exposure on thyroid function of pregnant women from South-West England: A cohort study. Thyroid Research. 2018;11:9. doi: 10.1186/s13044-018-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Jia Z, Wang T, Zhang H. Screening of estrogenic endocrine-disrupting chemicals in meat products based on the detection of vitellogenin by enzyme-linked immunosorbent assay. Chemosphere. 2021;263:128251. doi: 10.1016/j.chemosphere.2020.128251. [DOI] [PubMed] [Google Scholar]
- Liu C-Y, Chou Y-C, Chao JC-J, Hsu C-Y, Cha T-L, Tsao C-W. The Association between Dietary Patterns and Semen Quality in a General Asian Population of 7282 Males. PLoS ONE. 2015;10(7):e0134224. doi: 10.1371/journal.pone.0134224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. American Journal of Epidemiology. 2002;155:313–322. doi: 10.1093/aje/155.4.313. [DOI] [PubMed] [Google Scholar]
- Ly TK, Ho TD, Behra P, Nhu-Trang TT. Determination of 400 pesticide residues in green tea leaves by UPLC-MS/MS and GC-MS/MS combined with QuEChERS extraction and mixed-mode SPE clean-up method. Food chemistry. 2020;326:126928. doi: 10.1016/j.foodchem.2020.126928. [DOI] [PubMed] [Google Scholar]
- Makrogianni DI, Tsistraki A, Karapanos IC, Passam HC. Nutritional value and antioxidant content of seed-containing and seedless eggplant fruits of two cultivars grown under protected cultivation during autumn-winter and spring-summer. Journal of the Science of Food and Agriculture. 2017;97(11):3752–3760. doi: 10.1002/jsfa.8238. [DOI] [PubMed] [Google Scholar]
- Mansour SR, Moustafa MAA, Saad BM, Hamed R, Moustafa AR. Impact of diet on human gut microbiome and disease risk. New Microbes and New Infections. 2021;41:100845. doi: 10.1016/j.nmni.2021.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez PF, Carvalho MR, Mendonça MLM, Okoshi MP, Oliveira-Junior SA. Antioxidant and Anti-Inflammatory Effects of Orange Juice. Arquivos Brasileiros De Cardiologia. 2021;116(6):1137–1138. doi: 10.36660/abc.20210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage R, Defarge N, De Vendomois JS, Seralini G. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food and Chemical Toxicology. 2015;84:133–153. doi: 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Mink PJ, Mandel JS, Sceurman BK, Lundin JI. Epidemiologic studies of glyphosate and cancer: A review. Regulatory Toxicology and Pharmacology. 2012;63:440–452. doi: 10.1016/j.yrtph.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Moreira H, Slezak A, Szyjka A, Oszmianski J, Gasiorowski K. Antioxidant And Cancer Chemopreventive Activities Of Cistus And Pomegranate Polyphenols. Acta Poloniae Pharmaceutica. 2017;74(2):688–698. [PubMed] [Google Scholar]
- Mukherjee R, Banerjee S, Joshi N, Singh PK, Baxi D, Ramachandran AV. A combination of melatonin and alpha lipoic acid has greater cardioprotective effect than either of them singly against cadmium-induced oxidative damage. Cardiovascular Toxicology. 2011;11(1):78–88. doi: 10.1007/s12012-010-9092-9. [DOI] [PubMed] [Google Scholar]
- Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environmental Health. 2016;15:1–13. doi: 10.1186/s12940-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Revised guidance document 150 on standardised test guidelines for evaluating chemicals for endocrine disruption. OECD Series on Testing and Assessment. 2018 doi: 10.1787/9789264304741-en. [DOI] [Google Scholar]
- Okada A, Sato T, Ohta Y, Buchanan D, Iguchi T. Effect of diethylstilbestrol on cell proliferation and expression of epidermal growth factor in the developing female rat reproductive tract. Journal of Endocrinology. 2001;170:539–554. doi: 10.1677/joe.0.1700539. [DOI] [PubMed] [Google Scholar]
- Okugbe EO, Zhang Songhe. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A Review. 2019 doi: 10.1016/j.sciaf.2019.e00135. [DOI] [Google Scholar]
- Pajurek M, Pietron W, Maszewski S, Mikolajczyk S, Piskorska-Pliszczynska J. Poultry eggs as a source of PCDD/Fs, PCBs, PBDEs and PBDD/Fs. Chemosphere. 2019;223:651–658. doi: 10.1016/j.chemosphere.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Park SR, Park SJ, Jeong MJ, Choi JC, Kim M. Fast and simple determination and exposure assessment of bisphenol A, phenol, p-tert-butylphenol, and diphenylcarbonate transferred from polycarbonate food-contact materials to food simulants. Chemosphere. 2018;203:300–306. doi: 10.1016/j.chemosphere.2018.03.185. [DOI] [PubMed] [Google Scholar]
- Pietron W, Pajurek M, Mikolajczyk S, Maszewski S, Warenik-Bany M, Piskorska-Pliszczynska J. Exposure to PBDEs associated with farm animal meat consumption. Chemosphere. 2019;224:58–64. doi: 10.1016/j.chemosphere.2019.02.067. [DOI] [PubMed] [Google Scholar]
- Ramachandran, A. V. 2003. Aftermath of Baroda Effluent Channel: Impact assessment along the channel and the Mahi estuary with Reference to Heavy Metals. Environment global changes and challenges. 15–49.
- Rezaei M, Khodayar MJ, Seydi E, Soheila A, Parsi IK. Acute, but not chronic, exposure to arsenic provokes glucose intolerance in rats: Possible roles for oxidative stress and the adrenergic pathway. Canadian Journal of Diabetes. 2017;41(3):273–280. doi: 10.1016/j.jcjd.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: A review of the literature. Reproductive Toxicology. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rubin R, et al. Maternal perchlorate exposure in pregnancy and altered birth outcomes. Environment Research. 2017;158:72–81. doi: 10.1016/j.envres.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary Pattern in Relation to the Risk of Alzheimer's Disease: A Systematic reviewNeurological Sciences. 2019;40(10):2031–2043. doi: 10.1007/s10072-019-03976-3. [DOI] [PubMed] [Google Scholar]
- Sandoval-Insausti H, Chiu YH, Lee DH, Wang S, Hart JE, Mínguez-Alarcón L, Chavarro JE. Intake of fruits and vegetables by pesticide residue status in relation to cancer risk. Environment International. 2021;156:106744. doi: 10.1016/j.envint.2021.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septembre-Malaterre A, Stanislas G, Douraguia E, Gonthier MP. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chemistry. 2016;1(212):225–233. doi: 10.1016/j.foodchem.2016.05.147. [DOI] [PubMed] [Google Scholar]
- Shaban NS, Abdou KA, Hassan NEHY. Impact of toxic heavy metals and pesticide residues in herbal products. Beni-Suef University Journal of Basic and Applied Sciences. 2016;5(1):102–106. [Google Scholar]
- Sharma BM, Bharat GK, Chakraborty P, Martiník J, Audy O, Kukučka P, Nizzetto L. A comprehensive assessment of endocrine-disrupting chemicals in an Indian food basket: Levels, dietary intakes, and comparison with European data. Environmental Pollution. 2021;288:117750. doi: 10.1016/j.envpol.2021.117750. [DOI] [PubMed] [Google Scholar]
- Silveira BKS, Oliveira TMS, Andrade PA, Hermsdorff HHM, Rosa COB, Franceschini SDCC. Dietary Pattern and Macronutrients Profile on the Variation of Inflammatory Biomarkers: Scientific Update. Cardiology Research and Practice. 2018;14(2018):4762575. doi: 10.1155/2018/4762575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits - A review. Food Chemistry. 2016;1(206):1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Siraj, J. 2021. Organochlorine pesticide residues in tea and their potential risks to consumers in Ethiopia. Heliyon, e07667. [DOI] [PMC free article] [PubMed]
- Steinmaus C, et al. Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in southern California. Environmental Health Perspectives. 2016;124:861–867. doi: 10.1289/ehp.1409614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson NL, Leu A, Abrahamson J, Wallet B. Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. Journal of Organic Systems. 2014;9:6–37. [Google Scholar]
- Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food and Chemical Toxicology. 2013;59:129–136. doi: 10.1016/j.fct.2013.05.057. [DOI] [PubMed] [Google Scholar]
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan MTS, Owen RW, Calatayud-Vernich P, Breuer A, Picó Y. Pesticide analysis in coffee leaves using a quick, easy, cheap, effective, rugged and safe approach and liquid chromatography tandem mass spectrometry: Optimization of the clean-up step. Journal of Chromatography A. 2017;1512:98–106. doi: 10.1016/j.chroma.2017.07.033. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Sharma P, Singh K, Purchase D, Chandra R. Translocation of heavy metals in medicinally important herbal plants growing on complex organometallic sludge of sugarcane molasses-based distillery waste. Environmental Technology & Innovation. 2021;22:101434. [Google Scholar]
- Troisi J, Richards S, Symes S, Ferretti V, Di Maio A, Amoresano A, De Castro O. A comparative assessment of metals and phthalates in commercial tea infusions: A starting point to evaluate their tolerance limits. Food Chemistry. 2019;288:193–200. doi: 10.1016/j.foodchem.2019.02.115. [DOI] [PubMed] [Google Scholar]
- Tuzovic S, Kabadayi S, Paluch S. To dine or not to dine? Collective wellbeing in hospitality in the COVID-19 era. International Journal of Hospitality Management. 2021;95:102892. doi: 10.1016/j.ijhm.2021.102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaid Hafiz, ur Rahman, Waqas Asghar, Wahab Nazir, Mansur Abdullah Sandhu, Anwaar Ahmed, Nauman Khalid. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: Evidence of mechanisms, exposures and mitigation strategies. Science of The Total Environment. 2021;755(2):142649. doi: 10.1016/j.scitotenv.2020.142649. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency. 2014. Framework for human health risk assessment to inform decision making. US EPA https://www.epa.gov/risk/framework-human-health-risk-assessment-inform-decision-making.
- Van Breda SGJ, de Kok TMCM. Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases. Molecular Nutrition & Food Research. 2018;62:1700597. doi: 10.1002/mnfr.201700597. [DOI] [PubMed] [Google Scholar]
- Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut microbiota, endocrine-disrupting chemicals, and the diabetes epidemic. Trends in Endocrinology & Metabolism. 2017;28(8):612–625. doi: 10.1016/j.tem.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Geng Z, Liu Y, Guo L, Xiao G. Spatial analysis of heavy metals in meat products in China during 2015–2017. Food Control. 2019;104:174–180. [Google Scholar]
- Wei JC, Wei B, Yang W, He CW, Su HX, Wan JB, Wang YT. Trace determination of carbamate pesticides in medicinal plants by a fluorescent technique. Food and Chemical Toxicology. 2018;119:430–437. doi: 10.1016/j.fct.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Williams DE, Wareham NJ, Cox BD, Byrne CD, Hales CN, Day NE. Frequent salad vegetable consumption is associated with a reduction in the risk of diabetes mellitus. Journal of Clinical Epidemiology. 1999;52(4):329–335. doi: 10.1016/s0895-4356(99)00006-2. [DOI] [PubMed] [Google Scholar]
- IARC.2012. Working Group on the Evaluation of Carcinogenic Risks to Humans. Pharmaceuticals. Volume 100 A. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 100: 1–401.
- Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Bäckhed F. The gut microbiota in prediabetes and diabetes: A population-based cross-sectional study. Cell Metabolism. 2020;32(3):379–390. doi: 10.1016/j.cmet.2020.06.011. [DOI] [PubMed] [Google Scholar]
- Xavier AA, Pérez-Gálvez A. Carotenoids as a Source of Antioxidants in the Diet. Subcellular Biochemistry. 2016;79:359–375. doi: 10.1007/978-3-319-39126-7_14. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Huang H, Zhang Y, Xiong G, Zhang Q, Li Y, Liu W. Evaluation of PAHs in edible parts of vegetables and their human health risks in Jinzhong City, Shanxi Province, China: A multimedia modeling approach. Science of The Total Environment. 2021;773:145076. doi: 10.1016/j.scitotenv.2021.145076. [DOI] [PubMed] [Google Scholar]