Abstract
The use of biopesticides in pest management and pre-harvest disease and crop pest control have been advocated in recent years. This is because of their eco-friendliness and suitability for pest control in the agricultural industry. The objective of this study was to determine the antibacterial and pesticidal potential against sugar ant of metabolites produced by Bacillus species. The species were B. proteolyticus, B. thuringensis, B. cereus and B. subtilis. Metabolite production was carried out in batch experimental setup. The inoculated flasks were incubated in an incubator shaker for 120 h at temperature of 37 °C ± 2 °C. Metabolite extraction was carried out using the acid precipitation method. The crude metabolites were characterized using Fourier Transform Infrared (FTIR) and Gas Chromatography Mass Spectroscopy (GC-MS). Antibacterial activity of the metabolites was carried out both in agar and broth media while pesticidal potential was carried out using the diet-fed approach. All the metabolites showed antibacterial activity against the test pathogens used for investigation. This was irrespective of whether they were used singly or in combination. Generally, the rate of kill of the sugar ants by the respective metabolites was directly proportional to metabolite concentration in the diet. In the control diet setup with no added metabolite, no mortality was recorded throughout the period of incubation. The study findings gave an indication of the potential of these metabolites for possible control of phytopathogens.
Keywords: Biopesticide, Metabolite, Antibacterial, Bacterial growth, Pest management, Phytopathogens
Biopesticide; Metabolite; Antibacterial; Bacterial growth; Pest management; Phytopathogens.
1. Introduction
With increasing world population, there is a concomitant increase in food demand. Increase in food demand, which will lead to increase in crop production, requiring improved crop variety, effective control of disease, use of fertilizers and pest management. (Birch et al., 2011).
The indiscriminate and excessive use of chemical pesticides is known to eliminate non-target insects and could persists in the environment, thus biomagnifying and bioaccumualating along a food chain. Their persistence could affect both microbial and pest ecology. Apart from deterioration of soil texture, the inappropriate or continuous use of chemical pesticides also deteriorates the soil texture, and could lead to microbial resistance and the destruction and elimination of natural occurring organisms which will have negative impacts human and environmental (Kumari et al., 2014). The use of biopesticides in pest management and pre-harvest disease and crop pest control have been advocated in recent years (Yadav et al., 2020). Biopesticides are suitable and appropriate because of their eco-friendliness as pest control in the agricultural industry. In addition to protection against target pests, biopesticides have the additional benefit of not causing toxicity to crops (Thakur et al., 2020; Kumar et al., 2021).
The screening of microbial metabolites has revealed their diversity with broad biological activities, such as antimicrobial, antiviral, immunosuppressive and antitumor activities that enable the microorganism to survive in its natural environment (Cherif et al., 2001; Lisboa et al., 2006). Bacillus species are relatively abundant source of antimicrobials and production secondary metabolites (Sansinenea and Ortiz, 2011). Although sugar ants (Camponotus consobrinus) are not indicated to be harmful, they are known to be nuisance in homes and their activities attract other pests and cause damage to foods. Although current traditional for the control of sugar ants are ecofriendly and less harmful, when compared to chemical application, there efficiency and cost implication is still not well documented. This study was therefore aimed at extracting metabolites produced by four Bacillus species for possible antibacterial activity against selected pathogens and pesticidal potentials against sugar ant.
2. Materials and methods
2.1. Isolates for metabolite production
A total of four Bacillus species were used for production of metabolites in this study. The species were B. proteolyticus, B. thuringensis, B. cereus and B. subtilis. The isolates, which were from soil were part of the laboratory stock in the Department of Biological Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria. The bacterial were isolated from soil in Landmark University Teaching and Research Farm. Prior to use, the isolates were first streaked on nutrient agar plate and incubated for 24 h at 37 °C ± 2 °C, to ascertain their purity. The pure cultures of the respective isolates were transferred to nutrient agar slants and stored at 4 °C ± 2 °C till when needed.
2.2. Metabolite production and microbial count
For metabolite production, the medium used consist of yeast extract (10 g/L), peptone (10 g/L), magnesium sulphate (0.5 g/L), sodium chloride (5 g/L) and sodium acetate (sodium acetate) at a concentration of 20 g/L. The different components of the medium were first weighed and dissolved separately before combination. The prepared medium was dispensed 200 mL quantity in 250 mL capacity conical flasks and sterilized in an autoclave for 15 min at a temperature 121 °C.
After sterilization, the medium was allowed to cool before inoculating with 1 mL of 18–24 h old broth cultures of the respective bacterial species. The inoculated flasks were incubated at an incubator shaker for 120 h at temperature of 37 °C ± 2 °C.
For spore microbial count, following incubation, every 24 h for a 120 h incubation period, 1 mL of medium was aseptically withdrawn from the respective flasks to carry out 10-fold serial dilutions viable and spore counts. Viable count was carried out using the pour plate technique. Using a known dilution of the 10-fold, serially diluted cultures, 1 mL was aseptically withdrawn and placed in sterile petri dishes. Sterile nutrient agar, which has been pre-prepared and allowed to cool in a water bath at 45 ± 2 °C dispensed in the petri dishes and swirled gently for 30 s for proper mixing. The plates were then allowed to solidify before incubating at 37 °C ± 2 °C for 24 h. Following incubation, colonies were counted in each of the respective plates and expressed in colony forming units per millilitres (CFU/mL).
For the spore count, following serial dilutions, test tubes containing known dilutions were incubated for 45 min in a water bath that has been preheated at 80 °C. At the expiration of the incubation period, the tubes were cooled in the refrigerator at 4 °C ± 2 °C for 45 min. After cooling, 1 mL of a known dilution was withdrawn for pour plating, as described for viable count. The plates were also incubated at 37 °C for 24 h before colonies were counted and expressed in CFU/mL.
2.3. Extraction of crude metabolite
The extraction of crude metabolites was done by using the acid precipitation method as reported by Abouseoud et al. (2010). Following the cultivation of the respective bacterial cells (as described earlier), at incubation duration for 120 h, the respective cells were separated from the broth by centrifugation, using a cold centrifuge at a speed of 5000 rmp at 4 °C for 10 min. After centrifugation, the cell-free supernatant was decanted into 1 L beakers and acidified by adjusting the pH to 2.0 ± 0.2, using 1 M HCl. The acidified supernatant was then incubated for 24 h in a refrigerator at 4 °C ± 2 °C. After incubation, crude metabolite was precipitated by addition of solvent mixture of methanol and ethyl acetate (3:1 v/v) to the acidified solution at a ratio of 2:1. The solvent was then separated from the broth, using a separating funnel, after which the liquid was evaporated by placing the beakers in a water bath at 80 °C to obtain the crude metabolites.
2.4. Determination of antibacterial activity
The antibacterial activity of the respective crude metabolites was determined using the agar well diffusion method and growth inhibition study in broth culture.
For the study, concentration of 200 mg/mL of each of the respective metabolites was prepared by dissolving 1.0 g of metabolite in 5 mL of distilled water. In this study, four metabolites were tested individually and as combinations, at a ratio of 1:1.
A total of five clinical and four typed bacterial isolates were used for inhibitory potential of the metabolites, using the agar well diffusion method. The clinical isolates were Escherichia coli (ECO 050102), Salmonella sp. (SAL PS049), Salmonella sp (STY AAA 0026), Escherichia coli (ECO 050335), and Salmonella sp (STY AAA 0039). The clinical isolates were obtained from the University College Hospital, Ibadan, Oyo State, Nigeria. The typed isolates used were Escherichia coli (ATCC 25922) Salmonella typhi (ATCC 20971), Klebsiella pneumoniae, and Staphylococcus aureus (ATCC 6538). All the isolates were first streaked on nutrient agar to ascertain their purity before they were used.
Prior to the study, 80 g of nutrient agar was prepared in 100 mL capacity conical flasks, sterilized in an autoclave and allowed to cool to 45 °C. After cooling, 1 mL of 18–24 broth cultures of the respective clinical and typed isolates was added to respective flasks and labelled according. The flasks containing the inoculated agar were swirled for proper homogenization of the isolate in the medium. The medium was then dispensed into Petri dishes in 20 mL quantities and allowed to solidify.
Using a sterile cork-borer, four holes each were bored into the inoculated agar and 0.5 mL of crude metabolite was added to a hole. The plates were left for the metabolites to diffuse into the agar, after which they were incubated at 37 °C for 24 h. Antibacterial activity of a metabolite against an isolate was detected by observing and measuring the zone of inhibition. All experimental setups were in duplicates.
For growth inhibition study in liquid medium, Escherichia coli and Staphylococcus aureus were used as test pathogens. In a 100 mL capacity flask, containing 80 mL of sterile peptone water, 1 mL of overnight broth culture of a respective test pathogens was inoculated. This was followed by the addition of 5 mL of 200 mg/mL of a respective metabolite. The inoculated flasks were incubated in a rotary shaker at 4 °C ± 2 °C for 10 h. Immediately after inoculation and every 1 h, for a 10 h duration, aliquot samples were aseptically withdrawn from each flask and absorbance read, using a spectrophotometer at a wavelength of 750 nm. An inoculated control that contained no metabolite was also setup. All experimental setups were in duplicate.
Minimum inhibitory concentration (MIC) was determined using the broth dilution method. To 9 mL of sterile peptone water in test tubes, 0.5 mL of respective concentrations of a metabolite was added, inoculated with 0.5 mL of broth and incubated for 24 h at 37 °C ± 2 °C. At the expiration of the 24 h incubation period, the inoculated flasks were observed for turbidity. MIC was taken as the minimum concentration of the metabolite that was used for inoculation, at which no growth of the organism occurred. For this study, eleven different concentrations were prepared, ranging from 20 mg/mL to 200 mg/mL.
2.5. Pesticidal potential study
The pesticidal potential of the metabolite was tested using the diet fed method against Camponotus consobrinus (sugar ant). A litre of the diet comprised of glucose (50 g), yeast (10 g), agar powder (1 g) and nipragen (1 g), to serve as preservative.
For preparation, the diet components, with the exception of the nipragen were first weighed and dissolved separately before combination. The different components of the diet were then mixed together and heated in a hot plate for 15 min. After heating, the diet was allowed to cool to 45 °C, before adding the nipragen and a known concentration of the metabolite. The diet was then dispensed in 10 mL quantity in 20 mL capacity universal bottles and slightly covered with the cap to permit oxygen diffusion.
For the study, a total of six active Camponotus consobrinus were placed in each universal bottle that contained a known concentration of the respective metabolites and observed daily for 120 h. A setup diet that contained no metabolite was used as control. All setups were in duplicate.
2.6. Characterization of crude metabolite
The respective crude metabolites were characterized, using Fourier Transform Infrared (FTIR) and Gas Chromatogram-Mass Spectrometry (GC-MS).
For FTIR analysis, an Infrared spectrometer Varian 660 MidIR Dual MCT/DTGS Bundle with ATR) was used. Prior to the analysis, the samples were first prepared by drying in a desiccator for 24 h. After drying, the samples were directly applied to a diamante crystal of ATR. The resulting spectra were corrected for background air absorbance. The spectra were recorded in a transmittance mode from 4000 to 500/400 cm−1 at a resolution of 4 cm. Infrared spectrum was Fourier transformed and recorded in the absorption mode.
For GC-MS, the samples were analysed using a Varian 3800 gas chromatograph equipped with an Agilent fused silica capillary CP-Sil 5 CB column (30 m × 0.25 mm) and connected to a Varian 4000 mass spectrometer. The carrier gas used was Itroge, at flow rate and split ratio of 1.0 ml/min and 1:10, respectively. The operating temperature was 60 °C for 1 min; rising at 3.0 °C/min to 240 °C and held for 1 min, with the injector and detector also held at 240 °C.
The components were detected and quantified by the mass spectrometer. Through comparison of their retention times and fragmentation pattern of mass with mass spectral libraries (Wiley 9 and NIST 08), the components were identified.
2.7. Statistical analysis
Statistical analysis was carried out using the Statistical Package for Social Scientists (SPSS version 21.0). Comparison of means was done using the One-Way Analysis of Variance (ANOVA) test while relationship was tested for using the Pearson Correlation test. All analysis were carried out at 95 % confidence interval.
3. Results
3.1. Viable and spore counts
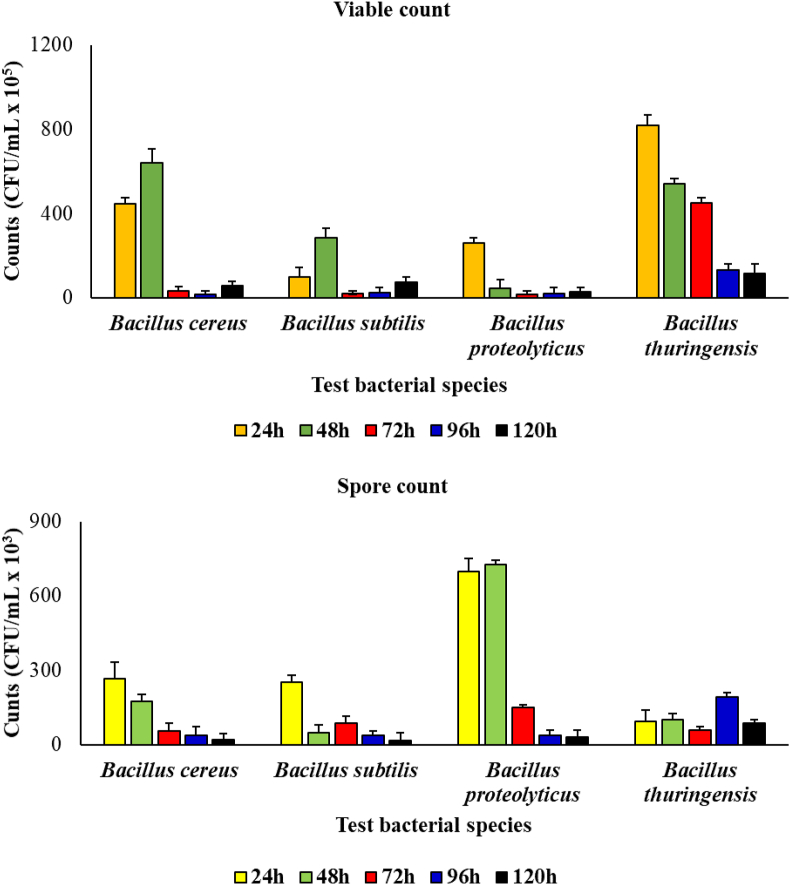
During the period of incubation, viable count of the bacterial species ranged from 5.8 × 106 to 4.5 × 107 CFU/mL, from 1.9 × 106 to 9.8 × 106 CFU/mL, 2.7 × 106 to 2.6 × 107 CFU/mL and from 1.3 × 107 to 8.2 × 107 CFU/mL, for the B. cereus, B. subtilis, B. proteolyticus and B. thuringensis, respectively. On the other hand, spore count in medium ce ranged from 2.0 × 104 to 2.7 × 105 CFU/mL, from 1.6 × 104 to 2.5 × 105, from 3.2 × 104 CFU/mL to 7.0 × 105 and from 5.7 × 104 to 1.0 × 105 CFU/mL for the B. cereus, B. subtilis, B. proteolyticus and B. thuringensis, respectively (Figure 1).
Figure 1.
Viable and spore counts of the Bacillus species during the period of incubation.
Generally, spore count showed significantly lower numbers than the viable count (p = 0.05). This observation was irrespective of the bacterial species.
3.2. Antibacterial potential of the metabolites
All the crude metabolites showed inhibition against the growth of the typed and clinical isolates examined, with the exception Salmonella sp (STY AAA 0026) that was not inhibited by the metabolite produced by Bacillus subtilis. Apart metabolite produced by the B. thuringiensis that showed highest zone of inhibition against the Salmonella sp (SAL PS049), all other metabolites showed highest zone of inhibition against Staphylococcus aureus (ATCC 6538), except for the metabolites produced by the (Table 1).
Table 1.
Antibacterial activity of the metabolites against selected clinical and typed pathogens.
| Metabolites |
||||
|---|---|---|---|---|
| A | B | C | D | |
| Clinical isolates | ||||
| Escherichia coli (ECO 050102) | + (17 mm) | +(29 mm) | +(30 mm) | +(29 mm) |
| Salmonella sp. (SAL PS049) | +(19 mm) | +(38 mm) | +(27 mm) | +(24 mm) |
| Salmonella sp (STY AAA 0026) | +(20 mm) | +(19 mm) | +(21 mm) | –– |
| Escherichia coli (ECO 050335) | +(14 mm) | +(25 mm) | +(26 mm) | +(26 mm) |
| Salmonella sp (STY AAA 0039) | +(16 mm) | +(21 mm) | +(19 mm) | +(29 mm) |
| Typed isolates | ||||
| Escherichia coli (ATCC 25922) | +(24 mm) | +(26 mm) | +(26 mm) | +(24mm) |
| Salmonella typhi (ATCC 20971) | +(20 mm) | +(27 mm) | +(28 mm) | +(20 mm) |
| Klebsiella pneumonia | +(28 mm) | +(29 mm) | +(32 mm) | +(30 mm) |
| Staphylococcus aureus (ATCC 6538) | +(35 mm) | +(31 mm) | +(33 mm) | +(31 mm) |
‘+’ and ‘-’ indicate sensitive and resistant, respectively. Values in parenthesis represent the zones of inhibition on the test bacteria. A, B, C and D represent metabolites produced by B. proteolyticus, B. thuringensis, B. cereus and B, subtilis, respectively.
As shown in Table 2, when the metabolites were combined, growth inhibition was observed against all the isolates tested with the exception of Salmonella sp (STY AAA 0026) that was not inhibited in presence of combined metabolites produced by B. thuringensis and B. cereus and by B. thuringensis and B. subtilis. Generally, highest zone of inhibition was observed against Salmonella typhi (ATCC 20971) in presence of the respective metabolites.
Table 2.
Antimicrobial activity of the combined metabolites against selected clinical and typed pathogens.
| Metabolites |
||||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Clinical isolates | ||||||
| Escherichia coli (ECO 050102) | +(22 mm) |
+(25 mm) | +(16 mm) | +(24 mm) | +(17 mm) | +(22 mm) |
| Salmonella sp. (SAL PS049) | +(21 mm) |
+(22 mm) | +(22 mm) | +(21 mm) | +(20 mm) | +(23 mm) |
| Salmonella sp. (STY AAA 0026) | +(25 mm) | +(21 mm) | –– | +(13 mm) | –– | +(14 mm) |
| Escherichia coli (ECO 050335) | +(18 mm) | +(19 mm) | +(16 mm) | +(20 mm) | +(19 mm) | +(24 mm) |
| Salmonella sp. (STY AAA 0631) | +(20 mm) | +(25 mm) | +(19 mm) | +(20 mm) | +(19 mm) | +(21 mm) |
| Typed isolates | ||||||
| Escherichia coli (ATCC 25922) | +(22 mm) | +(17 mm) | +(14 mm) | +(18 mm) | +(22 mm) | +(20 mm) |
| Salmonella typhi (ATCC 20971) | +(23 mm) | +(25 mm) | +(26 mm) | +(27 mm) | +(20 mm) | +(23 mm) |
| Klebsiella pneumonia | +(20 mm) | +(18 mm) | +(11 mm) | +(22 mm) | +(12 mm) | +(20 mm) |
| Staphylococcus aureus (ATCC 6538) | +(19 mm) | +(19 mm) | +(12 mm) | +(14 mm) | +(17 mm) | +(18 mm) |
‘+’ and ‘-’ indicates sensitivity and resistance, respectively. Values in parenthesis represent the zones of inhibition of the test bacteria. A = combination of metabolite from B. proteolyticus and B. thuringensis, B = combination of metabolite from B. proteolyticus and B. cereus, C = combination of metabolite from B. thuringensis and B. cereus, D = combination of metabolite from B. proteolyticus and B. subtilis, E = combination of metabolite from B. thuringensis and B. subtilis and F = combination of metabolite from B. cereus and B. subtilis.
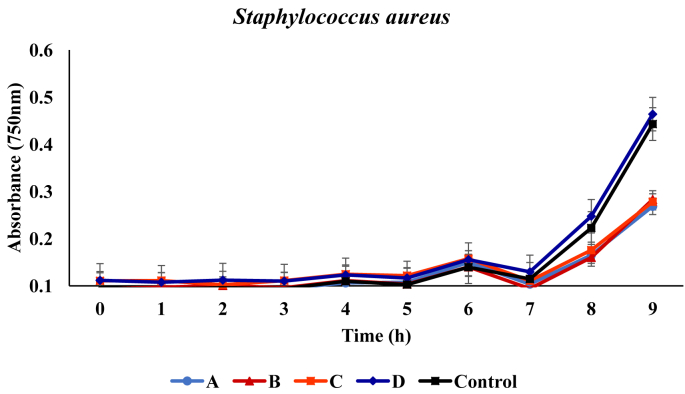
In liquid medium, growth of Staphylococcus aureus showed no inhibition in the presence of the respective metabolites. From the initial absorbance values of 0.092, 0.094, 0.111, 0.111 and 0.096, values of 0.414, 0.439, 0.455, 0.431 and 0.169 were recorded at the end of the incubation period in medium containing metabolites produced by B. proteolyticus, B. thurugiensis, B. cereus and B. subtilis respectively (Figure 2).
Figure 2.
Growth of Staphylococcus aureus in broth media in presence of the metabolites. A, B, C and D represents media with metabolites produced from Bacillus proteolyticus B. thuringiensis B. cereus B. subtilis. Control indicates the medium with no added metabolites.
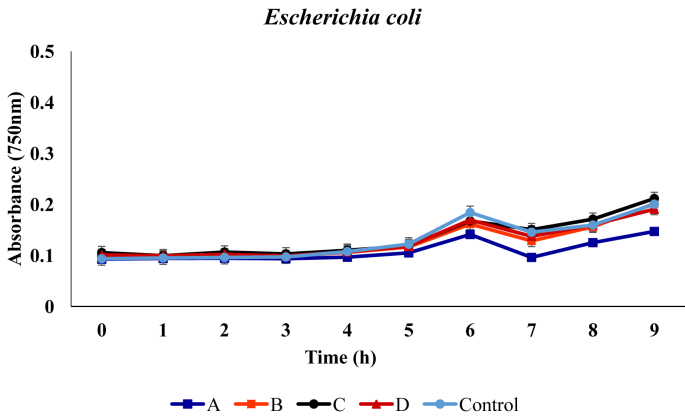
For the Escherichia coli, no remarkable inhibition in growth was observed was in presence of the respective metabolites, except for medium with metabolite that was produced by the B. proteolyticus, where growth inhibition was evident. From initial absorbance value of 0.093, 0.100, 0.105, 0.101 and 0.158, values of 0.256, 0.313, 0.327 and 0.301 were recorded at the end of incubation in medium containing metabolites produced by the B. proteolyticus, B. thurugiensis, B. cereus and B. subtilis, respectively (Figure 3).
Figure 3.
Growth of Escherichia coli in broth media in presence of the metabolites. A, B, C and D represents media with metabolites produced from Bacillus proteolyticus B. thuringensis B. cereus B. subtilis. Control indicates the medium with no added metabolites.
The minimum inhibitory concentration (MIC) of the respective metabolites against the test clinical and typed isolates was observed to be 200 mg/ml. This observation was irrespective of the isolates and metabolites used for investigation, except against Salmonella typhi (ATCC 20971), where MIC of 180 mg/mL was recorded for metabolite produced by B. proteolyticus.
3.3. Pesticidal activity
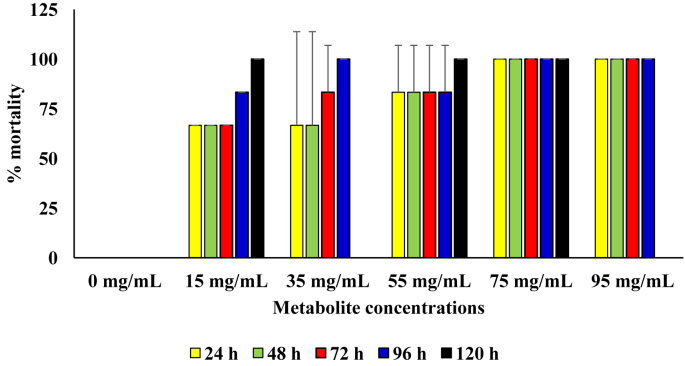
In diet containing the respective concentrations of the metabolite produced by the B. proteolyticus, 100 % mortality was attained after 24 h of incubation in diet with metabolite concentration of 75 mg/mL and above. Generally, the rate of kill of the sugar ants was directly proportional to metabolite concentration in the diet. The mortality rate in the diet with metabolite concentrations of 75 and 95 mg/L was were observed to be significantly higher those at other treatment setups (p = 0.05). In the control diet setup with no added metabolite, no mortality was recorded throughout the 120 h period of incubation (Figure 4).
Figure 4.
Pesticidal activity of the metabolite produced from the B. proteolyticus against sugar ants.
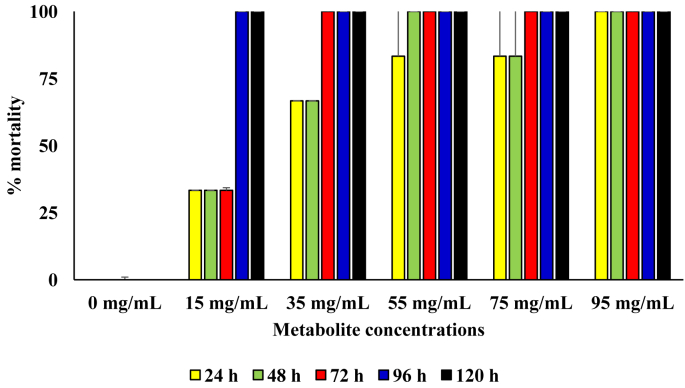
In diets that contained the metabolite produced by the B. thuringiensis, rate of kill was also observed to be concentration dependent, with significantly higher mortality in diets with metabolite concentrations of 75 and 95 mg/mL (p = 0.05). At metabolite concentration of 15 mg/mL and 35 mg/mL in the diet, 100 % mortality was recorded from 96 h and 72 h of incubation, respectively. Only in setup that contained 95 mg/mL of the metabolite was 100 % mortality recorded from 24 h of incubation (Figure 5).
Figure 5.
Pesticidal activity of the metabolite produced from the B. thuringensis against sugar ants.
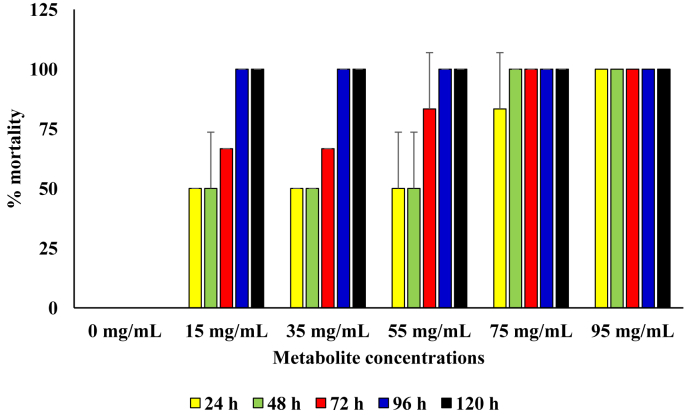
For the diets that contained the respective concentrations of the metabolite produced by the B. cereus, 100 % mortality was observed from 96 h of incubation in setups that contained metabolite concentrations of 15 mg/mL, 35 mg/mL and 55 mg/mL. Significantly higher mortality was only observed in diets that contained metabolite concentration of 95 mg/mL and those with concentrations lower than 55 mg/mL (p = 0.05). At metabolite concentrations of 75 mg/mL and 95 mg/mL, 100 % mortality was recorded from 48 h and 24 h of incubation, respectively (Figure 6).
Figure 6.
Pesticidal activity of the metabolite produced from the B. cereus against sugar ants.
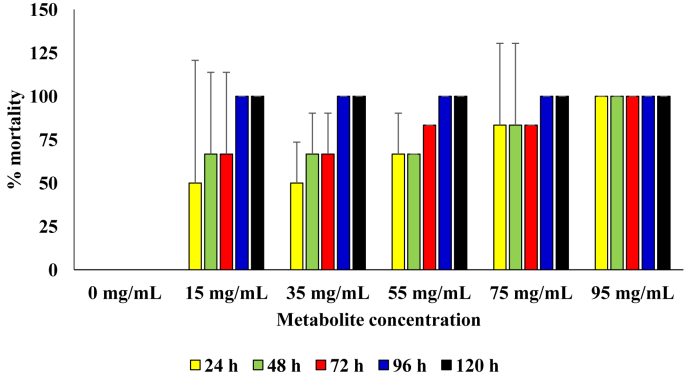
As shown in Figure 7, at the different concentrations of the metabolite produced by the B. subtilis, mortality rate of the sugar ant was also directly proportional to the concentration of metabolite in the diet. In the setup with metabolite concentration of 95 mg/mL, 100 % mortality was observed from 24 h of incubation. Significantly higher mortality was observed in diet with metabolite concentration of 95 mg/mL than those at concentrations below 55 mg/mL (p = 0.05).
Figure 7.
Pesticidal activity of the metabolite produced from the B. subtilis against sugar ants.
3.4. Metabolite characteristics
FTIR of the respective metabolites detected 15, 13, 16 and 16 peaks for the metabolites produced by the B. proteolyticus, B. thruringensis, B. cereus and B. subtilis, respectively. In all the metabolites O–H stretching vibration and C–H symmetric stretching vibration of the connection of the CH2 group were evident. The C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids was also observed to be common among the respectively metabolites (Tables 3, 4, 5, and 6).
Table 3.
Assigned peaks from the FTIR Spectra of the Metabolite Produced by the Bacillus proteolyticus.
| Run | Peak | Trans. | Assignment |
|---|---|---|---|
| 1 | 3582.74 | 86.21 | O–H stretching vibration |
| 2 | 3122.40 | 25.73 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 3 | 2598.51 | 36.05 | C–H stretching vibration, of aliphatic Alkane |
| 4 | 2496.73 | 57.11 | O–H stretching vibration |
| 5 | 2296.43 | 80.26 | CH3 group stretching vibrations |
| 6 | 1750.64 | 84.37 | C=O stretching vibration of a carbonyl bond |
| 7 | 1398.41 | 85.72 | CH3 symmetric bending vibrations |
| 8 | 1200.08 | 72.14 | C–O–C asymmetric stretching vibration |
| 9 | 1126.28 | 81.92 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 10 | 1000.73 | 52.17 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 11 | 984.15 | 20.00 | C–C–O stretching vibration |
| 12 | 862.40 | 64.38 | C–O Stretching vibration |
| 13 | 800.24 | 84.49 | CH bending vibration |
| 14 | 700.06 | 76.82 | Rocking CH2 mode due to the accumulation of polyesters |
| 15 | 550.31 | 92.71 | C–Cl C–Br, I Stretching vibrations |
Trans. represent transmittance (%). Peak is cm−1
Table 4.
Assigned peaks from the FTIR spectra of the metabolite produced by the Bacillus thuringiensis.
| Run | Peak | Trans | Assignment |
|---|---|---|---|
| 1 | 3805.31 | 83.97 | O–H stretching vibration |
| 2 | 3251.06 | 4.01 | Strong N–H stretching vibration of H-bonding (due to water) and possible metal binding of amide groups in proteins |
| 3 | 3018.23 | 48.15 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 4 | 2639.73 | 82.76 | O–H stretching vibration |
| 5 | 1672.40 | 80.51 | RCH = N=N Stretching vibration |
| 6 | 1522.09 | 36.28 | N–H stretching vibration of H-bonding and/or possible metal binding of amide groups in cellular proteins |
| 7 | 1499.98 | 76.85 | CH2 symmetric deformation |
| 8 | 1278.53 | 72.03 | C–O–C asymmetric stretching vibration |
| 9 | 1148.50 | 68.00 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 10 | 1092.27 | 84.00 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 11 | 1019.66 | 83.61 | C–OH stretching vibration |
| 12 | 890.11 | 56.50 | C–O – C ring vibration |
| 13 | 698.71 | 72.13 | C–Cl, Br, I Stretching vibrations |
Trans. represent transmittance (%). Peak is cm−1
Table 5.
Assigned peaks from the FTIR spectra of the metabolite produced by the Bacillus cereus.
| Run | Peak | Trans | Assignment |
|---|---|---|---|
| 1 | 3998.26 | 78.64 | O–H stretching vibration |
| 2 | 3308.41 | 29.73 | Strong N–H stretching vibration of H-bonding (due to water) and possible metal binding of amide groups in proteins |
| 3 | 3199.74 | 22.37 | O–H stretching vibration of phenol groups, |
| 4 | 3021.58 | 44.15 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 5 | 2999.58 | 48.26 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 6 | 2982.55 | 52.31 | C–H stretching vibration |
| 7 | 2843.62 | 60.00 | RCH = N=N stretching vibration |
| 8 | 2228.71 | 72.54 | C=C–C aromatic ring stretch |
| 9 | 1400.97 | 37.15 | CH2 symmetric bending vibration |
| 10 | 1200.04 | 43.97 | C–O–C asymmetric stretching vibration |
| 11 | 1026.48 | 46.02 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 12 | 898.27 | 73.14 | C–C Stretching vibration of amide group |
| 13 | 800.00 | 63.96 | CH bending vibration of alkenes |
| 14 | 747.35 | 64.25 | Rocking CH2 mode due to the accumulation of polyesters |
| 15 | 632.07 | 62.71 | C–O Stretching vibration |
| 16 | 530.73 | 52.94 | C–Cl C–Br, I Stretching vibrations |
Trans. represent transmittance (%). Peak is cm−1
Table 6.
Assigned peaks from the FTIR spectra of the metabolite produced by the Bacillus subtilis.
| Run | Peak | Trans | Assignment |
|---|---|---|---|
| 1 | 3998.26 | 56.19 | O–H stretching vibration |
| 2 | 3308.41 | 22.84 | Strong N–H stretching vibration of H-bonding (due to water) and possible metal binding of amide groups in proteins |
| 3 | 3199.74 | 14.94 | C–H stretching vibration, of aliphatic Alkane |
| 4 | 3021.58 | 32.71 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 5 | 2999.58 | 36.28 | C–H symmetric stretching vibration of the connection of the in CH2 group |
| 6 | 2982.55 | 38.49 | C–H stretching vibration |
| 7 | 2843.62 | 43.21 | RCH = N=N stretching vibration |
| 8 | 2228.71 | 52.70 | C≡N stretching vibrations |
| 9 | 1400.97 | 28.15 | CH2 symmetric bending vibration |
| 10 | 1200.04 | 31.57 | C–O–C asymmetric stretching vibration |
| 11 | 1026.48 | 32.20 | C–O–C and C–C–O Stretching vibration due to the accumulation of polyester compounds (poly-3-hydroxybutyrate and probably phospholipids (PLs)) |
| 12 | 898.27 | 52.10 | C–C Stretching vibration of amide group |
| 13 | 800.00 | 48.76 | CH bending vibration of alkenes |
| 14 | 747.35 | 48.75 | Rocking CH2 mode due to the accumulation of polyesters |
| 15 | 632.07 | 46.21 | C–O Stretching vibration |
| 16 | 530.73 | 38.94 | C–Cl C–Br, I Stretching vibrations |
Trans. represent transmittance (%). Peak is cm−1
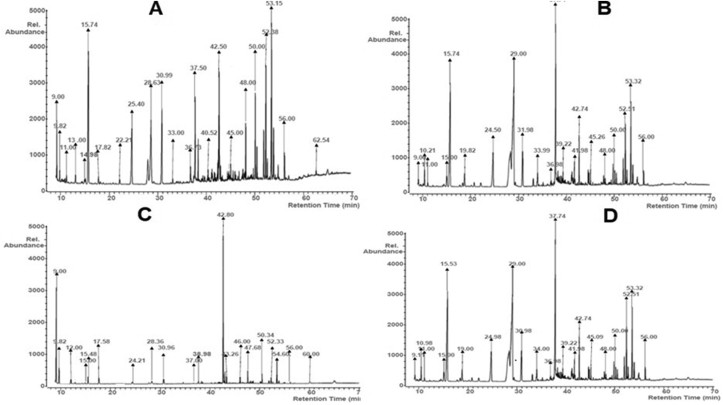
Gas chromatogram of metabolite produced by the Bacillus proteolyticus revealed the presence of the following: Methyl lactate, Benzoic acid, 2-amino-5-chloro-, methyl ester Ethyl 2,4-dihydroxy-6-methylnicotinate, Butanoic acid, 2-hydroxy-, ethyl ester, Dimethyl fumarateBenzoic acid, 1-methylethyl ester, Pentanedioic acid, 2-oxo-, dimethyl ester Gluconic acid, 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-Glucose, n-Hexadecanoic acid, Pentadecanoic acid, Heptadecanoic acid, Pentadecanoic acid, 13-methyl-, methyl ester, Monolinolenin, Maltose, Stigmasterol, Octadecanoic acid, 2,3-dihydroxypropyl ester 9-Octadecenoic acid, (E)-, Sucrose, Heptadecanoic acid, 16-methyl-, methyl este, Glycerol 1-palmitate, α-Tocospiro B (Figure 8a).
Figure 8.
Gas chromatogram of the metabolite produced by the B. proteolyticus (a), B. thuringiensis (b), B. cereus (c) and B. subtilis (d).
For the metabolite produced by the B. thuringiensis, compounds that were detected by the GC analysis included Methyl lactate, Benzoic acid, 2-amino-5-chloro-, methyl ester Ethyl 2,4-dihydroxy-6-methylnicotinate Butanoic acid, 2-hydroxy-, ethyl ester Benzoic acid, 1-methylethyl ester, Pentanedioic acid, 2-oxo-, dimethyl ester Gluconic acid, Pentadecanoic acid, 13-methyl-, methyl ester 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-Glucose Pentadecanoic acid, Sucrose, Monolinolenin, n-Hexadecanoic acid Heptadecanoic acid, Maltose, Stigmasterol Octadecanoic acid, 2,3-dihydroxypropyl ester 9-Octadecenoic acid, (E)-, Heptadecanoic acid, 16-methyl-, methyl ester and α-Tocospiro B (Figure 8b).
In the case of the metabolite produced by the B. cereus, Hexanoic acid, 2-methyl-, Methyl lactate Ethyl 2,4-dihydroxy-6-methylnicotinate, Benzoic acid, 2-amino-5-chloro-, methyl ester Benzoic acid, 1-methylethyl ester, Pentanedioic acid, 2-oxo-, dimethyl ester Gluconic acid, 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-Pentadecanoic acid, 13-methyl-, methyl ester Glucose, Pentadecanoic acid, Sucrose, Monolinolenin, n-Hexadecanoic acid, Heptadecanoic acid, 16-methyl-, methyl ester, Maltose, Stigmasterol, 1-Aminononadecane, N-trifluoro acetyl- 9-Octadecenoic acid, (E)- and α-Tocospiro B were detected from the GC analysis (Figure 8c).
For the metabolite produced by the B. subtilis, from the GC analysis, the following were detected: Methyl lactate, Benzoic acid, 2-amino-5-chloro-, methyl esterEthyl 2,4-dihydroxy-6-methylnicotinate Butanoic acid, 2-hydroxy-, ethyl ester, Benzoic acid, 1-methylethyl ester Pentanedioic acid, 2-oxo-, dimethyl ester, Gluconic acid, Pentadecanoic acid, 13-methyl-, methyl ester 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- Glucose Pentadecanoic acid, Sucrose, Monolinolenin, n-Hexadecanoic acid, Heptadecanoic acid, Maltose, Stigmasterol, Octadecanoic acid, 2,3-dihydroxypropyl ester 9-Octadecenoic acid, (E)-, Heptadecanoic acid, 16-methyl-, methyl ester and α-Tocospiro B (Figure 8d).
4. Discussion
All the metabolites effectively inhibited the growth of the test bacterial species. Besides, screening of the metabolites revealed the presence of various compound with antimicrobial activity. Similar observation was reported by Al-Ajlani and Hasnain (2010), who in indicated that the bacitracin produced by Bacillus sp inhibited the growth of Escherichia coli and Staphylococcus aureus. In a related study on the antibacterial action of secondary metabolites from soil Bacillus species, Al-Saraireh et al. (2015) reported the crude extract being only active against Gram positive bacteria: Bacillus subtilis, Micrococcus luteus and Staphylococcus aureus. Antibacterial activity of metabolite produced by B. cereus strain against most Gram-positive bacteria have been reported previously (Oscariz et al., 1999). It is posited that a large number of antimicrobial peptides with different chemical structures, such as bacteriocins, iturin A and surfactin are produced by a number Bacillus species (Al-Saraireh et al., 2015). Ortiz and Sansinenea (2019) have reported the presence natural compounds with large structural diversity with broad biological activities. The effectiveness of Bacillus subtilis against tomato Fusarium wilt under invitro conditions has been reported earlier by Ramyabharathi and Raguchander (2014). In that study, B. subtilis was observed to effectively reduce the growth of the fungal mycelial by 46.04 %.
It is reported that Bacillus species have the ability of secreting varying metabolites that can trigger plant growth and prevent infection by pathogen. While controlling pathogen population, Bacillus species also produce exopolysaccharides and siderophores that inhibit the movement of toxic anions and cations and adjust ionic balance and water transport in plant tissues (Radhakrishnan et al., 2017). Bacillus thuringiensis is indicated to be ubiquitous in soil, dust from stored grains, sand, dead larvae water and leaves. Their wild strains from environmental samples are reported to display high activity against insect pests (Konecka et al., 2012). The secretion of antibiotics, microbial lipopeptides and a number of hydrolytic enzymes, such as chitinases and proteases have been reported in Bacillus species. These substances from Bacillus subtilis, in particular, is characterized by the extracellular secretion of a number of antibiotics, microbial lipopeptides and hydrolytic enzymes such as chitinases and proteases.
All the Bacillus species used for this investigation were able to produce metabolites under the experimental conditions. Some of the components of the crude metabolites have been reported by earlier researchers (Mondol et al., 2013). It is opined that despite the fact that a number of microorganisms (bacteria, fungi and nematodes) are either commercially available or in their developmental stages for implementation as biopesticides, the host killing and pathogenicity is attained by the metabolites such organisms either secrete or produce. It is therefore imperative that the selection of any microbial strain for pesticidal use is a function of such strain to produce metabolites that have pesticidal action against a target pest (Subbanna et al., 2020).
In this study, the metabolites were observed to have pesticidal activity against the target sugar ants. The efficacy of the metabolites was found to be dosage dependent. Metabolites produced by B. cereus has been found to be pathogenic to insects on several occasions (Ehling-Schulz et al., 2019). Bacillus thuringiensis has been widely reported to synthesize proteins that accumulate in crystals that possess pesticidal properties. Some of the pests it has been reported against include Lepidoptera, Coleoptera, and Diptera (Valtierra-de-Luis et al., 2020). Similarly, in a study of insecticidal activity of several Bacillus species isolated from soil, it was indicated that 98.7 % and 89.3 % mortality was achieved for B alvei and B megaterium, respectively (Shakoori et al., 1998).
From the findings of this study, the respective metabolites produced by the four Bacillus species showed strong antibacterial action against the Gram negative and Gram positive pathogens that were used for investigation. In addition, the metabolites showed great pesticidal action against the target pest.
The study findings could give an indication that the metabolites from the B. subtilis, B. thuringiensis, B. proteolyticus and B. cereus could be successfully employed as an eco-friendly strategy for the control of Camponotus consobrinus.
Declarations
Author contribution statement
Oghenerobor Akpor: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Oluwafunto D. Akinwusi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tolulope A. Ogunnusi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abouseoud M., Yataghene A., Amrane A., Maachi R. Effect of pH and salinity on the emulsifying capacity and naphthalene solubility of a biosurfactant produced by Pseudomonas fluorescens. J. Hazard Mater. 2010;180(1-3):131–136. doi: 10.1016/j.jhazmat.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Al-Ajlani M.M., Hasnain S. Bacteria exhibiting antimicrobial activities; screening for antibiotics and the associated genetic studies. Open Conf. Proc. J. 2010;1:230–238. [Google Scholar]
- Al-Saraireh H., Al-Zereini W.A., Tarawneh K.A. Antimicrobial activity of secondary metabolites from a soil Bacillus sp. 7B1 isolated from South Al-Karak, Jordan. Jordan J. Biol. Sci. 2015;8(2):127–132. [Google Scholar]
- Birch A.N.E., Begg G.S., Squire G.R. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. J. Exp. Bot. 2011;62(10):3251–3261. doi: 10.1093/jxb/err064. [DOI] [PubMed] [Google Scholar]
- Cherif A., Ouzari H., Daffonchio D., Cherif H., Slama K.B., Hassen A., Jaoua S., Boudabous A. Thuricin 7: a novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett. Appl. Microbiol. 2001;32:243–247. doi: 10.1046/j.1472-765x.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- Ehling-Schulz M., Lereclus D., Koehler T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019;7(3):7–10. doi: 10.1128/microbiolspec.gpp3-0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecka E., Baranek J., Hrycak A., Kaznowski A. Vol. 2012. The Scientific World Journal; 2012. Insecticidal Activity of Bacillus Thuringiensis Strains Isolated from Soil and Water. Article ID 71050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J., Ramlal A., Mallick D., Mishra V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants. 2021;10(6):1185. doi: 10.3390/plants10061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S., Kumar S.C., Jha M.N., Kant R., Upendra S., Kumar P. Microbial pesticide: a boom for sustainable agriculture. Int. J. Sci. Eng. Res. 2014;5(6):1394–1397. [Google Scholar]
- Lisboa M.P., Bonatto D., Bizani D., Henriques J.A.P., Brandelli A. Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic Forest. Int. Microbiol. 2006;9:111–118. [PubMed] [Google Scholar]
- Mondol M.A., Shin H.J., Islam M.T. Diversity of secondary metabolites from marine Bacillus species: chemistry and biological activity. Mar. Drugs. 2013;11(8):2846–2872. doi: 10.3390/md11082846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A., Sansinenea E. Chemical compounds produced by Bacillus sp. factories and their role in nature. Mini Rev. Med. Chem. 2019;19(5):373–380. doi: 10.2174/1389557518666180829113612. [DOI] [PubMed] [Google Scholar]
- Oscariz J.C., Lasa I., Pisabarro A.G. Detection and characterization of cerein 7, a new bacteriocin produced by Bacillus cereus with a broad spectrum of activity. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 1999;178:337–341. doi: 10.1111/j.1574-6968.1999.tb08696.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R., Hashem A., Abd_Allah E.F. Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramyabharathi S.A., Raguchander T. Mode of action of Bacillus subtilis EPCO16 against tomato Fusarium wilt. Biochem. Cell. Arch. 2014;14(1):47–50. [Google Scholar]
- Sansinenea E., Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011;33(8):1523–1538. doi: 10.1007/s10529-011-0617-5. [DOI] [PubMed] [Google Scholar]
- Shakoori A.R., Afroz S., Khurshid N. Insecticidal activity of Bacillus spp. from soil samples in Pakistan against the house fly, Musca domestica. Int. J. Trop. Insect Sci. 1998;18(4):301–306. [Google Scholar]
- Subbanna A.R.N.S., Stanley J., Rajasekhara H., Mishra K.K., Pattanayak A., Bhowmick R. In: Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry. Mérillon J.M., Ramawat K., editors. Springer; Cham: 2020. Perspectives of microbial metabolites as pesticides in agricultural pest management. [Google Scholar]
- Thakur N., Kaur S., Tomar P., Thakur S., Yadav A.N. Microbial biopesticides: current status and advancement for sustainable agriculture and environment. New and Future Developments in Microbial Biotechnology and Bioengineering. 2020;1:243–282. [Google Scholar]
- Valtierra-de-Luis D., Villanueva M., Berry C., Caballero P. Potential for Bacillus thuringiensis and other bacterial toxins as biological control agents to combat dipteran pests of medical and agronomic importance. Toxins. 2020;12(12):773. doi: 10.3390/toxins12120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A.N., Kour D., Kaur T., Devi R., Guleria G., Rana K.L., Yadav N., Rastegari A.A. Microbial biotechnology for sustainable agriculture: current research and future challenges. New and Future Developments in Microbial Biotechnology and Bioengineering. 2020;1:331–344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.