Abstract
Background:
Exposure to repetitive head impacts (RHI) is associated with an increased risk of later-life neurobehavioral dysregulation and neurodegenerative disease. The underlying pathomechanisms are largely unknown.
Purpose:
To investigate whether RHI exposure is associated with later-life corpus callosum (CC) microstructure and whether CC microstructure is associated with plasma total tau and neuropsychological/neuropsychiatric functioning.
Study Type:
Retrospective cohort study.
Population:
75 former professional American football players (age 55.2+/−8.0 years) with cognitive, behavioral, and mood symptoms.
Field Strength/Sequence:
Diffusion-weighted echo-planar MRI (dMRI) at 3T.
Assessment:
Subjects underwent dMRI, venous puncture, neuropsychological testing, and completed self-report measures of neurobehavioral dysregulation. RHI exposure was assessed using the Cumulative Head Impact Index (CHII). dMRI measures of CC microstructure, (i.e., free-water corrected fractional anisotropy (FA), trace, radial diffusivity (RD) and axial diffusivity (AD)), were extracted from seven segments of the CC (CC1–7), using a tractography clustering algorithm. Neuropsychological tests were selected: Trail Making Test Part A (TMT-A) and Part B (TMT-B), Controlled Oral Word Association Test (COWAT), Stroop Interference Test, the Behavioral Regulation Index (BRI) from the Behavior Rating Inventory of Executive Function, Adult version (BRIEF-A).
Statistical Tests:
dMRI metrics were tested for associations with RHI exposure, plasma total tau, neuropsychological performance, and neurobehavioral dysregulation using generalized linear models for repeated measures.
Results:
RHI exposure was associated with increased AD of CC1 (correlation coefficient (r)=0.32, p<0.05) and with increased plasma total tau (r=0.34, p<0.05). AD of the anterior CC1 was associated with increased plasma total tau (CC1: r=0.30, p<0.05; CC2: r=0.29, p<0.05). Higher trace, AD, and RD of CC1 were associated with better performance (p<0.05) in TMT-A (trace, r=0.33; AD, r=0.31; and RD, r=0.28) and TMT-B (trace, r=0.31; RD, r=0.34). Higher FA and AD of CC2 were associated with better performance (p<0.05) in TMT-A (FA, r=0.36; AD, r=0.28)/TMT-B (FA, r=0.36; AD, r=0.27), COWAT (FA, r=0.3; AD, r=0.32)., and BRI (AD, r=0.2858).
Data Conclusion:
These results suggest an association between RHI exposure, CC microstructure, plasma total tau, and clinical functioning in former professional American football players.
Keywords: Sport-related brain injury, diffusion MR imaging, tractography, neurodegeneration, chronic traumatic encephalopathy, neuropsychological testing
INTRODUCTION
Exposure to repetitive head impacts (RHI) is commonly observed in contact sports such as American football. Exposure to RHI may lead to symptomatic brain injury such as concussion, as well as the more common asymptomatic subconcussive injuries (1). RHI exposure sustained over longer periods of time is associated with an increased risk of later-life cognitive impairment, neurobehavioral dysregulation, and neurodegenerative disease (2). The underlying pathomechanisms of potential cumulative effects are, however, not fully understood.
RHI exposure has previously been linked to brain alterations (3). Structural alterations of the brain can be non-invasively characterized using advanced neuroimaging. Diffusion MRI (dMRI) is particularly sensitive to subtle microstructural alterations of brain tissue following RHI (3). More specifically, studies have revealed decreased fractional anisotropy (FA) and increased radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD) (4, 5) following RHI. Of note, the corpus callosum (CC) is predominantly affected, likely due to its central location and increased vulnerability to shear strain (6). Importantly, the CC is involved in brain functions that are often impaired following brain injury, such as cognition, mood and behavior (7). A previous study found an association between younger age at first exposure to American football and later-life microstructural alterations of the anterior CC (5). However, whether exposure to RHI while participating in professional American football leads to later-life alterations in CC microstructure is not known. Moreover, the pathomechanism underlying microstructural alterations following exposure to RHI is not fully understood.
Exposure to RHI has been associated with increased levels of total tau in former National Football League (NFL) players (8). Total tau is a non-specific marker of general neurodegeneration and axonal damage (9), mainly expressed in neuronal axons where it regulates the stability of microtubules and supports axonal transportation (10). Additionally, tau crosses the blood-brain barrier (BBB) and, thus, can be detected and measured in blood plasma (11). There are few studies that have investigated the association between brain structure and total tau in former contact-sport athletes. One such study reported higher cerebrospinal fluid (CSF) total tau in a group of 22 former professional contact-sport athletes (age: 55.9 +/− 12.2 years) (American football (n=12), ice hockey (n=9), and snowboarding (n=1)) compared to five community control participants (9). Athletes with higher CSF total tau showed lower FA, as well as higher RD and MD across several white matter (WM) tracts and impaired neuropsychological performance on the Trail Making Test Part B (9).
RHI has also been linked to impaired neuropsychological and neuropsychiatric functioning in former professional American football players (2). Progressive cognitive impairment (specifically episodic memory deficits and/or executive dysfunction) and neurobehavioral dysregulation (e.g., explosiveness, impulsivity, rage, “short fuse”) are the core clinical features of the NINDS Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome (TES), the clinical syndrome associated with underlying pathology of the neurodegenerative disease, chronic traumatic encephalopathy (CTE) (12). However, it is not clear whether or not RHI exposure is associated with CC microstructure in former professional American football players. It also remains to be elucidated whether or not later-life CC microstructure is associated with increased later-life plasma total tau and with clinical functioning following exposure to RHI.
Thus the aim of this study was 1) to investigate whether or not RHI exposure is associated with alterations in CC microstructure, 2) to determine whether or not CC microstructure is associated with plasma total tau levels, and 3) to assess the association between CC microstructure and neuropsychological functioning and neurobehavioral dysregulation in former professional American football players.
METHODS
Study Design
This study was part of the Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT) project. The aim of this project was to develop in vivo biomarkers to diagnose CTE during life. Between 2011 and 2015, participants were tested in a single two- to three-day visit. The test protocol included an interview to quantify previous exposure to RHI from football, as well as neuropsychological testing, completion of self-report measures of neuropsychiatric functioning, collection of blood, and MRI acquisition. More details regarding the study design can be found elsewhere (13). The study was approved by the respective Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
Study participants
The DETECT sample includes 96 symptomatic former NFL players. Inclusion criteria were: 1) male sex, 2) age between 40 and 69 years, 3) minimum of twelve years of participation in organized American football, 4) playing at least two years in the NFL, and 5) self-reported (at time of telephone screening) cognitive, behavioral, and mood symptoms for a minimum of six months prior study enrollment. Exclusion criteria were: 1) having sustained a concussion or other traumatic brain injuries (TBI) within one year prior to study enrollment, 2) contraindications for MRI, 3) presence of another disease affecting the central nervous system, and 4) a primary language other than English.
For the present study, 15 of the 96 former NFL players were excluded because of missing dMRI data. Another six participants were excluded due to artifacts in imaging data (e.g., motion artefacts). Thus, the final sample consisted of 75 former NFL players. Cohort characteristics and demographics of these participants are shown in Table 1.
Table 1:
Cohort characteristics (n=75)
| Mean | StdDev | Range | |
|---|---|---|---|
| Sample Characteristics | |||
| Age | 55.16 | 7.98 | 40 – 69 |
| Body mass index (kg/m2) | 33.15 | 5.18 | 25.2 – 52.1 |
| Education (Years) | 16.49 | 0.98 | 15 – 20 |
| CHII | 20352.3 | 7236.02 | 6860.4 – 48218.3 |
| Race | W 44; B/A 23; NA 8 | ||
| Fluid Biomarkers | |||
| Plasma Total Tau (pg/mL) | 2.56 | 0.98 | 0.77 – 5.68 |
| Neuropsychological Tests | |||
| Trail Making Test A | 49.37 | 11.52 | 20 – 66 |
| Trail Making Test B | 45.21 | 15.75 | 20 – 74 |
| Stroop Test | 10.99 | 2.63 | 2 – 15 |
| COWAT | 49.86 | 11.41 | 24 – 80 |
| BRI | 63.01 | 12.32 | 37 – 95 |
Summary of demographical data, fluid biomarkers and neuropsychological test performance represented as mean, standard deviation (StdDev), range and numeric variables.
Abbreviations: CHII = Cumulative Head Impact Index; Race: W = White, B/A = Black or African American, NA = No data available; BRI = Behavioral Regulation Index; COWAT = Controlled Oral Word Association Test; StdDev = Standard Deviation.
Exposure to RHI
The Cumulative Head Impact Index (CHII) was used to quantify the players’ estimated exposure to RHI (2). The CHII is based on information about the individual’s football history, including the number of seasons played, position(s) played, and levels played (youth, high school, college), as well as an estimation of head impact frequencies established based on helmet accelerometer studies (2). Because helmet accelerometer data at the professional level has not been published or made available, head impact frequencies from college level studies were extrapolated to estimate participants’ post-college head impact frequencies.
Assessment of Plasma Total Tau
Blood was drawn by conventional venous puncture. Plastic dipotassium ethylenediamine tetraacetic acid (EDTA) tubes were used for the collection. Plasma was centrifuged, aliquoted, and stored at a temperature of −80 °C. The frozen plasma tubes were sent out for analysis. Tests were performed in duplicate from each sample using the Simoa HD-1 analyzer (Quanterix, Lexington, MA, USA). For the procedure, two monoclonal antibodies were used binding specific parts of human tau. The detection antibody recognizes the N-terminus, while the capture antibody connects to the mid-domain. Samples were tested in triplicate calculating standard curves and individual sample measurements. On average, a coefficient of variation of 4% across all samples was detected. More details concerning the procedure can be found elsewhere (14).
Neuropsychological and Neuropsychiatric Assessment
All participants were administered a neuropsychological test battery and completed self-report measures of neuropsychiatric symptoms. We chose the following tests to include in the present study because they are both sensitive to frontal system executive functioning (15) and frequently affected in people exposed to RHI (2, 13): Trail Making Test A and B (TMT-A, TMT-B), measuring visual scanning, psychomotor speed, and cognitive flexibility (16); Controlled Oral Word Association Test (COWAT), measuring phonemic fluency (17); the Stroop Interference Test, measuring selective attention and inhibition (18); and the Behavioral Regulation Index (BRI) from the Behavior Rating Inventory of Executive Function, Adult version (BRIEF-A), measuring neurobehavioral dysregulation (19). Raw scores of the tests were transformed into T scores using normative data that account for age, sex and education (13).
Magnetic Resonance Imaging
Image Acquisition
Diffusion MRI data were collected using a 3-Tesla MRI scanner (Magnetom Verio, Siemens Healthineers AG, Erlangen, Germany) with a 32-channel head coil. A diffusion-weighted echo-planar imaging (EPI) sequence was acquired with the following parameters: repetition time (TR) = 11,700 msec, echo time (TE) = 85 msec, matrix = 128*128, field of view = 256*256 mm2, slice thickness = 2 mm, and parallel imaging using GRAPPA with an acceleration factor of 3. In total, 73 slices were acquired using 64 diffusion directions, consisting of 59 diffusion-weighted images with multiple b-values from 80 s/mm2 to 3000 s/mm2 and 5 images with a b-value of 0 s/mm2 for anatomical reference.
Image Processing
Visual inspection of the raw dMRI data (LO, FZ, JK with 20, 10, and 3 years of experience in the analysis of MRI data) was followed by generating whole brain tractography maps using a two-tensor model. We applied the Unscented Kalman Filter (UKF, part of the ukftractography package (https://github.com/pnlbwh/ukftractography)) method (20) which has previously been shown to sensitively and consistently trace fibers across various populations (21). This two-tensor model accounts for crossing fibers (20) which are particularly relevant in the context of tracing the CC fibers crossing the corticospinal tract. This two-tensor model associates the first tensor with the main direction of the fiber tract that is being traced. The second tensor represents fibers crossing through the tract of interest. Whole brain tractography was then again visually inspected and also quantitatively assessed for quality using the quality control tool in the whitematteranalysis (WMA) software (https://github.com/SlicerDMRI/whitematteranalysis).
The CC was identified in each subject by applying a fiber clustering pipeline in combination with an anatomical tract atlas (22). This approach applies machine learning to dMRI data to identify WM tracts of each individual based on a neuroanatomist-curated WM atlas that was trained using tractography data from 100 healthy young adults stemming from the Human Connectome Project (23). Using the anatomical atlas, the CC was automatically divided into seven anatomical subregions (24): CC1 = rostrum, CC2 = genu, CC3+4 = anterior half of the body, CC5 + 6 = posterior half of the body, and CC7 = splenium (Figure 1). The division into seven subregions is based on a segmentation scheme by Witelson et al. (25). The CC tractography of each individual case was visually inspected for quality and number of fibers was quantitatively assessed (Figure 2). 3D Slicer (The SlicerDMRI project (http://dmri.slicer.org)) (26) was used to extract free-water corrected diffusion parameters (FA, trace, AD, and RD) of these seven subregions of the CC. Of note, to quantify the total amount of diffusion, one can either calculate the mean of the three eigenvalues (MD) or compute the sum (trace).
Figure 1:

White matter (WM) fibers passing through corpus callosum (CC) subregions; green = CC1 (rostrum), red = CC2 (genu), white and yellow = CC3 + CC4 (anterior half of the body), purple and pink = CC5 + CC6 (posterior half of the body), turquoise = CC7 (splenium). WM tracts consists of grouped fiber clusters. Predefined subregions of the CC (CC1-CC7) were selected and WM fibers passing through each subregions are depicted in different colors.
Figure 2:

Violin plots depicting number of fibers for each of the corpus callosum segments.
Statistical Analyses
Statistical analyses were performed using the Statistical Analysis System (SAS version 9.4; SAS Institute Inc., North Carolina, USA). Three sets of generalized linear models (GLM) for repeated measures with an unstructured covariance matrix were used to investigate associations between RHI exposure, CC microstructure, plasma total tau, and neuropsychological/neuropsychiatric test scores. The first set of GLM tested for associations between RHI (independent variable) and each dMRI parameter (FA, trace, AD, and RD) extracted from the seven CC subregions (dependent variables). To reduce model complexity, each dMRI parameter was tested separately. The second set of GLM investigated associations between each dMRI parameter (FA, trace, AD, and RD) from the seven CC subregions (dependent variables) with plasma total tau levels (independent variable). The third set of GLM investigated associations between neuropsychological/neuropsychiatric test scores (dependent variables) and each dMRI parameter (FA, trace, AD, and RD) from each CC subregion that revealed significant effects in previous GLM tests (independent variables). For all three sets of GLM, covariates included body mass index (BMI = weight divided by height squared [kg/m²]) and age (in years), in addition to years of education for the third set of GLM investigating neuropsychological/neuropsychiatric measures. BMI was included as a covariate due to evidence for a negative correlation between BMI and CC microstructure (27). Results from the GLM were adjusted for multiple comparisons across the number of CC subregions tested using a false discovery rate (FDR) of 5%. We set the level of statistical significance at p < 0.05 (FDR-corrected).
RESULTS
Cohort Characteristics
Demographics, neuropsychological test scores, and total tau in blood plasma of the 75 former American football players included in this study are shown in Table 1.
Association between RHI Exposure and Diffusion Measures
The average CHII was 20,352.3 ± 7,236.02 (range: 6,860.4 – 48,218.3). Among the seven subregions of the CC, AD of CC1 was associated with RHI exposure (r=0.32, p<0.05; Figure 3). Thus, the higher CHII (indicating greater exposure to RHI), the higher AD in CC1. No other subregional dMRI parameters were significantly associated with CHII (p ≥ 0.05) (FA: CC1 p=0.2831, CC2 p=0.2831, CC3 p=0.3198, CC4 p=0.582, CC5 p=0.4396, CC6 p=3189, CC7 p=0.522; Trace: CC1 p=0.2331, CC2 p=0.7622, CC3 p=0.7622, CC4 p=0.2331, CC5 p=0.7688, CC6 p=0.6465, CC7 p=0.7622; AD: CC2 p=0.2402, CC3 p=0.9462, CC4 p=0.2402, CC5 p=0.9462, CC6 p=0.8669, CC7 p=0.9462; RD: CC1 p=0.8361, CC2 p=0.5558, CC3 p=0.5558, CC4 p=0.5558, CC5 p=0.5558, CC6 p=0.8361, CC7 p=0.5558)
Figure 3:

Scatterplot displaying the association between the Cumulative Head Impact Index (CHII) and axial diffusivity (AD) of rostrum of corpus callosum (CC1).
Association between Diffusion Measures and Plasma Total Tau
AD of CC1 and CC2 was associated with plasma total tau (CC1: r=0.30; CC2: r=0.29, both p<0.05; Table 2 and Figure 4). AD in CC2 from one participant represents an outlier that seems to drive the regression line (Figure 4, indicated by arrow in the right panel) and was therefore reevaluated for data quality. Of note, data quality of this particular case is sufficiently high and therefore we did not exclude this participant in the statistical analysis. Other subregional dMRI parameters are not significantly associated with plasma total tau (see Table 2 for absolute p values).
Table 2:
Corpus Callosum Diffusion MRI Measures and Plasma Total Tau
| Region | Mean | StdDev | Partial R | P Value | |
|---|---|---|---|---|---|
| FA | CC1 | 0.6096 | 0.0406 | 0.1584 | 0.4374 |
| CC2 | 0.6738 | 0.0366 | 0.2093 | 0.4374 | |
| CC3 | 0.7069 | 0.0367 | 0.1528 | 0.4374 | |
| CC4 | 0.7306 | 0.0323 | 0.0249 | 0.8705 | |
| CC5 | 0.7346 | 0.0373 | 0.0733 | 0.8705 | |
| CC6 | 0.7281 | 0.0242 | 0.0190 | 0.8705 | |
| CC7 | 0.7391 | 0.0238 | −0.0557 | 0.8705 | |
| Trace (mm²/s) | CC1 | 0.0014 | 0.0001 | 0.2424 | 0.2439 |
| CC2 | 0.0016 | 0.0001 | 0.1387 | 0.4064 | |
| CC3 | 0.0015 | 0.0001 | −0.0535 | 0.7536 | |
| CC4 | 0.0015 | 0.0001 | −0.1565 | 0.4064 | |
| CC5 | 0.0016 | 0.0001 | −0.0121 | 0.9171 | |
| CC6 | 0.0016 | 0.0001 | −0.1412 | 0.4064 | |
| CC7 | 0.0016 | 0.0001 | −0.0979 | 0.5602 | |
| AD (mm²/s) | CC1 | 0.0009 | 0.0001 | 0.2996 | 0.0440 |
| CC2 | 0.0011 | 0.0001 | 0.2850 | 0.0440 | |
| CC3 | 0.0011 | 0.0001 | 0.0499 | 0.7797 | |
| CC4 | 0.0011 | 0.0001 | −0.1268 | 0.5544 | |
| CC5 | 0.0012 | 0.0001 | 0.0129 | 0.9119 | |
| CC6 | 0.0012 | 0.0001 | −0.1164 | 0.5544 | |
| CC7 | 0.0012 | 0.0001 | −0.0890 | 0.6227 | |
| RD (mm²/s) | CC1 | 0.0003 | <0.0000 | 0.09706 | 0.7583 |
| CC2 | 0.0003 | <0.0000 | −0.1013 | 0.7583 | |
| CC3 | 0.0002 | <0.0000 | −0.1340 | 0.7583 | |
| CC4 | 0.0002 | <0.0000 | −0.0711 | 0.7583 | |
| CC5 | 0.0002 | <0.0000 | −0.0142 | 0.9034 | |
| CC6 | 0.0003 | <0.0000 | 0.0804 | 0.7583 | |
| CC7 | 0.0002 | <0.0000 | −0.0342 | 0.8973 |
Associations of plasma total tau with diffusion parameters of corpus callosum (CC) subregions. Mean, standard deviation (StdDev), partial r and p values are presented. Significant results are marked in bold.
For CC1 there were only 74 subjects included since one data point was missing after clustering.
Abbreviations: FA = Fractional Anisotropy; AD = Axial Diffusivity; RD = Radial Diffusivity; StdDev = Standard Deviation.
Figure 4:
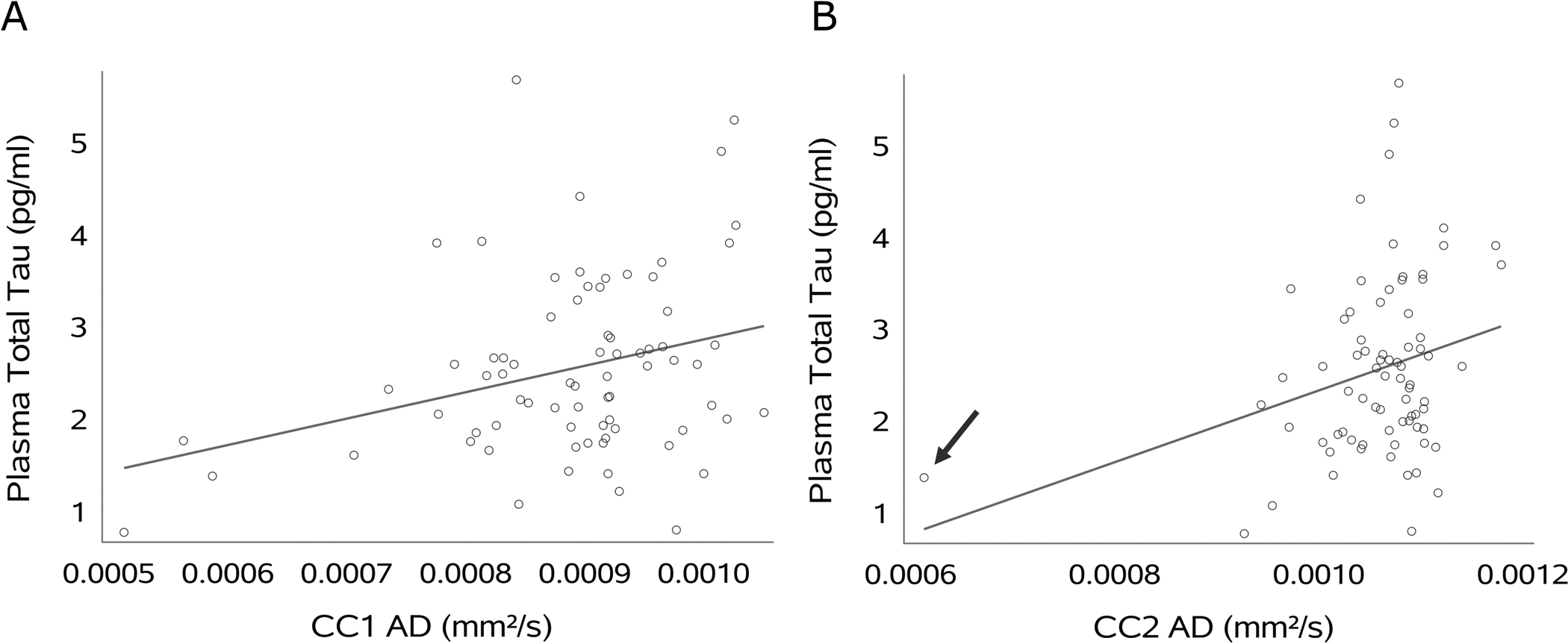
Scatterplots displaying the association between plasma total tau and axial diffusivity (AD) of rostrum (CC1) and genu (CC2) of the corpus callosum (CC). Arrow indicates outlier.
Association between Neuropsychological Function and Diffusion Measures
In light of significant associations between CHII and plasma total tau levels with WM microstructure in the anterior regions of the CC (i.e., CC1 and CC2), dMRI parameters in these specific callosal subregions were examined for associations with neuropsychological test scores and a measure of neurobehavioral dysregulation (Table 3 and Supplement Figure 1).
Table 3:
Results Neuropsychological Tests
| Region | DTI | Test | Estimate | StdErr | Partial R | P Value |
|---|---|---|---|---|---|---|
| CC1 | FA | TMT-A | 63.40 | 25.92 | 0.2752 | 0.0657 |
| TMT-B | 64.25 | 36.96 | 0.1993 | 0.1440 | ||
| STROOP | 9.09 | 7.76 | 0.1358 | 0.2452 | ||
| COWAT | 60.75 | 26.78 | 0.2566 | 0.0657 | ||
| BRI | 36.88 | 27.61 | 0.1544 | 0.2324 | ||
| Trace (mm²/s) | TMT-A | 25950.00 | 8649.23 | 0.3313 | 0.0184 | |
| TMT-B | 34753.00 | 12675.00 | 0.3056 | 0.0192 | ||
| STROOP | 3472.81 | 2469.56 | 0.1624 | 0.2049 | ||
| COWAT | 10628.00 | 9368.05 | 0.1316 | 0.2603 | ||
| BRI | 15057.00 | 9696.93 | 0.1788 | 0.2049 | ||
| AD (mm²/s) | TMT-A | 33384.00 | 12142.00 | 0.3063 | 0.0376 | |
| TMT-B | 37878.00 | 17933.00 | 0.2400 | 0.0952 | ||
| STROOP | 4289.61 | 3318.86 | 0.1496 | 0.2003 | ||
| COWAT | 17530.00 | 12985.00 | 0.1561 | 0.2003 | ||
| BRI | 24962.00 | 13331.00 | 0.2141 | 0.1086 | ||
| RD (mm²/s) | TMT-A | 116384.00 | 45840.00 | 0.2848 | 0.0331 | |
| TMT-B | 199840.00 | 64483.00 | 0.3410 | 0.0138 | ||
| STROOP | 14044.00 | 12597.00 | 0.1294 | 0.4476 | ||
| COWAT | 18110.00 | 49205.00 | 0.0430 | 0.7139 | ||
| BRI | 25984.00 | 50898.00 | 0.0596 | 0.7139 | ||
| CC2 | FA | TMT-A | 87.56 | 26.27 | 0.3613 | 0.0024 |
| TMT-B | 121.35 | 36.67 | 0.3590 | 0.0024 | ||
| STROOP | 10.77 | 8.61 | 0.1439 | 0.2150 | ||
| COWAT | 88.37 | 26.63 | 0.3599 | 0.0024 | ||
| BRI | 39.41 | 28.86 | 0.1568 | 0.2150 | ||
| Trace (mm²/s) | TMT-A | 19528.00 | 11525.00 | 0.1933 | 0.1246 | |
| TMT-B | 29599.00 | 17755.00 | 0.1903 | 0.1246 | ||
| STROOP | −2438.43 | 4546.17 | −0.0622 | 0.5933 | ||
| COWAT | 21430.00 | 11654.00 | 0.2090 | 0.1246 | ||
| BRI | 21122.00 | 12289.00 | 0.1959 | 0.1246 | ||
| AD (mm²/s) | TMT-A | 39231.00 | 15393.00 | 0.2841 | 0.0215 | |
| TMT-B | 59037.00 | 24880.00 | 0.2659 | 0.0253 | ||
| STROOP | −1143.55 | 6289.49 | −0.0211 | 0.8562 | ||
| COWAT | 44808.00 | 15418.00 | 0.3201 | 0.0215 | ||
| BRI | 42016.00 | 16377.00 | 0.2858 | 0.0215 | ||
| RD (mm²/s) | TMT-A | 3135.01 | 48670.00 | 0.0075 | 0.9846 | |
| TMT-B | 5916.46 | 68358.00 | 0.0101 | 0.9846 | ||
| STROOP | −7448.39 | 12534.00 | −0.0689 | 0.9846 | ||
| COWAT | −20847.00 | 49322.00 | −0.0491 | 0.9846 | ||
| BRI | −1004.26 | 51702.00 | −0.0023 | 0.9846 |
Association between TMT-A, TMT-B, COWAT, Stroop Test and BRI, with diffusion parameters of the anterior corpus callosum (CC; rostrum (CC1) and genu (CC2)). Estimate, standard error (StdErr), partial r and p value are listed for every item. Significant results are marked with bold letters.
For CC1 there were only 74 subjects included since one data point was missing after clustering.
Test scores were partly incomplete. Number of individuals with missing test scores: TMT-A 2; TMT-B 2; COWAT 1.
Abbreviations: TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; COWAT = Controlled Oral Word Association Test; BRI = Behavioral Regulation Index from the Behavior Rating Inventory of Executive Function, Adult version (BRIEF-A); StdErr = Standard Error.
More specifically, CC1 microstructure was associated with better performance (p<0.05) on TMT-A (trace, r=0.33; AD, r=0.31; and RD, r=0.28) and better performance (p<0.05) on TMT-B (trace, r=0.31; RD, r=0.34). CC2 microstructure was associated with better performance (p<0.05) on TMT-A (FA, r=0.36; AD, r=0.28) and on TMT-B (FA, r=0.36; AD, r=0.27). Higher scores on COWAT were significantly (p<0.05) and positively associated with FA (r=0.36) and AD (r=0.32). Moreover, the BRIEF BRI was associated with higher AD (r=0.2858, p<0.05) of CC2. There were no significant associations between Stroop Test scores with dMRI parameters CC1 or CC2 (Table 3).
DISCUSSION
This study revealed that greater estimated RHI exposure is associated with CC microstructure in former professional Amercian football players. CC microstructure and plasma total tau are associated, possibly reflects direct effects of RHI (while playing football) on WM microstructure or secondary effects caused by later-life neurodegeneration. Measures of CC microstructure were also associated with features of neuropsychological performance, including psychomotor speed, cognitive flexibility, and phonemic fluency, as well as neurobehavioral dysregulation.
Association between RHI Exposure, WM Microstructure and Neuropsychological Function
Greater exposure to RHI was associated with higher AD in the rostrum of the CC. The CC has previously been shown to be particularly susceptible to RHI (3). Findings from this study confirm previous studies that observed that within the CC, the anterior parts seem to be most often affected by exposure to RHI (5). The anterior part of the CC is characterized by densely packed, thin axons (28) and may therefore be especially vulnerable to shear forces (29).
Our results indicated that greater exposure to RHI may lead to longterm microstructural alterations in the anterior CC in former professional American football players. It has previously been hypothesized that higher AD may reflect persistence of axonal injury in patients with mild traumatic brain injury (30). Such axonal damage may be expressed by axonal swelling and edema. However, whether AD also captures neuroinflammation, neurodegeneration, or axonal injury needs further investigation.
The interpretation of diffusion metrics remains challenging because a given voxel contains various structures and types of cells that influence diffusion characteristics. Additionally, animal models that use both dMRI and histology to inform the interpretation of dMRI metrics are sparse (3). In human neuroimaging, a decrease in white matter FA has often been interpreted as damage to axon and myelin sheath leading to less directed diffusion. An increase in trace or MD, on the other hand, is thought to reflect an increase in diffusion and one cause for such an increase could be neuroinflammation. A decrease in AD has been associated with axonal injury and dysfunction, whereas an increase in RD has been associated with myelin injury (31).
Our results indicated that higher diffusion measures (FA, trace, RD, and AD) were also associated with better cognitive performance as assessed using the TMT-A, TMT-B, and COWAT, and with fewer symptoms of neurobehavioral dysregulation. While the positive association between FA and better cognitive function is in line with the literature (3), in our study, higher AD also correlated with better performance. Importantly, the cross-sectional study design of this study limits the interpretation of this finding. Accordingly, future studies need to include a longitudinal study design to capture individual changes of cognitive and neuropsychitric functioning associated with WM microstructure.
Association between CC Microstructure and Plasma Total Tau
In this study, we found an association between higher plasma total tau and higher AD in the anterior regions of the CC. A previous study in a cohort of 17 male high-school football players (age range 16–17) has shown that higher plasma total tau was associated with both higher mean diffusivity and lower fiber density in the anterior part of the CC (32). The authors hypothesized that RHI may disrupt axons and lead to axonal degeneration and at the same time may release tau from its microtubule bindings.
The pathophysiological processes underlying the association of later-life plasma total tau and WM microstructure are unclear. Higher levels of t-tau have been linked to lower levels of FA, and higher levels of MD, AD, and RD across the brain. This was the case for preclinical neurodegenerative disease stages (33) as well as in cohorts of cognitive impairment (34). Moreover, a longitudinal study on patients with mild cognitive impairment (MCI) reported a significant decrease in FA and an increase in RD in multiple brain regions in a high-tau group of MCI patients compared to controls (35).
In regard of RHI, possible explanations include that exposure to RHI directly leads to injury of WM microstructure. As a result, there may be neuronal death and subsequently elevated plasma total tau. However, it is unlikely that increased plasma total tau levels due to direct effects of shear injury would persist years and even decades following RHI exposure as it did in our sample of former professional American football players in their 40s, 50s, and 60s. Another pathway may be that exposure to RHI sets in motion a progressive neurodegenerative disease that results in neuronal death and elevations in plasma total tau. Microglial activation has also been discussed as a potential underlying mechanism (36). More specifically, activated microglia release cytokines, particularly proinflammatory cytokines and excitotoxins that are purported to play a role in the development of neurodegeneration (36).
We suggest that the underlying pathophysiology of WM alteration and increased plasma total tau is a combination of both direct injury and secondary neurodegenerative and neuroinflammatory processes. Moreover, there may be additional pathomechanisms at play. For example, in other tauopathies (e.g., Alzheimer’s disease), an increase in plasma total tau has been associated with cerebral hypoperfusion resulting from cerebrovascular disease (37). Hypoperfusion is also thought to increase perfusion of small molecules such as tau through the axonal membrane (37). Thus, tau may accumulate in extravasal space and likely also in plasma given its ability to cross the BBB (11). Interestingly, cerebrovascular dysfunction, including hypoperfusion, has also been described in the context of RHI exposure (38). In Alzheimer’s disease, chronic neuroinflammation and BBB dysregulation have been associated with increased plasma total tau (39). Of further note, RHI exposure has been related to BBB dysregulation occurring predominantly in regions with high density of perivascular phosphorylated tau depositions (40). Taken together, we hypothesize that the association between WM microstructure of the CC and plasma total tau may be due to a combination of pathomechanisms including direct injury, chronic inflammatory and neurodegenerative processes, as well as cerebrovascular dysfunction.
Limitations
First, this is a cross-sectional study which limits the interpretation of our results and thus a causal relationship cannot be determined. More specifically, effects of acute or chronic injury cannot be differentiated from progressive neurodegeneration. Future studies using a longitudinal design are needed to further our understanding of changes in brain structure associated with exposure to RHI. Second, results from this study cannot be generalized beyond the specific sample investigated, i.e., former professional American football players who played in the NFL during the 1970s to 1990s. Third, the lack of a comparison group with asymptomatic former professional football players reflects a limitation. Fourth, our estimation of exposure to RHI approximates the real head impacts of the players. Fifth, interpretation of diffusion metrics remains challenging because a given voxel contains various structures and types of cells that influence diffusion characteristics. Moreover, to date, there are no normative values of diffusion measures in the corpus callosum. Despite these important limitations, our study contributes to understanding better the relationship between RHI exposure from American football, WM alterations, plasma total tau, and clinical measures of executive functioning and neurobehavioral dysregulation.
CONCLUSION
This study identified an association between RHI exposure and WM microstructure as well as an association between WM microstructure, plasma total tau, and measures of executive functioning and neurobehavioral dysregulation in former professional American football players. WM alterations potentially reflect a combination of pathomechanisms including shear injury, chronic neuroinflammatory and neurodegenerative processes, as well as cerebral hypoperfusion following exposure to RHI. Longitudinal studies are, however, needed to determine causal relationships between exposure to RHI and alterations in WM microstructure.
Supplementary Material
Supplement - Figure 1: Scatterplots displaying the association between diffusion parameters of the rostrum (CC1) and genu (CC2) of the corpus callosum (CC) and neuropsychological tests. In detail, panel A to E display test scores of the Trail Making Test Part A (TMT-A) and Part B (TMT-B) that are associated with diffusion measures of CC1, while panels F to L illustrate associations of TMT-A, TMT-B, Controlled Oral Word Association Test (COWAT) and the Behavioral Regulation Index from the Behavior Rating Inventory of Executive Function (BRIEF BRI) with diffusion measures of CC2.
Acknowledgements and Grant Support
This study was funded by the National Institute of Neurologic Disorders and Stroke (R01 NS078337, PI: R.A. Stern). This work was also supported by research grants from NIH (R01 NS100952, I.K. Koerte; R01 HD090641, S. Bouix). Plasma total tau assays were provided by Quanterix (Billerica, MA, USA).
REFERENCES
- 1.Bailes JE, Petraglia AL, Omalu BI, et al. : Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 2013; 119:1235–45. [DOI] [PubMed] [Google Scholar]
- 2.Montenigro PH, Alosco ML, Martin BM, et al. : Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J Neurotrauma 2017; 34:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koerte IK, Lin AP, Willems A, et al. : A review of neuroimaging findings in repetitive brain trauma. Brain Pathol 2015; 25:318–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koerte IK, Kaufmann D, Hartl E, et al. : A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus 2012; 33:E3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamm JM, Koerte IK, Muehlmann M, et al. : Age at First Exposure to Football Is Associated with Altered Corpus Callosum White Matter Microstructure in Former Professional Football Players. J Neurotrauma 2015; 32:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King AI, Ruan JS, Zhou C, Hardy WN, Khalil TB: Recent Advances in Biomechanics of Brain Injury Research: A Review. Volume 12. Mary Ann Liebert, Inc; 1995. [DOI] [PubMed] [Google Scholar]
- 7.Popoola O, Olayinka O, Azizi H, et al. : Neuropsychiatric manifestations of partial agenesis of the corpus callosum: A case report and literature review. Case Rep Psychiatry 2019; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alosco ML, Tripodis Y, Jarnagin J, et al. : Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2017; 7:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taghdiri F, Multani N, Tarazi A, et al. : Elevated cerebrospinal fluid total tau in former professional athletes with multiple concussions. Neurology 2019; 92:e2717–e2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo T, Noble W, Hanger DP: Roles of tau protein in health and disease. Acta Neuropathol 2017; 133:665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks WA, Kovac A, Majerova P, Bullock KM, Shi M, Zhang J: Tau proteins cross the blood-brain barrier. J Alzheimer’s Dis 2016; 55:411–419. [DOI] [PubMed] [Google Scholar]
- 12.Katz DI, Bernick CB, Dodick D, et al. : NINDS Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 2021:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alosco ML, Jarnagin J, Tripodis Y, et al. : Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma 2017; 34:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dage JL, Wennberg AM, Airey DC, et al. : Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population based elderly cohort. Alzheimers Dement 2016; 12:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg E, Bougakov D: Neuropsychologic assessment of frontal lobe dysfunction. Psychiatr Clin North Am 2005(3 SPEC. ISS.):567–580. [DOI] [PubMed] [Google Scholar]
- 16.Reitan RM: Trail Making Test. Manual for Administration and Scoring. Tucson: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 17.Borkowski JG, Benton AL, Spreen O: Word Fluency and Brain Damage. Volume 5. Pergamon Press Ltd; 1967. [Google Scholar]
- 18.Golden CJ, Freshwater SM: Stroop Color and Word Test. Stoelting; Chicago; 1878. [Google Scholar]
- 19.Roth RM, Isquith PKGGA: Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A). Psychol Assess Resour 2005. [Google Scholar]
- 20.Reddy CP, Rathi Y: Joint multi-fiber NODDI parameter estimation and tractography using the unscented information filter. Front Neurosci 2016; 10(APR):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao R, Ning L, Chen Z, et al. : Performance of unscented Kalman filter tractography in edema: Analysis of the two-tensor model. NeuroImage Clin 2017; 15:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell LJ, Westin CF: Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans Med Imaging 2007; 26:1562–1575. [DOI] [PubMed] [Google Scholar]
- 23.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K: The WU-Minn Human Connectome Project: An overview. Neuroimage 2013; 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS: MRI-based topographic parcellation of human cerebral white matter and nuclei: II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 1999; 9:18–45. [DOI] [PubMed] [Google Scholar]
- 25.Witelson SF: Hand and Sex Differences in the Isthmus and Genu of the Human Corpus Callosum: A Postmortem Morphological Study. Volume 112; 1989. [DOI] [PubMed] [Google Scholar]
- 26.Norton I, Essayed WI, Zhang F, et al. : SlicerDMRI: Open source diffusion MRI software for brain cancer research. Cancer Res 2017; 77:e101–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Li Y, Lin H, Sinha R, Potenza MN: Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: A diffusion tensor imaging study. Hum Brain Mapp 2013; 34:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E: Fiber Composition of the Human Corpus Callosum. Volume 598; 1992. [DOI] [PubMed] [Google Scholar]
- 29.Dollé J-P, Jaye A, Anderson SA, et al. : Newfound sex differences in axonal structure underlie differential outcomes from in vitro traumatic axonal injury HHS Public Access. Exp Neurol 2018; 300:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasahara K, Hashimoto K, Abo M, Senoo A: Voxel- and atlas-based analysis of diffusion tensor imaging may reveal focal axonal injuries in mild traumatic brain injury - comparison with diffuse axonal injury. Magn Reson Imaging 2012; 30:496–505. [DOI] [PubMed] [Google Scholar]
- 31.Shenton MEM, Hamoda H, Schneiderman J, et al. : A Review of Magnetic Resonance Imaging and Diffusion Tensor Imaging Findings in Mild Traumatic Brain Injury. Brain Imaging Behav 2012; 6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawata K, Steinfeldt JA, Huibregtse ME, et al. : Association Between Proteomic Blood Biomarkers and DTI/NODDI Metrics in Adolescent Football Players: A Pilot Study. Front Neurol 2020; 11:581781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bendlin BB, Carlsson CM, Johnson SC, et al. : CSF T-TAU/Aβ 42 predicts white matter microstructure in healthy adults at risk for alzheimer’s disease. PLoS One 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenset V, Bjørnerud A, Fjell AM, et al. : Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging 2011; 32:581–589. [DOI] [PubMed] [Google Scholar]
- 35.Amlien IK, Fjell AM, Walhovd KB, et al. : Mild cognitive impairment: Cerebrospinal fluid tau biomarker pathologic levels and longitudinal changes in white matter integrity. Radiology 2013; 266:295–303. [DOI] [PubMed] [Google Scholar]
- 36.Blaylock R, Maroon J: Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int 2011; 2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laing KK, Simoes S, Baena-Caldas GP, et al. : Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun 2020; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey DM, Jones DW, Sinnott A, et al. : Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci 2013; 124:177–189. [DOI] [PubMed] [Google Scholar]
- 39.Michalicova A, Majerova P, Kovac A: Tau Protein and Its Role in Blood–Brain Barrier Dysfunction. Front Mol Neurosci 2020; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell M, Aherne S, O’Riordan S, O’Keeffe E, Greene C, Campbell M: Blood-brain barrier dysfunction in a boxer with chronic traumatic encephalopathy and schizophrenia. Clin Neuropathol 2019; 38:51–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement - Figure 1: Scatterplots displaying the association between diffusion parameters of the rostrum (CC1) and genu (CC2) of the corpus callosum (CC) and neuropsychological tests. In detail, panel A to E display test scores of the Trail Making Test Part A (TMT-A) and Part B (TMT-B) that are associated with diffusion measures of CC1, while panels F to L illustrate associations of TMT-A, TMT-B, Controlled Oral Word Association Test (COWAT) and the Behavioral Regulation Index from the Behavior Rating Inventory of Executive Function (BRIEF BRI) with diffusion measures of CC2.


