Abstract
Microbial resistance is a serious threat to human health worldwide. Among the World Health Organisation’s list of priority resistant bacteria, three are listed as critical—the highest level of concern—and all three are Gram-negative. Gram-negative resistance has spread worldwide via a variety of mechanisms, the most problematic being via AmpC enzymes, extended-spectrum β-lactamases, and carbapenemases. A combination of older drugs, many with high levels of toxicity, and newer agents are being used to combat multidrug resistance, with varying degrees of success. This review discusses the current treatments for multidrug-resistant Gram-negative bacteria, including new agents, older compounds, and new combinations of both, and some new treatment targets that are currently under investigation.
Introduction
Antimicrobial resistance is a complex and dynamic phenomenon, mostly relying on a complicated interaction between direct factors, such as misuse of antimicrobials in humans and agricultural animals, indirect factors, such as environmental pollution and poor sanitation, and the innate characteristics of the bacteria themselves.1 Previous antibiotic exposure, underlying diseases, and invasive procedures have been identified by some researchers as the risk factors most associated with resistance.2 However, the risk factors for spread of resistance vary by geography: according to the WHO, antimicrobial resistance in developing countries is more likely to be spread through poor sanitation and lack of clean drinking water,3 whereas data from the United States (US) indicate that one in five resistant infections are caused by exposure to contaminated food or animals.4 In Europe, factors for spread of antimicrobial resistance have been cited as cross-border transfer of patients carrying MDR bacteria, transmission of MDR pathogens in and between healthcare settings, antimicrobial over-use and misuse, and inconsistent infection control practices.5 The Asia-Pacific region, home to two-thirds of the world’s population, is highly vulnerable to increased antimicrobial resistance. Here, the spread of resistance is more likely driven by factors such as rapidly growing and densely populated cities and increasing wealth and the associated increase in mass-farming practices.6 Clearly, detailed information on the relative contribution of the various factors to the overall global problem of MDR infections has not been adequately researched and is yet to be fully elucidated.1 There is a need, therefore, to address the multiple factors associated with MDR infections both across the globe and locally based on the different epidemiological and societal scenarios.
The specific mechanisms by which pathogens become resistant to antimicrobials may be innate, adaptive or acquired by the organism, and include mechanisms that limit drug penetration into, or increase drug removal from, the bacteria, modification of the drug targets through mutation selection, or enzymatic inactivation of drugs. Regardless of the mechanism, antimicrobial resistance is already limiting our ability to successfully treat infections,7 and thus poses a serious threat to human health.8 MDR Gram-negative organisms, particularly carbapenem-resistant Enterobacterales (formerly known as Enterobacteriaceae), carbapenem-resistant Pseudomonas aeruginosa, and extensively-drug-resistant (XDR) Acinetobacter baumannii, present a particularly grave threat worldwide.9
This review discusses the current and future burden of MDR Gram-negative infections, treatment options—existing and potential—and other considerations in the overall management of MDR Gram-negative infections, including the importance of understanding local epidemiology and enabling rapid diagnosis.
The current and future burden of MDR Gram-negative infections
The increased threat from Gram-negative MDR species is widely acknowledged by global and national organizations including the WHO,8 European Centre for Disease Prevention and Control,10 Infectious Diseases Society of America (IDSA),4 and the US CDC.11 Indeed, among the WHO’s list of priority resistant bacteria for 2016–17, three are described as critical—the highest level of concern—and all three are Gram-negative, namely carbapenem-resistant Enterobacterales, carbapenem-resistant A. baumannii, and carbapenem-resistant P. aeruginosa.8 According the 2013 CDC report, 6.6% of the 140 000 most serious healthcare-related Enterobacterales infections occurring annually in the US are resistant to carbapenems, while 63% of the 12 000 Acinetobacter infections, and 13% of the estimated 51 000 Pseudomonas infections are multidrug resistant.12 While the 2019 report describes a relatively reduced incidence of many of these infections, the incidence of carbapenem-resistant infections has remained stable, and MDR organisms are still considered a global critical threat.11 In Europe, the highest levels of MDR infections were reported for P. aeruginosa,13 with carbapenem resistance in 2017 reportedly as high as 63% in some countries in Southern and South Eastern Europe.14
In a 2016 analysis of 175 studies conducted in several countries in Southeast Asia, carbapenem-resistant Enterobacterales rates were relatively low (2.8%), while carbapenem-resistant A. baumannii and carbapenem-resistant P. aeruginosa rates were 73.0% and 29.8%, respectively; however, the prevalence of all three species of resistant bacteria was rising.15 In China, data from the China Antimicrobial Surveillance Network showed that 71.4% of Acinetobacter spp. strains, 10% of Enterobacterales strains and 20%–30% of P. aeruginosa strains isolated in 2017 were resistant to carbapenems.16
The most serious outcomes of Gram-negative MDR occur in critically ill and other high-risk patients, and MDR is associated with high levels of mortality and inappropriate use of antibacterial treatment in patients with MDR infections. For example, in neutropenic patients, carbapenem resistance is increasing, particularly among Pseudomonas species, and mortality rates for neutropenic patients (primarily those with haematological malignancies) with carbapenem-resistant bloodstream infections (BSI) range from 33.3% to 71.4%.17 In haematopoietic stem cell recipients, inappropriate empirical antibacterial therapy was reportedly given in 46.2% of cases of MDR Gram-negative infection.18
Mechanisms of resistance
Broadly, organisms develop resistance to multiple antimicrobials via successive mutations, dissemination of multiresistance plasmids or transposons, or a combination of both processes.19
Specific mechanisms of resistance developed by organisms are more complex. Among the most problematic and relevant resistance mechanism developed by Gram-negative bacteria is that of β-lactamases, enzymes that transfer resistance to β-lactam (BL) antibiotics, a broad range of highly useful compounds that includes penicillin derivatives, cephalosporins, monobactams, and carbapenems. There are two main classification systems for β-lactamases: the Ambler classification system in which enzymes are classified according to their protein sequences (Ambler classes A, B, C and D; Table 1)20 and the Bush–Jacoby system, which classifies the enzymes according to their clinical phenotypes.21
Table 1.
Ambler classification of β-lactamases by main antibacterial substrate21
| β-Lactamase enzyme | Main antibacterial substrate | Inhibited by |
|---|---|---|
| Ambler class A | ||
| PC 1 | Penicillins | Clavulanic acid or tazobactam |
| TEM-1, TEM-2, SHV-1 | Penicillins, early cephalosporins | Clavulanic acid or tazobactam |
| TEM-3, SHV-2, CTX-M-15, PER-1, VEB-1 | Extended-spectrum cephalosporins, monobactams | Clavulanic acid or tazobactam |
| TEM-30, SHV-10 | Penicillins | |
| TEM-50 | Extended-spectrum cephalosporins, monobactams | |
| PSE-1, CARB-3 | Carbenicillin | Clavulanic acid or tazobactam |
| RTG-4 | Carbenicillin, cefepime | Clavulanic acid or tazobactam |
| CepA | Extended-spectrum cephalosporins | Clavulanic acid or tazobactam |
| KPC-2, IMI-1, SME-1 | Carbapenems | Clavulanic acid or tazobactam (variable) |
| Ambler class B | ||
| IMP-1, VIM-1, CcrA, IND-1, L1, CAU-1, GOB-1, FEZ-1 | Carbapenems (not monobactams) | EDTA |
| CphA, Sfh-1 | Carbapenems | EDTA |
| Ambler class C | ||
| E. coli AmpC, P99, ACT-1, CMY-2, FOX-1, MIR-1, GC1, CMY-37 | Cephalosporins | |
| Ambler class D | ||
| OXA-1, OXA-10 | Cloxacillin | Clavulanic acid or tazobactam (variable) |
| OXA-11, OXA-15 | Extended-spectrum cephalosporins | Clavulanic acid or tazobactam (variable) |
| OXA-23, OXA-48 | Carbapenems | Clavulanic acid or tazobactam (variable) |
Carbapenem resistance is particularly serious given that carbapenems are often the last resort in treating infections resistant to other drugs. Carbapenem resistance mechanisms have spread across the world and between organisms, and a wide range of enzymes have been identified among carbapenemase-producing Enterobacterales. These include the serine β-lactamases Klebsiella pneumoniae carbapenemase (KPC) (Ambler class A), metallo-β-lactamase (MBL) including New Delhi MBL (NDM) or Verona integron-encoded MBL (VIM), imipenemase (IMP) (Ambler class B) and OXA-48-like carbapenemases (Ambler class D).22 KPCs hydrolyse penicillins, cephalosporins, monobactams and carbapenems.23,24 KPC, NDM and OXA-48 enzymes are among the carbapenem resistance mechanisms of greatest concern.25
In addition to carbapenemase production, which is a common mechanism of carbapenem resistance in Enterobacterales, other such mechanisms include porin mutations and efflux pump upregulation.26 For example, in P. aeruginosa, carbapenem resistance occurs as a result of the loss of porin OprD or increased expression of MexAB-OprM, MexXY-OprM or MexCD-Opr efflux pumps, or a combination of the two. In A. baumannii, in addition to Ambler class D carbapenemases, such as OXA-23, OXA-40 and OXA-58, carbapenem resistance can result from AdeABC efflux pump overexpression.26
Resistance can also be viewed in terms of the specific antibacterials or antibacterial classes that are affected by these mechanisms. Examples include fluoroquinolone resistance, which occurs via mutations in DNA gyrase genes gyrA and gyrB; resistance to tigecycline, stemming from mutational upregulation of arcA/B-mediated efflux; and resistance to third-generation cephalosporins, which occurs via mutational de-repression of AmpC β-lactamases in certain species, including Enterobacter spp.19
Conversely, different bacteria also exhibit different levels of resistance to the same antimicrobial. In Canada, nitrofurantoin resistance rates were reportedly 16% in ESBL-producing E. coli, 71% in nosocomial ESBL-producing Klebsiella spp. and 93% in non-nosocomial ESBL-producing Klebsiella spp.19
Choice of antibacterial agent also differs between countries, both in terms of empirical therapy and targeted therapy against known pathogens.27 For example, a 2017 post hoc analysis of the INCREMENT study of treatment of BSI caused by MDR Enterobacterales found that carbapenems are more commonly used as empirical therapy in the USA and Taiwan, while empirical use of a β-lactam + β-lactamase inhibitor (BL/BLI) combination is more widespread in Italy and Turkey. For targeted treatment, regimens comprising a carbapenem plus at least one other agent were used in 17.1% (82/479) of cases overall, and most commonly in Italy (31/115; 27.0%), Greece (16/89; 18.0%) and Turkey (5/27; 18.5%); this despite the high levels of carbapenem resistance in Italy and Greece.28 Importantly, therefore, the INCREMENT study found that the differences in antibacterial use in the context of MDR Enterobacterales were not always explained by geographical variations in resistance patterns, and are influenced by historical practices and the clinical presentation and severity of these infections. Overall, however, countries with more carbapenem resistance tend to use more combination therapies.28 Robust surveillance systems and high-quality evidence of the efficacy of new treatments are needed to develop antibiotic stewardship protocols in MDR infections, and thereby reduce mortality and morbidity.
Attempts are ongoing to overcome antibacterial resistance by using new agents and combinations of new plus old agents. For example, both old (clavulanic acid, tazobactam) and new (avibactam, vaborbactam, relebactam) BLIs are being used in combination with other agents to counteract β-lactamases, and a number of BL/BLI combinations are now available.29,30 Interestingly, some BLIs also have a BL-enhancing mechanism that is independent of the BLI mechanism.29 Another analysis of data from the INCREMENT study showed that BL/BLI combinations with in vitro activity were as effective as carbapenems for the empirical or targeted treatment of ESBL Enterobacterales BSI.31
Therapeutic approaches for MDR Gram-negative infections
The most serious MDR clinical scenarios
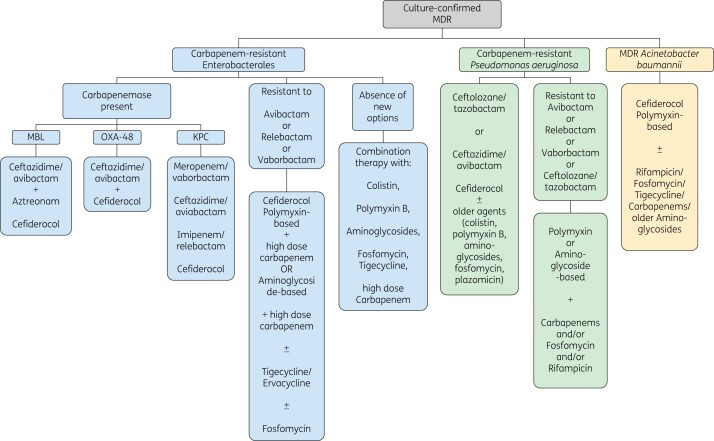
The threat of MDR Gram-negative infection is most serious among the critically ill, who often have multiple comorbidities. Recommendations for managing them are organized in one of two ways. Most treatment guidelines for managing MDR Gram-negative infections address broad clinical and epidemiological scenarios, rather than specific MDR Gram-negative pathogens. Furthermore, useful guidelines must address local resistance patterns and accommodate the potential need for rapid changes in recommendations. There are, however, published studies and reviews that contain recommendations presented by MDR pathogen, by antibacterial agent/class, or by disease. Bassetti et al.32 proposed a treatment algorithm for critically ill patients in the ICU according to MDR pathogen. Broadly, and allowing for local resistance patterns, their first-line recommendations, based on non-clinical and clinical evidence, are: ceftazidime/avibactam (Ambler classes A, C and D), meropenem/vaborbactam (A and C), imipenem/relebactam (A and C), aztreonam/avibactam (A, B and C) or cefiderocol (A, B and D) for carbapenem-resistant Enterobacterales; ceftolozane/tazobactam, imipenem/relebactam (A and C) or cefiderocol (A, B and D) for carbapenem-resistant P. aeruginosa; and cefiderocol-based (A, B and D) treatment for carbapenem-resistant A. baumannii.32,33 Similarly, a recent review by Peri et al.34 proposed a set of recommendations specifically for treating carbapenem-resistant Enterobacterales (CRE) and MDR A. baumannii and P. aeruginosa (Figure 1). In 2018, Hawkey et al.19 proposed recommendations for the use of specific antibiotics, but the guidance was not organized by indication. In contrast, the 2020 IDSA guidelines provide indication-specific recommendations for infections caused by different classes of MDR (Tables 2 and 3).35
Figure 1.
Suggested treatments for carbapenem-resistant Enterobacterales, multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter baumannii.32,34 Treatment choice in each case should also depend on local epidemiology and bacterial susceptibility, and any potential additional toxicity when combining therapy. BL/BLI, β-lactam/β-lactamase inhibitor; KPC, Klebsiella pneumoniae carbapenemase; MDR, multidrug-resistant.
Table 2.
Antibiotic treatment options recommended by the Infectious Diseases Society of America (IDSA) for ESBL-E and P. aeruginosa with difficult-to-treat resistance35
| Source of infection | ESBL-E |
P. aeruginosa with difficult-to-treat resistance |
||
|---|---|---|---|---|
| Preferred treatment | Alternative treatmenta | Preferred treatment | Alternative treatmenta | |
| Cystitis | Nitrofurantoin, trimethoprim/sulfamethoxazole. | Amoxicillin/clavulanate, single-dose aminogycosides, fosfomycin (E. coli only). | Ceftolozane/tazobactam, ceftazidime/avibactam, imipenem/relebactam, cefiderocol, or a single-dose of an aminoglycoside. | Colistin |
| Pyelonephritis or cUTI | Ertapenem, meropenem, imipenem/cilastatin, ciprofloxacin, levofloxacin, or trimethoprim/sulfamethoxazole. | Ceftolozane/tazobactam, ceftazidime/avibactam, imipenem/cilastatin/relebactam, and cefiderocol. | Once-daily aminoglycoside. | |
| Infections outside the urinary tract |
Meropenem, imipenem- cilastatin, ertapenem Oral step-down therapy to ciprofloxacin, levofloxacin, or trimethoprim/sulfamethoxazole can be considered.b |
Ceftolozane/tazobactam, ceftazidime/avibactam, or imipenem/cilastatin/relebactam. |
Cediferocol Aminoglycoside monotherapy: limited to uncomplicated BSI with complete source control.c |
|
This Table is adapted, with permission, from Table 2 in Tamma et al.35
BSI, blood stream infection; cUTI, complicated urinary tract infection (UTI occurring in association with a structural or functional abnormality of the genitourinary tract, or any UTI in a male patient); ESBL-E, extended-spectrum β-lactamase-producing Enterobacterales.
If first-line options are not available or tolerated.
Oral step-down therapy can be considered after (i) susceptibility to the oral agent is demonstrated, (ii) patients are afebrile and haemodynamically stable, (iii) appropriate source control is achieved, and (iv) there are no issues with intestinal absorption.
Uncomplicated BSIs include a BSI due to a urinary source or a catheter-related BSI with removal of the infected vascular catheter.
Table 3.
Antibiotic treatment options recommended by the Infectious Diseases Society of America for carbapenem-resistant Enterobacterales35
| Source of infection | Preferred treatment | Alternative treatmenta |
|---|---|---|
| Cystitis |
Ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, nitrofurantoin, or a single-dose of an aminoglycoside. Meropenemb (standard infusion): only if ertapenem resistant, meropenem susceptible, AND carbapenemase testing results are either not available or negative. |
Ceftazidime/avibactam, meropenem-vaborbactam, imipenem/cilastatin/ relebactam, and cefiderocol. Colistin (only when no alternative options are available). |
| Pyelonephritis or cUTI |
Ceftazidime/avibactam, meropenem/ vaborbactam, imipenem/cilastatin/ relebactam, and cefiderocol. Meropenemb (extended-infusion): only if ertapenem resistant, meropenem susceptible, AND carbapenemase testing results are either not available or negative. |
Once-daily aminoglycosides. |
| Infections outside the urinary tract if resistant to ertapenem, susceptible to meropenem, AND carbapenemase testing results are either not available or negative. | Meropenem (extended infusion). | Cetazidime/avibactam. |
| Infections outside the urinary tract if resistant to ertapenem and meropenem, AND carbapenemase testing results are either not available or negative. | Ceftazidime/avibactam, meropenem/ vaborbactam, and imipenem/ cilastatin/relebactam. |
Cefiderocol. Tigecycline, eravacycline (intra-abdominal infections). |
| KPC identified (or carbapenemase positive but identity of carbapenemase is unknown). | Ceftazidime/avibactam, meropenem/ vaborbactam, and imipenem/ cilastatin/relebactam. |
Cefiderocol. Tigecycline, eravacycline (intra-abdominal infections). |
| Metallo-β-lactamase (ie. NDM, VIM or IMP) carbapenemase identified. | Ceftazidime/avibactam + aztreonam, cefiderocol. | Tigecycline, eravacycline (intra-abdominal infections). |
| OXA-48-like carbapenemase identified. | Ceftazidime/avibactam. |
Cefiderocol. Tigecycline, eravacycline (intra-abdominal infections). |
This Table is adapted, with permission, from Table 3 in Tamma et al.35
CRE, carbapenemase-resistant Enterobacterales; cUTI, complicated urinary tract infection (UTI occurring in association with a structural or functional abnormality of the genitourinary tract, or any UTI in a male patient); IMP, imipenemase; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; VIM, Verona integron-encoded metallo β-lactamases.
If first-line options are not available or tolerated.
The vast majority of carbapenemase-producing Enterobacterales infections in the United States are due to bacteria that produce KPC. If a disease-causing Enterobacterales is carbapenemase-producing but the specific carbapenemase enzyme is unknown, it is reasonable to treat as if the strain is a KPC-producer. If a patient is infected with a CRE strain with an unknown carbapenemase status and the patient has recently travelled from an area where metallo-β-lactamases are endemic (e.g. Middle East, South Asia, Mediterranean), treatment with ceftazidime/avibactam plus aztreonam, or cefiderocol monotherapy are recommended. Preferred treatment approaches for infections caused by metallo-β-lactamase producers also provide activity against KPC and OXA-48-like enzymes.
In real-world conditions, several factors prevent the widespread adoption of novel antibiotics, such as higher costs and lack of comparative data versus older drugs, since comparative studies have either not been conducted or were non-inferiority studies.19,36–40 In addition, to be effective in critically ill patients, antibiotic treatment must be administered as early as possible, and conducting antibiotic susceptibility tests can result in delays.32 Therefore, specific guidelines for empirical treatment have been developed based on the type of infection. Some of the more serious specific MDR clinical scenarios are discussed here. It is important to point out, however, that the local epidemiological resistance pattern should always be considered.
Bloodstream infections
BSIs are associated with high morbidity and mortality, with risk factors for MDR BSI including liver disease, diabetes, male sex, age ≥60 years, indwelling catheters, previous therapeutic antimicrobial use and K. pneumoniae bacteraemia.41,42 According to Spanish guidelines for managing catheter-related BSI, Gram-negative bacilli are present in 17%–25% of such infections, particularly in patients with special conditions, including spinal cord injuries, femoral catheters, neutropenia and haematological malignancy, or diabetes.43 As such, these guidelines recommend empirical antibiotic therapy that includes Gram-negative coverage and must include an anti-pseudomonal agent; however, they do not stipulate how to address resistant organisms.43 An Italian surveillance programme demonstrated the rapid increase in carbapenem-resistant K. pneumoniae causing BSI, which rose from 1.3% in 2009 to 34.3% in 2013.44
In an Italian study of carbapenem-resistant K. pneumoniae BSI in critically ill patients (including those with septic shock, chronic renal failure, or neutropenia), one of the factors associated with reduced mortality was receiving an antimicrobial combination that included high-dose meropenem (hazard ratio for death 0.64, 95% CI 0.43–0.95, P = 0.03).45 Another Italian study assessed the efficacy of combination therapy containing high-dose continuous meropenem infusion in which steady-state meropenem concentrations were optimized with therapeutic drug monitoring in patients with KPC-producing K. pneumoniae infections, 60% BSIs and 53% meropenem-resistant.46 Successful clinical outcomes were achieved in 73% of cases, suggesting that optimizing steady-state meropenem concentrations improves outcomes for KPC-producing K. pneumoniae infections with meropenem MIC ≤64 mg/L.
The literature generally appears to support the use of carbapenem-sparing treatment of ESBL BSI, including possible de-escalation to piperacillin/tazobactam or cefepime in non-critically ill patients with BSIs susceptible to these therapies.47 However, the international prospective, randomized MERINO study published in 2018 did not establish non-inferiority of piperacillin/tazobactam compared with meropenem for patients with E. coli or K. pneumoniae BSI and ceftriaxone resistance, with 30 day mortality rates of 12.3% and 3.7%, respectively.48 In that study, patients in the carbapenem group were arguably at higher risk, with a higher APACHE II score and prevalence of diabetes.48
A survey of 616 infectious disease specialists from 56 countries conducted between 2016 and 2017 showed that BSI management practices vary significantly from institution to institution.49 The authors pointed out that such variations pose a threat to antimicrobial stewardship (AMS) programmes, and that evidence-based guidelines for the management of BSIs are urgently needed so that AMS can be implemented effectively at a local level to harmonize treatment.49
Hospital-acquired and ventilator-associated pneumonia
The increase in MDR organisms complicating hospital-acquired pneumonia (HAP) is of great concern. HAP is one of the most common infections in the ICU. According to a recent review of the international literature, there are reportedly as many as 20 cases per 1000 hospital admissions; 44% of all HAP cases are acquired in the ICU with up to 90% requiring ventilation.7 European and US data from 2014 showed that P. aeruginosa was the Gram-negative pathogen most commonly implicated in HAP and ventilator-associated pneumonia (VAP), accounting for 21% of all cases of HAP in 2014.50 Importantly, that study also showed that P. aeruginosa had reduced susceptibility to most antimicrobials tested, including ceftazidime (68.7%/79.6% susceptibility in Europe/US), meropenem (65.8%/76.3%), and piperacillin/tazobactam (63.9%/72.9%).50
The 2016 IDSA guidelines for managing HAP/VAP strongly recommend the use of individual hospital antibiograms to reduce patient exposure to unnecessary antibiotics and reduce the development of antibiotic resistance, and in particular to reduce the use of dual Gram-negative and empirical MRSA antibiotic treatment.51–53 Treatment depends largely on the causative MDR pathogen. Watkins et al.54 recommended the use of carbapenems first line in HAP caused by ESBL-producing Enterobacterales, and newer agents such as meropenem/vaborbactam or ceftazidime/avibactam in carbapenem-resistant Enterobacterales pneumonia. Potential initial treatment options for pneumonia caused by MDR P. aeruginosa include antipseudomonal cephalosporins, carbapenems, fluoroquinolones or BL/BLIs, while colistin combination therapy is recommended for pneumonia due to MDR A. baumannii.54
Complicated UTIs
Urinary tract infections (UTIs) represent the highest proportion of healthcare-acquired infections, at approximately 40%.55 Resistant Gram-negative bacteria are increasingly causing complicated UTIs (cUTIs), mainly due to the spread of ESBL-producing bacteria.56,57E. coli and other common Enterobacterales, including Klebsiella and Pseudomonas spp, are common causes of cUTIs.57,58 Scottish guidelines for treating UTIs include guidance for MDR organisms, recommending nitrofurantoin, pivmecillinam, trimethoprim or fosfomycin.59 The 2020 IDSA guidelines provide detailed recommendations for cUTI depending on the type of MDR, with separate recommendations for ESBL-producing Enterobacterales, CRE and P. aeruginosa with difficult-to-treat resistance (Tables 2 and 3).35 In severe UTIs, the following options are recommended by Muntean et al.55: cefepime, ceftazidime, imipenem, doripenem, meropenem and piperacillin/tazobactam. The latter also stress that empirical treatment must consider risk factors for resistant infections, namely duration of hospitalization, previous administration of antibiotics, and local resistance patterns.55
Other clinical scenarios
Guidelines for the treatment of complicated intra-abdominal infections (cIAI) have been published by the World Society of Emergency Surgery.60 Piperacillin/tazobactam is the common treatment of choice in this indication, followed by a carbapenem. However, the use of piperacillin/tazobactam to treat infections caused by ESBL-producing Enterobacterales remains controversial, and it should be reserved for stable, rather than critically ill, patients.60 Ceftolozane/tazobactam or ceftazidime/avibactam are recommended, as part of a carbapenem-sparing strategy, in critically ill patients with hospital-acquired IAIs; however, these agents must be used in combination with metronidazole60 as they have limited or variable activity against anaerobic bacteria.60–62 In contrast, the in vitro anaerobic activity of meropenem/vaborbactam is similar to that of meropenem alone,63 and this agent is approved as monotherapy for treatment of adults with cIAI in Europe.64
Main therapies currently used for MDR Gram-negative bacterial infections
Older treatments
Older antimicrobials are still commonly used for treating Gram-negative infections, usually as combinations, especially where certain types of MBL-producing organisms are common, and for carbapenem-resistant P. aeruginosa and A. baumannii, although their use in infections caused by KPC-producing CRE is more questionable.33 These treatment combinations have not been studied in well-designed clinical studies, thus evidence supporting their use is based on data from retrospective studies, and pharmacokinetic and pharmacodynamic characteristics have been derived from empirical data. The safety profiles and related limitations of these agents are well known and often negatively impact patient outcomes, particularly when used to treat MDR infections. These agents include colistin, fosfomycin, tigecycline, aminoglycosides, piperacillin/tazobactam and high-dose carbapenems.
Colistin
The optimal use of colistin in MDR Gram-negative infections is subject to debate. Despite its association with nephrotoxicity and neurotoxicity, there has been a resurgence in its use because of the increasing prevalence of carbapenem-resistant species.65 In MDR Gram-negative nosocomial pneumonia, colistin is used in an effort to address the high morbidity and mortality rates in patients hospitalized for pneumonia.54 A recent position statement by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) noted that colistin needs to be used at a dose that achieves steady-state levels in excess of 2 mg/L, yet less than half of patients achieve this level of colistin exposure because of concerns about nephrotoxicity.66 In an Italian cross-sectional study assessing colistin use in high-risk adults (including those with recent hospitalization, and multiple comorbidities), colistin was given most often in combination with agents for MDR Gram-negative organisms, mainly for the targeted therapy of lower respiratory tract infections and BSIs caused by carbapenem-resistant organisms.67 However, 30 day mortality in patients with pneumonia (mostly caused by Klebsiella) who were treated with colistin was three-fold higher than in those treated with ceftazidime/avibactam (32% versus 9%; absolute difference 23%, 95% CI 9%–35%; P = 0.001); patients in both groups received add-on anti-CRE agents.68 Furthermore, resistance to colistin is now emerging, as evidenced by a highly virulent strain of Escherichia coli found to be resistant to colistin via the mcr-1 gene.65 The same E. coli strain is resistant to numerous other antibiotics, including most BLs and all non-BLs.65 Furthermore, evidence suggests that colistin, alone or in combination, has no impact on clinical outcomes or mortality,69 and is often associated with nephrotoxicity in severely ill patients.70
Fosfomycin
Fosfomycin was discovered in the 1960s and has been used for many years, particularly in the treatment of UTIs.71 It has a unique mechanism of action, inhibiting UDP-GlcNAc enolpyruvyl transferase, the first step of the synthesis of bacterial cell walls, rendering it useful in the treatment of resistant Gram-negative infections.71 Indeed, resistance is the driver of the recent increase in its use.72 Resistance to fosfomycin itself is most commonly via amino acid replacement or peptidoglycan recycling in the formation of the bacterial wall, and cross-resistance is uncommon.73 Fosfomycin is particularly active against E. coli as well as some carbapenem-resistant bacteria.71 In the ZEUS study, which compared injectable fosfomycin with piperacillin/tazobactam in patients with cUTIs caused mostly by E. coli, fosfomycin was non-inferior in the primary outcome of clinical cure and microbial eradication.74E. coli eradication was 100% with fosfomycin.74 Hypokalaemia was more common in the fosfomycin group.74 In fact, intravenous (IV) fosfomycin is known to be associated with sodium overload and hypokalaemia, and therefore, administration of potassium supplements is recommended.75 Furthermore, because fosfomycin is eliminated mostly by the kidneys, it should be administered with caution in patients with renal impairment and dose adjustments may be necessary.75 A 2008 review evaluated numerous case studies and clinical trials of fosfomycin against a variety of non-UTI or gastrointestinal infections involving Gram-negative bacilli (most commonly P. aeruginosa), usually in combination with other antimicrobials.76 The overall results showed a cure rate of 81.1%, indicating that the usefulness of fosfomycin may be extended beyond UTIs.76 Fosfomycin is generally well tolerated.76
Tigecycline
While active in vitro against carbapenem-resistant Enterobacterales and carbapenem-resistant A. baumannii, but not carbapenem-resistant P. aeruginosa,77 tigecycline is generally used in combination with other agents.32,78 It is recommended for use in MDR skin and soft tissue infections (SSTIs) and abdominal infections, and in combination with other agents for hospital-acquired respiratory infections,19 although not for VAP.79 While one study found high-dose tigecycline to be the only independent predictor of clinical cure in critically ill patients with VAP and MDR bacterial infections (carbapenem-resistant A. baumannii or carbapenem-resistant K. pneumoniae),80 a meta-analysis of clinical studies found an increased risk of mortality with tigecycline versus active comparators,81 which led to a Black Box warning and change in the US labelling against its use in VAP.79 There is evidence of A. baumannii resistance to tigecycline via overexpression of the AdeABC efflux pump,82 and of breakthrough infection; furthermore, tigecycline may not achieve the tissue levels necessary to treat pneumonia, meaning high-dose therapy is often needed.83 Therefore, high-dose tigecycline-based combinations should be reserved for critically ill patients with carbapenem-resistant Enterobacterales infections and limited treatment options.84
Aminoglycosides
Aminoglycosides have been used for many decades to treat Gram-negative nosocomial pneumonia.85 and more recently have been used to treat infections caused by carbapenem-resistant Gram-negative bacteria.32 Traditionally used in combination, aminoglycosides such as amikacin, gentamicin and tobramycin are used as monotherapy in UTIs only,78 where they demonstrate considerable effectiveness. In carbapenem-resistant Enterobacterales-associated conditions other than UTIs, they are associated with unacceptably high mortality (up to 80%) when given alone.78 They are used in cases of polymyxin resistance, but aminoglycosides are susceptible to several resistance mechanisms, including reduced uptake, target modification through mutation, and enzymatic inactivation.32 Furthermore, when given in combination with IV BLs, IV aminoglycosides increase the risk of nephrotoxicity compared with a BL alone in patients with VAP.7 Inhaled amikacin initially showed promise, but the recent IASIS and INHALE studies have shown no clinical benefit in adding inhaled amikacin to IV standard of care for VAP.86
Of note, aminoglycosides are known to cause nephrotoxicity.32 In addition, they have decreased activity at lower pH of airway linings and the concentrations of aminoglycosides detected in the lung tissues may not be sufficient to effectively treat VAP.32,87
Piperacillin/tazobactam
The BL/BLI piperacillin/tazobactam is a broad-spectrum antibiotic with activity against multiple Gram-negative pathogens and is one of the few agents that is active against Pseudomonas spp.88,89 In patients with E. coli or K. pneumoniae BSI and ceftriaxone resistance, piperacillin/tazobactam showed no benefit over meropenem in terms of 30 day mortality.47 The ZEUS study found piperacillin/tazobactam to be somewhat less effective than fosfomycin in patients with UTIs.74 However, it was noted that the dose of the former may have been sub-optimal in that study.72
In many critically ill patients, as well as patients with mild or moderate renal impairment, the typical dose of piperacillin/tazobactam (4.5 mg three times daily) is insufficient to achieve effective bactericidal concentrations, and dose adjustments may be required.90
Newer treatment options
Ceftazidime/avibactam
Ceftazidime/avibactam is a novel combination of the third-generation cephalosporin ceftazidime and the BLI avibactam, with indications including HAP and VAP.91 Ceftazidime/avibactam was approved in the USA in 2015 for the treatment of cIAIs (in combination with metronidazole) and cUTIs, including pyelonephritis, in patients aged ≥18 years.92 In addition to these indications, ceftazidime/avibactam was approved in Europe in 2016 for the treatment of HAP, including VAP93 and, as of June 2020, for the treatment of bacteraemia associated with, or suspected to be associated with any of the above infections.93 The International Network For Optimal Resistance Monitoring global surveillance programme (2012–15) demonstrated 99.4% susceptibility to ceftazidime/avibactam for all Enterobacterales isolates and 98.5% susceptibility for meropenem-non-susceptible, MBL-negative isolates.78 In a study of antimicrobial activity against carbapenem-resistant Enterobacterales isolated from ICUs in Taiwan, ceftazidime/avibactam demonstrated susceptibility rates of 99% for E. coli, 100% for K. pneumoniae and 91% for P. aeruginosa.94 Ceftazidime/avibactam given as monotherapy or in combination with other agents was superior to other treatment regimens, including carbapenem plus aminoglycoside, colistin and other regimens, against carbapenem-resistant K. pneumoniae bacteraemia.95 In patients with KPC-producing K. pneumoniae infections, ceftazidime/avibactam proved to be a reasonable alternative treatment option to colistin, and was associated with a lower risk of nephrotoxicity.68 In a retrospective study that included 138 patients with KPC-producing K. pneumoniae infections, ceftazidime/avibactam was effective as salvage therapy following first-line treatment with other antimicrobials.96 Given ceftazidime’s inhibitory profile against OXA-48-like enzymes and its stability in the presence of these hydrolysing enzymes, ceftazidime/avibactam is the preferred agent for the treatment of infections caused by OXA-48-producing carbapenem-resistant Enterobacterales.97
The REPROVE study confirmed that ceftazidime/avibactam is non-inferior to meropenem in HAP, including VAP (clinical cure rate 68.8% versus 73.0%).98 However, the cure rate with ceftazidime/avibactam was lower than expected based on preclinical pharmacokinetic and pharmacodynamic data.99 Moreover, cases of K. pneumoniae resistance emerged during a clinical trial with ceftazidime/avibactam; the resistance was found to be caused by plasmid-borne mutations (D179Y/T243M) at position 243 in the blaKPC-3 genes.100
A key, currently unaddressed question is the optimal therapeutic regimen of ceftazidime/avibactam for carbapenem-resistant Enterobacterales (i.e. whether it should be given as monotherapy or in combination with other agents). Pharmacokinetic/pharmacodynamic optimization by administering prolonged (>2 h) or continuous infusions could be a key strategy to prevent treatment failure with ceftazidime/avibactam and subsequent development of resistance,101 but this approach has not yet received regulatory approval.
Meropenem/vaborbactam
Meropenem/vaborbactam has been approved in the USA for the treatment of patients aged 18 years and older with cUTIs, including pyelonephritis, since 2017.102 In Europe, meropenem/vaborbactam was approved in 2018 for the treatment of adults with cUTIs, including pyelonephritis, cIAIs and HAP, including VAP.64 Meropenem/vaborbactam is also indicated for the treatment of patients with bacteraemia that occurs in association with, or suspected to be associated with, any of the above infections and for treatment of infections due to bacterial organisms in adults with limited treatment options.64 The combination of the well-known, broad-spectrum carbapenem meropenem with vaborbactam, a first-in-class boronic acid inhibitor of class A and class C β-lactamases, had excellent in vitro activity against KPC-producing Enterobacterales isolates from around the world collected in 2014 and 2015 (99.0% susceptibility).103 Meropenem/vaborbactam demonstrated marked activity against Enterobacterales strains producing KPC carbapenemases, with less but still notable activity against those that produce MBLs or OXA-48-like enzymes.98,104,105 It is administered as a high dose prolonged infusion (2 g meropenem, 2 g vaborbactam over 3 h) every 8 h to optimize pharmacokinetic/pharmacodynamic exposures, resulting in enhanced bacterial killing and EUCAST species-related breakpoints for Enterobacterales and P. aeruginosa of susceptible ≤8 mg/L and resistant >8 mg/L.106P. aeruginosa is considered a clinically relevant pathogen for meropenem/vaborbactam in Europe but not in the US.
Meropenem/vaborbactam was approved in the USA in 2017 for the treatment of cUTIs and acute pyelonephritis91 based on the results of the TANGO 1 study, which showed non-inferiority of meropenem/vaborbactam to piperacillin/tazobactam.107 In the Phase III TANGO 2 study, meropenem/vaborbactam as monotherapy was compared with best available therapy in a representative group of patients with CRE infections (bacteraemia 36.0%, cUTI/acute pyelonephritis 45.3%, HAP/VAP 9.3% and cIAIs 9.3%), including those with multiple comorbidities, compromised immune systems and moderate-to-severe renal impairment.108 Meropenem/vaborbactam was associated with significantly higher rates of clinical cure than best available therapy [65.6% (21/32) versus 33.3% (5/15); difference, 32.3%; 95% CI 3.3%–61.3%, P = 0.03] at the end of treatment.108 Meropenem/vaborbactam was also associated with numerically lower 28 day mortality (15.6% versus 33.3%) and fewer renal-related adverse events (4.0% versus 24.0%) compared with best available therapy. The study was concluded early in favour of meropenem/vaborbactam based on a risk/benefit analysis by the Data Safety Monitoring Board.108 Early real-world experience with meropenem/vaborbactam further supports the effectiveness of meropenem/vaborbactam demonstrated in clinical studies, showing that the combination was able to achieve clinical success in 70% of severely ill patients with Gram-negative CRE infections, including nosocomial pneumonia, cUTI, intra-abdominal and SSTIs.109
Ceftolozane/tazobactam
Ceftolozane/tazobactam is another BL/BLI combination.91 It was approved in the USA in 2014 for the treatment of cIAIs (in combination with metronidazole) and cUTIs, including pyelonephritis.110 In Europe, ceftolozane/tazobactam was approved in 2015 for the treatment of cIAIs, acute pyelonephritis, cUTIs and HAP, including VAP.111 Unlike some other cephalosporins, it is active against AmpC β-lactamases, especially P. aeruginosa.91 In the ASPECT-NP study, ceftolozane/tazobactam was non-inferior to meropenem for treating Gram-negative nosocomial VAP.112 However, it should be noted that in this study, ceftolozane/tazobactam was administered at twice its first approval’s recommended dose (3 g versus 1.5 g every 8 h).112 Cure rates with ceftolozane/tazobactam have been found to be lower in patients with renal impairment, so dose adjustment may be required in patients with impaired renal function91 UK clinical practice guidelines recommend ceftolozane/tazobactam for the treatment of cUTI caused by resistant Gram-negative infections.58
In cIAIs, overall clinical cure rates were 83.0% with ceftolozane/tazobactam plus metronidazole and 87.3% with meropenem.113 When stratified by pathogen, the clinical cure rates for all patients with ESBL-producing Enterobacterales were 95.8% and 88.5%, respectively.113
It may be a useful treatment option for severe infections caused by carbapenem-resistant P. aeruginosa provided susceptibility is confirmed. A real-world study of patients infected with carbapenem-resistant P. aeruginosa reported a clinical cure rate of 74%.114
Imipenem/relebactam
Relebactam is a novel, IV class A and C BLI which, when combined with imipenem, restores the latter’s activity against the KPC-producing CREs, K. pneumoniae and P. aeruginosa, but not A. baumannii.33,91 Similarly, imipenem/relebactam has shown in vitro activity against KPC-producing Enterobacterales and MDR P. aeruginosa, but was not active against A. baumannii clinical isolates.115 In 2019, imipenem/relebactam was approved in the USA for the treatment of cUTIs, including pyelonephritis, and cIAIs in patients aged ≥18 years with limited or no alternative treatment options.116 In Europe, it was approved in 2020 for the treatment of infections caused by aerobic Gram-negative organisms in adults with limited treatment options.117 Imipenem/relebactam has been investigated in the treatment of imipenem-resistant HAP, VAP, cIAI and cUTI.91 In cUTI, imipenem/cilastatin + relebactam was non-inferior to imipenem/cilastatin alone, with over 95% of patients treated with either imipenem/cilastatin + relebactam 250 mg, imipenem/cilastatin + relebactam 125 mg or imipenem/cilastatin + placebo having favourable microbiological responses.118 RESTORE-IMI 1, conducted in patients with HAP/VAP, cIAI or cUTI caused by imipenem-resistant Enterobacterales, reported favourable responses in 71% of patients receiving imipenem/relebactam and 70% of those receiving colistin + imipenem overall; favourable responses were not found in patients with cIAI.119
Cefoperazone/sulbactam
Cefoperazone/sulbactam was found to have in vitro activity against 91.6% of Enterobacterales according to recent data published on behalf of the SENTRY antimicrobial surveillance programme, meaning it is one of the most active compounds in vitro.120 Susceptibility rates varied by region, ranging from 94.4% in Western Europe to 82.0% in Eastern Europe.120 In a study comparing cefoperazone/sulbactam with tigecycline for BSI due to carbapenem-resistant A. baumannii, 28 day mortality was significantly higher with tigecycline.121 In patients with BSI due to ESBL-producing Enterobacterales, there were no statistically significant differences between patients treated with cefoperazone/sulbactam and those treated with a carbapenem in terms of success rates (70.6% versus 73.9%, odds ratio 0.847, P = 0.761), sepsis-related mortality or 14 day mortality.122 In HAP or healthcare-associated pneumonia, cefoperazone/sulbactam demonstrated non-inferiority to cefepime, with a similar number of patients defined as cured at the end of the study.123 Cefoperazone/sulbactam is currently approved in some European countries (Bulgaria, Czech Republic, Italy, Lithuania, Poland, and Slovakia), but not in the USA.
Eravacycline
Eravacycline is a tetracycline antibiotic that is effective in vitro against many microorganisms that are resistant to other tetracyclines, including MDR Acinetobacter spp. and ESBL-producing Enterobacterales spp.124,125 In 2018, eravacycline was approved in the USA and Europe for the treatment of cIAIs in patients aged ≥18 years.126,127 Two randomized, double-blind studies [Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE)] demonstrated that, in terms of clinical cure rates, eravacycline was non-inferior to ertapenem (IGNITE1) and to meropenem (IGNITE4) in patients with cIAIs.128,129 Eravacycline is generally well tolerated; the most common adverse events are nausea, vomiting and infusion site reactions.126 Eravacycline is expected to provide a valuable therapeutic option for patients who cannot tolerate β-lactams or fluoroquinolones, and may help reduce the use of quinolones, carbapenems and BLIs.125
Plazomicin
The novel semisynthetic aminoglycoside plazomicin is active against Enterobacterales, but is less active against non-fermenting Gram-negative bacteria,91 due to its vulnerability to ribosomal ribonucleic acid methyltransferases.78 Plazomicin is currently approved by the US Food and Drug Administration for the treatment of cUTI; however, the application for marketing authorization for plazomicin in Europe was withdrawn in 2020.91,130 Plazomicin was evaluated in patients with serious carbapenem-resistant Enterobacterales infections in the CARE study, which, although it was terminated early because of low study enrolment, found that plazomicin was associated with reduced all-cause mortality at 28 days compared with colistin (24% versus 50% of patients, 95% CI –55 to 6).131
Plazomicin was found to be more active in vitro than traditional aminoglycosides.132 In the EPIC study, it was non-inferior to meropenem in the treatment of patients with cUTI.133 These results, taken together with the encouraging results from the CARE study,131 suggest that plazomicin may have an important role in the management of carbapenem-resistant Enterobacterales infections, particularly cUTIs.133
Cefiderocol
The novel siderophore cephalosporin cefiderocol is active in vitro against a variety of Ambler class A, C and D β-lactamases, and it is the first agent with activity versus class B β-lactamases. This confers activity against MDR Gram-negative bacilli, including MDR Enterobacterales, P. aeruginosa, A. baumannii and Stenotrophomonas maltophilia, while possessing a safety and tolerability profile similar to that of other cephalosporins.91 Cefiderocol was assessed in the Phase III study, CREDIBLE-CR, to compare its effectiveness with that of best available therapy in patients with CRE Gram-negative pneumonia, cUTI or BSI/sepsis.134 The results showed respective clinical cure rates for cefiderocol versus best available therapy of 50.0% versus 52.6%, 70.6% versus 60.0%, and 43.5% versus 42.9%.135 However, all-cause mortality was higher in patients who received cefiderocol than in those who received best available therapy.136,137 Cefiderocol was approved in the USA in 2019 and in Europe in 2020 for the treatment of infections, including pyelonephritis, caused by Gram-negative microorganisms in patients aged ≥18 years; however, the indication is limited to patients who have limited or no alternative treatment options.136,137
Potential future antibiotics
Cefepime/zidebactam
Cefepime/zidebactam combines the diazabicyclooctane (DBO) zidebactam, a second-generation BLI, with the broad-spectrum cephalosporin cefepime. In vitro, zidebactam demonstrated higher potency than avibactam or relebactam against class C β-lactamases.29 When available, cefepime/zidebactam could provide a much-needed antibacterial agent in the fight against MDR Gram-negative pathogens. A study assessing the safety, tolerability and pharmacokinetics of IV cefepime/zidebactam in healthy volunteers has been completed (NCT02707107).138
Meropenem/nacubactam
Nacubactam is another DBO BLI. Combined with meropenem, it has demonstrated in vivo effectiveness against carbapenem-resistant K. pneumoniae, E. coli and AmpC-depressed P. aeruginosa.139 A study examining the intrapulmonary penetration of nacubactam combined with meropenem in healthy volunteers has recently been completed (NCT03182504).140
Cefepime/enmetazobactam
Another potential agent for the treatment of ESBL-expressing Enterobacterales is cefepime/enmetazobactam. Enmetazobactam has been shown to restore the activity of cefepime and piperacillin against selected ESBL-producing strains more potently than tazobactam.141In vitro, cefepime/enmetazobactam was as effective as meropenem and imipenem against the same ESBL-producing strains.141
Long-term view: new targets for antimicrobials
Phages
Bacteriophages are viruses that specifically target bacteria by disrupting almost all bacterial cellular processes.82 They have several advantages over conventional antibiotics in that they are highly species- or strain-specific, and as such are less likely to cause dysbacteriosis and secondary infections.82 However, they are also susceptible to bacterial resistance and may themselves contribute to resistance by acting as vehicles for the acquisition, maintenance and spread of antibiotic resistance genes.82
Odilorhabdins
Odilorhabdins are a new class of modified peptide antibiotics produced by enzymes encoded in an identified non-ribosomal peptide synthetase gene cluster present in the genome of Xenorhabdus nematophila.142 Odilorhabdins have a unique mechanism of action in that they target a site on the small bacterial ribosomal subunit not targeted by any known ribosome-targeting antibiotic. NOSO-95179 is a synthetic version of naturally occurring odilorhabdin and has demonstrated activity against a wide range of Gram-negative pathogens including K. pneumoniae, E. coli and difficult-to-treat CRE.142
Major considerations for future management of resistant infections
Important broad principles for the future management of antimicrobial resistance include improvement in diagnostic and prescribing practices, reduction of antimicrobial use in agriculture, development of new antimicrobials, antimicrobial stewardship programmes, more equitable access to medications, and improved surveillance and infection control programmes.25 This list is extensive and challenging, but implementing these principles is fundamental to the continuing management of antimicrobial resistance worldwide.
One of the most important anticipated developments in the management of antibiotic-resistant infections is the introduction of novel diagnostic tools.143 At present, empirical treatment continues to be the most common approach, but contributes to the misuse of antibiotics and, therefore, the spread of antibiotic resistance. Furthermore, traditional growth-based techniques for assessing antibiotic susceptibility are time-consuming and require pure cultures. On the other hand, novel diagnostic techniques that rely on nucleic acid amplification, nucleic acid hybridization or immunodiagnostic methods can be applied to non-purified samples. Such techniques promise to provide rapid, point-of-care diagnosis and antibiotic susceptibility testing. This is expected to reduce treatment delays and enable the shift to evidence-based treatment, thereby preventing unnecessary use of antibiotics and the spread of antibiotic resistance. They may also reduce the overall cost of treatment by eliminating the need to purify and grow cultures.143
Antimicrobial stewardship programmes must include leadership commitment by infection experts, collaboration between stewardship teams and primary care physicians, and treatment algorithms for appropriate dosing and de-escalation of antibiotics according to culture and susceptibility results.9 With the steady increase in carbapenem resistance, and the continued high use of these agents, carbapenems must be utilized appropriately, particularly in hospitals.9 The WHO priority list of pathogens144 provides a framework to continue development of new antimicrobial agents and combinations of new and older agents, including some of those discussed here. However, many newer agents are not yet ready for clinical use despite showing high levels of activity in vitro, and bringing them to the approval stage takes time.145 It is not always possible to conduct randomized controlled studies involving the required number of patients in a timely manner, given the relatively small number of patients with certain MDR infections.34 In particular, in order to determine the place of new agents in treatment algorithms, these agents must undergo comparative studies with established agents.34 Newer agents may be more effective and better tolerated, but are also more costly; costs can be reduced through de-escalation protocols, when applicable, and these factors also need to be considered in any AMS programme.34 There is also a need for government-based financial incentives to promote the research and development of new antimicrobial agents, which may help combat MDR infections.146,147 Reimbursement decisions should consider the unique properties of novel antimicrobial agents in order to improve their market use and create incentives for pharmaceutical development of these agents.147
Geographic differences in the rates of resistance highlight the need to adapt empirical treatment to local epidemiology, patient risk stratification and local stewardship protocols. Rapid diagnostics are needed to guide management, including targeting treatments appropriately and rapid de-escalation from broad-spectrum agents when possible.
Conclusions
The WHO priority list of pathogens144 provides an impetus and a framework to continue development of new antimicrobials and combinations of new and older agents in order to combat the increase in MDR Gram-negative pathogens. The success of new antimicrobial agents depends upon increased efforts to promote research and development by governments around the world, as well as robust antimicrobial stewardship programmes and detailed local knowledge of resistance. There is also a need to put procedures in place to reduce inappropriate prescribing and misuse of antimicrobial agents in agriculture in order to ensure the success of future antimicrobial treatment.
Transparency declarations
Outside the submitted work, M.B. has participated in advisory boards and/or received speaker honoraria and/or has received study grants from Angelini, Astellas, Bayer, BioMérieux, Cidara, Gilead, Menarini, MSD, Pfizer and Shionogi. J.G. has received speaker honoraria and participated in Advisory Boards from Menarini, Pfizer, MSD, Navriba, Paratek, Pfizer, Shionogi, and VenatoRx.
Marion Barnett prepared a first draft of this manuscript on behalf of Springer Healthcare Communications. This medical writing assistance was funded by A. Menarini Farmaceutica Internazionale.
This paper was published as part of a Supplement sponsored and financially supported by A. Menarini Farmaceutica Internazionale.
References
- 1. Knight GM, Costelloe C, Murray KA. et al. Addressing the unknowns of antimicrobial resistance: quantifying and mapping the drivers of burden. Clin Infect Dis 2018; 66: 612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chatterjee A, Modarai M, Naylor NR. et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis 2018; 18: e368–78. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. An update on the fight against antimicrobial resistance. https://www.who.int/news-room/feature-stories/detail/an-update-on-the-fight-against-antimicrobial-resistance.
- 4.Infectious Diseases Society of America. Antimicrobial resistance. https://www.idsociety.org/globalassets/idsa/policy–advocacy/current_topics_and_issues/antimicrobial_resistance/10x20/background/amr-fact-sheet-2019-final.pdf.
- 5. Lepape A, Jean A, De Waele J. et al. European intensive care physicians' experience of infections due to antibiotic-resistant bacteria. Antimicrob Resist Infect Control 2020; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yam ELY, Hsu LY, Yap EP. et al. Antimicrobial resistance in the Asia Pacific region: a meeting report. Antimicrob Resist Infect Control 2019; 8: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassetti M, Welte T, Wunderink RG.. Treatment of Gram-negative pneumonia in the critical care setting: is the β-lactam antibiotic backbone broken beyond repair? Crit Care 2016; 20: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation. Global Antimicrobial Surveillance System (GLASS) report: early implementation 2016-2017. 2017. https://www.who.int/docs/default-source/searo/amr/global-antimicrobial-resistance-surveillance-system-(glass)-report-early-implementation-2016-2017.pdfsfvrsn=ea19cc4a_2.
- 9. Jean SS, Gould IM, Lee WS. et al. New drugs for multidrug-resistant Gram-negative organisms: time for stewardship. Drugs 2019; 79: 705–14. [DOI] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe: 2015. https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf.
- 11.US Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 12.US Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 13. Cassini A, Hogberg LD, Plachouras D. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe. 2017. https://www.ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-EARS-Net-2017.pdf.
- 15. Suwantarat N, Carroll KC.. Epidemiology and molecular characterization of multidrug-resistant Gram-negative bacteria in Southeast Asia. Antimicrob Resist Infect Control 2016; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu J, Huang Y, Lv X.. Crisis of Antimicrobial Resistance in China: now and the Future. Front Microbiol 2019; 10: 2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Righi E, Peri AM, Harris PN. et al. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis. J Antimicrob Chemother 2017; 72: 668–77. [DOI] [PubMed] [Google Scholar]
- 18. Averbuch D, Tridello G, Hoek J. et al. Antimicrobial resistance in Gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: intercontinental prospective study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis 2017; 65: 1819–28. [DOI] [PubMed] [Google Scholar]
- 19. Hawkey PM, Warren RE, Livermore DM. et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 2018; 73: iii2–iii78. [DOI] [PubMed] [Google Scholar]
- 20. Karaiskos I, Galani I, Souli M. et al. Novel β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant Gram-negative pathogens. Expert Opin Drug Metab Toxicol 2019; 15: 133–49. [DOI] [PubMed] [Google Scholar]
- 21. Bush K, Jacoby GA.. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 2010; 54: 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frohlich C, Sorum V, Thomassen AM. et al. OXA-48-mediated ceftazidime-avibactam resistance is associated with evolutionary trade-offs. mSphere 2019; 4: e00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bush K. The ABCD's of β-lactamase nomenclature. J Infect Chemother 2013; 19: 549–59. [DOI] [PubMed] [Google Scholar]
- 24. Munoz-Price LS, Poirel L, Bonomo RA. et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13: 785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes AH, Moore LS, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 26. Eichenberger EM, Thaden JT.. Epidemiology and mechanisms of resistance of extensively drug resistant Gram-negative bacteria. Antibiotics (Basel) 2019; 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander EL, Loutit J, Tumbarello M. et al. Carbapenem-resistant Enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis 2017; 4: ofx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PNA, Pezzani MD, Gutierrez-Gutierrez B. et al. Geographical variation in therapy for bloodstream infections due to multidrug-resistant Enterobacteriaceae: a post-hoc analysis of the INCREMENT study. Int J Antimicrob Agents 2017; 50: 664–72. [DOI] [PubMed] [Google Scholar]
- 29. Papp-Wallace KM, Nguyen NQ, Jacobs MR. et al. Strategic approaches to overcome resistance against Gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 2018; 61: 4067–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhanel GG, Lawrence CK, Adam H. et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018; 78: 65–98. [DOI] [PubMed] [Google Scholar]
- 31. Gutierrez-Gutierrez B, Perez-Galera S, Salamanca E. et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2016; 60: 4159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassetti M, Peghin M, Vena A. et al. Treatment of infections due to MDR Gram-negative bacteria. Front Med (Lausanne) 2019; 6: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassetti M, Peghin M.. How to manage KPC infections. Ther Adv Infect Dis 2020; 7: 2049936120912049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peri AM, Doi Y, Potoski BA. et al. Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis 2019; 94: 413–25. [DOI] [PubMed] [Google Scholar]
- 35. Tamma PD, Aitken SL, Bonomo RA. et al. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis 2021; 72: e169–83. [DOI] [PubMed] [Google Scholar]
- 36. Hughes S, Gilchrist M, Heard K. et al. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): a pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC Antimicrobiol Resist 2020; 2: dlaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satlin MJ. Languid uptake of ceftazidime-avibactam for carbapenem-resistant Gram-negative infections and continued reliance on polymyxins. Clin Infect Dis 2021; 72: 622–5. [DOI] [PubMed] [Google Scholar]
- 38. Strich JR, Ricotta E, Warner S. et al. Pharmacoepidemiology of ceftazidime-avibactam use: a retrospective cohort analysis of 210 US hospitals. Clin Infect Dis 2021; 72: 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tacconelli E, Carrara E, Savoldi A. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. [DOI] [PubMed] [Google Scholar]
- 40. Vickers RJ, Bassetti M, Clancy CJ. et al. Combating resistance while maintaining innovation: the future of antimicrobial stewardship. Future Microbiol 2019; 14: 1331–41. [DOI] [PubMed] [Google Scholar]
- 41. Leal HF, Azevedo J, Silva GEO. et al. Bloodstream infections caused by multidrug-resistant Gram-negative bacteria: epidemiological, clinical and microbiological features. BMC Infect Dis 2019; 19: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patolia S, Abate G, Patel N. et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacilli bacteremia. Ther Adv Infect Dis 2018; 5: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chaves F, Garnacho-Montero J, Del Pozo JL. et al. Diagnosis and treatment of catheter-related bloodstream infection: clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med Intensiva (Engl Ed) 2018; 42: 5–36. [DOI] [PubMed] [Google Scholar]
- 44. Iacchini S, Sabbatucci M, Gagliotti C. et al. Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: results from nationwide surveillance, 2014 to 2017. Euro Surveill 2019; 24: pii=1800159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giannella M, Trecarichi EM, Giacobbe DR. et al. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents 2018; 51: 244–8. [DOI] [PubMed] [Google Scholar]
- 46. Pea F, Della Siega P, Cojutti P. et al. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int J Antimicrob Agents 2017; 49: 255–8. [DOI] [PubMed] [Google Scholar]
- 47. Chastain DB, White BP, Cretella DA. et al. Is it time to rethink the notion of carbapenem-sparing therapy against extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections? A critical review. Ann Pharmacother 2018; 52: 484–92. [DOI] [PubMed] [Google Scholar]
- 48. Harris PNA, Tambyah PA, Lye DC. et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320: 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diallo K, Thilly N, Luc A. et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents 2018; 51: 794–8. [DOI] [PubMed] [Google Scholar]
- 50. Sader HS, Farrell DJ, Flamm RK. et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents 2014; 43: 328–34. [DOI] [PubMed] [Google Scholar]
- 51. Kalil AC, Metersky ML, Klompas M. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torres A, Niederman MS, Chastre J. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 2017; 50: 1700582. [DOI] [PubMed] [Google Scholar]
- 53. Torres A, Niederman MS, Chastre J. et al. Summary of the international clinical guidelines for the management of hospital-acquired and ventilator-acquired pneumonia. ERJ Open Res 2018; doi:10.1183/23120541.00028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watkins RR, Van Duin D.. Current trends in the treatment of pneumonia due to multidrug-resistant Gram-negative bacteria. F1000Res 2019; doi:10.12688/f1000research.16517.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muntean D, Licker M.. Infections and multidrug-resistant pathogens in ICU patients. In: Erbay RH, ed. Current Topics in Intensive Care Medicine. InTech Open, 2018; doi:10.5772/intechopen.79229. [Google Scholar]
- 56. Pallett A, Hand K.. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 2010; 65 Suppl 3: iii25–33. [DOI] [PubMed] [Google Scholar]
- 57. Bonkat G, Cai T, Veeratterapillay R. et al. Management of urosepsis in 2018. Eur Urol Focus 2019; 5: 5–9. [DOI] [PubMed] [Google Scholar]
- 58. NICE Guidance - Complicated urinary tract infections: ceftolozane/tazobactam: (c) NICE (2016) Complicated urinary tract infections: ceftolozane/tazobactam. BJU Int 2018; 121: 825–34. [DOI] [PubMed] [Google Scholar]
- 59.Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. A national clinical guideline. SIGN 88. Updated July 2012. https://www.sign.ac.uk/media/1051/sign88.pdf.
- 60. Sartelli M, Chichom-Mefire A, Labricciosa FM. et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg 2017; 12: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Armstrong ES, Farrell DJ, Palchak M. et al. In vitro activity of ceftolozane-tazobactam against anaerobic organisms identified during the ASPECT-cIAI study. Antimicrob Agents Chemother 2016; 60: 666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stone GG, Newell P, Bradford PA.. In vitro activity of ceftazidime-avibactam against isolates from patients in a phase 3 clinical trial for treatment of complicated intra-abdominal infections. Antimicrob Agents Chemother 2018; 62: e02584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel TS, Pogue JM, Mills JP. et al. Meropenem-vaborbactam: a new weapon in the war against infections due to resistant Gram-negative bacteria. Future Microbiol 2018; 13: 971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Medicines Agency. Vaborem: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/vaborem-epar-product-information_en.pdf.
- 65. Forde BM, Zowawi HM, Harris PNA. et al. Discovery of mcr-1-mediated colistin resistance in a highly virulent Escherichia coli lineage. mSphere 2018; 3: e00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Satlin MJ, Lewis JS, Weinstein MP. et al. Clinical and Laboratory Standards Institute and European Committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin Infect Dis 2020; 71: e523–9. [DOI] [PubMed] [Google Scholar]
- 67. Giacobbe DR, Saffioti C, Losito AR. et al. Use of colistin in adult patients: a cross-sectional study. J Glob Antimicrob Resist 2020; 20: 43–9. [DOI] [PubMed] [Google Scholar]
- 68. van Duin D, Lok JJ, Earley M. et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perez F, El Chakhtoura NG, Yasmin M. et al. Polymyxins: to combine or not to combine? Antibiotics (Basel) 2019; 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ordooei Javan A, Shokouhi S, Sahraei Z.. A review on colistin nephrotoxicity. Eur J Clin Pharmacol 2015; 71: 801–10. [DOI] [PubMed] [Google Scholar]
- 71. Avent ML, Rogers BA, Cheng AC. et al. Fosfomycin: what was old is new again. Intern Med J 2018; 48: 1425–9. [DOI] [PubMed] [Google Scholar]
- 72. Harris PNA. By ZEUS! Can We Use Intravenous Fosfomycin for Complicated Urinary Tract Infections? Clin Infect Dis 2019; 69: 2057–8. [DOI] [PubMed] [Google Scholar]
- 73. Lopez-Montesinos I, Horcajada JP.. Oral and intravenous fosfomycin in complicated urinary tract infections. Rev Esp Quimioter 2019; 32 Suppl 1: 37–44. [PMC free article] [PubMed] [Google Scholar]
- 74. Kaye KS, Rice LB, Dane AL. et al. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin Infect Dis 2019; 69: 2045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Falagas ME, Vouloumanou EK, Samonis G. et al. Fosfomycin. Clin Microbiol Rev 2016; 29: 321–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Falagas ME, Giannopoulou KP, Kokolakis GN. et al. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 2008; 46: 1069–77. [DOI] [PubMed] [Google Scholar]
- 77. Yaghoubi S, Zekiy AO, Krutova M. et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis 2021; 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karaiskos I, Lagou S, Pontikis K. et al. The "Old" and the "New" antibiotics for MDR Gram-negative pathogens: for whom, when, and how. Front Public Health 2019; 7: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.US Food and Drug Administration. Tygacil (tigecycline): prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021821s049lbl.pdf.
- 80. De Pascale G, Montini L, Pennisi M. et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014; 18: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Prasad P, Sun J, Danner RL. et al. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54: 1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Breijyeh Z, Jubeh B, Karaman R.. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020; 25: 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. El Chakhtoura NG, Saade E, Iovleva A. et al. Therapies for multidrug resistant and extensively drug-resistant non-fermenting Gram-negative bacteria causing nosocomial infections: a perilous journey toward ‘molecularly targeted’ therapy. Expert Rev Anti Infect Ther 2018; 16: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sheu CC, Chang YT, SY L. et al. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol 2019; 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kashuba AD, Nafziger AN, Drusano GL. et al. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by Gram-negative bacteria. Antimicrob Agents Chemother 1999; 43: 623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Torres A, Motos A, Battaglini D. et al. Inhaled amikacin for severe Gram-negative pulmonary infections in the intensive care unit: current status and future prospects. Crit Care 2018; 22: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Panidis D, Markantonis SL, Boutzouka E. et al. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 2005; 128: 545–52. [DOI] [PubMed] [Google Scholar]
- 88. Bassetti M, Vena A, Croxatto A. et al. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018; 7: 212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shah PJ, Ryzner KL.. Evaluating the appropriate use of piperacillin/tazobactam in a community health system: a retrospective chart review. P T 2013; 38: 462–83. [PMC free article] [PubMed] [Google Scholar]
- 90. Zander J, Dobbeler G, Nagel D. et al. Piperacillin concentration in relation to therapeutic range in critically ill patients–a prospective observational study. Crit Care 2016; 20: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bassetti M, Righi E, Russo A. et al. New Antibiotics for Pneumonia. Clin Chest Med 2018; 39: 853–69. [DOI] [PubMed] [Google Scholar]
- 92.US Food and Drug Administration. Avycaz: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206494s000lbl.pdf.
- 93.European Medicines Agency. Zavicefta: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf.
- 94. Liao CH, Lee NY, Tang HJ. et al. Antimicrobial activities of ceftazidime-avibactam, ceftolozane-tazobactam, and other agents against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa isolated from intensive care units in Taiwan: results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan in 2016. Infect Drug Resist 2019; 12: 545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shields RK, Nguyen MH, Chen L. et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61: e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tumbarello M, Trecarichi EM, Corona A. et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 2019; 68: 355–64. [DOI] [PubMed] [Google Scholar]
- 97. Pogue JM, Bonomo RA, Kaye KS.. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 2019; 68: 519–24. [DOI] [PubMed] [Google Scholar]
- 98. Torres A, Zhong N, Pachl J. et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 2018; 18: 285–95. [DOI] [PubMed] [Google Scholar]
- 99. Falcone M, Viale P, Tiseo G. et al. Pharmacokinetic drug evaluation of avibactam + ceftazidime for the treatment of hospital-acquired pneumonia. Expert Opin Drug Metab Toxicol 2018; 14: 331–40. [DOI] [PubMed] [Google Scholar]
- 100. Shields RK, Chen L, Cheng S. et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017; 61: e02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dietl B, Martinez LM, Calbo E. et al. Update on the role of ceftazidime-avibactam in the management of carbapenemase-producing Enterobacterales. Future Microbiol 2020; 15: 473–84. [DOI] [PubMed] [Google Scholar]
- 102.US Food and Drug Administration. Vabomere: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209776s003lbl.pdf.
- 103. Hackel MA, Lomovskaya O, Dudley MN. et al. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62: e01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Castanheira M, Doyle TB, Kantro V. et al. Meropenem-vaborbactam activity against carbapenem-resistant Enterobacterales isolates collected in U.S. hospitals during 2016 to 2018. Antimicrob Agents Chemother 2020; 64: e00305-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnston BD, Thuras P, Porter SB. et al. Activity of meropenem/vaborbactam against international carbapenem-resistant Escherichia coli isolates in relation to clonal background, resistance genes, resistance to comparators and region. J Glob Antimicrob Resist 2021; 24: 190–7. [DOI] [PubMed] [Google Scholar]
- 106.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Meropenem-Vaborbactam: Rationale for EUCAST Clinical Breakpoints. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Meropenem-Vaborbactam_Rationale_Document_1.0_20210101.pdf.
- 107. Kaye KS, Bhowmick T, Metallidis S. et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319: 788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G. et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7: 439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alosaimy S, Jorgensen SCJ, Lagnf AM. et al. Real-world multicenter analysis of clinical outcomes and safety of meropenem-vaborbactam in patients treated for serious Gram-negative bacterial infections. Open Forum Infect Dis 2020; 7: ofaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.US Food and Drug Administration. Zerbaxa: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206829lbl.pdf.
- 111.European Medicines Agency. Zerbaxa: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/zerbaxa-epar-product-information_en.pdf.
- 112. Kollef MH, Novacek M, Kivistik U. et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2019; 19: 1299–311. [DOI] [PubMed] [Google Scholar]
- 113. Solomkin J, Hershberger E, Miller B. et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60: 1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Munita JM, Aitken SL, Miller WR. et al. Multicenter Evaluation of Ceftolozane/Tazobactam for Serious Infections Caused by Carbapenem-Resistant Pseudomonas aeruginosa. Clin Infect Dis 2017; 65: 158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lapuebla A, Abdallah M, Olafisoye O. et al. Activity of imipenem with relebactam against Gram-negative pathogens from New York City. Antimicrob Agents Chemother 2015; 59: 5029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.US Food and Drug Administration. Recarbrio: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212819s000lbl.pdf.
- 117.European Medicines Agency. Recarbrio: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/recarbrio-epar-product-information_en.pdf.
- 118. Sims M, Mariyanovski V, McLeroth P. et al. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother 2017; 72: 2616–26. [DOI] [PubMed] [Google Scholar]
- 119. Motsch J, Murta de Oliveira C, Stus V. et al. RESTORE-IMI 1: a Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis 2020; 70: 1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sader HS, Carvalhaes CG, Streit JM. et al. Antimicrobial activity of cefoperazone-sulbactam tested against Gram-negative organisms from Europe, Asia-Pacific, and Latin America. Int J Infect Dis 2020; 91: 32–7. [DOI] [PubMed] [Google Scholar]
- 121. Niu T, Luo Q, Li Y. et al. Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control 2019; 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Su J, Guo Q, Li Y. et al. Comparison of empirical therapy with cefoperazone/sulbactam or a carbapenem for bloodstream infections due to ESBL-producing Enterobacteriaceae. J Antimicrob Chemother 2018; 73: 3176–80. [DOI] [PubMed] [Google Scholar]
- 123. Liu JW, Chen YH, Lee WS. et al. Randomized noninferiority trial of cefoperazone-sulbactam versus cefepime in the treatment of hospital-acquired and healthcare-associated pneumonia. Antimicrob Agents Chemother 2019; 63: e00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lee YR, Burton CE.. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis 2019; 38: 1787–94. [DOI] [PubMed] [Google Scholar]
- 125. Alosaimy S, Abdul-Mutakabbir JC, Kebriaei R. et al. Evaluation of eravacycline: a novel fluorocycline. Pharmacotherapy 2020; 40: 221–38. [DOI] [PubMed] [Google Scholar]
- 126.US Food and Drug Administration. Xerava: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf.
- 127.European Medicines Agency. Xerava: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/xerava-epar-product-information_en.pdf.
- 128. Solomkin J, Evans D, Slepavicius A. et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 2017; 152: 224–32. [DOI] [PubMed] [Google Scholar]
- 129. Solomkin JS, Gardovskis J, Lawrence K. et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis 2019; 69: 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.European Medicines Agency. Zemdri: withdrawal of the marketing authorisation application. https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/zemdri.
- 131. McKinnell JA, Dwyer JP, Talbot GH. et al. Plazomicin for infections caused by carbapenem-resistant enterobacteriaceae. N Engl J Med 2019; 380: 791–3. [DOI] [PubMed] [Google Scholar]
- 132. Eljaaly K, Alharbi A, Alshehri S. et al. Plazomicin: a novel aminoglycoside for the treatment of resistant Gram-negative bacterial infections. Drugs 2019; 79: 243–69. [DOI] [PubMed] [Google Scholar]
- 133. Wagenlehner FME, Cloutier DJ, Komirenko AS. et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 2019; 380: 729–40. [DOI] [PubMed] [Google Scholar]
- 134. Bassetti M, Ariyasu M, Binkowitz B. et al. Designing a pathogen-focused study to address the high unmet medical need represented by carbapenem-resistant Gram-negative pathogens - the international, multicenter, randomized, open-label, phase 3 CREDIBLE-CR study. Infect Drug Resist 2019; 12: 3607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.US Food and Drug Administration. Cefiderocol injection: meeting of the antimicrobial drugs advisory committee (AMDAC). FDA briefing document. https://www.fda.gov/media/131703/download.
- 136.European Medicines Agency. Fetcroja: EPAR - product information. https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf.
- 137.US Food and Drug Administration. Fetcroja: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209445s000lbl.pdf.
- 138.ClinicalTrials.gov. A randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability and pharmacokinetics of multiple escalating doses of intravenous WCK 5222 (zidebactam and cefepime) in healthy adult human subjects (ClinicalTrials.gov record: NCT02707107). https://clinicaltrials.gov/ct2/show/NCT02707107.
- 139. Barnes MD, Taracila MA, Good CE. et al. Nacubactam enhances meropenem activity against carbapenem-resistant Klebsiella pneumoniae producing KPC. Antimicrob Agents Chemother 2019; 63: e00432-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Roche H-L, A non-randomized, open label, one treatment, one group study to investigate the intrapulmonary lung penetration of RO7079901 in healthy volunteers (ClinicalTrials.gov record: NCT03182504). https://clinicaltrials.gov/ct2/show/NCT03182504.
- 141. Papp-Wallace KM, Bethel CR, Caillon J. et al. Beyond piperacillin-tazobactam: cefepime and AAI101 as a potent β-lactam-β-lactamase inhibitor combination. Antimicrob Agents Chemother 2019; 63: e00105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pantel L, Florin T, Dobosz-Bartoszek M. et al. Odilorhabdins, antibacterial agents that cause miscoding by binding at a new ribosomal site. Mol Cell 2018; 70: 83–94.e7. [DOI] [PubMed] [Google Scholar]
- 143. Vasala A, Hytonen VP, Laitinen OH.. Modern tools for rapid diagnostics of antimicrobial resistance. Front Cell Infect Microbiol 2020; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1
- 145. O'Meara S. Antimicrobial resistance. Nature 2020; 586: S49. [Google Scholar]
- 146. Plackett B. Why big pharma has abandoned antibiotics. Nature 2020; 586: S50–S2. [Google Scholar]
- 147. Morton A, Colson A, Leporowski A. et al. How should the value attributes of novel antibiotics be considered in reimbursement decision making? MDM Policy Pract 2019; doi:10.1177/2381468319892237. [DOI] [PMC free article] [PubMed] [Google Scholar]