Abstract
Background
The Prevention of Arrhythmia Device Infection Trial (PADIT) investigated whether intensification of perioperative prophylaxis could prevent cardiac implantable electronic device (CIED) infections. Compared with a single dose of cefazolin, the perioperative administration of cefazolin, vancomycin, bacitracin, and cephalexin did not significantly decrease the risk of infection. Our objective was to compare the microbiology of infections between study arms in PADIT.
Methods
This was a post hoc analysis. Differences between study arms in the microbiology of infections were assessed at the level of individual patients and at the level of microorganisms using the Fisher exact test.
Results
Overall, 209 microorganisms were reported from 177 patients. The most common microorganisms were coagulase-negative staphylococci (CoNS; 82/209 [39.2%]) and S. aureus (75/209 [35.9%]). There was a significantly lower proportion of CoNS in the incremental arm compared with the standard arm (30.1% vs 46.6%; P = .04). However, there was no significant difference between study arms in the frequency of recovery of other microorganisms. In terms of antimicrobial susceptibility, 26.5% of microorganisms were resistant to cefazolin. CoNS were more likely to be cefazolin-resistant in the incremental arm (52.2% vs 26.8%, respectively; P = .05). However, there was no difference between study arms in terms of infections in which the main pathogen was sensitive to cefazolin (77.8% vs 64.3%; P = .10) or vancomycin (90.8% vs 90.2%; P = .90).
Conclusions
Intensification of the prophylaxis led to significant changes in the microbiology of infections, despite the absence of a decrease in the overall risk of infections. These findings provide important insight on the physiopathology of CIED infections.
Trial registration
Keywords: cardiac electronic implantable device, infection, microbiology, prevention, prophylaxis
Cardiac implantable electronic device (CIED) infections are serious complications causing significant morbidity and mortality [1]. They can affect between 1% and 3.4% of all CIED implantations, with higher infection rates during replacements, revisions, and upgrades [2, 3]. From a pathophysiology perspective, most of these infections (in particular, those occurring within 6 months of implantation) are believed to occur mainly intraoperatively following local contamination during implantation [4–6]. This hypothesis is supported by the observation that microorganisms can frequently be recovered from the CIED pocket immediately after implantation and before wound closure [4]. Consequently, intensifying the perioperative prophylactic regimen has drawn significant interest to prevent these complications.
Recently, we reported on a large-scale cluster randomized crossover trial (Prevention of Arrhythmia Device Infection Trial [PADIT]) to investigate the benefits of intensifying the perioperative antimicrobial prophylaxis in terms of the risk of CIED infection compared with a standard single preoperative dose of cefazolin [7–9]. The incremental bundle was composed of preoperative administration of cefazolin and vancomycin, incisional wound irrigation with topical bacitracin before skin closure [10], and the administration of a 2-day postoperative course of oral cephalexin [10, 11]. The selection of this regimen was based, among other considerations, on the fact that the current standard of care (preoperative cefazolin) does not provide coverage against up to 45% of pathogens causing CIED infection (mainly methicillin-resistant staphylococci and enterococci) [12]. The vast majority of these pathogens, however, remain sensitive to vancomycin [12].
PADIT enrolled 19603 patients from 28 institutions in Canada and the Netherlands. Hospitalization for infection was reduced by a nonsignificant 23% in the incremental therapy arm (odd ratio, 0.77; 95% CI, 0.56–1.05; P = .10). At the moment, the reasons underlying the failure of the incremental regimen to prevent CIED infections are incompletely understood. The lower-than-expected infection rate in both arms may have contributed to a decrease in study power [13].
Whether the choice of prophylaxis had any influence on the microbiology of CIED infections has not been fully investigated [7]. Hence, we conducted a study to (1) describe the microbiology of infections that occurred in the context of PADIT and (2) compare the microbiology and antimicrobial susceptibility profiles between the 2 treatment arms.
METHODS
Study Design and Setting
This study is a post hoc analysis of data collected prospectively. The design and primary results of the original PADIT trial have been published [7–9]. Briefly, the primary outcome included hospitalization for device infection (pocket infection or infective endocarditis), pocket erosion and device exposure (with or without overt infection), or infective endocarditis/bloodstream infections within 1 year of the procedure. Although the main outcome of PADIT focused on 12826 high-risk individuals (eg, those with repeat procedures on an existing pocket or recipients of cardiac resynchronization therapy), this analysis includes infections in both high-risk and low-risk patients enrolled in the study (n = 19559). Bloodstream infections were defined according to 2008 National Healthcare Safety Network and US Centers for Disease Control and Prevention definitions for primary bloodstream infections [14]. Common skin contaminants had to be cultured from 2 or more blood cultures drawn on separate occasions to be considered significant [14]. Adjudication was performed by 2 investigators (Y.L. and P.G.) blinded to treatment received, with all discrepancies resolved by an adjudication committee.
Microbiological Methods
Microbiological samples (either pocket or wound cultures, blood cultures, vegetation, or CIED lead cultures) were processed by each participating hospital as per their routine laboratory standard operating procedures [8]. In this pragmatic approach, there was no standardized protocol for sample collection, transportation and handling, processing, and reporting. Up to 3 microorganisms could be reported per patient [8].
Sensitivity to cefazolin was collected for each microorganism whenever reported by the microbiology laboratory. In case of missing information, inferred sensitivity was conducted when possible (eg, inferred resistance to cefazolin for Enterococcus spp.; inferred sensitivity to cefazolin for methicillin-sensitive S. aureus). Similarly, sensitivity to vancomycin was inferred for most microorganisms, as susceptibility to this antibiotic is generally predictable. Sensitivity to bacitracin was not recorded or inferred. In case of polymicrobial infection, a single blinded assessor (Y.L.) determined the most likely pathogen for the infection based on speciation and relative potential virulence.
Analyses
All the analyses were performed among patients who had an adjudicated infection event. Categorical variables were summarized as frequencies and percentages. Missing data were excluded from the denominators. The following variables were compared between study arms at the patient level using the chi-square test or Fisher exact test, as appropriate: monomicrobial vs polymicrobial infection, proportion of main pathogens that are sensitive to cefazolin and vancomycin, and whether at least 1 (or all) of the reported pathogens isolated from a single patient were sensitive to cefazolin and/or vancomycin. Types of microorganisms as well as their sensitivity to cefazolin were analyzed at the level of reported microorganisms using logistic mixed-effects models with patients as random effects to account for the potential underlying correlation among multiple microorganisms in the same patient. P values calculated from mixed-effects models were reported for variables with at least 10 cases. The overall distribution of microorganisms was compared between treatment arms using the Fisher exact test to account for small cell counts. Analyses were conducted in SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA). A 2-tailed P value <.05 was considered to indicate statistical significance. Adjustments for multiple comparisons were not performed considering the exploratory nature of the study.
Sensitivity Analyses
CIED infections can occur for up to a year and even longer after insertion, but infections that occur later are increasingly likely to be due to postinsertion contamination due to wound dehiscence or hematogenous seeding. Consequently, in order to explore the impact of the incremental antibiotic on early infections (which are more likely to be insertion-related), we conducted a sensitivity analysis using only infections that occurred within 90 days of insertion.
Patient Consent
The study was approved by each local research ethics committee (REC). A waiver of individual written informed consent was approved by each REC. The rationale for obtaining a waiver of consent has been described previously [8].
RESULTS
Between December 2012 and September 2016, a total of 19603 patients were enrolled. Rehospitalization for the primary outcome within 1 year of follow-up occurred in 99 patients (1.03%) receiving standard treatment and in 78 (0.78%) receiving enhanced treatment (odds ratio, 0.77; 95% CI, 0.56–1.05; P = .10) [7]. The most frequent primary outcomes were skin, subcutaneous/pocket infections (n = 151), bloodstream infections (n = 52), infective endocarditis (n = 50), and erosion of the pocket without overt signs of infection (n = 4). Most patients with bloodstream infections (46/52, 88%) also met the case definition for skin infections, subcutaneous/pocket infections, and/or infective endocarditis. In terms of timing of infections, most infections (119/177 [67.2%]) occurred within 3 months of insertion, 24 (13.6%) occurred >3–6 months after insertion, 20 (11.3%) occurred >6–9 months after insertion, and 14 (7.9%) occurred >9–12 months after insertion.
In terms of infected patients, most infections (103/177 [58.2%]) were monomicrobial (Table 1). No microorganism was reported in 37 patients (20.9%). There was no difference between the conventional and incremental study arms in the proportion of infections that were monomicrobial, polymicrobial, or without pathogens reported (P > .05 for each comparison). In terms of antimicrobial susceptibility, there were no significant differences between the study arms in terms of proportion of infected patients for whom the main pathogen was sensitive to cefazolin or vancomycin. Likewise, no differences were detected in the proportion of infections in which at least 1 pathogen was sensitive to cefazolin or vancomycin. Finally, the proportion of infections in which all microorganisms were sensitive to cefazolin or in which all were sensitive to vancomycin was similar between the intervention arms.
Table 1.
Comparison of Characteristics of CIED Infections Between Treatment Arms (Patient-Level Analyses)
| Characteristic | Total (n = 177), No. (%) | Conventional (n = 99), No. (%) | Incremental (n = 78), No. (%) | P Value |
|---|---|---|---|---|
| Microbiology of infections | ||||
| Monomicrobial infections | 103 (58.2) | 54 (54.5) | 49 (62.8) | .27 |
| Polymicrobial infections | 37 (20.9) | 23 (23.2) | 14 (17.9) | .39 |
| No pathogen identified/reported | 37 (20.9) | 22 (22.2) | 15 (19.2) | .63 |
| Susceptibility of recovered pathogens | ||||
| Main pathogen sensitive to cefazolin | 85/119 (71.4) | 49/63 (77.8) | 36/56 (64.2) | .10 |
| Main pathogen sensitive to vancomycin | 124/137 (90.5) | 69/76 (90.7) | 55/61 (90.2) | .90 |
| At least 1 pathogen sensitive to cefazolin | 92/120 (76.7) | 52/63 (82.5) | 40/57 (70.2) | .11 |
| At least 1 pathogen sensitive to vancomycin | 130/138 (94.2) | 74/77 (96.1) | 56/61 (91.8) | .47 |
| All pathogens sensitive to cefazolin | 74/114 (64.9) | 42/60 (70.0) | 32/54 (59.3) | .23 |
| All pathogens sensitive to vancomycin | 121/138 (87.7) | 68/77 (88.3) | 53/61 (86.9) | .80 |
Abbreviation: CIED, cardiac implantable electronic device.
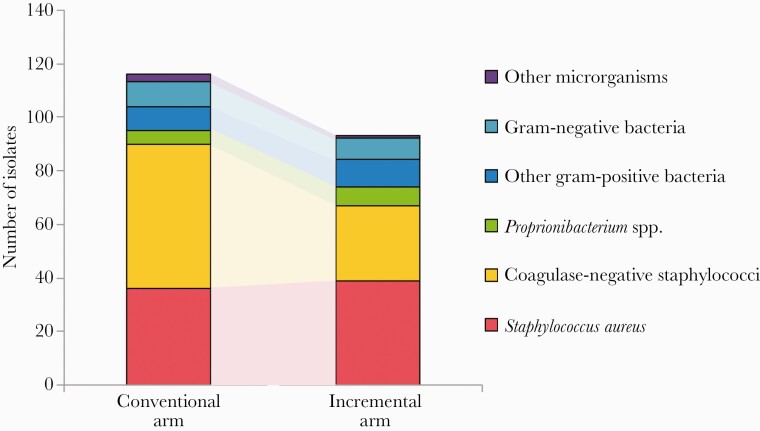
In terms of microorganisms, 209 microorganisms were reported (116 [55.5%] in the conventional arm and 93 [44.5%] in the incremental arm) (Table 2). Most microorganisms were reported from wound cultures or CIED lead cultures (149 [71.3%]), whereas 60 were from blood cultures (28.7%). Gram-positive bacteria represented 90% of all reported microorganisms. The most common types of microorganisms were S. aureus (35.9%) and coagulase-negative staphylococci (CoNS; 39.2%), of which 9.7% (7/72) and 35.9% (23/64) were methicillin-resistant, respectively. Proprionibacterium spp. and gram-negative bacteria represented 5.7% and 8.1% of all microorganisms, respectively. The overall distribution of microorganisms was significantly different between treatment arms (P = .006). In terms of specific pathogens, there was a lower number of CoNS reported in the incremental arm compared with the conventional arm (Figure 1) and a significantly lower proportion of CoNS in the incremental arm compared with the standard arm (30.1% vs 46.6%; P = .04). The distribution of other microorganisms was otherwise similar between treatment arms. For example, there was no significant difference between study arms in terms of frequency of recovery of S. aureus, methicillin-resistant S. aureus (MRSA), Proprionibacterium spp., gram-negative bacteria, and fungi.
Table 2.
Microbiology of Organisms Reported in the PADIT Trial
| Microorganism | Total
(n = 209), No. (%) |
Conventional (n = 116), No. (%) | Incremental (n = 93), No. (%) | P Valuea |
|---|---|---|---|---|
| Gram-positive bacteria | 188 (90.0) | 104 (89.7) | 84 (90.3) | .82 |
| Staphylococcus aureus | 75 (35.9) | 36 (31.0) | 39 (41.9) | .36 |
| Coagulase-negative Staphylococcus | 82 (39.2) | 54 (46.6) | 28 (30.1) | .04 |
| Streptococcus pneumoniae | 1 (0.5) | 1 (0.9) | 0 | - |
| Enterococcus spp. | 4 (1.9) | 0 | 4 (4.3) | - |
| Viridans streptococci | 5 (2.4) | 5 (4.3) | 0 | - |
| Other streptococci | 2 (1.0) | 0 | 2 (2.2) | - |
| Proprionibacterium spp.b | 12 (5.7) | 5 (4.3) | 7 (7.5) | .29 |
| Other gram-positive bacteria | 7 (3.3) | 3 (2.6) | 4 (4.3) | - |
| Gram-negative bacteria | 17 (8.1) | 9 (7.8) | 8 (8.6) | .67 |
| Escherichia coli | 1 (0.5) | 1 (0.9) | 0 | - |
| Klebsiella spp. | 1 (0.5) | 1 (0.9) | 0 | - |
| Serratia spp. | 2 (1.0) | 2 (1.7) | 0 | - |
| Enterobacter spp. | 4 (1.9) | 1 (0.9) | 3 (3.2) | - |
| Other Enterobacteriaceae | 1 (0.5) | 0 | 1 (1.1) | - |
| Pseudomonas aeruginosa | 5 (2.4) | 2 (1.7) | 3 (3.2) | - |
| Nonfermenting gram-negative bacteria | 2 (1.0) | 2 (1.7) | 0 | - |
| Other gram-negative bacteria | 1 (0.5) | 0 | 1 (1.1) | - |
| Other pathogens | 4 (1.9) | 3 (2.6) | 1 (1.1) | - |
| Anaerobic bacteria (other than Proprionibacterium) | 2 (1.0) | 2 (1.7) | 0 | - |
| Candida albicans | 2 (1.0) | 1 (0.9) | 1 (1.1) | - |
Abbreviation: PADIT, Prevention of Arrhythmia Device Infection Trial.
P values were calculated for the variables with at least 10 cases using a logistic mixed model to account for underlying correlation among microorganisms detected in the same patient.
Includes Proprionibacterium spp. and Cutibacterium acnes (formerly known as P. acnes) (Table 3).
Figure 1.
Number of bacterial isolates reported in the conventional and incremental arms of the PADIT cluster randomized crossover trial. Abbreviation: PADIT, Prevention of Arrhythmia Device Infection Trial.
Overall, one-quarter (26.5%) of reported strains were resistant to cefazolin (Table 3). Resistance to cefazolin was not different between the conventional and incremental arms (22.1% vs 31.6%, respectively; P = .24). However, cefazolin resistance among CoNS was more common in the incremental arm than the conventional arm (52.1% vs 26.8%; P = .05).
Table 3.
Cefazolin Resistance of all Microorganisms Reported in the PADIT Trial
| Microorganism | Resistance to Cefazolin | P Valuea | ||
|---|---|---|---|---|
| Both Arms, n/N (%) | Conventional, n/N (%) | Incremental, n/N (%) | ||
| Staphylococcus aureus | 7/72 (9.7) | 3/35 (8.6) | 4/37 (10.8) | - |
| Coagulase-negative Staphylococcus | 23/64 (35.9) | 11/41 (26.8) | 12/23 (52.2) | .05 |
| Other gram-positive microorganisms | 4/14 (28.6) | 0/5 (0) | 4/9 (44.4) | - |
| Other microorganisms | 9/12 (75.0) | 5/5 (100) | 4/7 (57.1) | - |
| Total | 43/162 (26.5) | 19/86 (22.1) | 24/76 (31.6) | .24 |
Abbreviation: PADIT, Prevention of Arrhythmia Device Infection Trial.
P values were calculated for the variables with at least 10 cases using a logistic mixed model to account for underlying correlation among microorganisms detected in the same patient.
Sensitivity Analyses
Sensitivity analyses that included only early (ie, <90 days) infections were concordant with the main analyses (Supplementary Data). They confirmed the lack of impact of the incremental strategy on the proportion of infections that are monomicrobial, polymicrobial, and of unknown etiology (P > .05) (Supplementary Table 1). In terms of microbial etiology, it confirmed that the overall distribution of pathogens was significantly different between treatment arms (P = .003) and that CoNS were significantly less likely to be recovered in the incremental arm compared with the conventional arm (25.0% vs 51.2%, respectively; P = .01) (Supplementary Table 2). It also showed that cefazolin resistance was numerically more likely to be reported from CoNS in the incremental vs conventional arm, although this analysis did not reach statistical significance (53.3% vs 26.7%, respectively; P = .10) (Supplementary Table 3).
DISCUSSION
CIED infections can occur during insertion [15]. They can also occur postoperatively due to wound infection, wound dehiscence, or hematogenous seeding of the device from a remote focus [15]. Even though the relative contribution of each of these routes of contamination is currently unknown, it is believed that most infections occur during implantation rather than postoperatively [15].
Overall, the microbiology of CIED infections, bloodstream infections, and endocarditis in PADIT was consistent with the literature, as S. aureus and CoNS were the most common pathogens [1]. The lower incidence of MRSA in our cohort compared with the United States (where up to 15% of CIED infections are due to MRSA) is likely the consequence of enrolling Canadian and Dutch hospitals, which have lower MRSA prevalence [1]. On the other hand, the proportion of microorganisms that were resistant to cefazolin in PADIT (26.5%) and, more specifically, the proportion of CoNS that were resistant to cefazolin in the conventional arm (26.8%) were lower than what is reported in the literature. Resistance to cefazolin among microorganisms causing CIED infections in previous studies ranges from 33% to 50% [5, 16, 17]. In a cohort of 816 CIED infections in the United States, nearly half of CoNS- and half of S. aureus–causing CIED infections were resistant to cefazolin [16]. This finding is important as it could explain in part the lower-than-expected infection rate in the conventional arm and could have led to a loss in study power.
Preliminary analyses published in the original manuscript did not detect any difference in the microbiology between the 2 treatment arms [7]. However, the more detailed analyses presented in the current study identified differences in the microbiology of infections between the study groups. Infections in the incremental arm were less likely to be due to CoNS and more likely to be due to cefazolin-resistant CoNS. This suggests that intensifying the prophylactic regimen altered the microbiology of infections, even though it did not significantly decrease the overall risk of infections.
The mechanism or mechanisms through which intensification can lead to a change in the microbiology without significantly altering the overall risk of infection remain unclear. The 2-day course of cephalexin postimplantation could have prevented some infections due to cefazolin-sensitive CoNS in the incremental arm (thereby leading to an overall decrease in the number of CoNS recovered and a relative increase in the proportion of CoNS that are resistant to cefazolin).
Many studies have investigated the benefit of perioperative antibiotic to prevent CIED infections, but few have compared various regimens or have compared the benefits of single-dose vs prolonged prophylaxis. Among those that have investigated these questions, many were of variable quality, with many being retrospective and/or single-center, with inconsistent definitions of device infection [6]. Furthermore, data regarding the microbiology of CIED infections are often not reported [6]. Thus, our study provides valuable insight by investigating the association between the choice and duration of perioperative antibiotic and the microbiology of infection.
The historically high rate of recovery of methicillin-resistant organisms in CIED infections in patients who receive preoperative cefazolin has been widely perceived to reflect breakthrough infections that occurred during device implantation, which led many experts to hypothesize that adding vancomycin to the prophylactic regimen could decrease the risk of infection. However, our study indicates otherwise. It is possible that the historically high frequency of cefazolin-resistant infections in patients who receive cefazolin monotherapy is not the consequence of intra-operative contamination with cefazolin-resistant organisms, but rather the result of infections that occur postoperatively among patients whose skin microbial flora has been modified by the administration of cefazolin. This hypothesis is supported by evidence that a single dose of preoperative cefazolin can significantly alter the skin microbiome in healthy humans and increase colonization with cefazolin-resistant strains even in the absence of infection [18]. Hence, even postimplantation infections (ie, infections in which microorganisms gain access to the device after its implantation and completion of the perioperative prophylaxis) are at increased risk of being due to cefazolin-resistant microorganisms. However, these infections would not be preventable by the addition of vancomycin to the perioperative antibiotics regimen.
Even the incremental prophylaxis, with its 2 days of cephalexin postimplantation, provided short-term coverage to prevent CIED infections considering that the primary outcome was followed for up to 12 months postimplantation. The lack of significant effect of the intensive prophylaxis on the overall risk of infection suggests that many infections may have their onset postimplantation. This notion is also supported by the fact that in 87% of infections in the incremental arm, all the pathogens recovered were susceptible to vancomycin despite the inclusion of vancomycin in the prophylactic regimen. In many of these infections, the bacteria may have gained access to the device after the end of the periprocedural prophylaxis (or, alternatively, the local tissue vancomycin concentration may have been insufficient to prevent infection). Hence, intensification of periprocedural prophylaxis with vancomycin may not be warranted considering the potential risks associated with this nephrotoxic antibiotic [19].
By contrast, a recent metanalysis of 5 prospective trials totaling >4000 patients on the impact of an absorbable, antibiotic eluting envelope to prevent CIED infections showed a >60% relative risk reduction [20]. A subsequent large-scale trial of >6000 patients also concluded that such a device could prevent 60% of infections [21]. Similar to our study, this trial also showed a lower proportion of infections due to CoNS in the enveloped arm (1/25 vs 9/42), although the magnitude of the decrease was more marked than in our study (81% decrease vs 30% decrease, respectively) [21].
The fact that a relatively short (48-hour) administration of very broad-spectrum perioperative antibiotics did not significantly impact the incidence of infections in PADIT, but that a drug-eluting envelope that releases antibiotics for >7 days was successful in preventing CIED infections, reinforces the notion that many CIED infections may have their onset >48 hours postimplantation (a period that the intensive arm of PADIT could not influence, but that a long-acting antibiotic eluting envelop could impact). Hence, we hypothesize that a large proportion of CIED infections that occur despite administration of a single preoperative dose of cefazolin may have their onset postimplantation, and thus are not preventable with intensification of the perioperative prophylaxis. These findings suggest that intraoperative contamination can be optimally prevented by a single dose of preoperative cefazolin and that preventing later-onset infections may require other strategies such as improved dressing and wound care, prevention of hematogenous seeding of the CIED, or prolonged administration of antibiotics (eg, through drug-eluting envelopes).
The study has strengths. It identifies that the proportion of cefazolin resistance was lower in our overall population, which could explain the lower-than-expected incidence of infection in PADIT. It also provides novel insights that could fundamentally alter our understanding of the pathogenesis of CIED infections. This could influence the development of future strategies by reorienting our focus on the prevention of infections whose onset occurs postoperatively rather than intra-operatively. It also has limitations. The surveillance period in PADIT was for 1 year postimplantation, but some late infections (in particular, those that were detected >6 months after implantation) may not have been related to the insertion process [22]. However, the sensitivity analyses performed using only infections that occurred within 90 days of insertion confirmed our main findings. The laboratory protocol to process samples was not standardized, and a maximum of 3 microorganisms could be reported. Susceptibility to cefazolin was not always reported and could not always be inferred with confidence. Determining the most likely pathogen in the case of polymicrobial infection was complex. Data regarding exposure to antibiotics during the 365-day follow-up period were not available. Still, we believe that these limitations should be equivalent between study arms in this randomized trial. Also, no adjustment of multiple testing was made given the exploratory nature of the study, which could increase the chance of false positives. To decrease the risk of false-positive associations, we limited the number of comparisons to variables with at least 10 cases. Finally, the potential benefit of adding vancomycin in regions with high prevalence of methicillin-resistance remains uncertain [1].
CONCLUSIONS
An intensive but relatively short perioperative prophylaxis significantly altered the microbiology of CIED infections without significantly decreasing the overall risk of CIED infections. We hypothesize that many infections that occur despite a single dose of preoperative cefazolin occur due to postimplantation contamination. Taken as a whole, these findings challenge the common perception that most infections occur intraoperatively [4] and suggest that future investigations should focus on preventing postimplantation contamination rather than intensifying perioperative measures.
Supplementary Material
Acknowledgments
We are indebted to the tireless work of the study coordinators and to our patients, who advance our understanding of device infection and prevention.
Disclaimer. The sponsors had no role in the design or conduct of the study; collection management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author contributions. Study conceptualization: Y.L., P.G., D.H.B., M.A., F.P., R.P., J.M., P.A., C.R., B.C., R.A.L., V.E., C.M., D.R., S.To., G.B., M.D., B.T., E.C., S.Tu., J.L., O.S., M.B., J.B., F.A.P., L.R., M.E.W.H., L.H.R.B., D.V.E., P.D., S.J.C., A.D.K. Data acquisition: D.H.B., M.A., F.P., R.P., J.M., P.A., C.R., B.C., R.A.L., V.E., C.M., D.R., S.To., G.B., M.D., B.T., E.C., S.Tu., J.L., O.S., M.B., J.B., F.A.P., L.R., M.E.W.H., L.H.R.B., D.V.E., P.D., S.J.C., A.D.K. Formal analysis: Y.L., J.W., A.D.K., P.D., S.J.C. Funding acquisition: A.D.K. Writing of original draft: Y.L., A.D.K., P.D., P.G., J.W. Supervision: Y.L., A.D.K. Writing—review and editing: D.H.B., M.A., F.P., R.P., J.M., P.A., C.R., B.C., R.A.L., V.E., C.M., D.R., S.To., G.B., M.D., B.T., E.C., S.Tu., J.L., O.S., M.B., J.B., F.A.P., L.R., M.E.W.H., L.H.R.B., D.V.E., S.J.C. Approval of final manuscript before submission: all authors.
Access to data. Yves Longtin had full access to all the data in the study and takes responsibility for the integrity and accuracy of the data analysis. The data are not publicly available.
Financial support. Dr. Krahn receives support from the Heart and Stroke Foundation of Canada, the Sauder Family and Heart and Stroke Foundation Chair in Cardiology, and the Paul Brunes Chair in Heart Rhythm Disorders. This work was supported by the Canadian Network and Centre for Trials Internationally (CANNeCTIN) network (Canadian Institutes of Health Research [CIHR] grant number 88370 to A.K.) and a clinical trial grant (CIHR grant number 119442 to A.K.).
Potential conflicts of interest. Yves Longtin declares receiving research grants from Merck, Gojo, and Becton Dickinson, outside of the submitted work. All the other authors have no conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Arnold CJ, Chu VH.. Cardiovascular implantable electronic device infections. Infect Dis Clin North Am 2018; 32:811–25. [DOI] [PubMed] [Google Scholar]
- 2. Olsen T, Jørgensen OD, Nielsen JC, et al. Incidence of device-related infection in 97750 patients: clinical data from the complete Danish device-cohort (1982-2018). Eur Heart J 2019; 40:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prutkin JM, Reynolds MR, Bao H, et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation 2014; 130:1037–43. [DOI] [PubMed] [Google Scholar]
- 4. Da Costa A, Lelièvre H, Kirkorian G, et al. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation 1998; 97:1791–5. [DOI] [PubMed] [Google Scholar]
- 5. Sandoe JA, Barlow G, Chambers JB, et al. ; British Society for Antimicrobial Chemotherapy; British Heart Rhythm Society; British Cardiovascular Society; British Heart Valve Society; British Society for Echocardiography. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015; 70:325–59. [DOI] [PubMed] [Google Scholar]
- 6. Korantzopoulos P, Sideris S, Dilaveris P, et al. Infection control in implantation of cardiac implantable electronic devices: current evidence, controversial points, and unresolved issues. Europace 2016; 18:473–8. [DOI] [PubMed] [Google Scholar]
- 7. Krahn AD, Longtin Y, Philippon F, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018; 72:3098–109. [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Philippon F, Longtin Y, et al. Randomized cluster crossover trials for reliable, efficient, comparative effectiveness testing: design of the Prevention of Arrhythmia Device Infection Trial (PADIT). Can J Cardiol 2013; 29:652–8. [DOI] [PubMed] [Google Scholar]
- 9. Birnie DH, Wang J, Alings M, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019; 74:2845–54. [DOI] [PubMed] [Google Scholar]
- 10. Allegranzi B, Zayed B, Bischoff P, et al. ; WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016; 16:e288–303. [DOI] [PubMed] [Google Scholar]
- 11. Tamayo E, Gualis J, Flórez S, et al. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J Thorac Cardiovasc Surg 2008; 136:1522–7. [DOI] [PubMed] [Google Scholar]
- 12. Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–7. [DOI] [PubMed] [Google Scholar]
- 13. Baddour LM, DeSimone DC, Sohail MR.. Interventions to prevent CIED infections: more or less? J Am Coll Cardiol 2018; 72:3110–1. [DOI] [PubMed] [Google Scholar]
- 14. Horan TC, Andrus M, Dudeck MA.. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 15. Leung S, Danik S.. Prevention, diagnosis, and treatment of cardiac implantable electronic device infections. Curr Cardiol Rep 2016; 18:58. [DOI] [PubMed] [Google Scholar]
- 16. Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of cardiac implantable electronic device infections. JACC Clin Electrophysiol 2016; 2:498–505. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez DJ, Afzal A, Evonich R, Haines DE.. The prevalence of methicillin resistant organisms among pacemaker and defibrillator implant recipients. Am J Cardiovasc Dis 2012; 2:116–22. [PMC free article] [PubMed] [Google Scholar]
- 18. Kernodle DS, Barg NL, Kaiser AB.. Low-level colonization of hospitalized patients with methicillin-resistant coagulase-negative staphylococci and emergence of the organisms during surgical antimicrobial prophylaxis. Antimicrob Agents Chemother 1988; 32:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asundi A, Stanislawski M, Mehta P, et al. Prolonged antimicrobial prophylaxis following cardiac device procedures increases preventable harm: insights from the VA CART program. Infect Control Hosp Epidemiol 2018; 39:1030–6. [DOI] [PubMed] [Google Scholar]
- 20. Koerber SM, Turagam MK, Winterfield J, et al. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: a meta-analysis. J Cardiovasc Electrophysiol 2018; 29:609–15. [DOI] [PubMed] [Google Scholar]
- 21. Tarakji KG, Mittal S, Kennergren C, et al. ; WRAP-IT Investigators. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019; 380:1895–905. [DOI] [PubMed] [Google Scholar]
- 22. Asundi A, Stanislawski M, Mehta P, et al. Real-world effectiveness of infection prevention interventions for reducing procedure-related cardiac device infections: Insights from the Veterans Affairs Clinical Assessment Reporting and Tracking Program. Infect Control Hosp Epidemiol 2019; 40:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.