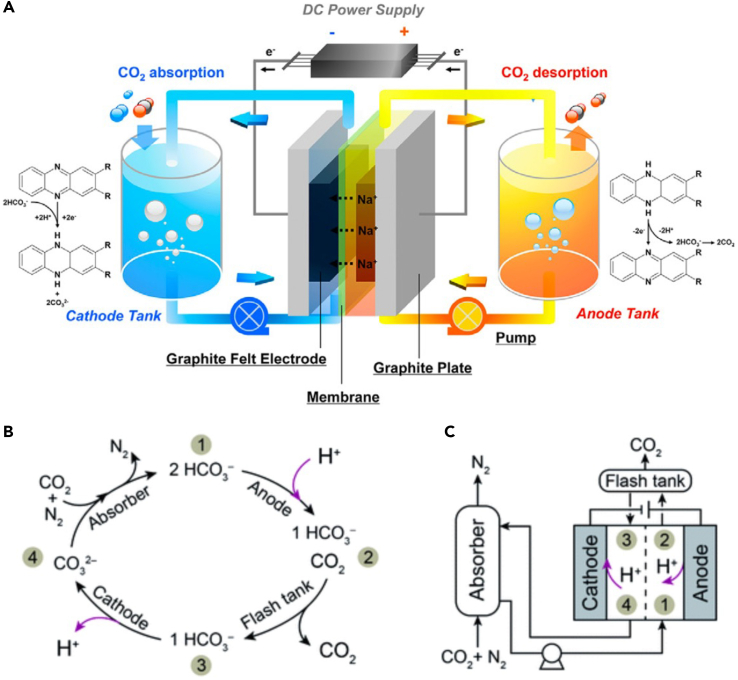
Summary
The desire toward decarbonization and renewable energy has sparked research interests in reactive CO2 separations, such as direct air capture that utilize electricity as opposed to conventional thermal and pressure swing processes, which are energy-intensive, cost-prohibitive, and fossil-fuel dependent. Although the electrochemical approaches in CO2 capture that support negative emissions technologies are promising in terms of modularity, smaller footprint, mild reaction conditions, and possibility to integrate into conversion processes, their practice depends on the wider availability of renewable electricity. This perspective discusses key advances made in electrolytes and electrodes with redox-active moieties that reversibly capture CO2 or facilitate its transport from a CO2-rich side to a CO2-lean side within the last decade. In support of the discovery of new heterogeneous electrode materials and electrolytes with redox carriers, the role of computational chemistry is also discussed.
Subject areas: Electrochemical energy conversion, Energy sustainability, Materials science, Materials chemistry, Computational materials science, Energy materials
Graphical abstract

Electrochemical energy conversion; Energy sustainability; Materials science; Materials chemistry; Computational materials science; Energy materials
Introduction
The atmospheric concentration of CO2 has been recorded to fluctuate for 800,000 years between 200 and 300 ppm, oscillating with the glacial and interglacial periods of the planet. In recent years, the concentration had a sharp increase to 415 ppm (Dlugokencky and Tans, 2021). The International Panel on Climate Change released its sixth Assessment Report in the summer of 2021, in which they review the unequivocal evidence that human activity, particularly anthropogenic greenhouse gas (GHG) emission, is the main contributor to global climate change (IPCC, 2021). The report indicates that the 1.5°C increase in average global temperature, established by the Paris Agreement, is approaching much faster than initially thought. The likely hood of surpassing 2°C of average global temperature warming within the next two decades is virtually certain unless significant GHG emission reduction efforts are implemented. One of the most effective ways of combatting this temperature increase is by achieving net-zero emissions of the GHGs, especially from large emitters such as fossil fuel burning power plants. CO2 is responsible for more than half of the warming imbalance and thus has been a common target of GHG capture research for the past few decades (Tans et al., 2020).
CO2 can be captured in a number of ways depending on its source. Many of these processes involve feeding CO2-rich gas to an absorbent or adsorbent material that selectively captures CO2, followed by the regeneration of the sorbent via thermal or pressure swing. The regeneration step is often the most energy-intensive step in the process, and aging of capture materials or solvents under the long-term swing conditions is another challenge. Although more than 70 years of research and some examples of implementation have been focused on technologies that capture CO2 from post-combustion flue gas, capturing CO2 from atmospheric air has recently gained more interest. Direct air capture (DAC) technology aims to separate CO2 from the atmosphere, where the CO2 concentration is much lower compared to point sources. However, DAC is currently a high-cost technology with 5–10 GJ energy requirements to capture a ton of atmospheric CO2. For DAC to be truly a negative emission technology (NET), the energy requirement should be lowered with alternative resources to fossil fuels and the captured CO2 should be geologically stored. Therefore, materials with high selectivity, significant CO2 capture capacity, and stability under a wide range of temperatures and humidities are needed for DAC. Furthermore, it would be highly desirable to regenerate DAC materials with low-cost zero-carbon energy. Currently, 28% of the electricity generated globally comes from renewables which is a limiting factor to electrify carbon capture technologies.
Removal of CO2 as a means of revitalizing breathing air has been an active research area since the 1940s (i.e., submarines and spacecraft, and more recently for sustainability in space) (Kammermeyer, 1966; Knox, July 2018; Blum et al., 1952). The concept of DAC to mitigate the rise in CO2 levels in the atmosphere was introduced by Lackner in 1999 (Lackner et al., 1999). Approaches to DAC include aqueous alkaline bases (Stolaroff et al., 2008), humidity-swing quaternary ammonium-based anion-exchange resins (Shi et al., 2020a; Wang et al., 2011), and solid-supported amines (Bali et al., 2015; Didas et al., 2015; Sujan et al., 2019) with fewer technologies based on electrochemical and membrane separations (Sanz-Pérez et al., 2016). Causticization with aqueous alkaline bases requires regeneration temperatures of 800–900°C, which constitutes the majority of the energy required for CO2 removal (6–9 GJ per ton of CO2). On a purely thermodynamic basis, the conversion of CO2 to calcium carbonate salt has a reaction enthalpy of approximately −109 kJ/mol, but the intermediate regeneration step of converting calcium carbonate to calcium oxide has a reaction enthalpy of +179 kJ/mol, resulting in an unavoidable energy penalty of at least 70 kJ/mol of CO2 before factoring in system inefficiencies. Aqueous amines (El Hadri et al., 2017; Yu et al., 2012; Nguyen et al., 2010) have milder regeneration temperatures (∼120°C); however, their reaction enthalpies, on the order of −80 kJ/mol of CO2 (Kim and Svendsen, 2007), are still demanding, especially when coupled with the evaporation of both the amine and water. The resulting vapor must be re-condensed to prevent the loss of active material, leading to an even greater energy penalty (Nguyen et al., 2010). The volatility and toxicity of amines further eliminate their use in open systems. Solid-supported amines are, therefore, more promising, but these systems present slow transport of CO2 and performance degradation over time owing to oxidation and moisture, similar to metal-organic frameworks (MOFs) and zeolites. The current state of the field necessitates improved solvents/sorbents with high CO2 selectivities and capacities, fast transport, new regeneration mechanisms or improved energy requirements for thermal regeneration, and long-term stability, as underscored by the 2018 National Academy of Sciences report on Negative Emission Technologies and Reliable Sequestration (National Academies of Sciences et al., 2018).
Humidity swing (Shi et al., 2020b), dielectric heating by electromagnetic field (Tsubaki et al., 2020), and electrochemically mediated separations (Liu et al., 2020b) are some of the more recent examples of alternative regeneration processes. The use of moisture or humidity is an attractive mechanism to drive the absorption–desorption cycle of CO2, specifically in comparison to the requirement of heat in thermal-swing and mechanical work in pressure-swing. Humidity-swing processes involve the absorption of CO2 with the hydroxide ion resin to form bicarbonate. As the resin uptakes CO2 under low humidity conditions (10–40% RH), some water is released owing to the difference in the hydration of the ions present. The flow of moist air (>70% RH) over the CO2-saturated resin during desorption results in the hydration of the ions which provides the free energy for CO2 release. To maximize energy efficiency, humidity-swing could be coupled with other driving forces such as thermal boost as increasing the temperature of a feed with a certain water content lowers the relative humidity (Lackner et al., 2020). CO2 regeneration by dielectric heating via electromagnetic field stimulation and Joule heating via electric-current stimulation are relatively new concepts with very few studies demonstrated to date for aqueous amines (McGurk et al., 2017; Tsubaki et al., 2020) and carbonaceous adsorbents (Sevanthi et al., 2016), respectively. On the other hand, electric stimuli for CO2 separations have been studied in a number of systems by Hatton and co-workers. One of the electrochemically mediated CO2 capture systems involves the use of redox-active carriers and another involves the use of amine absorbers that release CO2 in the presence of cupric ions. In these isothermal systems, the need for thermal energy is eliminated and the energy requirement is dependent on the overpotentials; additional voltage is required to perform the redox reaction resulting from kinetic and mass transport limitations. Design of the electrochemical cell and materials can help to minimize these overpotentials and the associated energy penalty. Furthermore, steam is not needed, thus enabling modularity in CO2 capture. Using renewable energy sources for these processes supports the distributed nature of electrochemical processes. In this perspective, we discuss the recent electrochemical approaches in CO2 separations (Table 1) with a specific focus on multifunctional electrolytes, modified electrodes, and the role of quantum chemistry in the design space of active materials, as summarized in Figure 1.
Table 1.
Examples of electrochemical approaches to reactive CO2 separations with reported current densities () and faradaic efficiencies ()
| E (kJ/mol CO2) | i (mA/cm2) | Advantages | Disadvantages | Ref | ||
|---|---|---|---|---|---|---|
| Amine-Based Systems | ||||||
| EMARa | 45 | 2.5 | 0.42 | Suppressed solvent volatility (compared to thermal swing amines) | Electrode dissolution and plating causes material imbalance in electrolytes | (Stern et al., 2013) (Wang et al., 2020) |
| CRABb | −8.2h | 3 | 0.45 | Harvests binding energy of CO2-sorbent complex | Requires thermal amine regeneration with low-pressure steam | (Li et al., 2020) |
| Redox-Active CO2Carriers | ||||||
| Quinones | 43 | 0.5 | 0.95 | Natural compounds with a large structural design space | Unstable in aqueous media; Highly susceptible to protonation in reduced form Slow diffusion in non-aqueous solvents |
(Gurkan et al., 2015; Scovazzo et al., 2003) |
| Disulfides | 200 | 3.03c | .9934c | Less basic/susceptible to protonation than quinones | Sluggish oxidation kinetics Large potential difference between reduction (CO2 capture) and oxidation (CO2 release) leading to inefficiencies |
(Singh et al., 2017a, 2017b) |
| Biomimetic Proton Carriers for PCETd | ||||||
| Phenazine derivatives | 21.6 | 10 | 0.958 | Moisture stable High faradaic efficiency |
Vulnerable to oxidizing gases (e.g., O2 in DAC) | (Xie et al., 2020b) |
| Tiron | 105.6 | 18 | 0.55e | High diffusion coefficient in aqueous media | Poor cycle performance owing to loss of alkalinity over multiple cycles | (Huang et al., 2019) |
| FMNf | 9.8 | 10 | 0.943 | Low cost redox-active species | Slow and consistent capacity fade over multiple cycles | (Xie et al., 2020a) |
| Membrane-Based Processes (pH swing) | ||||||
| KOH Absorption/K2CO3 Electrolysis | 290–350 | 100 | 0.908 | CO2 and H2 produced, can be coupled with utilization technology | Large overpotentials at high pH gradients | (Stucki et al., 1995) |
| Electro-dialysis membrane stacks coupled with synthesis of Methionine | 1109 | 30 | 0.864 | CO2 product recycled into amino acid synthesis | Difficult to integrate with existing power plant infrastructure | (Jiang et al., 2017) |
| Hydroxide exchange membrane cell | 350 | 20 | 0.95g | Can be coupled with fuel cell technology O2 presence improves efficiency |
Low CO2 product purity | (Eisaman et al., 2009; Landon and Kitchin, 2010; Matz et al., 2021) |
Energy consumptions (E) in kJ per mole of CO2 desorbed were theoretically estimated using the experimental cell voltages via the formulation where is the Faraday's constant, is the cell voltage required to release CO2 and is the electron utilization term. It was assumed that the electron utilization term is 0.9 although this may depend on the actual temperature, pressure and the active material concentration of the system.
Electrochemically mediated amine regeneration.
CO2- regenerative amine-based battery.
obtained from the computer model.
PCET = proton-coupled electron transfer.
in mol CO2 per mole e−.
riboflavin 5′-monophosphate sodium salt hydrate.
CO2 removal efficiency from 1000 sccm air containing 400 ppm CO2.
Theoretical energy created by the battery; the calculation did not account for the energy use associated with the low-pressure steam and the overpotentials.
Figure 1.
Summary of electrochemical approaches to CO2 separations discussed in this perspective with an outlook of possible integration with conversion processes through multifunctional electrolytes that can maintain high CO2 solubility at the electrode surface.
Electrochemical gas separation
Electrochemical gas separations involve the selective reduction of the target gas, followed by the transport of the gas in ion form from one electrode to the other. In the counter electrode, the ion gets selectively oxidized, releasing the gas. This process can be envisioned as pumping the target gas from the catholyte side to the anolyte side in an electrochemical cell with gas diffusion electrodes. In an alkaline solution, CO2, being an acidic gas, is absorbed from the feed gas in contact with the catholyte. It then reacts with the hydroxide ion to form bicarbonate (). Bicarbonate can then further react with another hydroxide to form a carbonate ion and water in pH's higher than 8 (). In an electric field, the anions move toward the anode where the pH is lowered owing to the oxygen evolution reaction (), thus reversing the catholyte reactions and releasing CO2. The large dependence of the solubility of CO2 on pH enables the reversible capture and release of CO2 even with a small pH swing between pH 6 and 8 (Datta et al., 2013).
pH-swing by electrodialysis and membranes
A variety of electrochemical pH-swing methodologies was devised to enable CO2 separation under ambient temperature and pressure, such as direct electrolysis (Mehmood et al., 2016; Datta et al., 2013), bipolar membrane electrodialysis (Eisaman et al., 2011a), and membrane capacitive deionization (Legrand et al., 2020; Sharifian et al., 2021). In 1996 (Xiao and Li, 1997), an electrodialysis system was reported for pH-swing CO2 separation by an electrochemical membrane module with polyamide soaked with aqueous potassium carbonate for air revitalization in confined spaces. Increased CO2 removal rate by achieved by increasing current density, which was obtained at high voltages (i.e., 3 V). However, at voltages above 1.23 V, water splitting occurs and the efficiency of CO2 removal is significantly reduced. In an effort to reduce voltage requirements, another study in 2010 (Landon and Kitchin, 2010), utilized an anion exchange membrane which reduced the ohmic resistance compared to the previous studies with thick separators. The anion exchange membrane transports CO2 in the form of bicarbonate. Bicarbonate forms as a result of CO2 reacting with the hydroxide ions that are released during oxygen reduction reaction (ORR) at the cathode (Equations 1 and 2). The reactions are reversed at the anode releasing CO2 and O2 (Equations 3 and 4).
Cathode:
| O2 + 2 H2O + 4 e− → 4 OH− | (Equation 1) |
| 4 CO2 + 4 OH− → 4HCO3− | (Equation 2) |
Anode:
| 4 HCO3− → 4 CO2 + 4 OH− | (Equation 3) |
| 4 OH− → O2 + 2 H2O + 4 e− | (Equation 4) |
Although these systems appear economically promising with consideration of potential improvements in cell design such as higher activity electrocatalysts and lower resistance membranes, they may not be ideal end-units in power plants to capture and sequester CO2, as the separated CO2 always contains a side gas such as O2. The US Department of Energy's target of a 90% CO2 capture rate with less than 35% cost of electricity requires such systems to operate at as small as 0.5 V with at least a 3.5 separation ratio of CO2:O2. Therefore, these systems need a valorization process to follow where O2 or other impurities are tolerable. Most recently, it was demonstrated (Matz et al., 2021) the removal of CO2 from the air upstream of a hydroxide exchange membrane fuel cell. In this electrochemically driven CO2 capture process associated with the fuel cell, high purity of CO2 could be produced by suppressing the generation of O2 at the anode by the flow of hydrogen gas (2 OH− + H2 → 2 H2O + 2 e−).
pH-swing by proton-coupled electron transfer reactions
Different from the previous approaches, proton carriers undergoing redox reactions are also utilized in pH-swing (Xu et al., 2010). It has been reported that the theoretical energy requirement of PCET is small compared to the general electrodialysis mechanism involving water-splitting (Renfrew et al., 2020). Quinone chemistry has been typically used for CO2 capture by PCET reactions (Watkins et al., 2015). In 2020, sodium 3,3′-(phenazine-2,3-diylbis(oxy))bis(propane-1-sulfonate) (DSPZ) was studied as a redox-active organic proton carrier (Jin et al., 2020). By utilizing the redox activity of 3,3'-(phenazine-2,3-diylbis(oxy))bis(propane-1-sulfonate) as a pH mediator, it was possible to capture CO2 by forming an alkaline solution via the reduction of the redox molecule and to release CO2 through acidification by re-oxidation. In another study, biomimetic phenazine derivatives were also used as the proton carrier (Figure 2A) (Xie et al., 2020b). The low solubility of organic proton carriers in aqueous solutions is one of the main obstacles that limit the CO2 capture and release rates. To solubilize the phenazine-based molecules, sulfonic group modification was conducted, and the product 7,8-dihydroxyphenazine-2-sulfonic acid exhibited excellent kinetics and cyclability. This sulfonic group modification has also been applied to quinones (i.e., tiron), taking advantage of the reduced quinone's sensitivity to protonation to make a pH mediator (Huang et al., 2019). Loss of alkalinity over time resulted in poor cycle performance for this system. Lower pH and lowered quinone concentration were shown to improve cyclability at the cost of lower CO2 capacity (Luo et al., 2021). The water-soluble form of vitamin B2, riboflavin 5′-monophosphate sodium salt, is the most recent example of a biomimetic proton carrier for pH swing by PCET, sporting an extremely low energy requirement for regeneration at 9.8 kJ/mol CO2 (Xie et al., 2020a).
Figure 2.
Proton-coupled electron transfer reactions for CO2 capture processes
(A–C) (A) Schematics of the system studied by Xie et al. that utilizes phenazine derivatives to drive the CO2 absorption by pH modulation in aqueous HCO3–/CO32−. Reprinted with permission from Ref (Xie et al., 2020b). Copyright 2020, Elsevier; (B) CO2 capture with K2CO3 as the absorbent (cation not shown for simplicity) and (C) a schematic diagram of the process. The proton deintercalation from the electrode shifts the CO2 (aq)/HCO3−(aq)/CO32−(aq) equilibrium toward CO2 formation at the anode and this is followed by gas separation through a flash tank. Subsequently, the proton intercalation in the cathode aids the regeneration of the absorbent.
It was also attempted to modulate pH using electrodes by applying proton intercalating MnO2 for CO2 capture (Rahimi et al., 2020a). MnO2 stored and released protons by the redox reaction of Mn(IV)/Mn(III) and the corresponding intercalation/deintercalation of H+. This work showed not only the effective CO2 separation via pH modulation with reversible MnO2 protonation, but also the practical feasibility of this system by the development of a thermodynamic model integrating a K2CO3-based CO2 absorption and electrochemically mediated pH swing (Figures 2B and 2C). Bench-scale experiments with this system were also demonstrated, focusing on the continuous desorption of CO2 from a K2CO3 solution (Rahimi et al., 2020b). Overall, the PCET process is still in the early stages of development, and the low stability of the organic proton carriers or redox electrodes is the current limiting issue. Further works to improve both the conductivity and the cyclability would advance this strategy into a viable and scalable CO2 separation system.
Electrochemically mediated CO2 separation
Slightly different than the electrochemical gas separations discussed above, electrochemically mediated CO2 separations involve electrochemically generated nucleophiles that act as CO2 carriers or electrochemically generated metal ions which disrupt the CO2 binding of the amine absorbers. The carrier molecules have no affinity to CO2 at their neutral state but bind with CO2 at their reduced state. The redox mechanism facilitates the pumping of CO2 from the cathode to the anode side as the carrier compound gets reduced at the cathode and oxidized at the anode. DuBois and coworkers (Bell et al., 1988) in 1988 examined electroactive species in quest of regenerable CO2 removal systems for the National Aeronautics and Space Administration's long space missions. Simpson et al. (Comeau Simpson and Durand, 1990) electrochemically studied several quinone species as redox-active carriers in an organic solvent. Quinones are natural compounds that undergo oxidation and reduction at potentials that depend on the pH of the media. Several studies to date have utilized quinones for energy harvesting and storage, with specific examples including their use in electrode design for Na-ion batteries (Gurkan et al., 2017) and in electrolyte formulation for organic redox flow batteries (Huskinson et al., 2014). In the case of electrochemically generated metal ions, the amine-CO2 bond is replaced with the interaction with the amine and the metal ions (i.e., cupric ions) thereby regenerating the amine and releasing CO2. We discuss CO2 separation by redox carriers studied to date and the metal electroplating/stripping to facilitate absorber regeneration later in discussion.
Electrochemically generated nucleophiles as CO2 carriers
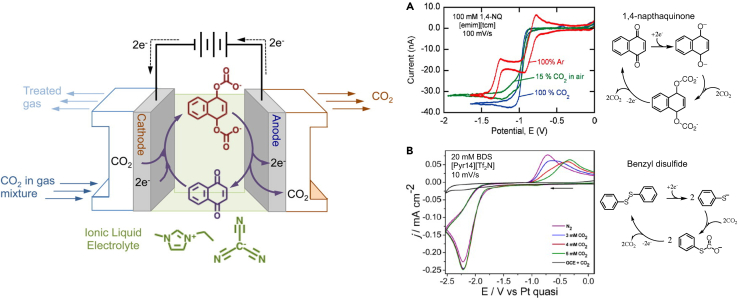
One common example of a CO2 carrier is quinones that contain carbonyl groups. Upon reduction, they yield nucleophiles that bind with CO2. The concept of electrochemically mediated CO2 separation with the use of a quinone species was demonstrated with an imidazolium hexafluorophosphate ionic liquid (IL) (Scovazzo et al., 2003). However, the solubility of the quinone in the IL was not significant which resulted in low separation capacity. Furthermore, significant overpotentials were present that led to low Faradaic efficiency. In 2015, it was demonstrated (Gurkan et al., 2015) that with the choice of substitution in quinone structure and an IL with high polarity, the net CO2 separation capacity can be improved while also improving the efficiency (Figure 3A). The reduction and the consecutive CO2-binding potential of 1,4-naphthaquinone (1,4-NQ) in an imidazolium tricyanomethanide ([emim][tcm]) IL translated to favorable energetics over the amine solvent regeneration by thermal-swing.
Figure 3.
Redox carriers for electrochemically mediated CO2 separation
Left: Schematic illustration of electrochemical CO2 separation using redox carriers. Figure reprinted with permission from Ref (Gurkan et al., 2015). Copyright 2015, American Chemical Society. Electrochemically generated nucleophiles bind with CO2 at the cathode and diffuse to the anode owing to the concentration gradient and finally release CO2 upon oxidation at the anode. Right: (A) 1,4-NQ reduction on Pt microelectrode and binding with CO2 in ionic liquid [emim][tcm]. Potentials with respect to internal Fc|Fc+ (Gurkan et al., 2015), (B) BDS reduction on glassy carbon and binding with CO2 in [Pyr14][Tf2N]; Singh et al. Reprinted with permission from Ref (Singh et al., 2017a). Copyright 2017, American Chemical Society.
In addition to quinones, sulfur-containing redox carriers have been developed by Buttry and colleagues (Singh et al., 2017a). Benzyldisulfide (BDS), upon reduction forms two benzothiolate anions, which can react with CO2 to form thiocarbonate. Thiolates are less basic than quinone nucleophiles and more stable in the presence of water. Although the electrochemically generated nucleophile is promising for CO2 scavenging, the oxidation potential of the thiocarbonate to disulfide and simultaneous CO2 release is far separated from the reduction potential with slow e-transfer rates (Rheinhardt et al., 2017). Furthermore, the highly negative reduction potential of BDS once again requires the use of a nonaqueous electrolyte. Figure 3B shows the microelectrode voltammetry of the CO2 redox carrier 1,4-NQ in [emim][tcm] from the study by (Gurkan et al., 2015). It is clearly seen that the reduction of quinone (Q) to the dianion (Q2−) via the radical anion formation (Q•–) under argon occurs by a 2-step single electron transfer reaction (QQ•–Q2-) whereas, under CO2 there is a single wave that corresponds to the two-electron reduction and complexation with CO2. This concerted mechanism at the same potential is enabled by the solvent media. The bottom panel of Figure 3C from a later study by (Singh et al., 2017a) shows the reduction of another CO2 redox carrier BDS in 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([Pyr14][Tf2N]) IL. In the case of BDS, the reaction is not reversible (too far apart reduction and oxidation); the electron transfer reaction involves a significant reorganization for the creation of the nucleophile. The S-S bond dissociation energy makes up a significant portion of the intrinsic energy barrier. Therefore, unless structural designs of diaryl sulfides that enable improved kinetics of electron injection are demonstrated, this approach will unlikely be practical in consideration of the energetics. Quinones, although having a more robust redox reaction, are not as stable especially in the presence of water.
Bipyridine-based compounds can also be reduced to become nucleophilic as demonstrated in 1994 (Ishida et al., 1994). As these compounds in their reduced form, such as quinones, are susceptible to protonation, an aprotic solvent is required. Later it was shown that owing to the stability of the radical anion CO2 adduct, a one-electron reduction step of bipyridine is possible (Ranjan et al., 2015). This leads to improved current efficiency of bipyridine over quinone systems. Monoalkylation of bipyridine reverts to a two-electron reduction to bind one CO2 (Singh et al., 2020). DAC applications of these compounds are unlikely owing to their high susceptibility to reaction with oxygen gas.
Another example of redox-active molecules that binds with CO2 at their reduced state is the vat dyes which are types of organic pigments (Apaydin et al., 2014). CO2 capture and release capacity of quinacridone was demonstrated. In the study, quinacridone was coated as a thin film over the electrode. Although the neutral form of the dye was not soluble in water, its reduced form was soluble in the aqueous electrolyte and the loss of active material from the electrode limited its cycling. Furthermore, the redox potential of quinacridone was outside of the electrochemical window of water and this resulted in significant hydrogen evolution. Therefore, this particular technology is in need of stable redox carriers with reversible and robust redox reactions in the electrolyte media.
Electrochemically generated metal ions
The concept of regenerating an amine absorber by breaking the CO2-amine complex through electrochemically generated metal ions has been demonstrated in a process referred to as electrochemically mediated amine regeneration (EMAR) (Figures 4A and 4B), which was developed by Hatton and colleagues in 2013 (Stern et al., 2013). The process uses ethylene diamine to capture CO2. Once the sorbent is saturated, it is pumped to a desorption chamber which is essentially an electrochemical cell that generates cupric ions from a Cu anode. Cu2+ competitively interacts with the amine group in the diamine, thus releasing CO2. Once CO2 is removed from the solution, the Cu2+ is plated out onto the cathode and the solution is ready to be used again. Thermodynamic analysis of this process indicates that the energy requirement for regeneration is approximately ∼35–40 kJ/mol CO2 (Wang et al., 2019, 2020), which is comparable to the energy requirement of thermal swing processes using monoethanolamine, MEA (40 kJ/mol CO2), and piperazine (38 kJ/mol CO2). The true advantage of the EMAR system is its “plug-and-play” nature. With only a need for electrical energy, the EMAR process can be installed without steam integration, significantly reducing installation costs and greatly expanding the range of applications for the device. The challenges in the EMAR system have been the volatility of the amine solvent and the gas accumulation on the electrode surface. These challenges have been addressed with some success by the electrolyte design and formulation. For example, an addition of a less volatile amine to the ethylenediamine (EDA) electrolyte could suppress the vapor pressure maintaining the CO2 capture efficiency, and an addition of an anionic surfactant, sodium dodecyl sulfate, could notably reduce the overall cell resistance (Rahimi et al., 2020c, 2020d). Very recently, a process similar to EMAR was demonstrated (Li et al., 2020), except the system produces electrical power from the energy of the absorption process that would be otherwise lost to heat dissipation. Although the majority of research has been on reducing the regeneration energy requirement, the heat that is produced as a result of the exothermic binding of CO2 to amine has not been utilized. In fact, to keep the absorber column temperature from rising, interstage cooling is applied. It is challenging to utilize such low-quality heat owing to the moderate temperatures of the absorber (i.e., 50°C). However, it was possible with a battery cell integration as shown in Figure 4C, referred to as the CO2-regenerative amine-based battery (CRAB), to produce 8.2 kJ per mol of CO2 (Figure 4D). As Cu is stripped in the anode, it binds with the amine in the CO2-lean solution. As this solution flows through the absorber next, it picks up CO2 then flows into the cathode where Cu electrodeposition takes place. Finally, the CO2-rich and Cu-lean solution gets replenished in terms of the amine in the desorber. This process unlike the previous approaches still relies on some level of integration with a power plant. Furthermore, the estimated thermodynamics path does not take into account the overpotentials that are likely present in practice owing to electrolyte resistance and electrode kinetics. CRAB is a very early-stage technology with a lot of moving parts that need further optimization in terms of the active components like the amine and the metal to address the material imbalance in the electrochemical cell and the redox kinetics.
Figure 4.
Electrochemically generated metal ions to regenerate amines in CO2 capture
(A) EMAR process schematics by Wang et al.64. The CO2-complexed amine solution is pumped from the absorber to the anode chamber of the regeneration unit where the electrochemically generated Cu2+ from the Cu anode coordinates with the amine. The formation of this complex desorbs CO2. Once Cu2+ is reduced at the cathode hence electroplates, amine is released and pumped back to the absorber column. Reprinted with permission from Ref.64 Copyright 2020, American Chemical Society.
(B) Thermodynamic cycle of the EMAR system where blue and red lines correspond to the constant CO2 partial pressure and constant amine loading, respectively. Reprinted with permission from Ref.64 Copyright 2020, American Chemical Society.
(C) Schematics of the Cu-mediated CRAB process by Li et al.28. CO2-amine reaction enthalpy is converted to electrical power by the electro dissolution and stripping of Cu. Reprinted with permission from Ref.28 Copyright 2020, Elsevier.
(D) Modeled thermodynamic pathway for the CRAB process with 2 M monoethanolamine at 25°C. Dashed lines indicate the endpoint of battery discharging in each cycle. For different Cu-loading, energy output per captured CO2 is indicated for three cases (the outmost black, red, and blue cycles). Reprinted with permission from Ref.28 Copyright 2020, Elsevier.
Heterogeneous electrodes for CO2 adsorption
The electrochemical separation of CO2 from post-combustion flue gas or air is also possible through the molecular engineering of selective electrodes. Compared to the approach of using a redox-active carrier in the electrolyte, electro-swing systems that involve redox electrodes have the advantage of not requiring a pumping system for the liquids (Voskian and Hatton, 2019). In an analogous fashion to liquid-phase redox reactions, quinone-based chemistries have been extensively applied to heterogeneous electrodes for CO2 capture and release (Q ↔ Q2− ↔ Q(CO2)2-).
Anthraquinone (AQ) was introduced for electrochemical CO2 capture in the form of thin-film electrodes (Wielend et al., 2018). The film electrodes, fabricated via an evaporation method, exhibited reversible capture and release of CO2 in aqueous solutions by forming an AQ-carbonate structure. Reversibility was confirmed by the observed stability in FTIR measurements upon fifty cycles of cyclic voltammetry experiments. CO2 capture from flue gas by the electro-swing system using AQ electrodes was also explored (Voskian and Hatton, 2019), using asymmetric redox systems. The system consisted of polyanthraquinone (PAQ)-carbon nanotube (CNT) composite cathodes, a polyvinylferrocene (PVF)–CNT composite anode, and an IL electrolyte. As shown in Figure 5A the reversible CO2 uptake and release take place at the PAQ-CNT electrode via carboxylation of quinone groups. This system achieved effective CO2 capture regardless of the CO2 feed concentration, and it exhibited exceptional recyclability in swing operation over 7000 cycles with high capacity and Faradaic efficiency retention. Moreover, this work demonstrated economic feasibility through a process model and energy analysis. However, the process still needs improvements in CO2 capacity and kinetics.
Figure 5.
Electro-swing-based CO2 separation approaches with heterogeneous electrodes modified with quinones
(A) PAQ-CNT composite cathode with PVF-CNT composite anode. Reprinted with permission from Ref (Voskian and Hatton, 2019). under CC BY-NC license.
(B) Controllable gas transport to the PAQ-CNT electrodes achieved by electrodeposition of metal in the gating membranes (M-layer as shown in the graphics). Reprinted with permission from Ref (Liu et al., 2020a). under CC BY-NC license.
Recently, a CO2 capture system using PAQ electrodes has been further improved (Liu et al., 2020a). The CO2 separation efficiency of the PAQ-CNT electrodes was demonstrated to be enhanced by introducing gating membranes to control the gas transport. As shown in Figure 5B, the gas transport was controllable by the electrodeposition of zinc on the conductive layer of the gating membranes, through which the feed or product streams could pass. Through this strategy, it was possible to modulate the gas composition effectively in both capture and release without pressurizing CO2. Another attempt to separate CO2 using quinone-based electrodes has recently been made by immobilizing quinone molecules to a support (Winter et al., 2021). A redox-responsive 2-aminoanthraquinone molecule was immobilized on core-shell particles, and the composite electrodes reversibly captured and released CO2. Therefore, for the development of CO2 separation using redox-active electrodes, new molecules based on the recent examples of 4,4′-bipyridine (Ranjan et al., 2015) and benzylthiolate (Singh et al., 2017a) could be investigated through polymerization or post-synthetic modification approaches.
Driving materials selection and discovery – role of quantum chemistry
High-throughput computational screening of several hundreds of materials to inform and aid experiments is now routine for applications ranging from energy storage to heterogeneous and electro-catalysis (Jain et al., 2016). When material performance is dictated primarily by the underlying electronic structure and/or nanoscale interactions with species of interest, quantum chemistry methods—which calculate the electronic structure by solving the approximate Schrodinger equation—are necessary to describe these characteristics. Density functional theory (DFT) is the most widely used quantum chemical modeling method for screening as it offers the most favorable cost-accuracy trade-off. This section highlights efforts toward using DFT calculations to calculate redox potentials of organic redox-active carriers, assess the feasibility of reversible CO2 binding, and solvent sensitivities of CO2 capture performance. There is vast untapped potential in the use of DFT-driven, high-throughput materials screening to accelerate the design and development of viable materials for electrochemical carbon capture and conversion.
Computational methods and frameworks
To select the appropriate level of theory for electrochemical systems of interest, one must rely on benchmarking studies (Neugebauer et al., 2020) because density functional approximations differ in their prediction accuracies for various material properties. A key shortcoming of DFT is spurious charge delocalization. While modeling interactions between radical/ionic redox species and CO2, for instance, this error can manifest in incorrect charge assignments to each of these interacting fragments. Constrained density functional theory (CDFT) overcomes this limitation as it enables the specification of excess charge and spin on each fragment and converges electronic structure calculations in a way that satisfies these constraints (Wu and Van Voorhis, 2006). Computational characterization methods such as energy decomposition analysis (EDA) break down interfragment interactions from electronic structure calculations into physically meaningful terms including dispersion, Pauli repulsions, electrostatics, polarization, and charge transfer (Hopffgarten and Frenking, 2012). Although rarely employed in probing characteristics of CO2 capture materials (Park et al., 2014), EDA is a powerful tool for characterizing redox-sorbent interactions, identifying dominant contributors to binding, and enabling systematic tuning of redox substituents. Solvent screening effects in CO2 electroreduction are also captured with the most recently developed version of EDA (Mao et al., 2021), to be used alongside implicit solvent models, such as polarizable continuum models (PCMs) and conductor-like screening models (COSMO), all of which treat the solvent as a dielectric continuum (Tomasi et al., 2005).
These DFT calculations based on implicit solvation models are combined with the harmonic oscillator approximation and thermochemical cycles to determine reduction potentials and their solvent sensitivity for redox-active species such as quinones (Bachman et al., 2014). Most computational studies focus on determining the favorability of CO2 binding to bipyridinium/pyrrolidinium compounds (Singh et al., 2020), benzyl thiolate (Singh et al., 2017a), and pyridinic nitrogen-doped CNT electrodes (Jiao et al., 2014). A notable exception is a work by Harris and Bushnell, who calculate reduction potentials as well as free energy changes associated with the complete cycle of capture and release for benzyl-disulfide, diselenide, and ditelluride compounds (Harris and Bushnell, 2019). This study finds that the most thermoneutral pathway, which is considered favorable for carbon capture, is offered by benzyl-ditelluride. Beyond these studies that probe CO2 binding to redox-active species, computational modeling can be employed to explore more complex aspects of reactive CO2 separations. For instance, CO2 affinities can be compared directly with those for potentially competing species present in air such as O2, H2O vapor, and acidic gases. DFT studies can also be extended to identifying possible electrode degradation pathways that are initiated by the binding of these competing species.
It is important to note that while implicit solvation models can be employed to generate reliable estimates of redox potentials, they are inadequate when solvent molecules interact chemically (and not just electrostatically) with the solute, typically in the form of hydrogen bonds (Kim et al., 2016). This can occur both in protic solvents as well as ILs, with the latter known to form hydrogen bonds with quinones in electrochemical CO2 capture (Gurkan et al., 2015). To capture the impact of these interactions on redox potentials and CO2 binding affinities, the explicit inclusion of solvent molecules becomes necessary. To the best of our knowledge, explicit models have yet to be applied to IL systems for CO2 separation, although studies are available that illustrate their use for the solvation of organic molecules by ILs (Payal et al., 2012).
High-throughput screening and discovery
DFT or CDFT-based descriptor-driven screening of properties of amines (such as pKa's) and amino-functionalized ILs is a promising means to guide the selection of viable starting candidates for developing CO2 capture methods from flue gas (Jing et al., 2018; Yang et al., 2017). The experimental studies described in this perspective motivate the future development of similar screening methods for redox-active quinones and benzyl chalcogenide derivatives for electrochemical DAC. Rapid screening and identification of viable CO2 carrier nucleophiles are possible by using DFT-based redox potentials and CO2 binding affinities (or for instance relative binding affinities of CO2 vis-à-vis O2) as descriptors. For heterogeneous electrodes, quinone carboxylation energies calculated from DFT can serve as descriptors that inform the choice of electrode material. High-throughput DFT calculations to facilitate such screening studies can now be streamlined using scientific workflows such as FireWorks and AiiDA (Jain et al., 2015; Huber et al., 2020).
With such descriptors as starting points for emerging genetic algorithms (Henault et al., 2020; Jensen, 2019) or reinforcement learning methods (Gómez-Bombarelli et al., 2018), the discovery of novel redox-active organic compounds with desired performance characteristics can be automated. Examples of the use of DFT-driven screening and discovery based on machine learning include the identification of organic chromophores for photovoltaic applications (Hachmann et al., 2011). Even though these studies can be limited in their focus on a few key parameters and uncertainties in calculated quantities, they provide means to rapidly identify the most promising candidates for desired applications and therefore accelerate experiments and further theoretical investigations. By combining DFT screening studies with recent advancements in machine learning, researchers can quickly gain insight into the chemical features that will promote efficient electrochemical DAC.
Outlook
The most widely studied electrochemical approach in reactive CO2 separations is electrolysis and bipolar membrane electrodialysis. In these systems, the persistent issue that has not been resolved is the overpotentials and specifically the ohmic losses which lead to electrical energy consumption that add to the energy penalty. More recent concepts that utilize redox-active carriers tend to require less energy than these approaches and the CO2 separation efficiency can be further improved by incorporating pH-swing mediators as in PCET systems. However, the stability issues in these systems have not been tackled for practical conditions (i.e., when oxygen and other volatiles are present). Technologies such as EMAR and CRAB present innovative ways to replace the thermal regeneration step of conventional systems while offering modularity and smaller footprint. Direct reporting of CO2 capture rates in addition to energy consumption can serve as another benchmark for future literature, as the rate of capture will significantly impact the implementation of these technologies. However, the improved design of electrolytes, electrodes, and the process is still necessary across all of the electrochemical approaches for CO2 capture to be economically feasible, as summarized later in discussion.
Electrode design
-
•
The continued development of redox-active molecules and redox-active materials for CO2 capture and release is needed to improve electrochemical capture systems. By utilizing the potential-responsive properties of redox materials in different configurations, it is feasible to switch the surface polarity with small energy input, and the selective interaction with specific molecules can be varied depending on their structural differences. For CO2 capture systems, a major challenge is to generate materials with high binding affinities, especially for dilute streams such as CO2 in DAC. At the same time, optimizing the regeneration efficiency and redox potentials to minimize the energetics of the potential swing step are also important considerations. Fundamentally, tailoring the electronic structure for optimal binding, thermodynamics, and even predictive control are future challenges envisioned in this field.
-
•
A second challenge is the presence of side reactions, which can lower current efficiency and cause degradation of the electrode. These can stem from factors such as competing acid gases, water, or oxygen content. Therefore, continued chemical design of both homogeneous and heterogeneous redox-responsive materials is expected to increase their chemical and electrochemical stability in the presence of various competing species, while still enhancing binding affinity toward CO2 across a range of concentrations. This will be a key goal in future materials design.
-
•
The creation of new hierarchical materials with electrochemically-responsive properties and high porosity can be envisioned. A major challenge with polymeric systems is their low surface area compared to ordered porous materials. Morphology and structural design, either through organic synthesis or composite material processing can be key to improving the performance of electrodes for electrochemical adsorption or permeation of CO2.
Electrochemical system design
-
•
It is desirable to reduce the overall cell resistance in the electrochemical process in order to lower the energy requirement. The most sensitive and costly component in this regard is the ion-exchange membrane which controls the mass transfer. Therefore, to improve the overall efficiency, it would be essential to develop thin, yet robust membranes that prevent co-ion crossover. Moreover, it will be important to control the gas bubbles inside the cell. The presence of gas bubbles can lead to blocking of electroactive surface area and an increase in resistance. In addition to interfacial engineering at the electrode/electrolyte to prevent bubble buildup, future studies should consider optimization of operational parameters such as the pressure of CO2 (Eisaman et al., 2011b).
-
•
The electrochemical engineering of the CO2 capture device is essential for efficient electrochemical gas capture systems. In order to enable continuous gas reaction in a flow cell configuration, gas-diffusion electrodes (GDEs) are used to facilitate the rapid access of the gas to the electrode (Pan and Yang, 2020; Higgins et al., 2019). Therefore, the development of more efficient GDEs capable of enlarging the gas-electrode contact area, along with the introduction of the hydrophobic gas diffusion layer and the formation of a composite with the redox material, will be the key for improving the performance of electrochemical CO2 separation, as well as for scale-up of the system.
-
•
In the as-described processes coupled with the amine sorbents or wet scrubbers, liquid formulation, and handling play a significant role in terms of both operational cost and sorption efficiency. Optimization of parameters such as volatility, viscosity, and composition will be crucial for the efficiency of the combined system.
-
•
Direct flue gas conversion could be a long-term carbon dioxide utilization strategy. Although the fabrication of electrodes for electrocatalytic CO2 conversion to high-value products has been extensively studied, low selectivity remains a significant issue. Furthermore, concentrated CO2 is required in many cases. The low concentration of CO2 and presence of impurities (Ko et al., 2020; Luc et al., 2019) in dilute feed gas streams present a barrier for direct conversion at this time. In order to solve these challenges, through improvements of catalytic electrode materials or gas introduction systems, notable results have been recently reported (Xu et al., 2020; Maina et al., 2021; Zhao et al., 2020). Through this process intensification that enables direct conversion from flue gas, in combination with the electrochemical CO2 capture strategy, significant cost reduction, and efficiency improvement can be envisioned.
Electrolyte design
-
•
Functional electrolytes with high loading of alcohol and amine moieties have superior CO2 solubilities compared to aqueous systems (Wang et al., 2010). Therefore, they can better separate CO2 from dilute mixtures and maintain CO2 at the electrode interface. However, for electrochemically mediated separations with redox carriers, very high CO2 solubilities are not desired, as this can result in CO2 transport in the opposite direction of the redox carrier transport; their concentration gradient profiles would work against each other. The solubilities of the redox carrier and CO2 should be optimized for a net CO2 separation.
-
•
It is important to examine the redox potentials and redox reversibility of the redox carriers in the electrolyte of interest as these potentials are highly influenced by the solvation media. Ideally, the reduction of the carrier and the oxidation of the generated nucleophile should be closer than 60 mV for the process to be reversible and robust.
-
•
It is desirable for the electrolytes to have low volatility to maintain adequate wetting of the electrodes and suppress solvent loss. Electrolytes such as ILs and deep eutectic solvents (DESs) (Garcia et al., 2015) have high salt concentrations and low volatility as well as tunable physical properties. Therefore they are potential multifunctional electrolytes for integrated capture and conversion processes. However, these electrolytes generally suffer from high viscosities and low conductivities which lead to transport rates that are much slower than desired for practical purposes. Formulations of these electrolytes with aqueous systems could be interesting.
-
•
The double-layer structure of the electrolyte, especially for more complex electrolytes must be investigated further, as the effects of the electrode-electrolyte interfacial structure and surface adsorbed species may be significant for the electron transfer kinetics which would ultimately control the CO2 separation or conversion rate.
Acknowledgments
The authors would like to acknowledge funding from Research Corporation for Science Advancement (award number: 27704) through the Scialog: Negative Emissions Science.
Author contributions
All authors jointly conceptualized the paper and contributed to the writing of the manuscript. B.G. led the discussions on the electrochemical and electrochemically mediated separations with a focus on the electrolyte. X.S. led the discussions with a focus on the electrodes and process design. A.K. constructed the review table and discussed the very early stage technologies. S.M.S. led the discussions on the role of quantum chemistry. A.R.K. and K.J.K. contributed to the discussions of the calculations with the density functional theory.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
References
- Apaydin D.H., Głowacki E.D., Portenkirchner E., Sariciftci N.S. Direct electrochemical capture and release of carbon dioxide using an industrial organic pigment: quinacridone. Angew. Chem. Int. Ed. 2014;53:6819–6822. doi: 10.1002/anie.201403618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman J.E., Curtiss L.A., Assary R.S. Investigation of the redox chemistry of anthraquinone derivatives using density functional theory. J. Phys. Chem. A. 2014;118:8852–8860. doi: 10.1021/jp5060777. [DOI] [PubMed] [Google Scholar]
- Bali S., Sakwa-Novak M.A., Jones C.W. Potassium incorporated alumina based CO2 capture sorbents: comparison with supported amine sorbents under ultra-dilute capture conditions. Colloids Surf. A: Physicochem. Eng. Aspect. 2015;486:78–85. [Google Scholar]
- Bell W.L., Miedaner A., Smart J.C., Dubois D.L., Verostko C.E. Synthesis and evaluation of electroactive CO₂ carriers. SAE Trans. 1988;97:544–552. [Google Scholar]
- Blum H.A., Stutzman L.F., Dodds W.S. Gas absorption - absorption of carbon dioxide from air by sodium and potassium hydroxides. Ind. Eng. Chem. 1952;44:2969–2974. [Google Scholar]
- Comeau Simpson T., Durand R.R. Reactivity of carbon dioxide with quinones. Electrochim. Acta. 1990;35:1399–1403. [Google Scholar]
- Datta S., Henry M.P., Lin Y.J., Fracaro A.T., Millard C.S., Snyder S.W., Stiles R.L., Shah J., Yuan J., Wesoloski L., et al. Electrochemical CO2 capture using resin-wafer electrodeionization. Ind. Eng. Chem. Res. 2013;52:15177–15186. [Google Scholar]
- Didas S.A., Choi S., Chaikittisilp W., Jones C.W. Amine–oxide hybrid materials for CO2 capture from ambient air. Acc. Chem. Res. 2015;48:2680–2687. doi: 10.1021/acs.accounts.5b00284. [DOI] [PubMed] [Google Scholar]
- Dlugokencky E., Tans P. Trends in CO2 [Online]. NOAA.gov: NOAA/GML. 2021. gml.noaa.gov/ccgg/trends/
- Eisaman M.D., Schwartz D.E., Amic S., Larner D., Zesch J., Torres F., Littau K. Energy-efficient electrochemical CO2 capture from the atmosphere. Clean. Technol. 2009:175–178. [Google Scholar]
- Eisaman M.D., Alvarado L., Larner D., Wang P., Garg B., Littau K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011;4:1319–1328. [Google Scholar]
- Eisaman M.D., Alvarado L., Larner D., Wang P., Littau K.A. CO2 desorption using high-pressure bipolar membrane electrodialysis. Energy Environ. Sci. 2011;4:4031–4037. [Google Scholar]
- El Hadri N., Quang D.V., Goetheer E.L.V., Abu Zahra M.R.M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy. 2017;185:1433–1449. [Google Scholar]
- Garcia G., Aparicio S., Ullah R., Atilhan M. Deep eutectic solvents: physicochemical properties and gas separation applications. Energy Fuels. 2015;29:2616–2644. [Google Scholar]
- Gurkan B., Simeon F., Hatton T.A. Quinone reduction in ionic liquids for electrochemical CO2 separation. ACS Sustain. Chem. Eng. 2015;3:1394–1405. [Google Scholar]
- Gurkan B., Qiang Z., Chen Y.-M., Zhu Y., Vogt B.D. Enhanced cycle performance of quinone-based anodes for sodium ion batteries by attachment to ordered mesoporous carbon and use of ionic liquid electrolyte. J. Electrochem. Soc. 2017;164:H5093–H5099. [Google Scholar]
- Gómez-Bombarelli R., Wei J.N., Duvenaud D., Hernández-Lobato J.M., Sánchez-Lengeling B., Sheberla D., Aguilera-Iparraguirre J., Hirzel T.D., Adams R.P., Aspuru-Guzik A. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 2018;4:268–276. doi: 10.1021/acscentsci.7b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann J., Olivares-Amaya R., Atahan-Evrenk S., Amador-Bedolla C., Sánchez-Carrera R.S., Gold-Parker A., Vogt L., Brockway A.M., Aspuru-Guzik A. The harvard clean energy project: large-scale computational screening and design of organic photovoltaics on the world community grid. J. Phys. Chem. Lett. 2011;2:2241–2251. [Google Scholar]
- Harris D., Bushnell E. Density functional theory study of the capture and release of carbon dioxide by Benzyl–Disulfide, −Diselenide, and −Ditelluride. J. Phys. Chem. A. 2019;123:3383–3388. doi: 10.1021/acs.jpca.9b01862. [DOI] [PubMed] [Google Scholar]
- Henault E.S., Rasmussen M.H., Jensen J.H. Chemical space exploration: how genetic algorithms find the needle in the haystack. Peerj Phys. Chem. 2020;2:e11. [Google Scholar]
- Higgins D., Hahn C., Xiang C., Jaramillo T.F., Weber A.Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 2019;4:317–324. [Google Scholar]
- Hopffgarten M.V., Frenking G. Energy decomposition analysis. Wires Comput. Mol. Sci. 2012;2:43–62. [Google Scholar]
- Huang C., Liu C., Wu K., Yue H., Tang S., Lu H., Liang B. CO2 capture from flue gas using an electrochemically reversible hydroquinone/quinone solution. Energy Fuels. 2019;33:3380–3389. [Google Scholar]
- Huber S.P., Zoupanos S., Uhrin M., Talirz L., Kahle L., Häuselmann R., Gresch D., Müller T., Yakutovich A.V., Andersen C.W., et al. AiiDA 1.0, a scalable computational infrastructure for automated reproducible workflows and data provenance. Sci. Data. 2020;7:300. doi: 10.1038/s41597-020-00638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson B., Marshak M.P., Suh C., Er S., Gerhardt M.R., Galvin C.J., Chen X., Aspuru-Guzik A., Gordon R.G., Aziz M.J. A metal-free organic–inorganic aqueous flow battery. Nature. 2014;505:195–198. doi: 10.1038/nature12909. [DOI] [PubMed] [Google Scholar]
- IPCC . In: Climate Change 2021: The Physical Science Basis. Masson-Delmotte V., Zhai P., Pirani A., Connors S.L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M.I., et al., editors. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; 2021. Summary for Policymakers. In: Masson-Delmotte. [Google Scholar]
- Ishida H., Ohba T., Yamaguchi T., Ohkubo K. Interaction between CO2 and electrochemically reduced species of N-propyl-4,4′-bipyridinium Cation. Chem. Lett. 1994;23:905–908. [Google Scholar]
- Jain A., Ong S.P., Chen W., Medasani B., Qu X., Kocher M., Brafman M., Petretto G., Rignanese G.-M., Hautier G., et al. FireWorks: a dynamic workflow system designed for high-throughput applications. Concurrency Comput. Pract. Ex. 2015;27:5037–5059. [Google Scholar]
- Jain A., Shin Y., Persson K.A. Computational predictions of energy materials using density functional theory. Nat. Rev. Mater. 2016;1:15004. [Google Scholar]
- Jensen J.H. A graph-based genetic algorithm and generative model/Monte Carlo tree search for the exploration of chemical space. Chem. Sci. 2019;10:3567–3572. doi: 10.1039/c8sc05372c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Zhang Y., Feng H., Wang Q., Wang Y., Xu T. Simultaneous CO2 capture and amino acid production using bipolar membrane electrodialysis (BMED) J. Membr. Sci. 2017;542:264–271. [Google Scholar]
- Jiao Y., Zheng Y., Smith S.C., Du A., Zhu Z. Electrocatalytically switchable CO2 capture: first principle computational exploration of carbon nanotubes with pyridinic nitrogen. ChemSusChem. 2014;7:435–441. doi: 10.1002/cssc.201300624. [DOI] [PubMed] [Google Scholar]
- Jin S., Wu M., Gordon R.G., Aziz M.J., Kwabi D.G. pH swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. Energy Environ. Sci. 2020;13:3706–3722. [Google Scholar]
- Jing G., Qian Y., Zhou X., Lv B., Zhou Z. Designing and screening of multi-amino-functionalized ionic liquid solution for CO2 capture by quantum chemical simulation. ACS Sustain. Chem. Eng. 2018;6:1182–1191. [Google Scholar]
- Kammermeyer K. In: Atmosphere in Space Cabins and Closed Environments. Kammermeyer K., editor. Springer US; 1966. Space technology — Today’s challenge to science. [Google Scholar]
- Kim I., Svendsen H.F. Heat of absorption of carbon dioxide (CO2) in monoethanolamine (MEA) and 2-(Aminoethyl)ethanolamine (AEEA) solutions. Ind. Eng. Chem. Res. 2007;46:5803–5809. [Google Scholar]
- Kim H., Goodson T., Zimmerman P.M. Achieving accurate reduction potential predictions for Anthraquinones in water and aprotic solvents: effects of inter- and intramolecular H-Bonding and ion pairing. J. Phys. Chem. C. 2016;120:22235–22247. [Google Scholar]
- Knox J.C. 48th International Conference on Environmental Systems Proceedings. July 2018. Development of carbon dioxide removal systems for NASA’s deep space human exploration missions 2017-2018. [Google Scholar]
- Ko B.H., Hasa B., Shin H., Jeng E., Overa S., Chen W., Jiao F. The impact of nitrogen oxides on electrochemical carbon dioxide reduction. Nat. Commun. 2020;11:5856. doi: 10.1038/s41467-020-19731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner K.S., Grimes P., Ziock H.J. Coal and Slurry Technology Association, Washington, DC (US); Los Alamos National Lab., NM (US); 1999. Carbon Dioxide Extraction from Air: Is it an Option? [Google Scholar]
- Lackner K.S., Kedia S., Carlson B., Page R., Choodamani V., Wright A. 2020. System and Method for Passive Collection of Atmospheric Carbon Dioxide. USA patent application 16/975110. [Google Scholar]
- Landon J., Kitchin J.R. Electrochemical concentration of carbon dioxide from an oxygen/carbon dioxide containing gas stream. J. Electrochem. Soc. 2010;157:B1149. [Google Scholar]
- Legrand L., Shu Q., Tedesco M., Dykstra J.E., Hamelers H.V.M. Role of ion exchange membranes and capacitive electrodes in membrane capacitive deionization (MCDI) for CO2 capture. J. Colloid Interf. Sci. 2020;564:478–490. doi: 10.1016/j.jcis.2019.12.039. [DOI] [PubMed] [Google Scholar]
- Li K., Feron P.H.M., Jones T.W., Jiang K., Bennett R.D., Hollenkamp A.F. Energy harvesting from amine-based CO2 capture: proof-of-concept based on mono-ethanolamine. Fuel. 2020;263:116661. [Google Scholar]
- Liu Y., Chow C.-M., Phillips K.R., Wang M., Voskian S., Hatton T.A. Electrochemically mediated gating membrane with dynamically controllable gas transport. Sci. Adv. 2020;6:eabc1741. doi: 10.1126/sciadv.abc1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.Y., Ye H.Z., Diederichsen K.M., Van Voorhis T., Hatton T.A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 2020;11:11. doi: 10.1038/s41467-020-16150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc W., Ko B.H., Kattel S., Li S., Su D., Chen J.G., Jiao F. SO2-induced selectivity change in CO2 electroreduction. J. Am. Chem. Soc. 2019;141:9902–9909. doi: 10.1021/jacs.9b03215. [DOI] [PubMed] [Google Scholar]
- Luo L., Hou L., Liu Y., Wu K., Zhu Y., Lu H., Liang B. Regeneration of Na2Q in an electrochemical CO2 capture system. Energy Fuels. 2021;35:12260–12269. [Google Scholar]
- Maina J.W., Pringle J.M., Razal J.M., Nunes S., Vega L., Gallucci F., Dumée L.F. Strategies for integrated capture and conversion of CO2 from dilute flue gases and the atmosphere. ChemSusChem. 2021;14:1805–1820. doi: 10.1002/cssc.202100010. [DOI] [PubMed] [Google Scholar]
- Mao Y., Loipersberger M., Kron K.J., Derrick J.S., Chang C.J., Sharada S.M., Head-Gordon M. Consistent inclusion of continuum solvation in energy decomposition analysis: theory and application to molecular CO2 reduction catalysts. Chem. Sci. 2021;12:1398–1414. doi: 10.1039/d0sc05327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz S., Setzler B.P., Weiss C.M., Shi L., Gottesfeld S., Yan Y. Demonstration of electrochemically-driven CO2 separation using hydroxide exchange membranes. J. Electrochem. Soc. 2021;168:014501. [Google Scholar]
- McGurk S.J., Martín C.F., Brandani S., Sweatman M.B., Fan X. Microwave swing regeneration of aqueous monoethanolamine for post-combustion CO2 capture. Appl. Energy. 2017;192:126–133. [Google Scholar]
- Mehmood A., Iqbal M.I., Lee J.-Y., Hwang J., Jung K.-D., Ha H.Y. A novel high performance configuration of electrochemical cell to produce alkali for sequestration of carbon dioxide. Electrochim. Acta. 2016;219:655–663. [Google Scholar]
- National Academies of Sciences E., Medicine, Division On E., Life S., Ocean Studies B., Board On Chemical S., Technology, Board On Earth S., Resources, Board On A., Natural R., Board On E., Environmental S., et al. National Academies Press (US); 2018. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Copyright 2019 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- Neugebauer H., Bohle F., Bursch M., Hansen A., Grimme S. Benchmark study of electrochemical redox potentials calculated with semiempirical and DFT methods. J. Phys. Chem. A. 2020;124:7166–7176. doi: 10.1021/acs.jpca.0c05052. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Hilliard M., Rochelle G.T. Amine volatility in CO2 capture. Int. J. Greenh. Gas Control. 2010;4:707–715. [Google Scholar]
- Pan F., Yang Y. Designing CO2 reduction electrode materials by morphology and interface engineering. Energy Environ. Sci. 2020;13:2275–2309. [Google Scholar]
- Park J.Y., Lee Y.S., Jung Y. Springer International Publishing; 2014. Local Intermolecular Interactions for Selective CO2 Capture by Zeolitic Imidazole Frameworks: Energy Decomposition Analysis; pp. 277–288. [Google Scholar]
- Payal R.S., Bharath R., Periyasamy G., Balasubramanian S. Density functional theory investigations on the structure and dissolution mechanisms for cellobiose and xylan in an ionic liquid: gas phase and cluster calculations. J. Phys. Chem. B. 2012;116:833–840. doi: 10.1021/jp207989w. [DOI] [PubMed] [Google Scholar]
- Rahimi M., Catalini G., Hariharan S., Wang M., Puccini M., Hatton T.A. Carbon dioxide capture using an electrochemically driven proton concentration process. Cell Rep. Phys. Sci. 2020;1:100033. [Google Scholar]
- Rahimi M., Catalini G., Puccini M., Hatton T.A. Bench-scale demonstration of CO2 capture with an electrochemically driven proton concentration process. RSC Adv. 2020;10:16832–16843. doi: 10.1039/d0ra02450c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi M., Diederichsen K.M., Ozbek N., Wang M., Choi W., Hatton T.A. An electrochemically mediated amine regeneration process with a mixed absorbent for postcombustion CO2 capture. Environ. Sci. Technol. 2020;54:8999–9007. doi: 10.1021/acs.est.0c02595. [DOI] [PubMed] [Google Scholar]
- Rahimi M., Zucchelli F., Puccini M., Alan Hatton T. Improved CO2 capture performance of electrochemically mediated amine regeneration processes with ionic surfactant additives. ACS Appl. Energy Mater. 2020;3:10823–10830. [Google Scholar]
- Ranjan R., Olson J., Singh P., Lorance E.D., Buttry D.A., Gould I.R. Reversible electrochemical Trapping of carbon dioxide using 4,4′-bipyridine that does not require thermal activation. J. Phys. Chem. Lett. 2015;6:4943–4946. doi: 10.1021/acs.jpclett.5b02220. [DOI] [PubMed] [Google Scholar]
- Renfrew S.E., Starr D.E., Strasser P. Electrochemical approaches toward CO2Capture and concentration. ACS Catal. 2020;10:13058–13074. [Google Scholar]
- Rheinhardt J.H., Singh P., Tarakeshwar P., Buttry D.A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2017;2:454–461. doi: 10.1021/jacs.6b10806. [DOI] [PubMed] [Google Scholar]
- Sanz-Pérez E.S., Murdock C.R., Didas S.A., Jones C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016;116:11840–11876. doi: 10.1021/acs.chemrev.6b00173. [DOI] [PubMed] [Google Scholar]
- Scovazzo P., Poshusta J., Dubois D., Koval C., Noble R. Electrochemical separation and concentration of <1% carbon dioxide from nitrogen. J. Electrochem. Soc. 2003;150:D91. [Google Scholar]
- Sevanthi R., Irin F., Parviz D., Andrew Jackson W., Green M.J. Electrical current stimulated desorption of carbon dioxide adsorbed on graphene based structures. RSC Adv. 2016;6:43401–43407. [Google Scholar]
- Sharifian R., Wagterveld R.M., Digdaya I.A., Xiang C., Vermaas D.A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021;14:781–814. [Google Scholar]
- Shi X., Xiao H., Azarabadi H., Song J., Wu X., Chen X., Lackner K.S. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020;59:6984–7006. doi: 10.1002/anie.201906756. [DOI] [PubMed] [Google Scholar]
- Shi X., Xiao H., Kanamori K., Yonezu A., Lackner K.S., Chen X. Moisture-driven CO2 sorbents. Joule. 2020;4:1823–1837. [Google Scholar]
- Singh P., Rheinhardt J.H., Olson J.Z., Tarakeshwar P., Mujica V., Buttry D.A. Electrochemical capture and release of carbon dioxide using a disulfide–thiocarbonate redox cycle. J. Am. Chem. Soc. 2017;139:1033–1036. doi: 10.1021/jacs.6b10806. [DOI] [PubMed] [Google Scholar]
- Singh S., Stechel E.B., Buttry D.A. Transient modeling of electrochemically assisted CO2 capture and release. J. Electroanal. Chem. 2017;799:156–166. [Google Scholar]
- Singh P., Tarakeshwar P., Buttry D.A. Experimental, simulation, and computational study of the interaction of reduced forms of N-Methyl-4,4’-Bipyridinium with CO2. ChemElectroChem. 2020;7:469–475. [Google Scholar]
- Stern M.C., Simeon F., Herzog H., Hatton T.A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 2013;6:2505–2517. [Google Scholar]
- Stolaroff J.K., Keith D.W., Lowry G.V. Carbon dioxide capture from atmospheric air using sodium hydroxide spray. Environ. Sci. Technol. 2008;42:2728–2735. doi: 10.1021/es702607w. [DOI] [PubMed] [Google Scholar]
- Stucki S., Schuler A., Constantinescu M. Coupled CO2 recovery from the atmosphere and water electrolysis: feasibility of a new process for hydrogen storage. Int. J. Hydrogen Energy. 1995;20:653–663. [Google Scholar]
- Sujan A.R., Pang S.H., Zhu G., Jones C.W., Lively R.P. Direct CO2 capture from air using poly(ethylenimine)-loaded polymer/silica fiber sorbents. ACS Sustain. Chem. Eng. 2019;7:5264–5273. [Google Scholar]
- Tans P., Dlugokencky E., Miller B. The power of greenhouse gases. 2020. https://gml.noaa.gov/ccgg/ghgpower/ NOAA Global Monitoring Laboratory.
- Tomasi J., Mennucci B., Cammi R. Quantum mechanical continuum solvation models. Chem. Rev. 2005;105:2999–3094. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- Tsubaki S., Furusawa K., Yamada H., Kato T., Higashii T., Fujii S., Wada Y. Insights into the dielectric-heating-enhanced regeneration of CO2-rich aqueous amine solutions. ACS Sustain. Chem. Eng. 2020;8:13593–13599. [Google Scholar]
- Voskian S., Hatton T.A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 2019;12:3530–3547. [Google Scholar]
- Wang C., Mahurin S.M., Luo H., Baker G.A., Li H., Dai S. Reversible and robust CO2 capture by equimolar task-specific ionic liquid–superbase mixtures. Green. Chem. 2010;12:870–874. [Google Scholar]
- Wang T., Lackner K.S., Wright A. Moisture swing sorbent for carbon dioxide capture from ambient air. Environ. Sci. Technol. 2011;45:6670–6675. doi: 10.1021/es201180v. [DOI] [PubMed] [Google Scholar]
- Wang M., Hariharan S., Shaw R.A., Hatton T.A. Energetics of electrochemically mediated amine regeneration process for flue gas CO2 capture. Int. J. Greenh. Gas Control. 2019;82:48–58. [Google Scholar]
- Wang M., Herzog H.J., Hatton T.A. CO2 capture using electrochemically mediated amine regeneration. Ind. Eng. Chem. Res. 2020;59:7087–7096. [Google Scholar]
- Watkins J.D., Siefert N.S., Zhou X., Myers C.R., Kitchin J.R., Hopkinson D.P., NULWALA H.B. Redox-mediated separation of carbon dioxide from flue gas. Energy Fuels. 2015;29:7508–7515. [Google Scholar]
- Wielend D., Apaydin D.H., Sariciftci N.S. Anthraquinone thin-film electrodes for reversible CO2 capture and release. J. Mater. Chem. A. 2018;6:15095–15101. [Google Scholar]
- Winter T., Bitsch M., Mller F., Voskian S., Hatton T.A., Jacobs K., Presser V., Gallei M. Redox-responsive 2-aminoanthraquinone core–shell particles for structural colors and carbon capture. ACS Appl. Polym. Mater. 2021;3:4651–4660. [Google Scholar]
- Wu Q., Van Voorhis T. Constrained density functional theory and its application in long-range electron transfer. J. Chem. Theor. Comput. 2006;2:765–774. doi: 10.1021/ct0503163. [DOI] [PubMed] [Google Scholar]
- Xiao S.Q., Li K.W. On the use of an electrochemical membrane module for removal of CO2 from a breathing gas mixture. Chem. Eng. Res. Des. 1997;75:438–446. [Google Scholar]
- Xie H., Jiang W., Liu T., Wu Y., Wang Y., Chen B., Niu D., Liang B. Low-energy electrochemical carbon dioxide capture based on a biological redox proton carrier. Cell Rep. Phys. Sci. 2020;1:100046. [Google Scholar]
- Xie H., Wu Y., Liu T., Wang F., Chen B., Liang B. Low-energy-consumption electrochemical CO2 capture driven by biomimetic phenazine derivatives redox medium. Appl. Energy. 2020;259:114119. [Google Scholar]
- Xu Y., Wen Y.-H., Cheng J., Cao G.-P., Yang Y.-S. A study of tiron in aqueous solutions for redox flow battery application. Electrochim. Acta. 2010;55:715–720. [Google Scholar]
- Xu Y., Edwards J.P., Zhong J., O'brien C.P., Gabardo C.M., Mccallum C., Li J., Dinh C.T., Sargent E.H., Sinton D. Oxygen-tolerant electroproduction of C2 products from simulated flue gas. Energy Environ. Sci. 2020;13:554–561. [Google Scholar]
- Yang X., Rees R.J., Conway W., Puxty G., Yang Q., Winkler D.A. Computational modeling and simulation of CO2 capture by aqueous amines. Chem. Rev. 2017;117:9524–9593. doi: 10.1021/acs.chemrev.6b00662. [DOI] [PubMed] [Google Scholar]
- Yu C.H., Huang C.H., Tan C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012;12:745–769. [Google Scholar]
- Zhao Y., Wang T., Wang Y., Hao R., Hui W. Simultaneous absorption and hydrogenation of CO2 from flue gas by KBH4 catalyzed by nickel nanoparticles supported on TiO2. Chem. Eng. J. 2020;380:122523. [Google Scholar]