Abstract
A case of the successful reconstruction of an extensive chest wall defect combined with a ventral hernia in a patient after multimodality treatment of breast cancer complicated by sternal and costal osteomyelitis is presented. To recover the chest mechanics, with emphasis on the supporting function, and to repair the hernial defect, customized reinforced “sandwich” TiNi rib endografts and knitted TiNi surgical mesh were used. A five-year follow-up indicated no recurrence of osteomyelitis or ventral hernia, and no failure/migration of the implants or instability of the thorax. Excellent clinical and functional outcomes were achieved pursuant to the Enneking score.
Keywords: Sternal and costal osteomyelitis, Chest wall reconstruction, Ventral hernia, TiNi implant
1. Introduction
Osteochondral chest wall defect plasty after surgical treatment of sternal and costal osteomyelitis is a topical challenge indeed in surgery. Extensive excision sternal and costal defects are often combined with rib cage instability and thoracoabdominal hernia, and this combination results in physiological and psychosocial disability in the patient [1]. The use of solid and mesh implants to reconstruct the thoracic cage deserves particular attention and has come to the forefront. These reconstructive techniques may reduce the trauma and duration of surgical interventions and help to standardize them. However, in the case of very large defects, there have been reports of metallic osteosynthesis failure and a large number of recurrences of osteomyelitis or herniated tissues evident in follow-up checks [2]. In the context of a reinforcing material, customized bioadaptive TiNi-based implants are quite promising. There have already been reports of the successful use of such an approach [[3], [4], [5]].
We report a case of the successful reconstruction of an extensive anterior chest wall defect aggravated with a ventral hernia in a former breast cancer patient who suffered from chronic sternal and costal osteomyelitis.
2. Case report
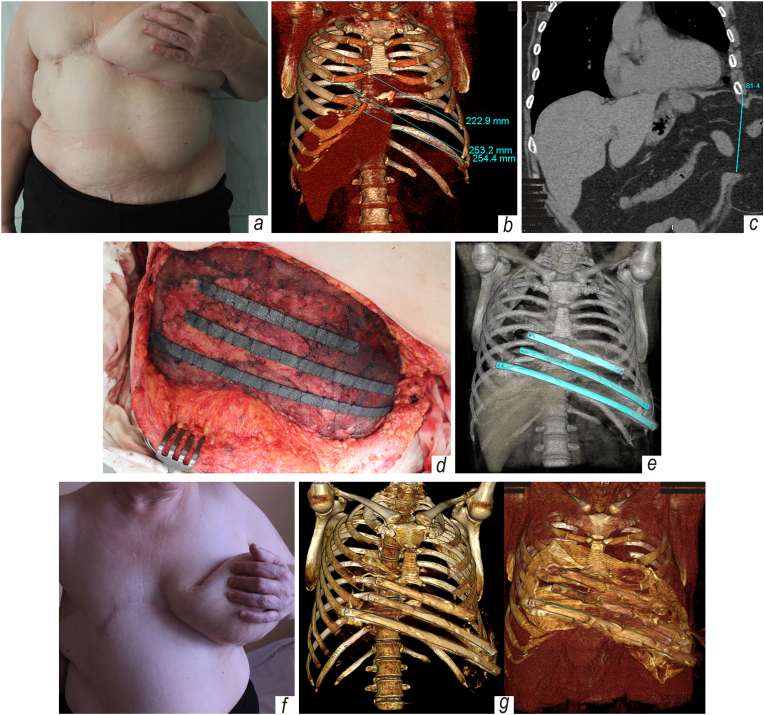
Patient, a 53-y.o. obese Caucasian female living in a rural area, was admitted to our department of thoracic surgery and treated in Nov 2014 for a very large post-excision chest wall defect combined with instability of the rib cage and a reducible ventral hernia, all of which resulted from failed surgical procedures in the treatment of chronic sternal and costal osteomyelitis (Fig. 1a).
Fig. 1.
Huge post-excision chest wall defect complicated by ventral hernia after right-side mastectomy and failed surgical care of sternal and costal osteomyelitis: a – appearance of the patient upon her admission, where lateral herniated tissues are evident; b – a 3D reconstruction of the thoracic osteochondral cage; c – MIP reconstruction, frontal view; d – view of surgical wound after implantation of customized TiNi devices; e – control post-surgical CT reconstruction; f-g – patient's appearance and images of 3-D reconstruction five years after combined repair of thoracoabdominal defect.
In 2011, the patient with a history of morphologically verified malignant tumor (T2N0M0) of the right breast received the multimodality treatment (radiotherapy, chemotherapy, and subsequent right-side radical mastectomy) in the oncology center located in another province, where the postoperative period was complicated by abscess of the surgical wound and sternal and costal osteomyelitis. After multimodality treatment performed at a local facility and, subsequently, at a thoracic surgery clinic, the sternal and costal osteomyelitis was believed to be successfully cured.
However, in mid-2012, she complained of pain in the sternum and left half of the chest, edema of soft tissues appeared in the scar area, and fistulas with purulent discharge were noted. Due to the recurrence of osteomyelitis, the body of the sternum and the xiphoid process and anterior segments of ribs 5, 6, and 7 on the right and ribs 5, 6, 7, 8, and 9 on the left were excised in a subsequent revision surgery at the same facility, and a huge defect was created in the anterior chest wall in combination with a reducible ventral hernia and instability of the rib cage. Histological examination of the excised material revealed signs of chronic purulent inflammation, without evidence of malignant cells.
After pre-surgery checkup, written consent was obtained, and the operation was scheduled. A multidisciplinary approach included input from thoracic and plastic surgeons, as well as clinical oncologist and anesthesiologist. Under general anesthesia with endotracheal intubation, the surgical scars and partially the left breast were dissected by a vertical incision, and the upper and lower fasciocutaneous flaps were then mobilized. The hernial sac up to 20 cm in diameter was separated from the surrounding tissues and abdominal wall, was positioned in the plane of the excised left costal margin. The contents of the hernial sac were the greater omentum, which had migrated into the abdominal cavity. The hernial defect was formed from below and laterally by the abdominal oblique muscles, medially by the left rectus abdominis muscle, and from above by what remained of the excised costal margin; the dimensions of the hernial defect were 8.5 × 10 cm. Through cicatricial atrophied tissue at the mediastinum level, we noted signs of transmitted cardiac pulsations. The thoracoabdominal defect presented as an osteochondral defect on the chest wall and an aponeurotic defect of the abdominal wall 576 cm2 in size.
A combined reconstractive plasty of the chest and abdominal wall was then performed (Fig. 1, d-e). The peritoneum in the area of the hernial defect was stitched using a simple interrupted suture. Overlapping the margins by at least 5 cm, a knitted TiNi mesh was placed over the thoracoabdominal defect and secured in the standard fashion, with outer edges of the mesh folded circumferentially. The surgical mesh used was knitted of the superelastic TiNi-based alloy filament, 60 μm in diameter, with bioinert oxycarbonitride surface studied by Zaworonkow et al. [4] and also reported by Anikeev et al. [6]. The mesh was fixed with maximum tension around the perimeter, using a simple continuous suture technique with atraumatic 3-0 prolene suture. This type of surgical mesh selection stemmed from the positive experience communicated previously by Muhamedov et al. [3] and Zaworonkow et al. [4].
To create a reliable chest framework, three reinforced artificial ribs of different lengths were used. Each artificial rib, depicted in Fig. 1d, was a customized sandwich 6 mm thick consisting of a medullary wrought superelastic TiNi plate between cortical plates of porous SHS-TiNi, the alloy fabricated by high-temperature self-propagating synthesis (SHS). To secure the sandwich, a thin TiNi wire 0.1 mm in diameter was used that wrapped around the device along its entire length. After the artificial ribs were placed in the surgical wound atop the mesh implant, they were not additionally fixed to the rib remnants, using metallic screws or wire twisted suture, in view of the highly developed specific surface activity of the SHS-TiNi alloy, which delivers extraordinary adhesive properties in vivo [5]. However, the rib prostheses bedded on the rib remnants were then linked with the mesh and subjacent tissues using atraumatic 3-0 prolene encircling stitch, therefore forming a reliable tent-like structure. The soft tissue lesion was reconstructed by draping the adjoining tissues and the left breast. The space under the flap was drained by placement of two drain tubes followed by active aspiration.
The patient was extubated in the operation room. The postoperative period was smooth and proceeded without complications. No clinical signs of respiratory failure were observed. The surgical wound healed by primary intention. Pain syndrome was assessed on a 10-point scale. On day 3, pain at rest and during movement did not exceed 2–3 points. After removing the drainage, the pain corresponded to one point, whereas that was zero upon discharge, and simple analgesia was stopped. On the 30th day after surgery, the pain syndrome was also zero even in varied body movements. The patient declined suggested mammoplasty and cosmetic procedures, preferring to conceal the chest defect with items of clothing. Two months after discharge, she continued her job as a milkmaid in a farming household.
In follow-up examinations, and thus far, no paradoxical breathing and no recurrence of osteomyelitis or hernia have been observed in the repaired area (Fig. 1 f). Also, there was no evidence of fibrous or fibrocartilaginous tissue formation. As a valid indicator of device consistency, functional outcomes, and patient status, we have resorted to the Enneking modified scoring system (physical function, social role, pain, emotional acceptance, dexterity, etc.). On a five-point scale, with zero being the lowest, the resulting score was five. Computed tomography images taken five years after the surgery also confirmed that the implants are well integrated without rejection, side effects, migration, or postoperative complications (Fig. 1 g).
3. Discussion
Despite the improved surgical techniques including modern implants, the reconstruction of extensive thoracic defects, regardless of etiology, is still challenging even for high-skilled surgeons [7]. A variety of techniques for surgical treatment of post-excision thoracic defects indicates that there is no versatile of the proposed methods. All of them are not devoid of certain disadvantages and risks. That requires thorough planning, customization, and a multidisciplinary approach. To date, the global clinical practice dictates an attitude towards reconstructive procedures which suggested a tolerable postoperative complication rate. In the treatment of patients requiring a rib-plasty procedure, in particular after extensive excisions, three key points are indispensable. First, it is to restore the rigidity of the osteochondral framework, minimizing its anatomical distortion and maintaining the anatomical and physiological volume of the mediastinum and pleural cavities. Second, it is to maintain pulmonary mechanics. Finally, third, it is to protect the vital organs of the thorax.
To reconstruct the thoracic cage, the use of synthetic materials and implants is known to come to the fore [2,8]. Synthetic biomaterials most often applied for these purposes are of polymer origin. Noted disadvantages of these implants include secondary wound infections in up to 6% of cases [9], inadequate thoracic cage rigidity, respiratory dysfunction, and seroma, which often leads to the revision surgery and removal of inconsistent devices [[10], [11], [12]]. Reports have appeared in recent years on the reconstruction of post-excision chest wall lesions using Ti meshes and customized titanium 3D-printed devices of various designs [1,2,10,[13], [14], [15], [16]]. However, even in the simple breathing cycle, the implanted device may suffer from complex loading, including tension, bending, and torsion. Of course, a good device used for thoracic wall repair should mimic the anisotropic compliance of chest wall tissues and should demonstrate a tolerant long-term stress-strain behavior without impairment of mechanical characteristics at higher loads. Considering а 3D-printed Ti construct, especially in the case of a long or sophisticated device, which mechanical characteristics differ from that shown by the rib to be substituted, we can assume that there is a noncoincidence in the context of elastic moduli. Eventually, the latter would result in implant-induced complications leading to pain, rejection, failure, fibrosis, and inflammation. Moreover, crucial issues and limitations regarding 3D-printed devices as cost, reproducibility of the microstructure and properties, and scalability of the fabrication processes to mass-production levels still remain.
The TiNi surgical mesh is double-knitted by a process that interlinks each filament junction and provides for superelasticity in any direction. The filament junctions are not subject to the same work fatigue demonstrating by other metallic meshes. The multidirectional compliance allows the mesh to be easily adapted to various loads that occurred in the body. The bio-mimic in vivo features of the mesh have been reported to resemble the behavior of host tissues [3,4,[17], [18], [19]], and that is why such design enabled us to stretch and manage the mesh intraoperatively without its unraveling.
Biocompatible porous and solid TiNi-based implants and their successful deployment in surgical treatments have encouraged insights for immediate and delayed rib-plasty. Experimental and clinical studies with good results have demonstrated successful integration of TiNi implants with the formation of regenerated tissues, which anatomically and physiologically restore the injured area [5,17,20]. Recently, porous SHS-TiNi alloys have been reported to have some particularities which significantly distinguish them from those manufactured by other methods of powder metallurgy [21]. It happens that the porous compound formation during the SHS-reaction is accompanied by the genesis of bio-active nonmetallics and nanocrystalline, corrosion-proof, amorphous superficial layers concealing the pore walls [22], which are of great interest for clinical applications. Moreover, the rheological similarity between the viscoelastic artificial TiNi sandwich and the rib imparts additional engineering benefits to this biomaterial. The distinctive feature of porous TiNi is conditioned by the lowest elastic modulus similar to that demonstrated by the bone tissue. Additionally, the rheological resemblance in terms of stress-strain allows the artificial rib to be congruentially deformed without failure and delamination, passing through a million cycles, as recently assessed by Yasenchuk et al. [23]. The rough, hydrophilic surface of porous SHS-TiNi was reported to sustain cell adhesion, growth, and proliferation via a system of interconnected macro-/micropores and grooves [[24], [25], [26]]. That explains why rapid bone ingrowth with no evidence of fibrous or fibrocartilaginous tissue formation occurred and accords well with findings emanating from the study reported by Aihara et al. [27]. In view of the above, TiNi-based devices seem to be a promising alternative to rib osteosynthesis using Ti-based implants which mechanical discrepancy with bone tissue, under alternating strain, and nontreated surfaces can be considered the severe disadvantages resulting in adverse corrosion effect, failure, and noted complications.
4. Conclusions
-
(i)
The method opted to repair the extensive thoracoabdominal defect using bio-adaptive TiNi-based devices combined in the tent-like structure is a reliable and promising alternative to existing surgical procedures, even though the case is aggravated with a ventral hernia or osteomyelitis.
-
(ii)
Availability of superelastic TiNi-based implants which show no foreign body response with the associated complications of fibrosis and patient discomfort seems to expand the capabilities for a surgeon to manage sizeable post-excision thoracic lesions with good functional and clinical outcomes.
-
(iii)
The reported case is believed to create a new insight for colleagues to suggest the optimal implant’ design for improved patient tolerance as compared to well-known surgical methods.
Ethical approval
Approval from an institutional board review was obtained for the case report.
Animal rights
This work does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient for publication of the case report and corresponding images.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation, project No. 0721-2020-0022.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to express our great thanks to Dr. Victor Gunther, Tomsk State University, for his guidance and enthusiastic efforts.
References
- 1.Berthet J., Canaud L., D'Annoville T., et al. Titanium plates and dualmesh: a modern combination for reconstructing very large chest wall defects. Ann. Thorac. Surg. 2011;91:1709–1716. doi: 10.1016/j.athoracsur.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Sanna S., Brandolini J., Pardolesi A., et al. Materials and techniques in chest wall reconstruction: a review. J. Vis. Surg. 2017;3:95. doi: 10.21037/jovs.2017.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muhamedov M., Kulbakin D., Gunther V., et al. Sparing surgery with the use of TiNi-based endografts in larynx cancer patients. J. Surg. Oncol. 2015;111:231–236. doi: 10.1002/jso.23779. [DOI] [PubMed] [Google Scholar]
- 4.Zaworonkow D., Chekan M., Kusnierz K., et al. Evaluation of TiNi-based wire mesh implant for abdominal wall defect management. Biomed. Phys. Eng. Express. 2018;4 [Google Scholar]
- 5.Yasenchuk Y., Marchenko E., Gunther V., et al. Biocompatibility and clinical application of porous TiNi alloys made by self-propagating high-temperature synthesis (SHS) Materials. 2019;12:2405. doi: 10.3390/ma12152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anikeev S., Hodorenko V., Gunther V., et al. The effect of mechano-chemical treatment on structural properties of the drawn TiNi-based alloy wire. Mater. Res. Express. 2018;5 [Google Scholar]
- 7.Losken A., Thourani V., Carlson G., et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br. J. Plast. Surg. 2004;57:295–302. doi: 10.1016/j.bjps.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller D., Force S., Pickens A., et al. Chest wall reconstruction using biomaterials. Ann. Thorac. Surg. 2013;95:1050–1056. doi: 10.1016/j.athoracsur.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Weyant M., Bains M., Venkatraman E., et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann. Thorac. Surg. 2006;81:279–285. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Ng C. Recent and future developments in chest wall reconstruction. Semin. Thorac. Cardiovasc. Surg. 2015;27:234–239. doi: 10.1053/j.semtcvs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Hazel K., Weyant M. Chest wall resection and reconstruction: management of complications. Thorac. Surg. Clin. 2015;25:517–521. doi: 10.1016/j.thorsurg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Rocco G., Martucci N., La Rocca A., et al. Postoperative local morbidity and the use of vacuum-assisted closure after complex chest wall reconstructions with new and conventional materials. Ann. Thorac. Surg. 2014;98:291–296. doi: 10.1016/j.athoracsur.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Tamburini N., Grossi W., Sanna S., et al. Chest wall reconstruction using a new titanium mesh: a multicenters experience. J. Thorac. Dis. 2019;11:3459–3466. doi: 10.21037/jtd.2019.07.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joalsen I., Christian D., Rosalie A., Angga M. Reconstruction with titanium mesh following wide excision in chest wall myxofibrosarcoma: a case report. Int. J. Surg. Case Rep. 2020;77:111–115. doi: 10.1016/j.ijscr.2020.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen X., Gao S., Feng J., et al. Chest-wall reconstruction with a customized titanium-alloy prosthesis fabricated by 3D printing and rapid prototyping. J. Cardiothorac. Surg. 2018;13:4. doi: 10.1186/s13019-017-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragon J., Perez Mendez I. Dynamic 3D printed titanium copy prosthesis: a novel design for large chest wall resection and reconstruction. J. Thorac. Dis. 2016;8:E385–E389. doi: 10.21037/jtd.2016.03.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunther V., Radkevich A., Kang S.B., et al. Study of the knitted TiNi mesh graft in a rabbit cranioplasty model. Biomed. Phys. Eng. Express. 2019;5 [Google Scholar]
- 18.Shtin V., Novikov V., Chekalkin T., et al. Repair of orbital post-traumatic wall defects by custom-made TiNi mesh endografts. J. Funct. Biomater. 2019;10:27. doi: 10.3390/jfb10030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernyshova A., Kolomiets L., Chekalkin T., et al. Fertility-sparing surgery using knitted TiNi mesh implants and sentinel lymph nodes: a 10-year experience. J. Invest. Surg. 2021;34:1110–1118. doi: 10.1080/08941939.2020.1745965. [DOI] [PubMed] [Google Scholar]
- 20.Kulbakin D., Chekalkin T., Muhamedov M., et al. Sparing surgery for the successful treatment of thyroid papillary carcinoma invading the trachea: a case report. Case Rep. Oncol. 2016;9:772–780. doi: 10.1159/000452790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunther V., Yasenchuk Y., Chekalkin T., et al. Formation of pores and amorphous-nanocrystalline phases in porous TiNi alloys made by self-propagating high-temperature synthesis (SHS) Adv. Powder Technol. 2019;30:673–680. doi: 10.3390/ma12152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasenchuk Y., Gunther V., Marchenko E., et al. Formation of mineral phases in self-propagating high-temperature synthesis (SHS) of porous TiNi alloy. Mater. Res. Express. 2019;6 doi: 10.3390/ma12152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasenchuk Y., Marchenko E., Baigonakova G., et al. Study on tensile, bending, fatigue, and in vivo behavior of porous SHS-TiNi alloy used as a bone substitute. Biomed. Mater. 2021;16 doi: 10.1088/1748-605X/aba327. [DOI] [PubMed] [Google Scholar]
- 24.Kokorev O., Hodorenko V., Chekalkin T., et al. In vitro and in vivo evaluation of porous TiNi-based alloy as a scaffold for cell tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016;44:704–709. doi: 10.3109/21691401.2014.982799. [DOI] [PubMed] [Google Scholar]
- 25.Kokorev O., Hodorenko V., Chekalkin T., et al. Evaluation of allogenic hepato-tissue engineered in porous TiNi-based scaffolds for liver regeneration in a CCl4-induced cirrhosis rat model. Biomed. Phys. Eng. Express. 2019;5 [Google Scholar]
- 26.Kokorev O., Chekalkin T., Marchenko E., et al. Exploring the role of surface modifications of TiNi-based alloys in evaluating in vitro cytocompatibility: a comparative study. Surf. Topogr. Metrol. Prop. 2020;8 [Google Scholar]
- 27.Aihara H., Zider J., Fanton G., Duerig T. Combustion synthesis porous Nitinol for biomedical applications. Int. J. Biomater. 2019;2019 doi: 10.1155/2019/4307461. [DOI] [PMC free article] [PubMed] [Google Scholar]