Abstract
Background
Multiplex immunoassays capture a comprehensive profile of the humoral response against SARS-CoV-2 and human endemic coronaviruses. We validated a multiplex panel (V-PLEX Panel 2) from Meso Scale Diagnostics targeting antibodies against nine coronavirus antigens. Performance was compared against alternative single- and multi-antigen immunoassays.
Methods
Sera collected for clinical or public health testing from 2018 to 2020 (n = 135) were used to compare all tested platforms, and inter-test agreement was assessed by Cohen's kappa coefficient. Sample category (positive/negative) was assigned based on collection date relative to the index case in Canada, and SARS-CoV-2 PCR and serology results. 117 out of the 135 samples (31 positive, 86 negative) were assigned a category and were used to calculate sensitivity and specificity, with MSD's test results based upon manufacturer-set cut-offs.
Results
We observed SARS-CoV-2 target sensitivities of 100% and specificities >94% for all antigens (RBD, Nucleocapsid, Spike) in V-PLEX Panel 2. When targets were combined, we found a SARS-CoV-2 sensitivity of 100% and specificity of 98.8% with no difference in performance compared to clinical assays, and Cohen's kappa ranging from 0.798 to 0.945 compared to surface plasmon resonance imaging (SPRi). Quantitative measurements of antibodies against the Spike protein of endemic human coronaviruses were concordant with SPRi.
Conclusion
Meso Scale Diagnostics’ V-PLEX Coronavirus Panel 2 allows for highly sensitive and specific detection of anti-coronavirus IgG, and is concordant with other serological assays for detection of antibodies against SARS-CoV-2 and the endemic human coronaviruses, making it a good tool for humoral response characterization after both infection and vaccination.
Keywords: SARS-CoV-2, COVID-19, Immunoassays, Multiplex serology, Human coronavirus
Abbreviations
- HC
Health Canada
- BCCDC PHL
British Columbia Centre for Disease Control Public Health Laboratory
- MSD
Meso Scale Diagnostics
- PCR
Polymerase chain reaction
- SPRi
Surface plasmon resonance imaging
- S
Spike
- NC
Nucleocapsid
- RBD
Receptor binding domain
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of COVID-19 and is part of the Betacoronavirus (Beta-CoV) genus that also includes two common cold endemic human coronaviruses (HCoVs), HCoV-HKU1 and HCoV-OC43. Interestingly, several studies have demonstrated elevated anti-Beta-HCoV IgG in COVID-19, particularly in those with severe disease, presenting it as a potential prognostic marker for disease severity [1], [2], [3], [4].
Serosurveys are crucial in informing public health practice and guidelines, providing information on disease incidence and prevalence, and humoral responses to vaccination. Serological assays, such as chemiluminescent immunoassays that measure immunoglobulins, can identify immune responses generated in response to both infection and vaccination by measurements of IgG. Immunoassays currently authorized by Health Canada (HC) for clinical use detect antibodies against one protein target per test. However, multiple targets must be evaluated to better understand the immune response against SARS-CoV-2 in natural infection and vaccination. Thus, multiplex immunoassays capable of simultaneously detecting multiple targets present an easily implementable solution for further investigations into the complex humoral immune responses against SARS-CoV-2.
Here, we have evaluated the performance of a novel pan-coronavirus (pan-CoV) immunoassay on residual sera collected for routine clinical testing at the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory (PHL) in Canada. The V-PLEX Coronavirus Panel 2 assay is a multiplex coronavirus immunoassay from Meso Scale Diagnostics (MSD, Rockville, USA) that utilizes electrochemiluminescence for detection of six coronaviruses. We compared the performance of this assay against surface plasmon resonance imaging (SPRi), another pan-CoV assay using changes in the refractive indices upon ligand binding for label-free, real-time detection of serum antibodies [5]. We have also compared the MSD assay's COVID-19 diagnostic performance against three HC-approved single-antigen serological tests. Together, we found that MSD's V-PLEX Coronavirus Panel 2 produced reliable quantification across SARS-CoV-2 and HCoV antibodies.
2. Material and methods
2.1. Serum samples
A total of 135 residual patient sera previously submitted to the BCCDC PHL for clinical and public health testing from 2018 to 2020 were used for validation studies. Of the 135, 117 well-defined samples were classified as either “COVID-19 negative” or “COVID-19 positive” for use in determining assay performance for detection of anti-SARS-CoV-2 antibodies. Presumptive negative samples (n = 86) were collected prior to the first index case of COVID-19 in Canada (January 23rd, 2020), and were additionally confirmed to be SARS-CoV-2-negative by PCR and HC-approved immunoassays in a clinical testing algorithm (Supplementary Figure 1). Presumptive positive samples (n = 31) were collected >14 days post-symptom onset from PCR-confirmed COVID-19 patients diagnosed after January 23rd, 2020, and tested positive for anti-SARS-CoV-2 antibodies by HC-approved serology. The remaining eighteen samples were all included to assess inter-assay agreement, but not to evaluate diagnostic performance. No category was assigned to these samples as the criteria above were not met. Ten samples were negative by both PCR and serology but were collected following the index case in Canada; four were PCR positive but negative by clinical serology; and four were PCR indeterminate and serology positive.
Testing and analyses were conducted under a public health laboratory mandate under the Public Health Act and were therefore exempt from research ethics board review.
2.2. Health Canada-approved single-antigen chemiluminescent immunoassays
ADVIA Centaur XP SARS-CoV-2 Total Antibody anti-Receptor Binding Domain (Siemens T; Siemens, USA), ARCHITECT SARS-CoV-2 IgG anti-Nucleocapsid (Abbott IgG; Abbott, USA), and VITROS Anti-SARS-CoV-2 Total Antibody anti-Spike (Ortho T; Ortho Clinical Diagnostics, USA) were used to benchmark the multi-antigen assays and were performed as per manufacturer recommendations.
2.3. Meso ScaleDdiagnostics V-PLEX Coronavirus Panel 2 for IgG
Reactivity against HCoVs, SARS-CoV-1 and SARS-CoV-2 was tested on the MSD's V-PLEX Coronavirus Panel 2 for IgG (K15369U). Each well on the 96-well plate contains multiple coronavirus-related targets spotted at the bottom of the well and include Spike (S) of Alpha-HCoVs HCoV-229E and HCoV-NL63; Spike of Beta-HCoVs HCoV-OC43 and HCoV-HKU1; Spike of SARS-CoV-1; and Spike, nucleocapsid (NC), and S1 receptor binding domain (RBD) of SARS-CoV-2. The assay was performed per manufacturer's instructions as described below .
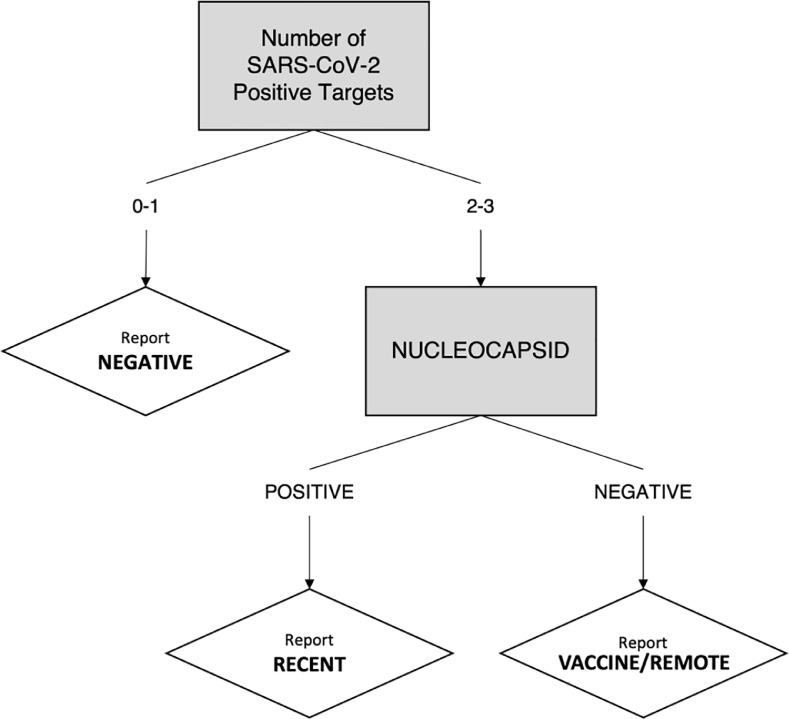
Samples diluted 1:5000 were added to the plate, and bound antibodies were then labelled with SULFO-TAG™ Anti-human IgG Antibody. Plates were read using MSD QuickPlex SQ120. Raw data was processed using MSD's Discovery Workbench version 4.0 and imported into R to interpret signal cut-off values. Quantification was reported in Arbitrary Units/mL (AU/mL). As per manufacturer guidelines, cut-off values were: Spike, 1960 AU/mL; NC, 5000 AU/mL; and S1 RBD, 538 AU/mL. To be considered serologically positive for SARS-CoV-2 via natural infection using this assay's interpretive algorithm, samples must be positive for targets as described in Fig. 1 .
Fig. 1.
Schematic of the interpretive algorithm used for classification. This algorithm aims to classify negatives from recent positive cases (Recent) and vaccine-induced/waned post-infection, or “remote”, responses (Vaccine/Remote) using three protein targets (Receptor binding domain (RBD), Spike, Nucleocapsid) on Meso Scale Diagnostics’ Coronavirus Panel 2.
Three serology controls (SC 1.1, SC 1.2, and SC 1.3) provided by the manufacturer containing known concentrations of human IgG against the targets in the panel (Supplementary Table 1) were run in duplicate on each plate. To assess assay precision and inter-assay variability, readings from 15 independent runs were used to calculate the coefficient of variation (CV). To further assess the precision around the manufacturer-recommended cut-offs, we re-analyzed three serum specimens previously found to be close to the cut-off from our validation panel. We first identified potential candidate sera lying within a pre-determined range (Spike: 1300–2300 AU/mL, RBD: 500–600 AU/mL, NC: 4500–5500 AU/mL), and then anonymized the container identifiers before randomly selecting three to assay. To note, ranges were expanded in increments of 100 AU/mL to ensure a minimum of six candidates per target. Readings from four independent runs were used to calculate CVs (Supplementary Table 2).
2.4. Surface plasmon resonance imaging
The SARS CoV-2 SPRi immunoassay uses a Molecular Affinity Screening System (MASS-2) (Bruker Daltonics, Billerica, MA) that employs high intensity laser light and high-speed optical scanning to monitor real time binding of antibodies against SARS-CoV-2 RBD and Spike of Beta-HCoVs from patient serum. Polyhistidine-tagged SARS-CoV-2 RBD and Spike of Beta-HCoVs OC43 and HKU1 were captured and immobilized onto a sensor chip, and sera diluted 1:10 in dilution buffer (3 mM HEPES buffered saline with EDTA, 0,05% P-20, 0.2 mg/mL BSA) was sequentially injected over the sensor surface, with binding events recorded as a function of time to generate a “sensorgram”. Mass changes on the surface of the chip due to antibody binding were translated into response units (RUs), with 1 RU equivalent to 1 pg/mL of bound antibody. Antibody responses were compared to a positivity cut-off defined as the mean value plus 3 standard deviations of five pre-pandemic healthy control sera run on each assay, and S1 RBD values above 10 RU were considered positive.
2.5. Statistical analysis
All analyses were conducted in R version 4.0.3 and RStudio Desktop version 1.3.1093. Calculations of diagnostic sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) alongside corresponding 95% confidence intervals constructed using the Wilson score method were performed by the meta package version 4.18.0. Cohen's kappa coefficient was used to assess inter-test reliability, and was calculated by the fmsb package version 0.7.0. Overlapping 95% confidence intervals were deemed statistically significant. Plots were generated using ggplot2 package version 3.3.2 and ggpubr package version 0.4.0.999. Comparisons between serology status in the assessment of endemic data were performed by Wilcoxon rank sum test with p<0.05 considered significant.
3. Results
3.1. Determination of MSD assay sensitivity and specificity for individual SARS-CoV-2 antigens
To determine the clinical sensitivity, specificity, PPV, and NPV of the MSD assay, we analyzed the results of the 117 serum specimens with defined categories from the original panel of 135. The 18 sera with no assigned category were excluded from this analysis.
Diagnostic performance is shown for both MSD's individual antigen targets and the three HC-approved chemiluminescent assays for clinical diagnosis in Table 1 . SARS-CoV-2 antigens RBD, S, and NC on the MSD assay were able to detect the presence of anti-SARS-CoV-2 IgG with 100% clinical sensitivity and clinical specificities of > 94%. To note, no statistically significant differences were seen in IgG detection between the targets on MSD's panel and when compared to HC-approved chemiluminescent assays across all parameters. Based on serology controls run in duplicate alongside the panel, inter-assay variation for NC and S was found to be between 6 to 15% while RBD ranged from 24 to 30% (Supplementary Table 1).
Table 1.
Diagnostic performance for each described assay using the 117 serum specimens from the validation panel.
| Presumed Positive (n = 31) | Presumed Negative (n = 86) | |||||||
|---|---|---|---|---|---|---|---|---|
| Assay Target | Positive | Negative | Sensitivity% (95% CI) | Positive | Negative | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) |
| MSD RBD | 31 | 0 | 100 (89.0–100) | 5 | 81 | 94.2 (87.1–97.5) | 86.1 (71.3–93.9) | 100 (95.7–100) |
| MSD Spike | 31 | 0 | 100 (89.0–100) | 0 | 86 | 100 (95.7–100) | 100 (89.0–100) | 100 (95.7–100) |
| MSD NC | 31 | 0 | 100 (89.0–100) | 1 | 85 | 98.8 (93.7–99.8) | 96.9 (84.3–100) | 100 (95.7–100) |
| Siemens Ta | 31 | 0 | 100 (89.0–100) | 0 | 86 | 100 (95.7–100) | 100 (89.0–100) | 100 (95.7–100) |
| Abbott IgGb | 31 | 0 | 100 (89.0–100) | 0 | 86 | 100 (95.7–100) | 100 (89.0–100) | 100 (95.7–100) |
| Ortho Tc | 31 | 0 | 100 (89.0–100) | 2 | 84 | 97.7 (91.9–99.4) | 93.9 (80.4–98.3) | 100 (95.7–100) |
ADVIA Centaur XP SARS-CoV-2 Total Antibody (Siemens, USA); target epitope: recombinant RBD of Spike protein.
ARCHITECT SARS-CoV-2 IgG (Abbott IgG; Abbott, USA); target epitope: recombinant NC protein.
VITROS Anti-SARS-CoV-2 Total Antibody (Ortho Clinical Diagnostics, USA); target epitope: recombinant S1 of Spike protein.
3.2. Sensitivity and specificity of assay testing algorithm
While currently approved vaccines are predominantly designed against Spike [7], [8], [9], [10], the absence of anti-NC in the presence of anti-Spike/RBD antibodies does not definitively arise in a post-vaccination setting, as anti-NC IgG is known to wane faster than anti-Spike/RBD IgG [11], [12], [13]. Thus, we sought to design an algorithm able to differentiate between a recent positive response (“Recent”) from a vaccine-induced/remote-infection response (“Vaccine/Remote”) based on positivity in anti-Spike/RBD and anti-NC antibodies as described in Fig. 1.
We assessed the performance of the algorithm in SARS-CoV-2 diagnosis using the same 117 serum specimens as before with results summarized in Table 2 . To note, all serum was collected prior to the start of immunization programs in Canada from patients diagnosed with SARS-CoV-2 infection within three months pre-collection, with no anticipated waning in humoral response. Thus, only “Recent” was defined as positive for the purpose of assessing the diagnostic performance of the algorithm.
Table 2.
Diagnostic performance of the proposed algorithm for the MSD assay's SARS-CoV-2 antigens.
| Presumed Positive (n = 31) | Presumed Negative (n = 86) | ||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Sensitivity% (95% CI) | Positive | Negative | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) |
| 31 | 0 | 100 (88.9–100) | 1 | 85 | 98.8 (93.7–100) | 96.9 (83.8–99.9) | 100 (95.8–100) |
We then included the 18 samples with no assigned category into the analysis to compare the agreement between our proposed algorithm with the BCCDC PHL's serological testing algorithm (Supplementary Figure 1). We found no significant difference in percent agreement between the two, with an observed agreement of 96.3% (130/135), and a Cohen's kappa of 0.926 (95% CI: 0.854–997) indicating almost perfect agreement.
3.3. Comparison of MSD V-PLEX coronavirus panel 2 performance against SPRi
We then compared the SARS-CoV-2 diagnostic agreement between MSD (SARS-CoV-2 antigen targets S1 RBD, NC, and S) and SPRi (RBD) using the full panel of 135 specimens (Table 3). Positivity was based upon manufacturer-set cut-offs as described in the Methods, and observed percent agreement and Cohen's kappa coefficient were calculated based on agreement between test interpretations.
Table 3.
Observed percent agreement (%; number of samples in agreement) and Cohen's kappa coefficient (κ; 95% CI) between the listed assays in the study's 135 samples.
| Assay | MSD Spike | MSD NC | SPRi RBD |
|---|---|---|---|
| MSD RBD | 94.1 (127) | 96.3 (130) | 91.1 (123) |
| κ=0.860 (0.766–0.954) | κ=0.913 (0.838–0.988) | κ =0.798 (0.688–0.907) | |
| MSD Spike | 97.8 (132) | 95.6 (129) | |
| κ=0.945 (0.885–1) | κ=0.895 (0.813–0.977) | ||
| MSD NC | 94.8 (128) | ||
| κ=0.878 (0.791–0.966) |
MSD's diagnosis by Spike and NC both exhibited almost perfect agreement with SPRi's RBD (κ = 0.895 and κ = 0.878, respectively), though only substantial agreement was seen when comparing MSD RBD to SPRi RBD (κ = 0.798). However, no significant difference in agreement was observed between all three of MSD's SARS-CoV-2 antigens and SPRi, with overlapping 95% confidence intervals across all measurements.
3.4. Endemic coronavirus assay performance
The clinical course of endemic HCoV infection is relatively short and self-resolving, and there also exists no current gold standard to discern true positives from true negatives. Furthermore, due to the endemic nature of HCoVs, it is estimated that all individuals 6 years or older would have acquired immunity against one of the four endemic HCoVs [14,15], making it difficult to validate an assay's ability to discern true positives from true negatives with sufficient sample sizes. Thus, we validated the detection of anti-HCoV IgG on the MSD assay by comparing the results to literature and against SPRi, which can detect antibodies against Beta-HCoVs OC43 and HKU1.
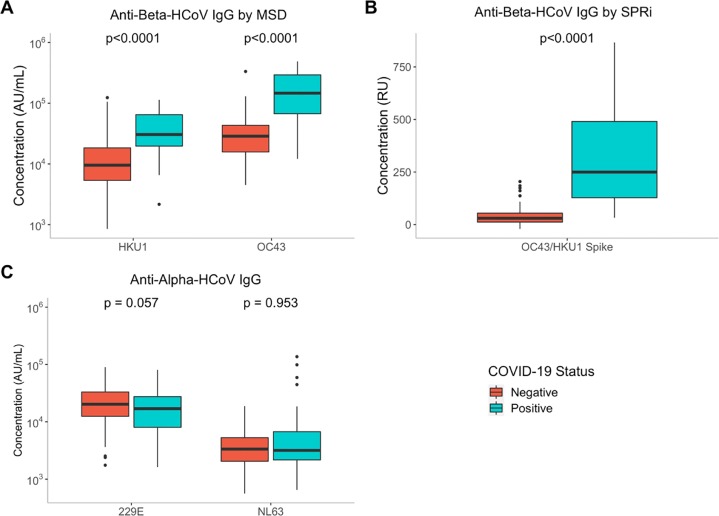
The 117 serum specimens with defined SARS-CoV-2 categories were used for this analysis, and similar distributions were observed across SPRi and MSD when stratified by COVID-19 status (Fig. 2A, B). Significantly higher anti-Beta-HCoV antibody levels were seen in COVID-19 positive serum samples compared to COVID-19 negative serum samples across both platforms (p < 0.0001), a trend which has also been previously described in literature [4,16]. Of note, one SARS-CoV-2 negative case came from a 4-year-old individual.
Fig. 2.
Assay signals for antibodies against Spike of HCoVs in 117 serum specimens stratified by COVID-19 status. (A) The MSD assay's determined concentrations (AU/mL) for anti-HKU1-S IgG and anti-OC43-S. (B) Relative signal (RU) for both anti-HKU1-S and anti-OC43-S for SPRi. C) The MSD assay's determined concentrations (AU/L) for anti-229E-S IgG and anti-NL63-S IgG. Boldfaced line indicates the median of the distribution, with ends of the box representing the first and third quantiles of the distribution. Comparisons between serology status had p < 0.0001 by Wilcoxon rank-sum test.
We also assessed the reliability of the MSD assay in the quantification of anti-Alpha-HCoVs NL63 and 229E, two other HCoVs in the Alphacoronavirus genus that can cause the common cold. While elevated titres have been reported in the Beta-HCoVs, studies have noted no significant difference in antibody titres against HCoV-NL63 and HCoV-229E when stratified by COVID-19 status [4,17]. Concordant with these findings, we did not detect a significant difference in serum IgG when stratified by COVID-19 status on the MSD assay (Fig. 2C).
4. Discussion
Serosurveillance is critical in understanding the prevalence of COVID-19 to inform public health measures to help stop the spread of infection. While previous studies have evaluated the MSD assay's SARS-CoV-2 diagnostic performance [18] and have tested its reactivity with HCoVs [19], there has yet to be literature validating the performance and reproducibility of the assay by comparison against other platforms. Here, we have demonstrated the reliability of MSD's V-PLEX Coronavirus Panel 2 in the detection of coronavirus antibodies by comparing its quantified readings against COVID-19 status, HC-authorized serological immunoassays, and SPRi.
We report diagnostic sensitivities and specificities greater than 94%, with no significant difference in performance observed between the V-PLEX Panel 2 and current clinically-approved chemiluminescent immunoassays when assessing by protein target or by algorithm, making it well suited for use in both individual diagnoses and population serosurveillance. Almost perfect agreement was also seen between SPRi RBD and MSD's NC and Spike, though the agreement dropped to substantial agreement when compared against MSD's RBD. This may have been due to favoring clinical sensitivity over specificity in both individual target cut-off and algorithm generation, with three of the five false positives close to the cut-off value of 538 AU/mL (545.7705 AU/mL, 578.6048 AU/mL, and 581.2465 AU/mL). Alternatively, RBD demonstrated an average inter-assay CV of 26.56% using serological controls and an average inter-assay CV of 17.28% using human serum within the range of 500–600 AU/mL, and it may have been fluctuations in assay performance that resulted in the misinterpretation. Nonetheless, no significant difference in performance was observed across the assays, with overlapping 95% confidence intervals between all comparisons.
Consistent with reported observations of elevated Beta-HCoV antibodies in SARS-CoV-2 infection [1], [2], [3], we found significantly higher Beta-HCoV titres in positive cases when compared to negative cases on both the MSD assay and SPRi. By validating MSD's panel's simultaneous quantification of anti-SARS-CoV-2 and anti-Beta-HCoVs, we have ascertained a suitable tool that can be used to investigate the possible correlations between COVID-19 disease severity and immune responses against the seasonal HCoVs.
A limitation in our study is the inability to validate the quantitative signals of anti-HCoV IgG due to the high prevalence of immune responses in the population. While we were able to compare MSD's panel's measurements against SPRi to allow for an additional level of validation, SPRi's platform pooled together equimolar HCoV-OC43 S and HCoV-HKU1 S to generate one combined signal, limiting our comparison to the genus level only. To validate at the virus species level, an SPRi panel with individual spots for each virus to generate independent signals should be utilized.
With vaccine rollout ramping up worldwide, seroprevalence of anti-SARS-CoV-2 S is expected to increase. As many of the vaccines generate immunity via Spike, NC may serve as a marker for natural infection. Here, we have proposed an interpretive algorithm using MSD's V-PLEX Coronavirus Panel 2 that can be used to differentiate recent infection responses from post-vaccination/remote infection responses, providing a valuable tool for serosurveillance studies.
MSD's platform allows for highly sensitive and specific detection of anti-SARS-CoV-2 IgG, and its performance at the algorithm and individual protein target levels is comparable to Health Canada-approved immunoassays in clinical use. MSD's V-PLEX Coronavirus Panel 2 is capable of accurate detection of anti-HCoVs and anti-SARS-CoV-2 IgG, thereby providing an invaluable tool in investigations of cross-reactivity and the contributions of pre-existing immune memory against the common cold HCoVs to COVID-19 infection or vaccination.
Role of funding source
This work was supported by the Michael Smith Foundation for Health Research [grant number: COV-2020–1120]. N.C. and E.G. report funding from the Digital Supercluster for their work in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Tamara Pidduck, Jesse Kustra, Annie Mak, Bonny So, Tahereh Valadbeigy, and Sydney Schwartz for providing technical assistance, and Ken Chu for curating the specimen database.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.105050.
Appendix. Supplementary materials
References
- 1.Aydillo T., Rombauts A., Stadlbauer D., Aslam S., Abelenda-Alonso G., Escalera A., Amanat F., Jiang K., Krammer F., Carratala J., García-Sastre A. Antibody immunological imprinting on COVID-19 patients. MedRxiv. 2020 doi: 10.1038/s41467-021-23977-1. 2020.10.14.20212662. 10.1101/2020.10.14.20212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Yan R., Liu M., Liu Z., Wang Y., Luan D., Wu K., Song Z., Sun T., Ma Y., Zhang Y., Wang Q., Li X., Ji P., Li Y., Li C., Wu Y., Ying T., Wen Y., Jiang S., Zhu T., Lu L., Zhang Y., Zhou Q., Huang J. Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: studies based on cellular and structural biology analysis. MedRxiv. 2020 2020.10.08.20209114. 10.1101/2020.10.08.20209114. [Google Scholar]
- 3.Shrock E., Fujimura E., Kula T., Timms R.T., Lee I.-.H., Leng Y., Robinson M.L., Sie B.M., Li M.Z., Chen Y., Logue J., Zuiani A., McCulloch D., Lelis F.J.N., Henson S., Monaco D.R., Travers M., Habibi S., Clarke W.A., Caturegli P., Laeyendecker O., Piechocka-Trocha A., Li J., Khatri A., Chu H.Y., Villani A.-.C., Kays K., Goldberg M.B., Hacohen N., Filbin M.R., Yu X.G., Walker B.D., Wesemann D.R., Larman H.B., Lederer J.A., Elledge S.J. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020 doi: 10.1126/science.abd4250. (80-.)eabd4250. 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J.E., Hicks P., Manzoni T.B., Oniyide O., Ramage H., Mathew D., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., D’Andrea K., Kuthuru O., Dougherty J., Pattekar A., Kim J., Han N., Apostolidis S.A., Huang A.C., Vella L.A., Kuri-Cervantes L., Pampena M.B., Betts M.R., Wherry E.J., Meyer N.J., Cherry S., Bates P., Rader D.J., Hensley S.E. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864. doi: 10.1016/j.cell.2021.02.010. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djaileb A., Charron B., Jodaylami M.H., Thibault V., Coutu J., Stevenson K., Forest S., Live L.S., Boudreau D., Pelletier J.N., Masson J.F. A rapid and quantitative serum test for SARS-CoV-2 antibodies with portable surface plasmon resonance sensing. ChemRxiv. 2020 10.26434/chemrxiv.12118914.v1. [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadoff J., Gars M.Le, Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., Berghmans P.-.J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson K.E., De Rosa S.C., Cohen K.W., McElrath M.J., Cormier E., Scheper G., Barouch D.H., Hendriks J., Struyf F., Douoguih M., Van Hoof J., Schuitemaker H. Interim results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. NEJMoa2034201. 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Elslande J., Gruwier L., Godderis L., Vermeersch P. Estimated Half-Life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab219. 10.1093/cid/ciab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S.A., Peters B., Krammer F., Simon V., Saphire E.O., Smith D.M., Weiskopf D., Sette A., Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;(80):371. doi: 10.1126/science.abf4063. 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumley S.F., Wei J., O'Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Peck L.J., Ritter T.G., de Toledo Z., Cornall R.J., Jones E.Y., Stuart D.I., Screaton G., Ebner D., Hoosdally S., Crook D.W., Conlon C.P., Pouwels K.B., Walker A.S., Peto T.E.A., Walker T.M., Jeffery K., Eyre D.W. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab004. 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaunt E.R., Hardie A., Claas E.C.J., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol. 2010;48:2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovi T., Kainulainen H., Ziola B., Salmi A. OC43 strain-related coronavirus antibodies in different age groups. J. Med. Virol. 1979;3:313–320. doi: 10.1002/jmv.1890030410. 10.1002/jmv.1890030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phuong N.-.C., Karim E.A., Preshetha K., A C.F., Hongmei Y., R B.A., J T.D., Y S.M., Ali E., Stacey S.-.C. S Protein-Reactive IgG and Memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. MBio. 2021;11 doi: 10.1128/mBio.01991-20. e01991-20. 10.1128/mBio.01991-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L., Wang Y., Kang L., Hu Y., Wang L., Zhong J., Chen H., Ren L., Gu X., Wang G., Wang C., Dong X., Wu C., Han L., Wang Y., Fan G., Zou X., Li H., Xu J., Jin Q., Cao B., Wang J. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg. Microbes Infect. 2021;10:664–676. doi: 10.1080/22221751.2021.1905488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson M., Wagstaffe H.R., Gilmour K.C., Mai A.L., Lewis J., Hunt A., Sirr J., Bengt C., Grandjean L., Goldblatt D. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J. Clin. Virol. 2020:130. doi: 10.1016/j.jcv.2020.104572. 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majdoubi A., Michalski C., O'Connell S.E., Dada S., Narpala S.R., Gelinas J.P., Mehta D., Cheung C., Winkler D.F., Basappa M., Liu A.C., Görges M., Barakauskas V.E., Irvine M.A., Mehalko J., Esposito D., Sekirov I., Jassem A.N., Goldfarb D.M., Pelech S., Douek D.C., McDermott A.B., Lavoie P.M. A majority of uninfected adults show pre-existing antibody reactivity against SARS-CoV-2. JCI Insight. 2021 doi: 10.1172/jci.insight.146316. 10.1172/jci.insight.146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.