Abstract
A multiplex PCR assay for detection of genes for staphylococcal enterotoxins A to E (entA, entB, entC, entD, and entE), toxic shock syndrome toxin 1 (tst), exfoliative toxins A and B (etaA and etaB), and intrinsic methicillin resistance (mecA) was developed. Detection of femA was used as an internal positive control. The multiplex PCR assay combined the primers for sea to see and femA in one set and those for eta, etb, tst, mecA, and femA in the other set. Validation of the assay was performed using 176 human isolates of Staphylococcus aureus. This assay offers a very specific, quick, reliable, and inexpensive alternative to conventional PCR assays used in clinical laboratories to identify various staphylococcal toxin genes.
It is well documented that strains of Staphylococcus aureus produce a variety of extracellular protein toxins, including enterotoxins, toxic shock syndrome toxin 1 (TSST-1), exfoliative toxin (ET), hemolysins, and coagulase (15). S. aureus is one of the most commonly found pathogenic bacteria and is hard to eliminate from the human environment. It is responsible for many nosocomial infections, besides being the main causative agent of food intoxication by virtue of its variety of enterotoxins (15).
According to serological classification, to date six staphylococcal enterotoxin (SE) groups have been recognized: SEA, SEB, SEC, SED, and SEE (15) and the recently described SEH (22, 27). These enterotoxins are small peptides (26 to 29 kDa) and have a great deal of similarity at the amino acid level (21). They are the main source of food poisoning and cause intensive intestinal peristalsis. The toxic shock syndrome of humans and animals is caused by the presence of S. aureus isolates producing TSST-1. The enterotoxins, as well as TSST-1, belong to a family of superantigens (25). The two ETs ETA and ETB, in conjunction or independently, are implicated in the cause of staphylococcal scalded-skin syndrome (15).
Over the last few decades, there has been an enormous increase and emergence of S. aureus strains resistant to the antibiotic methicillin (MRSA strains), particularly in nosocomial settings (13). The intrinsic resistance to these antibiotics is attributed to the presence of mecA, whose product is a 78-kDa protein called penicillin binding protein 2a (14, 28). Identification of mecA in such MRSA strains has led to some knowledge regarding the use of the antibiotic vancomycin (9). The femA gene encodes a factor which is essential for methicillin resistance and is universally present in all S. aureus isolates. The femA gene product, a 48-kDa protein, has been implicated in cell wall metabolism and is found in large amounts in actively growing cultures (17, 29).
For epidemiological surveillance, the methods most frequently used for the detection of staphylococcal toxins are immunodiffusion, agglutination, radioimmunoassay, and enzyme-linked immunosorbent assay (15, 18). Among the techniques used to identify toxin genotypes, DNA-DNA hybridization and PCR have been reported to be very successful and reliable, and our laboratory previously designed specific primers for the successful and reliable detection of SEs, TSST-1, and ETs by PCR (18).
There are several reports in the literature describing the use of multiplex PCR for detection of MRSA strains, but most of these techniques are designed to detect only two or three gene fragments (1, 8, 12, 24, 26, 29, 30). In this report, we describe two multiplex PCR primer sets which can detect the presence of 10 staphylococcal genes simultaneously, in just two reactions. As an internal positive control for each reaction, we incorporated primers specifically designated to amplify femA, which has been reported to be specific to S. aureus (29). Validation of the multiplex PCR primers was performed and interpreted using 176 isolates of S. aureus which were first characterized for their toxin gene profiles by using individual primers (18). We conclude that the multiplex primer sets described here are reliable and specific in detecting the toxin genes of S. aureus.
MATERIALS AND METHODS
Bacterial strains and culture media.
A total of 176 S. aureus strains were used in this study. Of these, 107 strains were isolated from nasal swabs of a control (healthy) population, 47 strains were from The Netherlands, and 19 MRSA strains were from a national surveillance study in Canada. The remaining three strains were isolated from clinical specimens. All strains were stored on suitable maintenance media in the culture collection in the National Laboratory for Bacteriology, Laboratory Center for Disease Control. The control strains had been previously defined in terms of toxigenicity with respect to enterotoxins, ETs, and TSST-1. The following strains were used as positive controls in this study: ATCC 13565 (SEA), ATCC 14458 (SEB), ATCC 19095 (SEC), 90-S-1025 (SED), ATCC 27664 (SEE), 88-S-8902 (ETA), 88-S-8620 (ETB), 92-S-1344 (TSST-1), and 95-S-739 (mecA). The non-ATCC strains mentioned above were obtained from the culture collection of the Laboratory Center for Disease Control and were characterized for their toxin production by using semiquantitative reversed passive latex agglutination toxin detection kits (SET-RPLA and TST-RPLA; Oxoid Ltd., Basingstoke, Hampshire, England). The two ET-producing strains were originally identified by immunodiffusion tests (18). Disk diffusion tests for MRSA isolates were performed as described previously (21a). Bacterial cultures were grown in brain heart infusion broth prior to extraction of total DNA.
DNA isolation.
Total DNA was isolated from 0.5 ml of brain heart infusion broth culture grown overnight for all the bacterial strains used in the study. The procedure used for DNA isolation has been described previously (18). DNA samples were dissolved in Tris-EDTA buffer (10 mM Tris chloride, 1 mM EDTA [pH 8.0]), and the concentration was determined as micrograms per milliliter according to A260 values. Template DNA in amounts ranging from 10 to 1,000 ng was used in the study.
Primers.
Oligonucleotides ranging from 18- to 24-mers were selected from the published DNA sequences of the S. aureus genes (Table 1) using Oligo software (version 3.4). Synthesis of oligonucleotides was carried out at the DNA Core Facility at the Laboratory Center for Disease Control. For multiplex PCRs, two primers sets were prepared: set A was designed to amplify sea, seb, sec, sed, see, and femA, whereas set B was designed to amplify mecA, eta, etb, and tst as well as femA. The primer sequences used in the multiplex PCRs are described in Table 1.
TABLE 1.
Nucleotide sequences, gene locations, and anticipated sizes of PCR products for the S. aureus gene-specific oligonucleotide primers used in this study
| Genea | Primer | Oligonucleotide sequence (5′-3′) | Location within gene | Size of amplified product (bp) | Multiplex PCR set |
|---|---|---|---|---|---|
| sea | GSEAR-1 | GGTTATCAATGTGCGGGTGG | 349–368 | 102 | A |
| GSEAR-2 | CGGCACTTTTTTCTCTTCGG | 431–450 | |||
| seb | GSEBR-1 | GTATGGTGGTGTAACTGAGC | 666–685 | 164 | A |
| GSEBR-2 | CCAAATAGTGACGAGTTAGG | 810–829 | |||
| sec | GSECR-1 | AGATGAAGTAGTTGATGTGTATGG | 432–455 | 451 | A |
| GSECR-2 | CACACTTTTAGAATCAACCG | 863–882 | |||
| sed | GSEDR-1 | CCAATAATAGGAGAAAATAAAAG | 492–514 | 278 | A |
| GSEDR-2 | ATTGGTATTTTTTTTCGTTC | 750–769 | |||
| see | GSEER-1 | AGGTTTTTTCACAGGTCATCC | 237–257 | 209 | A |
| GSEER-2 | CTTTTTTTTCTTCGGTCAATC | 425–445 | |||
| femA | GFEMAR-1 | AAAAAAGCACATAACAAGCG | 1444–1463 | 132 | A or B |
| GFEMAR-2 | GATAAAGAAGAAACCAGCAG | 1556–1575 | |||
| mecA | GMECAR-1 | ACTGCTATCCACCCTCAAAC | 1182–1201 | 163 | B |
| GMECAR-2 | CTGGTGAAGTTGTAATCTGG | 1325–1344 | |||
| eta | GETAR-1 | GCAGGTGTTGATTTAGCATT | 775–794 | 93 | B |
| GETAR-2 | AGATGTCCCTATTTTTGCTG | 848–867 | |||
| etb | GETBR-1 | ACAAGCAAAAGAATACAGCG | 509–528 | 226 | B |
| GETBR-2 | GTTTTTGGCTGCTTCTCTTG | 715–734 | |||
| tst | GTSSTR-1 | ACCCCTGTTCCCTTATCATC | 88–107 | 326 | B |
| GTSSTR-2 | TTTTCAGTATTTGTAACGCC | 394–113 |
Multiplex PCR conditions.
Two sets of primer mixes were prepared according to the master mixes of components from the GeneAmp kit (Perkin-Elmer, Norwalk, Conn.), with slight modifications to the given instructions. Multiplex primer set A contained 200 μM deoxynucleoside triphosphates; 5 μl of 10× reaction buffer (100 mM Tris-HCl [pH 8.3], 500 mM KCl); 1.5 mM MgCl2; 20 pmol (each) of sea, seb, sec, see, and femA primers; 40 pmol of sed primer; 2.5 U of Taq DNA polymerase (AmpliTaq DNA polymerase; Perkin-Elmer), and 10 to 1,000 ng of template DNA. The volume of this mix was adjusted to 50 μl with sterile water. Multiplex primer set B included the same constituents as in set A except for the MgCl2 concentration (2.0 mM) and the primers, which were used at 50 pmol for eta and 20 pmol each for etb, tst, mecA, and femA. Evaporation of the reaction mixture was prevented by addition of 100 μl of sterile mineral oil. DNA amplification was carried out in a Perkin-Elmer thermocycler with the following thermal cycling profile: an initial denaturation at 94°C for 5 min was followed by 35 cycles of amplification (denaturation at 94°C for 2 min, annealing at 57°C for 2 min, and extension at 72°C for 1 min), ending with a final extension at 72°C for 7 min.
RESULTS
Multiplex PCR for detection of selected staphylococcal genes.
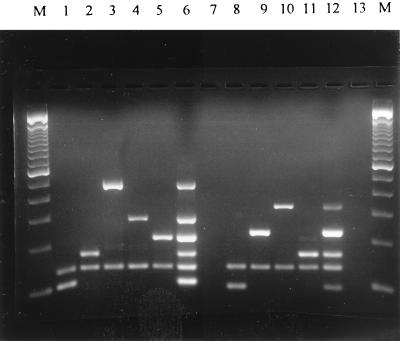
The reaction conditions for the multiplex PCR assay were optimized to ensure that all of the target gene sequences were satisfactorily amplified. The primers were designed to target the coding regions of the genes; care was taken to avoid areas of homology within the structural genes for the enterotoxins. The primers used in each set had almost equal annealing temperatures, which reduced the possibility of occurrence of unwanted bands originating from nonspecific amplification. Figure 1 shows the presence of the amplified products after agarose gel electrophoresis, when DNA extracted from a representative toxigenic S. aureus strain (positive control) was used as the template in the PCR using the multiplex primer sets. Reliable amplification of six bands in set A (for sea, seb, sec, sed, see, and femA) was obtained when a mixture of DNAs from the same strains was tested (Fig. 1). Similarly, five bands were obtained when a mixture of DNAs from the corresponding strains in set B (for eta, etb, tst, mecA, and femA) was tested (Fig. 1). The sizes of the amplicons obtained from the various control strains corresponded to the predicted sizes (Table 1). As a negative control, both sets were tested with sterile water, and no amplicons were observed (Fig. 1). Genomic DNA in amounts ranging from 10 to 1,000 ng/reaction was used, with no apparent change in sensitivity and ability to detect all of the genes in the sample (data not shown).
FIG. 1.
Agarose gel electrophoresis patterns showing multiplex PCR amplification products for the S. aureus genes. Lanes M, DNA molecular size marker (100-bp ladder; Bethesda Research Laboratories Inc., Gaithersburg, Md.); lanes 1 to 7, PCR amplicons from primer set A; lanes 8 to 13, PCR amplicons from set B. Lanes: 1, sea plus femA; 2, seb plus femA; 3, sec plus femA; 4, sed plus femA; 5, see plus femA; 6, sea, seb, sec, sed, see, and femA simultaneously; 7, negative control; 8, eta plus femA; 9, etb plus femA; 10, tst plus femA; 11, mecA plus femA; 12, eta, etb, tst, mecA, and femA; 13, negative control.
To further test the specificity of the assay, DNA templates from one Campylobacter jejuni strain and two Escherichia coli strains were used with the multiplex PCR primer sets (A and B), and no amplified products were obtained (data not shown).
To substantiate the multiplex PCR technique, 176 strains of S. aureus that were tested by multiplex PCR were also screened for the presence of individual toxin genes by using the method described previously (18). Full agreement of the two methods was observed. In order to test for 10 toxin genes individually, a total of 1,760 PCRs were required, whereas when multiplex primer sets were used, only 352 PCRs were needed. The multiplex primers sets were seen to be very reliable, since no discrepancy was observed in the results obtained using the two methods. An internal control of femA was present in all of the samples, confirming the presence of S. aureus and also validating the PCR conditions. Other workers have used primers for 16S rRNA as the internal control when designing multiplex primers to detect mecA (24, 26).
Primary validation of the amplicons.
The sizes of the amplicons obtained by the multiplex primer sets were identical to those predicted from the design of the primers (Tables 1 and 2). The amplicons from the control strains were subjected to further confirmation and characterization by digestion with restriction endonucleases with cleavage sites within the amplicon. The restriction enzymes used and the predicted product sizes are given in Table 2. Enzyme fragments with the anticipated sizes were obtained in each case (data not shown).
TABLE 2.
Predicted sizes of restriction fragments and enzymes used for restriction fragment length polymorphism analysis to validate the amplified products of multiplex PCR
| Gene | Amplicon size (bp) | Multiplex primer set | Restriction enzyme used | Expected sizes (bp) of restriction fragments |
|---|---|---|---|---|
| sea | 102 | A | AluI | 65, 37 |
| seb | 164 | A | MaeIII | 120, 31, 13 |
| sec | 451 | A | NdeI | 307, 144 |
| sed | 278 | A | MboII | 175, 103 |
| see | 209 | A | AccI | 119, 90 |
| femA | 132 | A or B | AccI | 78, 54 |
| eta | 93 | B | MboI | 57, 36 |
| etb | 226 | B | HpaII | 159, 67 |
| tst | 326 | B | MboI | 187, 139 |
| mecA | 163 | B | MaeIII | 84, 79 |
Analysis of the results.
Among the 176 strains tested, 107 strains were collected from nasal swabs of healthy humans. Of these 107 strains, 21 (19.6%) were positive for sea, 26 (24.3%) were positive for the TSST-1 gene (tst), 6 (5.6%) were found to be seb positive, 8 (7.5%) were positive for sec, and 2 (1.9%) contained the gene for sed. None were positive for see, eta, etb, or mecA. Of the 47 strains obtained from The Netherlands, 6 were positive for sea, 1 was positive for seb, 3 were positive for sec, 4 were positive for eta, and 11 were positive for tst. Among the three clinical isolates tested, all were positive for eta as well as etb.
Among the 19 strains obtained from the national MRSA surveillance study, 18 were mecA positive, indicating that mecA is responsible for methicillin resistance in those strains. The single remaining oxacillin-resistant isolate (which was mecA negative) must be methicillin resistant by virtue of some other mechanism (9). Of these MRSA isolates, three were positive for sea, two were positive for seb, three were positive for sec, two were positive for sed, three were positive for see, and one contained the gene for TSST-1. All of the 176 samples tested contained the femA gene.
DISCUSSION
We have described a multiplex PCR-based diagnostic protocol to detect the genes for enterotoxins A to E, ETA, ETB, and TSST-1 and the mecA gene in DNA extracted from human isolates of S. aureus. This procedure is an improvement over our previously described PCR protocols, where individual primers were used to identify the staphylococcal toxin genes (18). The multiplex PCR primer sets were shown to be very specific, reliable, and, most importantly, very efficient in detection of all 10 genes. As an internal control, femA was found to be present in all of the strains studied. The gene product of femA has been suggested to have a role in cell wall metabolism and is reported to be present in all S. aureus species during the active growth phase (17, 29).
Six pairs of primers were used in multiplex primer set A to target the structural genes for enterotoxins A to E (sea, seb, sec, sed, and see), along with femA. In the multiplex primer set B, five pairs of primers were mixed together to target the structural genes for mecA, eta, etb, and tst, along with femA. All of the primers were gene specific, as demonstrated by restriction fragment lengths obtained after specific restriction endonuclease digestion of the amplicons. The multiplex primers were shown to be specific for S. aureus, since no amplification product was obtained when either C. jejuni or E. coli DNA was used as the template.
In this study, the toxin genotypes of S. aureus strains isolated from healthy human carriers are also demonstrated. Of 107 such isolates tested, 24.3% possessed the gene for TSST-1 and 19.6% were positive for SEA. These results are in accordance with previous findings that many healthy individuals are carriers of toxin-producing strains of S. aureus (10).
The use of multiplex PCR to characterize staphylococcal strains and their resistance to methicillin has been well documented (1, 8, 12, 29, 30). Those reports focus on detection of the gene responsible for methicillin resistance (mecA) along with either the femA, 16S rRNA, nuc, or IS431 gene as a positive control(s) (1, 8, 12, 29). A recent study describes the use of two multiplex PCR assays for detection of S. aureus exotoxin genes: one is designed to detect the enterotoxin genes, and the other is designed to detect the tst, eta, and etb genes (3). Becker et al. have used DNA enzyme immunoassays to validate the specificity of the PCR products, using oligonucleotide probes derived from the sequences of the S. aureus toxin genes (3).
The study described in this paper provides detailed information about S. aureus toxin genes as well as mecA. The inclusion of an internal positive control (femA) in the reaction provides assurance against false-negative results. Use of this multiplex PCR assay will help provide the information required for appropriate therapy and infection control during outbreaks of S. aureus. It is important to recognize that this technique only will identify strains harboring the toxin genes and is independent of the expression and secretion of the toxin. To verify toxin production by any given isolate, time- and labor-intensive immunological methods may be used to detect the excreted toxins.
Considering the low cost and much shorter time required to detect the 10 genes of S. aureus by multiplex PCR, we believe this to be a powerful tool for studying the genotypes of staphylococcal isolates. This procedure was specially developed to fit into the daily work pattern of a routine clinical laboratory, since genotypic detection of drug resistance and the presence of toxin genes is becoming an important component of the diagnostic inventory of such laboratories.
ACKNOWLEDGMENTS
We are grateful to J. W. Cohen Tervaert for the strains from The Netherlands and to M. Mamelak for the strains from healthy controls. We also thank Russell Easy and Hugh Cai for technical assistance and M. R. Mulvey for critical review and reading of the manuscript.
M.M. was funded by a postdoctoral fellowship from a grant to W.M.J. by the Canadian Bacterial Disease Network.
REFERENCES
- 1.Barski P, Piechowicz L, Galinski J, Kur J. Rapid assay for detection of methicillin-resistant Staphylococcus aureus using multiplex PCR. Mol Cell Probes. 1996;10:471–475. doi: 10.1006/mcpr.1996.0066. [DOI] [PubMed] [Google Scholar]
- 2.Bayles K W, Iandolo J J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989;171:4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin gene. J Clin Microbiol. 1998;36:2548–2553. doi: 10.1128/jcm.36.9.2548-2553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger-Bachi B, Barberis-Maino L, Strassle A, Kayser F H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 5.Betley M J, Mekalanos J J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988;170:34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomster-Hautamaa D A, Kreiswirth B N, Kornblum J S, Novick R P, Schlievert P M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986;261:15783–15786. [PubMed] [Google Scholar]
- 7.Bohach G A, Schlievert P M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987;209:15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- 8.Brakstad O G, Maeland J A, Tveten Y. Multiplex polymerase chain reaction for detection of genes for Staphylococcus aureus thermonuclease and methicillin resistance and correlation with oxacillin resistance. APMIS. 1993;101:681–688. doi: 10.1111/j.1699-0463.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 9.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chance T D. Toxic shock syndrome: role of the environment, the host and the microorganism. Br J Biomed Sci. 1996;53:284–289. [PubMed] [Google Scholar]
- 11.Couch J L, Soltis M T, Betley M J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988;170:2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geha D L, Uhl J R, Gustaferro C A, Persing D H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol. 1994;32:1768–1772. doi: 10.1128/jcm.32.7.1768-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley R W, Hightower A W, Khabbaz R F, Thorsberry C, Martone W J, Allen J R, Hughes J M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Ann Intern Med. 1982;97:297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- 14.Hartman B J, Tomasz A. Low-affinity binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iandolo J J. Genetic analysis of extracellular toxins of Staphylococcus aureus. Annu Rev Microbiol. 1989;43:375–402. doi: 10.1146/annurev.mi.43.100189.002111. [DOI] [PubMed] [Google Scholar]
- 16.Jackson M P, Iandolo J J. Sequence of the exfoliative toxin B gene of Staphylococcus aureus. J Bacteriol. 1986;167:726–728. doi: 10.1128/jb.167.2.726-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S, Dominique K, Labischinski H. FemA of Staphylococcus aureus: isolation and immunodetection. FEMS Microbiol Lett. 1995;132:221–228. doi: 10.1111/j.1574-6968.1995.tb07837.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson W M, Tyler S D, Ewan E P, Ashton F E, Pollard D R, Rozee K R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones C L, Khan S A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166:29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C Y, Schmidt J J, Johnson-Winegar A D, Spero L, Iandolo J J. Sequence determination and comparison of the exfoliative toxin A and toxin B genes from Staphylococcus aureus. J Bacteriol. 1987;169:3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 21a.National Committee for Clinical Laboratory Standards. Document M2-A5. Performance standards for antimicrobial disk susceptibility tests. 5th ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 22.Ren K, Bannan J D, Pancholi V, Cheung A L, Robbins J C, Fischetti V A, Zabriskie J B. Characterization and biological properties of a new staphylococcal exotoxin. J Exp Med. 1994;180:1675–1683. doi: 10.1084/jem.180.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bachi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 24.Salisbury S M, Sabatini L M, Spiegel C A. Identification of methicillin-resistant staphylococci by multiplex polymerase chain reaction assay. Am J Clin Pathol. 1997;107:368–373. doi: 10.1093/ajcp/107.3.368. [DOI] [PubMed] [Google Scholar]
- 25.Schlievert P M. Role of superantigens in human diseases. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz F-J, Mackenzie C R, Hofmann B, Verhoef J, Finken-Eigen M, Heinz H-P, Kohrer K. Specific information concerning taxonomy, pathogenicity, and methicillin resistance of staphylococci obtained by a multiplex PCR. J Med Microbiol. 1997;46:773–778. doi: 10.1099/00222615-46-9-773. [DOI] [PubMed] [Google Scholar]
- 27.Su Y, Lee Wong A C. Identification and purification of a new staphylococcal enterotoxin, H. Appl Environ Microbiol. 1995;61:1438–1443. doi: 10.1128/aem.61.4.1438-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985;28:397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vannuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G, Gala J. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol. 1995;33:2864–2867. doi: 10.1128/jcm.33.11.2864-2867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambardi G, Reverdy M E, Bland S, Bes M, Freney J, Fleurette J. Laboratory diagnosis of oxacillin resistance in Staphylococcus aureus by a multiplex-polymerase chain reaction assay. Diagn Microbiol Infect Dis. 1994;19:25–31. doi: 10.1016/0732-8893(94)90047-7. [DOI] [PubMed] [Google Scholar]