Abstract
While COVID-19 is still ongoing and associated with more than 5 million deaths, the scope and speed of advances over the past year in terms of scientific discovery, data dissemination, and technology have been staggering. It is not a matter of “if” but “when” we will face the next pandemic, and how we leverage technology and data management effectively to create flexible ecosystems that facilitate collaboration, equitable care, and innovation will determine its severity and scale. The aim of this review is to address emerging challenges that came to light during the pandemic in health care and innovations that enabled us to adapt and continue to care for patients. The pandemic highlighted the need for seismic shifts in care paradigms and technology with considerations related to the digital divide and health literacy for digital health interventions to reach full potential and improve health outcomes. We discuss advances in telemedicine, remote patient monitoring, and emerging wearable technologies. Despite the promise of digital health, we emphasise the importance of addressing its limitations, including interpretation challenges, accuracy of findings, and artificial intelligence–driven algorithms. We summarise the most recent recommendation of the Virtual Care Task Force to scaling virtual medical services in Canada. Finally, we propose a model for optimal implementation of health digital innovations with 5 tenets including data management, data security, digital biomarkers, useful artificial intelligence, and clinical integration.
Résumé
Alors que la pandémie, toujours en cours, a causé plus de cinq millions de décès, la portée et la rapidité des avancées réalisées au cours de la dernière année en matière de découverte scientifique, de communication des données et sur le plan technologique ont été stupéfiantes. Il ne s’agit pas de savoir « si », mais plutôt « quand » nous devrons faire face à la prochaine pandémie, et son ampleur et sa gravité dépendront de l’efficacité avec laquelle nous exploiterons la technologie et la gestion des données pour créer des écosystèmes flexibles, qui faciliteront la collaboration, permettront d’offrir des soins équitables et favoriseront l’innovation. Le présent article de synthèse vise à cerner les défis émergents dans le domaine des soins de santé mis en lumière durant la pandémie et les innovations qui nous ont permis de nous adapter et de continuer à offrir des soins aux patients. La pandémie a révélé la nécessité de modifier radicalement les paradigmes de soins et l’utilisation des technologies en tenant compte de considérations liées à la fracture numérique et à la littératie en santé pour que les interventions numériques en santé atteignent leur plein potentiel et améliorent les résultats de santé. Nous aborderons les progrès de la télémédecine, la surveillance des patients à distance et les technologies portables émergentes. Malgré les promesses de la santé numérique, nous insistons sur l’importance de saisir ses limites, y compris les défis d’interprétation, l’exactitude des données et les algorithmes axés sur l’intelligence artificielle. Nous résumons les plus récentes recommandations du Groupe de travail sur les soins virtuels pour créer un cadre pancanadien. Finalement, nous proposons un modèle pour la mise en œuvre optimale des innovations en matière de santé numérique, lequel repose sur cinq principes, y compris la gestion des données, la sécurité des données, les biomarqueurs numériques, l’intelligence artificielle utile et l’intégration clinique.
Over the past century, the world has experienced a number of pandemics with varying impacts on health care and the global economy. The 1918 influenza outbreak was the most severe in recent history, resulting in an estimated 50 million deaths worldwide. A similar prevention approach was used during the beginning of the coronavirus 2019 (COVID-19) pandemic, with control efforts limited to isolation, quarantine, and reducing the size of public gatherings.1 COVID-19 is still ongoing and associated with more than 5 million deaths. During this time, the scope and speed of advances in terms of scientific discovery, data dissemination, and technology have been staggering. The World Health Organisation was first informed of the unusual rates of pneumonia in Wuhan, China, on December 30, 2019. Within 11 days, the genome of the coronavirus was sequenced and shared online for global collaboration efforts.2
In this review, we address emerging challenges that came to light during the pandemic in health care delivery and innovations that helped health services adapt to COVID-19. We discuss the promise that digital health (DH) technologies hold and the importance of addressing its limitations in order to harness these innovations. There have been a number of lessons learned from this pandemic that will prepare us for the next one. It is not a matter of if but when we will face the next pandemic; how we leverage technology and data management effectively to create flexible ecosystems that facilitate collaboration, equitable care, and innovation will determine the severity and scale of future threats to our provision of health care.
Disparities in Equitable Care Exposed: Addressing the Gap With Technology
The COVID-19 pandemic unmasked deep-rooted inequities in health access and outcomes globally. Many contemporary studies have found that when adjusted for confounders, such as socioeconomic status, insurance coverage, and site of care, the differences in race are largely attenuated. Black, Hispanic and Indigenous individuals are over-represented in COVID-19 hospitalisations and mortality. In the USA, 1 in 390 Indigenous Americans and 1 in 555 Black American have died compared with 1 in 665 White Americans.3 Members of these racialised communities are more likely to rely on higher-risk employment, delay seeking health care owing to financial constraints, reside in multigenerational housing, and receive unequal treatment once hospitalised.4 , 5 Despite stay-at-home orders to contain the spread of the virus, many essential workers were unable to work from home. Rogers et al. found that compared to Whites, Blacks were more likely to work essential jobs during COVID-19, including transportation, food preparation, health care support, and cleaning services, thereby increasing their risk of exposure from the workplace and transmission in their respective communities.6
Early on in the COVID-19 pandemic, reports demonstrated increased morbidity and mortality among patients with cardiovascular disease (CVD).7 These at-risk patients were advised to stay home as much as possible, to limit their chances of contracting COVID-19. However, this was not always possible, for the reasons described above. COVID-19 has seen a sharp reduction in hospital visits by patients with multiple comorbidities, those who historically had a higher utilisation of hospital care than the general population. This suggests that both missed and postponed care led to a greater disparity in health care and may lead to poorer outcomes in the longer term.8
The pandemic highlighted the need for seismic shifts in care paradigms and technology used as a means to deliver this change. In the next section we evaluate challenges that emerged during the COVID-19 era.
Challenges to Providing Pandemic Health Care
Digital divide
As there was a pivot to providing more care using telehealth technologies, both to protect patients by reducing their contact with in-person review and to mitigate extreme hospital workloads, a number of challenges related to uptake of digital solutions were encountered. These included access to reliable internet connections and a lack of readiness for adoption of virtual care in those from minority groups or older populations.9
Access to reliable internet
High-speed reliable internet has become an essential resource during this pandemic, whether to facilitate working from home, telemedicine, online purchases, or social communication. It has recently been recognised as an additional social determinant of health.10 Yet in Canada, only one-fourth of Indigenous communities have access to broadband internet, compared with 97% of urban households.11 Similarly, in the USA the digital disenfranchisement is highest among rural residents, Black and Hispanic communities, and those with the lowest income (< $50,000/year).12 The majority of access to internet services in Canada is through internet-enabled mobile devices. Access to the internet from home reduces sharply for Canadians aged > 65 years.11 Rural communities and First Nations reserves also suffer from a reduction in provision in broadband internet and LTE–mobile phone connectivity.13
Readiness for adoption
Access to the internet does not translate into adoption. In a recent cross-sectional study including 4,525 community adults, 38% of seniors aged > 65 years and 72% of those aged > 85 years were not able to undertake video consultations for clinic visits. Unreadiness was more prevalent among older single men, non-White counterparts, those with lower education status, and those who resided in nonurban areas. Lack of familiarity with the technology, lack of a stable internet connection, and difficulty with hearing/seeing, holding the device, and language were identified as barriers to video visits.14
Health literacy
In the Canadian context, health literacy is defined as “the ability to access, understand, evaluate, and communicate information as a way to promote, maintain and improve health in a variety of settings across the life-course.”15 Low health literacy is commonly associated with older age, lower levels of education, and minority status.16, 17, 18 Even when accounting for confounders, low health literacy is a key determinant in survival, with low health literacy associated with a 75% higher risk of mortality.18 The effects of limited health literacy can manifest in many ways, including more frequent drug errors and reduced patient understanding of their own health conditions, for example, not knowing when to seek assistance as their condition is deteriorating.17 , 19
Dealing with a fast-moving situation, such as a newly emerging pandemic, required patients to be adaptable, embracing new information, particularly digital information, as experience with the disease grew. Patients needed to become accustomed to new models of care at the same time. It is unclear how well these requirements were met during the COVID-19 era, using vaccination as an example, with the initial lag, particularly among low-income and rural Canadian residents, who were more likely to have lower levels of health literacy.20 , 21 Data from Ontario in 2016 showed that 47% of provincial residents had low indices of health literacy, reducing their ability to navigate the health care system and be proactive in managing their own care.22 These were important considerations in the evolving care paradigms seen during the COVID-19 pandemic.
Evolution of health care practices
To enable the evolution of patient care during a pandemic, there must be a change in how health care professionals (HCPs) deliver care. Few professional curricula include detailed education for doctors, nurses, and other HCPs on how to successfully use digital technologies. The uptake of health care innovation is often championed by a few enthusiasts without consideration of how such technology might be best integrated into the routine workings of clinics. There is little included in medical curricula at either undergraduate or postgraduate levels nor opportunities in continuing medical education to learn techniques required for providing routine care with the use of digital technologies. In a recent self-reported survey of Canadian cardiology residents, respondents reported a lack of comfort and a need for telemedicine-targeted education during medical training.23 Accelerated specialty-focused telemedicine curricula have been feasible in the ambulatory pediatric setting and would be readily transferrable to the adult setting. In that study, sessions delivered via Zoom included the basic principles of telemedicine communication, technical skills, and physical examination. Direct observation by attendees was also developed for evaluation purposes.24 There was a significant increase in residents’ self-reported efficacy in performing key components of telemedicine visits. Another study demonstrated that undergraduate medical students taught with the use of DH technologies were more familiar with them in their later clinical practice and more able to use such technologies in their career compared with peers who did not receive DH-based lessons during their training.25 Beyond simply incorporating important DH and digital learning competencies into educational frameworks, such models of education will need to incorporate rapid knowledge translation and dissemination of new concepts and care paradigms that emerge at great speed in response to fresh challenges.
It is reassuring that early reports show that a move to telehealth and virtual clinics did not increase overall morbidity or mortality.26 However, translating these digital innovations into improved outcomes compared with previous models of care in the longer term remains to be seen.27 For DH to reach full potential, we will need a workforce trained in DH delivery and leaders with a vision to deploy changes in practice.
Innovation Through COVID-19
Pivoting to telemedicine during COVID-19
Telemedicine has been in place for decades in Canada; long on promise, it has been short on delivery. According to the Canadian Institute for Health information, before COVID-19, virtual care represented 0.15% of 270.3 million billable services.28 Virtual care was mostly used in the private sector domain, which offered services directly to patients and providers for a fee. There was tension between patient benefits (increased access to care, cost efficiency, convenience) and provider barriers (lack of reimbursement, jurisdiction licensure restrictions, and lack of interoperability with various electronic health records).28 COVID-19 resulted in universal adoption of telemedicine, effectively overnight. This was due to the need for a steep decline in face-to-face ambulatory visits owing to pandemic restrictions, and was supported through the use of temporary provincial billing codes.29 In the post–COVID-19 era, it is anticipated that patients will derive benefit from “blended” care with both in-person and virtual care (including telemonitoring, advanced artificial intelligence (AI), and algorithm-based care) where appropriate.
The pivot to virtual visits during COVID-19 was necessary to provide appropriate and timely care. After COVID-19, we will need to better understand appropriate patient selection for virtual care and remote patient monitoring (RPM). Moreover, we will need to appropriately evaluate the outcomes and quality in order to define what is “good” virtual care. A recent perspective article entitled “Remote Patient Monitoring—Overdue or Overused” highlights that while RPM can reduce cost by reducing preventable admissions and offer convenience and heightened surveillance for clinical events, there is a desperate need for research to further differentiate which patients would most derive benefit from it after the COVID-19 era.30 In the next section, we will describe the pearls and pitfalls of RPM, using heart failure (HF) as the canonic example.
Telemonitoring in heart failure
The care of patients with HF requires frequent clinical assessments and laboratory investigations to initiate and titrate guideline-directed medical therapy (GDMT) as well as for the surveillance of acute decompensation. RPM engages patients as equal partners in their care and should therefore theoretically appeal to care providers and to patients with HF.31
Despite general enthusiasm, the majority of trials that depended on traditional physiologic metrics (weight, blood pressure, and heart rate) have not improved outcomes (Table 1 ). In the Telemonitoring to Improve Heart Failure Outcomes (Tele-HF) study, among 1653 high risk patients, collecting daily weights and symptoms and providing daily coaching did not result in a reduction in hospitalisation compared with usual care when monitored for weight and symptoms.32 In the first Telemedical Interventional Monitoring in Heart Failure (TIM-HF) study, despite improved adherence rates (80% of the interventional cohort had ≥ 70% of daily data transfers), there was no difference in all-cause mortality.33 Common themes in these neutral trials are low adherence to the technology, poor data accuracy, and either delays or lack of actionability on data received.34 For example, in the Tele-HF trial, 14% of patients in the active treatment arm never used the monitoring device. Furthermore, by the end of the study, only half of the patients in the active arm were using the device 3 times per week as instructed.32
Table 1.
Noninvasive heart failure remote patient monitoring trials
| Trial (country of origin) | Study population | Intervention | Results | Explanations given for results |
|---|---|---|---|---|
| Tele-HF (2010) Chaudhry et al. (USA)32 |
1653 HF patients enrolled within 30 days of hospitalisation for HF decompensation | Daily telephone call to automated interactive voice response system providing information on symptoms, clinical status, and weight | No difference in readmission or death from any cause within 180 days compared with usual care | Underuse of the telemonitoring system: only 86% of patients made any calls and only 55% making 3 calls weekly at 6 months |
| TIM-HF (2011) Koehler et al. (Germany)33 |
710 patients in NYHA II/III with LVEF ≤ 25%, or 25%-35% with decompensation requiring intravenous diuretics in previous 24 months | Patients used portable devices for ECG, blood pressure, and body weight measurements and reported self-assessed health status sent to telemedicine monitoring centre | No reduction in mortality, CV death, or HF hospitalisation compared with usual care | Stable and well managed group of patients in usual care group: only 10% experienced a cardiac event during the 24 months of the study |
| INH (2012) Angermann et al. (Germany)37 |
715 patients with signs and symptoms of HF decompensation and LVEF ≤ 40% | Nurse-delivered disease management programs of education, remote monitoring through structured telephone support and medical optimisation of GDMT | No reduction in primary composite end point of death or rehospitalisation compared with usual care; significant reduction in death from any cause (secondary end point) | Early hospitalisation may have allowed better care for patients in intervention group leading to a reduction in mortality |
| WISH (2012) Lynga et al. (Sweden)38 |
344 patients with NYHA III/IV symptoms, on diuretic and HF medication with LVEF < 50% | Daily electronic weight transmission to HF clinic vs standard scale and no automatic transmission of data; all patients advised to contact clinic if > 2 kg weight gain in 3 days | No reduction in all-cause hospitalisation or death, or composite cardiac hospitalisation or death | Despite better adherence to daily weight checking in intervention group (75% vs 32% in usual-care group), no difference, suggesting that weight alone may not be a useful monitoring metric |
| TEHAF (2012) Boyne et al. (The Netherlands)39 |
382 HF patients with NYHA II-IV symptoms, previous use of diuretics, and impaired cardiac function on echocardiography | Daily preset dialogue on symptoms, knowledge, and behaviour; device collected and provided tailored patient- and disease-specific information; no vital signs measured | No significant reduction in time to first HF hospitalisation | Trial underpowered for primary outcome and well treated and rather stable population |
| CHAT (2013) Krum et al. (Australia)40 |
405 patients with NYHA II-IV HF, LVEF < 40%, in rural and remote areas | At least monthly use of telephone-based automated telemedicine system which assessed clinical status and medical management of their condition, sending alerts to HF nurses | No reduction in primary end point of Packer clinical composite score; significant reduction in HF hospitalisation compared with usual care | Possible useful intervention for those in rural locations without local access to community-based multidisciplinary care |
| BEAT-HF (2016) Ong et al. (USA)41 |
1427 patients aged > 50 years discharged home after hospitalisation for HF | Coaching telephone calls and telemonitoring including blood pressure, heart rate, symptoms, and weight | No reduction in readmission for any cause | Limited efficacy in use of weight as a surrogate of HF deterioration |
| TIM-HF2 (2018) Koehler et al. (Germany)36 |
1571 patients in NYHA II/III, LVEF ≤ 45% (or > 45% treated with diuretic) and HF hospitalisation during previous 12 months | Web-based daily remote monitoring of weight, blood pressure, pulse, ECG, peripheral capillary oxygen saturation, and self-reported health status | Reduction in the weighted average of % of days lost due to unplanned CV hospital admissions or death: HR 0.80, 95% CI 0.65-1.00 | Very high usage rate among participants: 97% of patients were 70% compliant with daily data transfer |
BEAT-HF, Better Effectiveness After Transition-Heart Failure; BP, blood pressure; CHAT, Chronic Heart Failure Assessment by Telephone; CI, confidence interval; CV, cardiovascular; ECG, electrocardiography; GDMT, guideline-directed medical therapy; HF, heart failure; HR, hazard ratio; INH, Interdisciplinary Network for Heart Failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; TEHAF, Telemonitoring in Patients With Heart Failure; Tele-HF, Telemonitoring to Improve Heart Failure Outcomes; TIM-HF, Telemedical Interventional Monitoring in Heart Failure; TIM-HF2, Telemedical Interventional Monitoring in Heart Failure; WISH, Weight Monitoring in Patients with Severe Heart Failure.
Implant-based RPM technologies used in HF have been shown to be useful for monitoring patients, but there have been challenges with costs, workflow, and responding to alerts consistently across trial populations.35 There are often few designated protocols for action after an alert is received. This reduces the effectiveness of the system if appropriate alerts are not followed by equally appropriate alterations in patient management, including altering medications or reasserting the need for adherence to lifestyle measures such as limiting dietary intake of salt and water.
Successful remote monitoring requires adherence to schedules of data transmission, that is, it requires the patient to actually use the technology. For example, in one of the only positive telemonitoring trials, the TIM-HF2 trial, 97% of patients were 70% compliant with daily data transfer. In that trial, 1571 patients were randomised to either usual care or usual care supported by RPM. Overall, there was a borderline significant reduction in the primary end point of days lost to death or cardiovascular hospitalisation from 6.6% in the usual-care group to 4.9% in the RPM group (ratio 0.80, 95% confidence interval [CI] 0.65-1.00; P = 0.046) but no significant difference in mortality. Interestingly, in extended follow-up 1 year after RPM was stopped, there was no longer a difference between patients previously managed with RPM and those receiving usual care, suggesting that the effect of RPM only occurred while the technology was in use.36 This suggests that sustained benefits to patient care require ongoing engagement with RPM by both patients and clinicians.
COVID-19 heart failure RPM use case
During the COVID-19 pandemic, there was a decline in hospitalisations in patients with HF with each lockdown order.42 Many feared that this downtrend would result in an increase in mortality due to delayed care as an unintended consequence of COVID-19 fear and public messaging.43 To date this fear has not materialised; recent data have shown no differences in HF mortality (hazard ratio [HR] 0.97, 0.92-1.10) compared with 2019.26 It is possible that enhanced telemonitoring and widespread adoption mitigated the indirect death toll in patients with HF during the pandemic.
Medly is a Health Canada Class II licensed software as a medical device remote patient management program used to monitor HF patients at the University Health Network, Toronto, Canada.44 Medly uses an in-app rules-based algorithm that delivers personalised self-care messages based on daily input of weight, blood pressure, heart rate, and HF-specific symptoms.45 Examples of the self-care feedback messages run the gamut of confirming that everything is normal, to a change in diuretic medication dose, to suggesting when patients should contact their care providers or go to the emergency department.46 Clinical alerts are also managed by a nurse coordinator with rapid escalation to a cardiologist as needed. In a small pragmatic study, Medly was associated with a 50% decrease in HF-related hospitalisation (incidence relative ratio [IRR] 0.50; P < 0.001) and a 24% decrease in all-cause hospitalisations (IRR 0.76; P = 0.02).45 In a 2-arm randomised control pilot trial (n = 42 patients) comparing Medly-enabled remote titration vs standard of care, patients randomised to remote titration were more likely to be on target or maximally tolerated doses of GDMT (angiotensin receptor–neprilysin inhibitor/angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist) at a shorter median interval (11 weeks vs 18.8 weeks; P < 0.001). Within 6 months, 86% of the intervention group compared with 48% of the control group achieved optimal GDMT (P = 0.004).47
During the COVID-19 pandemic, Medly was leveraged to provide greater support to HF clinicians undertaking remote patient management. Program evaluation has demonstrated that both patients and clinicians valued care continuity through telemonitoring and the opportunity to foster a meaningful patient-provider relationship.48 This experience highlighted the importance of RPM being embedded in the context of the interaction with the clinic; patients who understood why they were being enrolled had more positive engagement with the technology than those who had not been introduced to the program by their usual cardiologist, highlighting the ongoing challenge of building relationships through remote methods.49
Emerging Technologies With Potential to Revolutionise Pandemic Care
As the realm of DH continues to grow, a number of opportunities for patient management are emerging. These technologies were not as widely used during the pandemic as RPM for HF, nor have they yet provided solutions to manage the workload associated with COVID-19, but they all have potential for improving care in the future. In this next section we review wearable devices, which can provide continuous monitoring of patients and the role of AI in patient management.
Wearables: the opportunity for continuous monitoring
The interest in using technologic innovations such as “smart wearables” to monitor health has grown significantly over the past 5 years.50 Smart wearables are consumer-grade devices with sensors that can be worn as an accessory or embedded into clothing. These devices include smartwatches, rings, wristbands, and pedometers. Common sensors in smart wearable devices are summarised in Table 2 . Data from these devices can be processed through software algorithms to provide potentially important insights into personal health. Contrary to traditional medical models, wearables have been introduced to consumers before validation of effectiveness, safety, and reliability in the health care setting.50 , 51
Table 2.
| Engineering sensor | Sensor type | Description | Measurement | Examples of consumer wearable |
|---|---|---|---|---|
| Activity | Triaxial accelerometer | Evaluates linear acceleration along 3 planes based on the principle of a seismic mass attached to a mechanical suspension system | Steps Activity intensity Activity minutes EE Posture when worn on torso |
Apple Watch SE, Series 3-6 Fitbit Flex, One, Charge, Sense Garmin Vivoactive, Venu Huawei Watch GT Omron HeartGuide Withings Steel HR, Move, Scan Oura Ring Motiv Ring |
| Gyroscope | Measures angular motion | |||
| GPS | Uses satellite system to identify precise orbital position | Distance | ||
| Barometer | Uses diaphragm on a vacuum chamber that compresses proportionally to pressure | Change in altitude, stair count, and detection of falls | ||
| Heart rate and rhythm | PPG | Measures the microvascular blood volume that translates into pulse waves and a tachygram recording | Arrhythmia HR HRV HRR Cuffless BP |
|
| Single-lead ECG | Contralateral finger on crown serves as negative electrode and back of the watch serves as positive electrode | Atrial fibrillation vs sinus rhythm | Apple Watch Series 4-6 Fitbit Sense KardiaMobile (AliveCor) Scanwatch (Withings) |
|
| Blood pressure | Oscillometry | Wrist-cuff BP | Ambulatory cuff BP monitoring | HeartGuide (Omron) |
| Fluid content | Cloth-based nanosensors | Phonocardiography, impedance cardiography, multichannel ECG, and accelerometer | Cardiac output Stroke volume HR HRV RR Thoracic impedance Activity Posture |
SimpleSENSE (Nanowear) |
BP, blood pressure; ECG, electrocardiography; EE, energy expenditure; GPS, Global Positioning System; HR, heart rate; HRR, heart rate recovery, HRV, heart rate variability; PPG, photoplethysmography; RR, respiratory rate.
Currently 21% of American adults regularly wear a smartwatch or wearable fitness tracker.52 Use is more common among women than men (25% vs 18%), among Blacks and Hispanics than Whites (23% and 25% vs 20%), and among those with higher income (31% > $75,000 vs 12% < $30,000).52
Application of wearables in routine cardiovascular care
Physical activity monitoring and cardiac rehabilitation
Physical inactivity is associated with an estimated 5 million deaths per year worldwide.54 Increasing physical activity levels at the population level could have a substantial effect on chronic disease and increase longevity. The lockdowns and stay-at-home orders in response to COVID-19 reduced the opportunity for the general public to participate in organised sport or even attend fitness centres. Early reports indicate that patients were enthusiastic in using DH solutions to maintain their activity levels during COVID-19, and it is likely that this will continue beyond the pandemic.55 However, pre-pandemic studies evaluating the efficacy of wearables have been mixed. In a randomised control trial conducted to address weight loss with the use of a wearable tracker in 470 young adults over 24 months, the intervention group lost less weight than the standard group.56 Simply wearing a device tracker may not offer an advantage over standard behavioural approaches. A wearable tracker coupled with gamification has shown more effective results in the short-term for improving physical activity. The Behavioral Economics Framingham Incentive Trial (BE FIT) was a noncompetitive team-based intervention that offered a coffee mug as prize after a 12-week intervention. The intervention group reached a higher step count compared with the control group, but the effects tapered during the postintervention period.57 A systematic review has shown that wearable physical activity monitors can increase cardiorespiratory fitness but have little effect on sedentary time of individuals, limiting its benefits.58
Cardiac rehabilitation, already an underutilised resource, saw further limitations to access imposed by the COVID-19 restrictions. More than 50% of Canadian programs ceased to provide any care during the first wave of the pandemic.59 Virtual care rehabilitation, however, became a priority during this time, with the rapid delivery of cardiac rehabilitation to high-risk populations. Successful programs were able to leverage home monitoring with individualised exercise prescriptions. Wearable devices were able to be used to monitor “moderate” activity, albeit slightly differently depending on the wearable.58 Using a baseline step count could support clinicians in determining weekly targeted increases in both step count and heart rate goals.60
Heart failure
Physical activity is an important assessment in patients with HF. Traditionally, clinicians used the New York Heart Association (NYHA) functional class. However, this profiling is subjective with poor clinician interobserver reliability.61 In a feasibility study, we found that a daily step count of 5000 can differentiate between patients with NYHA functional class 2 and 3 symptoms.62 Furthermore, a study of 170 patients with HF found a step count of ≤ 4889.4 steps/day to be a strong and independent predictor of prognosis (HR 2.28, 95% CI 1.31-6.30; P = 0.008).63 The Apple-CPET Ted Rogers Understanding Exacerbations of Heart Failure (TRUE-HF; NCT05008692) study will prospectively assess whether smartwatch physiologic and sensor data alone or in aggregate can predict objective cardiopulmonary exercise testing among 200 HF patients.64 Wearable technologies will also be instrumental in optimising GDMT, but their utility during the pandemic has been limited. The Nanowear Heart Failure Management Multi-sensor Algorithm (NanoSense) study (NCT03719079) is investigating the SimpleSENSE monitoring undergarment and closed-loop machine learning platform (Nanowear New York, NY) in 500 patients across 5 US centres to validate its ability to identify patients at risk of HF decompensation.65 The cloth-based nanosensor array incorporated into an undergarment allows monitoring of cardiac output and stroke volume alongside thoracic impedance, posture, and activity levels to provide a multiparametric signal of changes to a patient’s HF state. With further evidence to support their efficacy, wearables may play a more useful role in future pandemics.
Atrial fibrillation, photoplethysmography, and electrocardiographic monitoring
Atrial fibrillation (AF) lends itself well to remote monitoring owing to its paroxysmal nature and critical need for stroke prevention. Smartwatches with photoplethysmography (PPG) technology coupled with algorithms to detect irregular rhythms may become integral in the screening of AF in targeted populations. During the pandemic, TeleCheck-AF was a multicentre international project launched to maintain care for patients with AF in 25 European centres. By using teleconsultations supported by an on-demand PPG-based heart rate and rhythm monitoring app (FibriCheck, Hasselt, Belgium), clinicians were able to monitor and adjust medications in the follow-up of these patients. Initial deployment of this technology was reported to be uncomplicated, but long-term outcome data are still pending.66 Previous studies have shown some utility of wearables in detecting arrhythmia. The Huawei Heart Study enrolled 187,912 individuals to screen for AF with the use of a band or a wristwatch. Overall, 0.23% of participants received an irregular heart rhythm notification. Subsequent electrocardiography (ECG) resulted in a positive predictive value of 91.6%.67 The Apple Heart Study was a fully virtual study, enrolling 419,297 individuals to screen for AF with the use of a PPG-based smartwatch. An irregular pulse was found in 0.52% of participants. In patients who had an irregular heart alert and returned the ECG patches, 34% had confirmed AF.68
Hypertension and blood pressure monitoring
Hypertension remains an important risk factor in CVD, and early experience of the pandemic saw a drastic reduction in patients seeking review and optimisation of antihypertensive therapies. One study reported a 50% reduction in primary care office visits for blood pressure checks, with no difference between those with good blood pressure control and those with uncontrolled hypertension.69 Technologies allowing home monitoring of blood pressure could help to provide care to these patients for whom in-person care is deferred.
Unfortunately, a recent review of home blood pressure measurement devices found that only a small fraction of these devices are validated. The majority of these devices are sold in online marketplaces, with 972 unique devices identified from 59 individual businesses. Of the 532 wrist-wearable devices identified, none had been validated for accuracy or performance, thus reducing the potential effectiveness of blood pressure monitoring for users.70
Recommendations focus on development of blood pressure management programs, rather than the measurement devices themselves, advocating for a closed-loop monitoring system that can automatically send data to the clinic to inform ongoing patient management.71
New technologies are emerging, with promise shown in using smartphone video-capture to estimate blood pressure with the use of transdermal optical imaging. Although they are still developing, better ways to detect and intervene earlier may provide improvements in preventing subsequent complications of hypertension.72
Diabetes and blood glucose monitoring
Continuous blood glucose monitoring devices, with or without coupling to insulin delivery pumps have been approved by the US Food and Drug Administration for nearly a decade.73 Used to measure capillary blood glucose levels in patients without intercurrent illness, these devices allow patients with diabetes to assess their glycemic status, receive automatic alerts when out of range, and guide their diabetes management. There was no widespread deployment of these devices during the pandemic, but the review of data from these devices suggested better diabetes control during lockdowns. It remains unclear whether this was due to better diet and self-care generally, or whether lockdowns provided individuals with an opportunity to better focus on their own health.74
Artificial intelligence
AI is defined as the ability of a machine to imitate intelligent human behaviour, whereas machine learning (ML) is the application of AI that allows a system to automatically learn and improve from experience. Deep learning is a subset of ML that uses complex algorithms and deep neural networks to train a model.75 AI could provide support in a future pandemic through enhancing clinical assessment by the nonspecialist when access to specialist resources is limited by restrictions on travel or capacity at specialty providers. Technology-enhanced medical examination devices, such as the Eko (Oakland, CA, USA) digital stethoscope, provide an opportunity for enhanced clinical assessment. By training a deep neural network to identify murmurs from recording through the device coupled with underlying echocardiographic abnormalities, the device could identify common murmurs with an acceptable positive predictive value. Such tools could aid learners in developing their skills and assist nonexperts in obtaining useful clinical information to aid decision making from a distance.76
Within cardiology, echocardiography is a highly specialised field that is infrequently available in rural or remote settings. AI to guide the acquisition of images is a novel focus, particularly with the emergence of point-of-care ultrasound. A deep learning algorithm trained on 5 million examples of ultrasound probe movement was applied to provide real-time guidance to novice operators.77 Eight nurses were able to acquire 10-view transthoracic echocardiographic images at the level of sonographers. That study has significant implications for access to cardiac care in pandemic situations, in isolated communities, and in areas where travel may be restricted.
Challenges in adoption of digital technologies in health care
Interpretation challenges
There are no publicly available data on the accuracy and validation of most consumer wearables. Commercially available devices that are used to support a healthy lifestyle may not go through the same rigorous review as a medical-grade device. A recent (2020) systematic review, which included 158 articles, evaluated the accuracy of 9 different commercially available wearable device brands: Apple, Fitbit, Garmin, Mio, Misfit, Polar, Samsung, Withings, and Xiaomi. The metrics most commonly reported in the literature are mean percentage error (MPE) as defined by the actual observed values compared with the criterion standard. Devices had optimised accuracy in controlled laboratory settings. All devices measured heart rate within ± 3% on average in controlled settings; Apple Watch and Garmin were the most accurate for estimating heart rate. Fitbit, Apple Watch, and Samsung devices measured steps accurately. Energy expenditure was poorly estimated across all devices. One of the most relevant limitations of that review was the inclusion of discontinued and outdated devices, such as Apple Watch Series 2, which is several generations behind the currently available Apple Watch Series 6 device.78
Accuracy of findings
There have been a number of questions raised over the accuracy of wearable devices, particularly with PPG technology. In the SmartWATCHes for Detection of Atrial Fibrillation (WATCH AF) study, 22% of participants were excluded from analysis because of poor-quality PPG recordings.79 The detection of AF is always more challenging at higher heart rates, so the effectiveness is often context dependent, presenting a challenge in detecting arrhythmia, which has a low incidence across the general population while older populations who have a higher incidence of AF are less likely to be using wearable devices.80 Further areas of inaccuracies with PPG technology deriving heart rate and oxygen saturation relate to 1) skin tone, 2) motion artefacts, and 3) signal crossover.81 , 82 Future steps consist of defining who would most benefit from tracking, establishing modern standards for the evaluation of sensors, and collaborating with industry to ensure improvement of reach and accuracy in people of colour.
Clinical implications
The lack of robust evaluation of the clinical outcomes associated with wearable-detected abnormal findings and the uncertain impact of treating those findings is a significant challenge for clinicians. Using the example of pacing device–detected arrhythmia, for which recent studies have shown that limited episodes of asymptomatic AF do not necessarily warrant stroke prophylaxis treatment with anticoagulation, physicians remain unsure about what thresholds of wearable-detected arrhythmia are important and require treatment.83 , 84 The role of wearable-detected HF parameters in management of HF patients remains at the investigation stage. The only significant evidence of benefit arises from continuous glucose monitoring devices. Evidence of benefit of these technologies, in terms of both clinical results and acceptability to patients, are needed before wearable use can be generalised into mainstream cardiovascular patient management.
Artificial intelligence–driven algorithms
ML was used during the pandemic largely to identify early signs of COVID-19 illness. Many of the reported studies were hampered by methodologic flaws that prevented generalisability. Algorithms used small datasets with low-quality data and limited diversity, and in certain reports the same data set was used for training and validation.85 These limitations mirror the experience of ML-driven cardiology research. In 2020, nearly 1 in every 1000 new papers indexed on Medline was about AI or ML in cardiology.86 Despite the early excitement, even from the World Health Organisation, of using AI to deal with the pandemic, it provided few useful solutions.87 Patient populations included in training databases still do not accurately reflect the populations being treated in routine clinical practice, a feature that has affected conventional clinical trials for decades.88 Until these data sources are inclusive and bias free, the algorithms developed based on them will remain flawed. AI-enhanced clinical decision tools could help us achieve more meaningful and efficient interactions with patients. Unfortunately, though, current AI technologies in cardiology are still at the “hype” stage and will require rigorous validation with robust scientific research in diverse populations to demonstrate safety and generalisability.89
Implementation
There is a critical role for an implementation plan at the pre-prototype stage as a roadmap outlining the timelines, resources, and deliverables.90 As DH interventions (DHIs) emerge, they undergo an intervention maturation life cycle. Early stages of the prototype require evaluations related to end-user interfacing (usability) and feasibility of the technology. As the intervention produces consistent outputs, systematic evaluations of efficacy and effectiveness are necessary to assess the impact of the intervention. In later stages, a DHI may undergo economic and health technology assessments to appraise their affordability in the health care system. As the technology continues to mature, a DHI may then undergo wider-scale adoption within the health stream and policy environment (implementation science). Most DHIs do not reach the larger real-world implementation scale, and this maturation life cycle may take decades to evolve.90
Lessons Learned From COVID-19 and Future Aspirations
DH offers an opportunity to counter the effects of a future pandemic if lessons are learned from the role it played in dealing with the effects of COVID-19. Health care systems were quick to pivot to technology-enhanced solutions to patient management; although the success, or otherwise, of this approach has yet to be fully evaluated, several key factors have aided this change in the care paradigm. There are issues that need to be addressed to improve the utility of DH in future pandemic management, including mitigation of the disproportionate effects of future pandemics on vulnerable populations. Specific actions recommended to limit the spread of COVID-19 included addressing the intermediate layers of the determinants of health (ie, housing, job, and food security) and supporting broad access to computers and free internet to reduce the digital divide.4 , 5 , 28
Alterations in how care is provided, including increased use of telehealth and digital innovation, should be co-designed and include patients at the outset to ensure their needs are met. Often reinventing the wheel is not necessary; for example, in the months before COVID-19 took hold in Canada, the Virtual Care Task Force created by the Canadian Medical Association, the College of Family Physicians of Canada, and the Royal College of Physicians and Surgeons of Canada released its recommendations for scaling up virtual medical services.91 Their 5 key recommendations from February 2020 are summarised in Table 3 .
Table 3.
Recommendations of the Virtual Care Task Force91
| Develop national standards for patient health information access. |
| Support the efforts of the Federation of Medical Regulatory Authorities of Canada to simplify the registration and licensure processes for qualified physicians to provide virtual care across provincial and territorial boundaries. |
| Encourage provincial and territorial governments and provincial and territorial medical associations to develop fee schedules that are revenue neutral between in-person and virtual encounters. |
| Engage the CanMEDS consortium in incorporating and updating virtual care competencies for undergraduate, postgraduate, and continuing professional development learners. |
| Develop a standardised pan-Canadian lexicon for virtual care. |
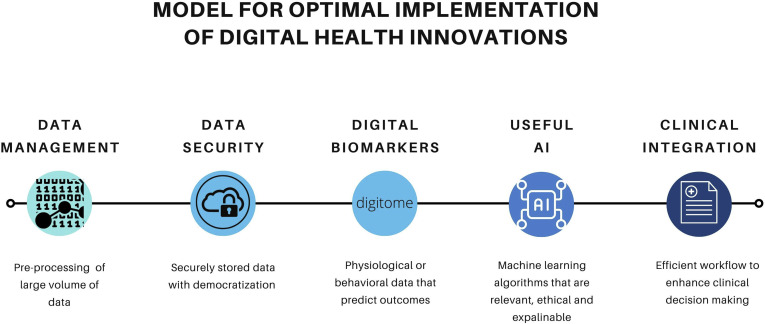
A rapid review of DH solutions and remote monitoring technologies used in response to COVID-19 identified perceived benefits, including a lower burden of care for hospitals and HCPs, reduced infection risk for patients, and support for vulnerable populations. At the same time, barriers to implementation included equity-related barriers (affordability of technology for users, poor internet connectivity, poor health literacy), the need for quality best-practice guidelines for use of RPM, and the need for additional resources to develop and support technology solutions.92 Innovation in patient management and alteration of paradigms of health care delivery were successful, but not all opportunities were utilised to their fullest extent. There remains a gap between use of DH innovation and its integration into routine clinical practice through evidence-based implementation practices. We propose 5 important steps required to bridge this gap, creating a model for how future virtual clinics could be organised (Fig. 1 ).
Figure 1.
Model for optimal implementation of digital health innovations. AI, artificial intelligence.
Data management
Many digital solutions currently involve transfer of data from patient devices to clinicians for review. However, often there is no automated means of analysing the large volume of data produced from wearable technologies. Any data that are submitted by patients for clinical review must be painstakingly analysed, often manually, with little in the way of a safety net to ensure correct analysis. This requires skills that are not always well developed and time on the part of the analyser to perform this task. These novel technologies should provide data that are organised with important findings highlighted by automated preprocessing. Without these filters in place, reliably identifying features of interest that clinicians can act on, HCPs will quickly lose interest and motivation in using novel technologies. Finally, current patient record systems are not geared toward archiving data received from wearables, making subsequent review to verify new findings against previous data challenging. For effective future deployment, these considerations need to be addressed as part of a system-wide approach.
Data security and access
Patients should be confident that data produced by innovative digital technologies is securely stored and can be accessed only by members of their health care teams. Furthermore, patients should have access to their own data, with appropriate support and empowerment to be able to alter their management should abnormal findings be present. This is a paradigm that has been successfully used in diabetes and HF management, where the clinical team takes on an advisory role rather than providing instruction on what to do in response to every measurement taken. These data flows will be reliant on storage in digital repositories, using cloud-based solutions. It is imperative that appropriate security standards are developed alongside interactivity frameworks to ensure meaningful use of this data while preserving privacy. Furthermore, data flows need to be effective, and this can only occur with inexpensive and readily available internet access in all parts of the country. The Canadian Radio-television and Telecommunications Commission have launched their “Closing the Digital Divide” program, aiming to achieve broadband speeds of at least 50 Mbps download and 10 Mbps upload with access to unlimited data for all Canadians. Such initiatives are key to promoting digital transformation of care.93
Identification of effective digital biomarkers (digitome)
An important application of data preprocessing includes digital biomarkers. Digital biomarkers are physiologic and behavioural measures that explain, influence, or predict health-related outcomes, creating a personalised digitome. It may be that in the future, particular motion and movements (eg, placing a hand to the mouth while smoking) or attendance at locations (visiting a fitness centre) will aid priming of risk models for routine care, leveraging data that are currently routinely collected by wearables and other smartphone technologies. Data from sensors need to be integrated and provide contextual information, processed to help understand the clinical phenomenon being measured, and matching this to clinical need to produce useful digital biomarkers.94 These digital biomarkers will need to demonstrate validity in each population that will be treated, including those from nonurban, underserved, and vulnerable backgrounds.
Useful artificial intelligence
The promise of AI requires a systematic approach to training algorithms and ML models underpinned by rigorous methodology. Despite enthusiasm related to ML and health care automation, there are inconsistencies related to the availability of labelled data and outcomes, bias injection (eg, introduction of racial/gender bias in nonrepresentative data sets), inaccurate measurements, reproducibility, and lack of external validation. To mitigate some of these issues, the American College of Cardiology Healthcare Council put forth “Proposed Recommendations for Cardiovascular Imaging–Related Machine Learning Evaluation” (PRIME).95 PRIME aims to provide a checklist for the ML community, with the goal of standardising the application of AI and ML. This framework provides recommendations, ranging from design to reporting the limitations phase that data scientists, researchers, and clinicians should adopt, to allow for the implementation of AI in clinical practice.
Furthermore, the concept of “ethical AI” is emerging, with due consideration being given to data privacy and ownership alongside the determination of accountability for decisions now made by computers that were previously made by humans.96 , 97 Although neural networks can offer important accurate diagnostic and prognostic information, algorithms should be explainable and interpretable.86 Highly complex neural networks may yield accurate diagnostics, but it may be difficult to generalise to real-life clinical situations.98 Moreover, health care data are particularly vulnerable to challenges where the underlying code and patient data are subject to privacy disclosure restrictions.
Coding for these algorithms should be freely available for scrutiny and external review, without compromising intellectual property rights or dissuading innovation in this field. If there is little appetite for development of such tools by the private sector, then it should be made a priority for national and international collaborations through research-funding bodies such as the Canadian Institutes of Health Research or US National Institutes of Health to ensure that patients have confidence in these AI-driven technologies.
Integration into the clinical care pathway
Without consideration of how these technologies can be integrated into the clinical pathway for patients, take-up of innovation will be suboptimal. Issues such as data management, storage, workflow for analysis, clinical decision making, and enacting changes in management plans need to be addressed. How data filters into the current model of multidisciplinary management of patients has yet to be tackled. It may be that current models of care are inadequate for dealing with future challenges, and these models will need to be proactive to maintain efficacious. The final, but no less important, driver of change in care pathways is the remuneration of clinical activity undertaken by both the HCP and the health care organisation. Without adequate reimbursement, there will always be barriers to implementation. By demonstrating the value of digital innovation to the health care economy through appropriately designed clinical evaluation studies, regulators and payers should be willing to embrace novel, evidence-based technologies.
Conclusion
COVID-19 will be remembered for the devastating number of associated deaths and long-term health consequences, and the sociodemographic divides that it unmasked. It will also be remembered as a time of opportunity, when unprecedented digital solutions were rapidly implemented into clinical practice and medical education. DH technology needs to be held to the same rigorous standards as current health care tools, with embedded quality measures to promote high-quality care, ensure that devices and digital biomarkers are valid and that AI is ethical and clear, and confirm that patient data can be securely stored and transferred to clinicians where it can provide a meaningful trigger to engage evidence-based interventions to change patient management. DH has all the potential to deliver safe, efficient, and effective care to the population as a whole, reaching parts of society who have previously been underserved to try to reduce health inequity at the same time.
Fast forward to 2025: We are now living in a post–COVID-19 era with nearly 6 billion people vaccinated. A “new normal” has emerged, including a transformed health care system. We follow more than half of our patients with the use of digital technologies as stand-alone or blended models of care. Reimbursement for virtual care is a permanent fixture. Technology is increasingly addressing health inequity and disparities in care. We hope.
Funding Sources
Dr Brahmbhatt is supported by postdoctoral fellowship funding from TRANSFORM HF.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 289 for disclosure information.
References
- 1.Centers for Disease Control and Prevention 1918 pandemic (H1N1 virus). Reviewed March 20, 2019. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html Available at:
- 2.Cohen J. Chinese researchers reveal draft genome of virus implicated in Wuhan pneumonia outbreak. Science Insider, January 11, 2020. https://www.sciencemag.org/news/2020/01/chinese-researchers-reveal-draft-genome-virus-implicated-wuhan-pneumonia-outbreak Available at:
- 3.APM Research Lab The color of coronavirus: COVID-19 deaths analyzed by race and ethnicity in the U.S. March 5, 2021. https://www.apmresearchlab.org/covid/deaths-by-race Available at:
- 4.Haynes N., Cooper L.A., Albert M.A., Association of Black Cardiologists At the heart of the matter: unmasking and addressing the toll of COVID-19 on diverse populations. Circulation. 2020;142:105–107. doi: 10.1161/CIRCULATIONAHA.120.048126. [DOI] [PubMed] [Google Scholar]
- 5.Chin-Hong P., Alexander K.M., Haynes N., Albert M.A., Association of Black Cardiologists Pulling at the heart: COVID-19, race/ethnicity and ongoing disparities. Nat Rev Cardiol. 2020;17:533–535. doi: 10.1038/s41569-020-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers T.N., Rogers C.R., VanSant-Webb E., et al. Racial Disparities in COVID-19 mortality among essential workers in the United States. World Med Health Policy. 2020;12:311–327. doi: 10.1002/wmh3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgson K., Peytrignet S., Marszalek K. How has hospital use among those clinically extremely vulnerable to COVID-19 been impacted by the pandemic? The Health Foundation, March 24, 2021. https://www.health.org.uk/news-and-comment/charts-and-infographics/hospital-use-clinically-extremely-vulnerable-population Available at:
- 9.Superina S., Malik A., Moayedi Y., McGillion M., Ross H.J. Digital health: the promise and peril. Can J Cardiol. 2022;38:145–148. doi: 10.1016/j.cjca.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early J., Hernandez A. Digital disenfranchisement and COVID-19: broadband internet access as a social determinant of health. Health Promot Pract. 2021;22:605–610. doi: 10.1177/15248399211014490. [DOI] [PubMed] [Google Scholar]
- 11.Innovation, Science and Economic Development Canada High-speed access for all: Canada’s connectivity strategy. 2019. https://www.ic.gc.ca/eic/site/139.nsf/vwapj/ISEDC_19-170_Connectivity_Strategy_E_Web.pdf/$file/ISEDC_19-170_Connectivity_Strategy_E_Web.pdf Available at:
- 12.Julien H.M., Eberly L.A., Adusumalli S. Telemedicine and the forgotten America. Circulation. 2020;142:312–314. doi: 10.1161/CIRCULATIONAHA.120.048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Radio-television, Telecommunications commission. communications monitoring report: LTE and broadband availability. Modified December 10. 2020. https://crtc.gc.ca/eng/publications/reports/policyMonitoring/2020/cmr4.htm Available at:
- 14.Lam K., Lu A.D., Shi Y., Covinsky K.E. Assessing telemedicine unreadiness among older adults in the United States during the COVID-19 pandemic. JAMA Intern Med. 2020;180:1389–1391. doi: 10.1001/jamainternmed.2020.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rootman I., Gordon–El-Bihbety D. A vision for a health literate Canada: report of the Expert Panel on Health Literacy. Executive summary. Canadian Public Health Association. 2008. https://www.cpha.ca/sites/default/files/uploads/resources/healthlit/execsum_e.pdf Available at: Accessed 28 November 2021.
- 16.Sudore R.L., Yaffe K., Satterfield S., et al. Limited literacy and mortality in the elderly: the health, aging, and body composition study. J Gen Intern Med. 2006;21:806–812. doi: 10.1111/j.1525-1497.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Council on Learning Health literacy in Canada: a healthy understanding. February 2008. http://www.en.copian.ca/library/research/ccl/health/health.pdf Available at:
- 18.Mitic W., Rootman I., editors. Public Health Association of BC. 2012. https://phabc.org/wp-content/uploads/2015/09/IntersectoralApproachforHealthLiteracy-FINAL.pdf Available at: [Google Scholar]
- 19.Williams M.V., Baker D.W., Parker R.M., Nurss J.R. Relationship of functional health literacy to patients’ knowledge of their chronic disease. A study of patients with hypertension and diabetes. Arch Intern Med. 1998;158:166–172. doi: 10.1001/archinte.158.2.166. [DOI] [PubMed] [Google Scholar]
- 20.Environics Analytics VaccineInsights: Where do we need to build confidence in vaccines? A data and analytics approach. March 26, 2021. https://storymaps.arcgis.com/stories/7aceed148261444d8cb9c909f1ef1969 Available at:
- 21.Paakkari L., Okan O. COVID-19: health literacy is an underestimated problem. Lancet Public Health. 2020;5:e249–e250. doi: 10.1016/S2468-2667(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz F., Filipov M. Health literacy. Public Health Ontario Grand Rounds, March 6, 2018. https://www.publichealthontario.ca/-/media/documents/H/2018/health-literacy.pdf Available at:
- 23.Almufleh A., Lee C., Tsang M.Y., Gin K., Tsang T.S.M., Nair P. The need for telemedicine integration into adult cardiology training curricula in Canada. Can J Cardiol. 2021;37:929–932. doi: 10.1016/j.cjca.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Costich M., Robbins-Milne L., Bracho-Sanchez E., Lane M., Friedman S. Design and implementation of an interactive, competency-based pilot pediatric telemedicine curriculum. Med Educ Online. 2021;26:1911019. doi: 10.1080/10872981.2021.1911019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waseh S., Dicker A.P. Telemedicine training in undergraduate medical education: mixed-methods review. JMIR Med Educ. 2019;5:e12515. doi: 10.2196/12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadhera R.K., Shen C., Gondi S., et al. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. 2021;77:159–169. doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoaib A., van Spall H.G.C., Wu J., et al. Substantial decline in hospital admissions for heart failure accompanied by increased community mortality during COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes. 2021;7:378–387. doi: 10.1093/ehjqcco/qcab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canadian Medical Association Virtual care in Canada: discussion paper. CMA Health Summit, August 2019. https://www.cma.ca/sites/default/files/pdf/News/Virtual_Care_discussionpaper_v2EN.pdf Available at:
- 29.Kronfli C. COVID-19 policy brief: Realizing the full potential of virtual care in Ontario. Ontario Chamber of Commerce. 2020. https://occ.ca/wp-content/uploads/COVID19-Policy-Brief-Virtual-Care-final.pdf Available at:
- 30.Mecklai K., Smith N., Stern A.D., Kramer D.B. Remote patient monitoring—overdue or overused? New Engl J Med. 2021;384:1384–1386. doi: 10.1056/NEJMp2033275. [DOI] [PubMed] [Google Scholar]
- 31.Mohebali D., Kittleson M.M. Remote monitoring in heart failure: current and emerging technologies in the context of the pandemic. Heart. 2021;107:366–372. doi: 10.1136/heartjnl-2020-318062. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry S.I., Mattera J.A., Curtis J.P., et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler F., Winkler S., Schieber M., et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 34.Moayedi Y., Ross H.J. Seizing opportunities in mobile health technologies and heart failure: empowering patients and informing clinicians. Can J Cardiol. 2021;37:1163–1164. doi: 10.1016/j.cjca.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Hindricks G., Varma N. Remote monitoring and heart failure: monitoring parameters, technology, and workflow. Eur Heart J. 2016;37:3164–3166. doi: 10.1093/eurheartj/ehw201. [DOI] [PubMed] [Google Scholar]
- 36.Koehler F., Koehler K., Deckwart O., et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392(10152):1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 37.Angermann C.E., Störk S., Gelbrich G., et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012;5:25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 38.Lyngå P., Persson H., Hägg-Martinell A., et al. Weight Monitoring in Patients with Severe Heart Failure (WISH). A randomized controlled trial. Eur J Heart Fail. 2012;14:438–444. doi: 10.1093/eurjhf/hfs023. [DOI] [PubMed] [Google Scholar]
- 39.Boyne J., Vrijhoef H.J., Nieman F.H., et al. Telemonitoring in patients with heart failure: results from a multicenter randomized controlled trial (the TEHAF study) J Am Coll Cardiol. 2011;57:e389. [Google Scholar]
- 40.Krum H., Forbes A., Yallop J., et al. Telephone support to rural and remote patients with heart failure: the Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther. 2013;31:230–237. doi: 10.1111/1755-5922.12009. [DOI] [PubMed] [Google Scholar]
- 41.Ong M.K., Romano P.S., Edgington S., et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition—Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J., Mamas M.A., de Belder M.A., Deanfield J.E., Gale C.P. Second decline in admissions with heart failure and myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2021;77:1141–1143. doi: 10.1016/j.jacc.2020.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moayedi Y., Alba A.C., Lee D.S., Wijeysundera H.C., Ross H.J. The Next wave of health care strain related to COVID-19: heart failure patients coming back in force—we must not fail them. Can J Cardiol. 2020;36:993–994. doi: 10.1016/j.cjca.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Government of Canada University Health Network. Medical Devices Active Listing, revised December 6, 2021. https://health-products.canada.ca/mdall-limh/information.do?companyId_idCompanie=147669 Available at:
- 45.Ware P., Ross H.J., Cafazzo J.A., et al. Outcomes of a heart failure telemonitoring program implemented as the standard of care in an outpatient heart function clinic: pretest-posttest pragmatic study. J Med Internet Res. 2020;22 doi: 10.2196/16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seto E., Leonard K.J., Cafazzo J.A., et al. Developing healthcare rule-based expert systems: case study of a heart failure telemonitoring system. Int J Med Inform. 2012;81:556–565. doi: 10.1016/j.ijmedinf.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Artanian V., Ross H.J., Rac V.E., et al. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4 doi: 10.2196/21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wali S., Guessi Margarido M., Shah A., et al. Expanding telemonitoring in a virtual world: a case study of the expansion of a heart failure telemonitoring program during the COVID-19 pandemic. J Med Internet Res. 2021;23 doi: 10.2196/26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah A., Guessi M., Wali S., et al. The Resilience of cardiac care through virtualized services during the COVID-19 pandemic: case study of a heart function clinic. JMIR Cardio. 2021;5 doi: 10.2196/25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayoumy K., Gaber M., Elshafeey A., et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18:581–599. doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarakji K.G., Silva J., Chen L.Y., et al. Digital health and the care of the patient with arrhythmia: what every electrophysiologist needs to know. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.007953. [DOI] [PubMed] [Google Scholar]
- 52.Vogels E.A. About one-in-five Americans use a smart watch or fitness tracker. Pew Research Center, January 9, 2020. https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/ Available at: Accessed November 28, 2021.
- 53.Dunn J., Kidzinski L., Runge R., et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat Med. 2021;27:1105–1112. doi: 10.1038/s41591-021-01339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bull F.C., Al-Ansari S.S., Biddle S., et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dohse H. Patient perspective: Wearable and digital health tools to support managing our health during the COVID-19 pandemic and beyond. Cardiovasc Digit Health J. 2021;2:88–90. doi: 10.1016/j.cvdhj.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakicic J.M., Davis K.K., Rogers R.J., et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA. 2016;316:1161–1171. doi: 10.1001/jama.2016.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel M.S., Benjamin E.J., Volpp K.G., et al. Effect of a game-based intervention designed to enhance social incentives to increase physical activity among families. JAMA Intern Med. 2017;177:1586. doi: 10.1001/jamainternmed.2017.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hannan A.L., Harders M.P., Hing W., et al. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: systematic review and meta-analysis. BMC Sports Sci Med Rehabil. 2019;11:14. doi: 10.1186/s13102-019-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moulson N., Bewick D., Selway T., et al. Cardiac Rehabilitation during the COVID-19 era: guidance on implementing virtual care. Can J Cardiol. 2020;36:1317–1321. doi: 10.1016/j.cjca.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Doherty A.F., Humphreys H., Dawkes S., et al. How has technology been used to deliver cardiac rehabilitation during the COVID-19 pandemic? An international cross-sectional survey of healthcare professionals conducted by the BACPR. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallagher A.M., Lucas R., Cowie M.R. Assessing health-related quality of life in heart failure patients attending an outpatient clinic: a pragmatic approach. ESC Heart Fail. 2019;6:3–9. doi: 10.1002/ehf2.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moayedi Y., Abdulmajeed R., Duero Posada J., et al. Assessing the use of wrist-worn devices in patients with heart failure: feasibility study. JMIR Cardio. 2017;1:e8. doi: 10.2196/cardio.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izawa K.P., Watanabe S., Oka K., et al. Usefulness of step counts to predict mortality in Japanese patients with heart failure. Am J Cardiol. 2013;111:1767–1771. doi: 10.1016/j.amjcard.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 64.Apple-CPET Ted Rogers Understanding Exacerbations of Heart Failure (TRUE-HF) https://clinicaltrials.gov/ct2/show/NCT05008692 First posted August 17, 2021; Updated September 29, 2021. Available at: Accessed November 28, 2021.
- 65.Nanowear Heart Failure Management Multi-sensor Algorithm (Nanosense) https://clinicaltrials.gov/ct2/show/NCT03719079 First posted October 25, 2018; updated October 19, 2021. Available at: Accessed November 28, 2021.
- 66.Gawałko M., Duncker D., Manninger M., et al. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace. 2021;23:1003–1015. doi: 10.1093/europace/euab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo Y., Wang H., Zhang H., et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beckman A.L., King J., Streat D.A. Bet al. Decreasing primary care use and blood pressure monitoring during COVID-19. Am J Manag Care. 2021;27:366–368. doi: 10.37765/ajmc.2021.88644. [DOI] [PubMed] [Google Scholar]
- 70.Picone D.S., Deshpande R.A., Schultz M.G., et al. Nonvalidated home blood pressure devices dominate the online marketplace in Australia: major implications for cardiovascular risk management. Hypertension. 2020;75:1593–1599. doi: 10.1161/HYPERTENSIONAHA.120.14719. [DOI] [PubMed] [Google Scholar]
- 71.Omboni S., McManus R.J., Bosworth H.B., et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. 2020;76:1368–1383. doi: 10.1161/HYPERTENSIONAHA.120.15873. [DOI] [PubMed] [Google Scholar]
- 72.Luo H., Yang D., Barszczyk A., et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.119.008857. [DOI] [PubMed] [Google Scholar]
- 73.US Food and Drug Administration Animas [OneTouch] Vibe Plus System. Premarket Approval, November 25, 2014. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P130007 Available at:
- 74.Maddaloni E., Coraggio L., Pieralice S. Effects of COVID-19 lockdown on glucose control: continuous glucose monitoring data from people with diabetes on intensive insulin therapy. Diabetes Care. 2020;43:e86–e87. doi: 10.2337/dc20-0954. [DOI] [PubMed] [Google Scholar]
- 75.Wikipedia Artificial intelligence. https://en.wikipedia.org/w/index.php?title=Artificial_intelligence Available at:
- 76.Chorba J.S., Shapiro A.M., Le L., et al. Deep learning algorithm for automated cardiac murmur detection via a digital stethoscope platform. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Narang A., Bae R., Hong H., et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 2021;6:624–632. doi: 10.1001/jamacardio.2021.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuller D., Colwell E., Low J., et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: systematic review. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/18694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dörr M., Nohturfft V., Brasier N., et al. The WATCH AF trial: Smartwatches for Detection of Atrial Fibrillation. JACC Clin Electrophysiol. 2019;5:199–208. doi: 10.1016/j.jacep.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Cheung C.C., Gin K.G., Andrade J.G. Watch out: the many limitations in smartwatch-driven AF detection. JACC Clin Electrophysiol. 2019;5:525–526. doi: 10.1016/j.jacep.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Colvonen P.J. Response to: Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit Med. 2021;4:38. doi: 10.1038/s41746-021-00408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shcherbina A., Mattsson C.M., Waggott D., et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017;7:3. doi: 10.3390/jpm7020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noseworthy P.A., Kaufman E.S., Chen L.Y., et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140:e944–e963. doi: 10.1161/CIR.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Svendsen J.H., Diederichsen S.Z., Højberg S., et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP study): a randomised controlled trial. Lancet. 2021;398(10310):1507–1516. doi: 10.1016/S0140-6736(21)01698-6. [DOI] [PubMed] [Google Scholar]
- 85.Ross C. Machine learning is booming in medicine. It’s also facing a credibility crisis. Stat+, June 2. 2021. https://www.statnews.com/2021/06/02/machine-learning-ai-methodology-research-flaws/ Available at:
- 86.Quer G., Arnaout R., Henne M., Arnaout R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:300–313. doi: 10.1016/j.jacc.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mariano B., Jr., Wu S., Muneene D. Artificial intelligence for covid-19 response. The BMJ Opinion, July 23, 2021. https://blogs.bmj.com/bmj/2021/07/23/artificial-intelligence-for-covid-19-response/ Available at:
- 88.Hanlon P., Hannigan L., Rodriguez-Perez J., et al. Representation of people with comorbidity and multimorbidity in clinical trials of novel drug therapies: an individual-level participant data analysis. BMC Med. 2019;17:201. doi: 10.1186/s12916-019-1427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antoniades C., Asselbergs F.W., Vardas P. The year in cardiovascular medicine 2020: digital health and innovation. Eur Heart J. 2021;42:732–739. doi: 10.1093/eurheartj/ehaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Health Organization Monitoring and evaluating digital health interventions: a practical guide to conducting research and assessment. 2016. https://saluddigital.com/wp-content/uploads/2019/06/WHO.-Monitoring-and-Evaluating-Digital-Health-Interventions.pdf Available at:
- 91.Canadian Medical Association Virtual care: recommendations for scaling up virtual medical services. Report of the Virtual Care Task Force. February 2020. https://policybase.cma.ca/en/viewer?file=/documents/PolicyPDF/PD20-07.pdf Available at:
- 92.Houlding E., Mate K.K.V., Engler K., et al. Barriers to use of remote monitoring technologies used to support patients with COVID-19: rapid review. JMIR Mhealth Uhealth. 2021;9 doi: 10.2196/24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Canadian Radio-television and Telecommunications Commission Broadband Fund: closing the digital divide in Canada. January 14, 2016. https://crtc.gc.ca/eng/internet/internet.htm Available at:
- 94.Sim I. Mobile devices and health. N Engl J Med. 2019;381:956–968. doi: 10.1056/NEJMra1806949. [DOI] [PubMed] [Google Scholar]
- 95.Sengupta P.P., Shrestha S., Berthon B., et al. Proposed Requirements for Cardiovascular Imaging–Related Machine Learning Evaluation (PRIME): a checklist. REVIEWED by the American College of Cardiology Healthcare Innovation Council. JACC Cardiovasc Imaging. 2020;13:2017–2035. doi: 10.1016/j.jcmg.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lang M., Bernier A., Knoppers B.M. Artificial intelligence in cardiovascular imaging: "unexplainable" legal and ethical challenges? Can J Cardiol. 2022;38:225–233. doi: 10.1016/j.cjca.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Cave S., Whittlestone J., Nyrup R., O hEigeartaigh S., Calvo R.A. Using AI ethically to tackle covid-19. BMJ. 2021;372:n364. doi: 10.1136/bmj.n364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nate Mach Intell. 2019;1:206–215. doi: 10.1038/s42256-019-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]