Abstract
The endothelial glycocalyx (eGC), a delicate carbohydrate-rich structure lining the luminal surface of the vascular endothelium, is vital for maintenance of microvascular homeostasis. In sepsis, damage of the eGC triggers the development of vascular hyperpermeability with consecutive edema formation and organ failure. While there is evidence that protection or rebuilding of the eGC might counteract sepsis-induced vascular leakage and improve outcome, approved therapeutics are not yet available. This narrative review aims to outline possible therapeutic strategies to ameliorate organ dysfunction caused by eGC impairment.
Keywords: Glycocalyx, Sepsis, Heparanase, Perfused boundary region, Glycosaminoglycan
Introduction
Sepsis, life-threatening organ dysfunction due to a dysregulated host response to infection, is a very common condition that often requires treatment in the intensive care unit (ICU). A central event in sepsis pathophysiology is the development of profound leakiness of the vascular barrier leading to tissue edema and subsequent multiple organ failure. Despite great efforts to refine the definition of sepsis and improve early screening approaches, mortality remained stable over the last decade [1], [2]. At the same time the current therapeutic approaches, including antibiotics, fluid resuscitation and vasopressors, not only fail to ameliorate the root problem of vascular leakiness, but may partially worsen it [3]. Both, current guidelines and clinical routine, lack specific therapeutic strategies to prevent the breakdown of the vascular barrier early on or rescue it in advanced stages of sepsis.
To identify possible therapeutic targets of vascular protection and rebuilding, it is necessary to take a closer look on the composition of the vascular barrier. Endothelial cells, the first cellular lining of the inner vessel wall, obviously play an important role in the development of its barrier function. Inter-endothelial junctions, composed of many different junctional proteins such as vascular endothelial (VE) cadherin, become loose upon harmful stimuli, enabling movement of plasma and leukocytes from the blood into the injured tissues. Although this complex mechanism is desirable to eliminate invading pathogens in a local infection, it is highly dangerous when dysregulated and generalized during sepsis. This cellular “gap formation” causes a distinctive increase in vascular permeability and further facilitates migration of immune cells from the vascular compartment to non-vascular tissues [4], [5], [6], [7]. So far, research on vascular integrity has focused mainly on endothelial cells and their junctions. However, recent data highlighted the key role of a delicate structure covering the endothelial cells and regulating the vascular homeostasis, the so called endothelial glycocalyx (eGC). This narrative review focuses on the protection and rebuilding of this neglected layer which is possibly affected even before the endothelial cells during sepsis.
Composition and function of the eGC
By the mid-1960s, a carbohydrate-rich surface layer on the apical side of vascular endothelium was visualized for the first time by electron microscopy [8]. The major physiological relevance and functional significance of this discovery was overlooked for a long time. Today, it is acknowledged that this complex and fragile eGC consists of various soluble and membrane bound components like glycosaminoglycans (GAGs) and proteoglycans, which form a negatively charged mesh, that facilitates frictionless blood flow through capillaries [9], [10], [11]. Along with various incorporated proteins of plasmatic and endothelial origin, the eGC attains a thickness of up to 2 µm and thus can be slightly thicker than the endothelial cells themselves [12], [13], [14], (Fig. 1, left panel). Meanwhile, many believe that this impressive layer holds the key to understanding the forces that regulate plasma filtration and the fundamental principles of edema [15], [16].
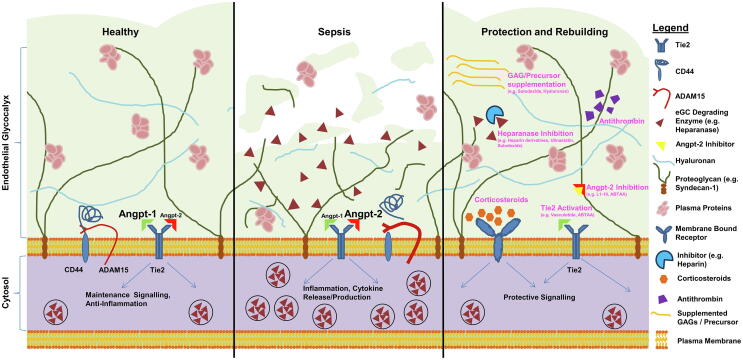
Fig. 1.
Protection and rebuilding of the endothelial glycocalyx (eGC): a schematic overview: The scheme summarizes potential approaches to prevent or ameliorate sepsis-induced eGC damage. Left: intact eGC with intact angiopoietin (Angpt)/Tie2 signalling promoting a quiescent state in healthy vessels. Middle: In sepsis, the Angpt-1/Angpt-2 ratio switches to Tie2 deactivation, which facilitates enzymatic degradation of the eGC via release of heparanase. Another eGC degrading mechanism is cleavage of the CD44 ectodomain, an important anchor for hyaluronan. Right: overview on possible therapeutic approaches to counteract eGC damage in sepsis.
Early studies of Van den Berg et al. already revealed that an intact eGC protects against edema formation [17]. Upon treatment of isolated rat hearts with hyaluronidase they observed a significant increase of pericapillary interstitial space while electron microscopy showed a concordant decrease in the eGC, indicating that loss of one specific eGC compound (in this case hyaluronan) leads to vascular barrier dysfunction. Elegant studies in perfused guinea pig hearts by Rehm et al. were the first to suggest, that the eGC acts as a competent barrier for water and colloids – independent of the endothelium itself [18]. Our lab further confirmed this finding using a modified Miles permeability assay in mouse skin. Specifically, peak permeability could not be triggered by histamine alone (which selectively opens the gaps), but was only observed after an additional damage to the eGC [19].
A growing number of sophisticated studies now shows, that not only the endothelial cell layer (as assumed by Ernest Starling in 1896 [20]), but also the gel-like extracellular eGC acts as a complex semi-permeable barrier along the vast surface area of the human vascular tree [21], [22]. Specifically, the carefully protected sub-glycocalyceal space – a protein-poor space between the eGC and the vascular endothelium – maintains a fairly high oncotic pressure gradient, thereby uncoupling the oncotic effect of interstitial concentration from the vessel lumen. Loss of the eGC, therefore, not only increases capillary permeability and interstitial edema formation, but also impairs “autotransfusion” capacity (i.e., the result of water moving into the circulation out of the non-circulating glycocalyx, which contributes about 25% of total intravascular volume [18]).
Besides its outstanding role for vascular permeability [15], [23], [24] several additional physiological properties were attributed to the eGC during the last years. Given its strategic location as the interface between blood and endothelium, the intact eGC controls leukocyte-endothelial interactions. Specifically, thinning of the eGC exposes previously hidden (because much smaller) endothelial surface adhesion molecules, including ICAM-1 and VCAM-1, allowing neutrophils to recognize and adhere to the endothelial surface [23], [25]. Beyond that, the eGC contributes to the regulation of the redox state and is crucially involved in the mediation of shear-induced nitric oxide release as well as physiologic anticoagulation [9], [13], [26]. Its structure is fairly stable but also in healthy endothelium subject to a permanent dynamic equilibrium between biosynthesis of new components and shear dependent removal, the so-called shedding, of existing constituents [27].
Visualization and measurement of the endothelial glycocalyx
As the eGC is composed of highly variable, membrane-bound as well as soluble components, its visualization and measurement has been technically challenging.
Characterization of eGC degrading products, in vitro approaches and preclinical visualization
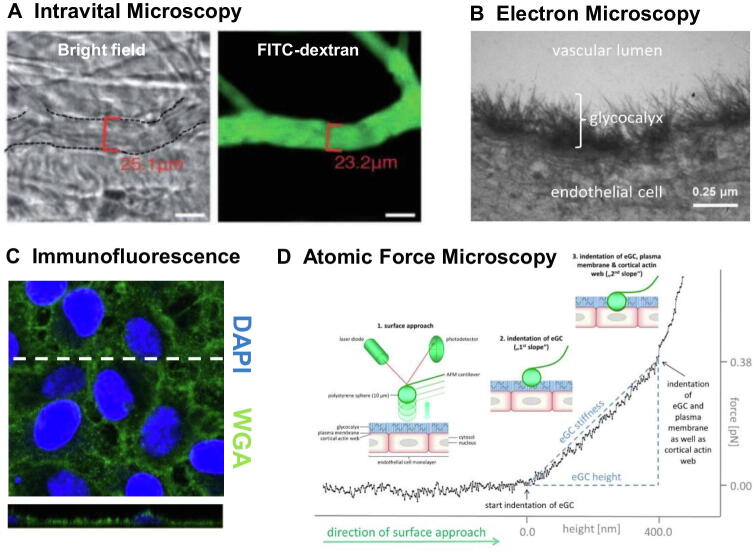
Assays for quantification of individual eGC components in e.g. blood, tissue fluids or cell culture supernatants can reveal information about turnover or degradation [28], [29], [30]. Intravital microscopy with e.g. FITC-labelled high-molecular dextran (which cannot enter the eGC) is one elegant method established in rodent models (Fig. 2). Indirect conclusions about the eGC can be drawn from changes in the vascular exclusion zone – i.e. the difference between FITC-positive capillary diameter before vs. after an eGC degrading event [31], [32], [33]. In living endothelial cells, Atomic Force Microscopy (AFM) approaches allow observation of differences in eGC thickness and stiffness, while immunostaining of fixated cells can also reveal changes in eGC composition [28], [34], [35]. In case of AFM, eGC thickness and stiffness are derived from a force versus distance curve upon nanoindentation (Fig. 2). Representing the eGC the first slope of the curve can be approximated for example via Young’s modulus [36], [37] or a simplified model of a linear fit [38]. Consequently, AFM allows estimation of eGC dimensions in cell culture or on vascular tissue. Nevertheless, absolute determination of eGC height should be regarded with reservation due to dependence of approximation modus and the methodical limitation of probably underestimating the less dense outer part of the eGC [15], [39]. Another method capable of providing high resolution images of the fragile luminal molecular structure is electron microscopy (EM). Classical EM staining protocols must be adapted to include heavy metal ions such as copper, ruthenium, or lanthanum. However, good results are only achieved by combined in vivo perfusion of fixative and stain [40].
Fig. 2.
Visualization and measurement of the endothelial glycocalyx (eGC): (A) Representative image of intravital microscopy in mouse cremaster model with FITC-dextran exclusion assay. The difference between bright field image vessel diameter and diameter of FITC-dextran (150 kDa), which cannot enter the eGC, allows estimation of eGC height in vivo. Image adapted from Yang et al. [32], modified. (B) Electron microscopy image of in vivo perfused/fixated and lanthanum-stained eGC in isolated rat aorta. Image adapted from Wiesinger et al. [38], modified. (C) Immunofluorescence wheat germ agglutinin (WGA)-staining of the eGC on endothelial cells with cross-sectional slide (indicated by the dashed line) along the stack. Image adapted from Drost et al. [59], modified. (D) Force versus distance curve generated upon nanoindentation on endothelial cells with atomic force microscopy. When indenting into the eGC the cantilever is deflected and linear fitting of the corresponding first slope allows deductions on eGC height. When indenting deeper the cantilever reaches the cell cortex resulting in the second slope as indicated in the schematic. Image adapted from Wiesinger et al. [38], modified.
Of note, in vitro approaches to characterize the eGC are limited as they can hardly reflect the highly dynamic changes of the eGC, which is subject to permanent modification in an intact organism. If a “mature” eGC, compared to in vivo conditions, can be detected in vitro must be questioned. Elegant studies by Potter et al. show that a hydrodynamically relevant eGC on human umbilical vein endothelial cells (HUVECs) could not be detected in standard cell culture conditions, especially in the absence of shear stress. While immunofluorescence staining frequently reveals the existence of eGC constituents like heparan sulfate or syndecan-1 in different cell lines [12], [28], [34], [35], electron microscopy studies of Chappell et al. show striking discrepancy of eGC height between HUVECs ex vivo (∼900 nm) and in vitro (∼30 nm) [12]. In contrast to those previous reports, our own AFM data provides evidence for the existence of a mature, enzyme-sensitive endothelial glycocalyx on both, freshly isolated rat/mouse aorta preparations and cultured endothelial cells [38]. A possible explanation for the unexpected eGC abundance in living endothelial cells in our in vitro experiments is that we examined glycocalyx dimensions directly (i.e. physically) without exposing cultured endothelial cells to potentially harmful fixation or staining procedures, thereby avoiding impairment of the fragile glycocalyx structure. These visualization approaches are essential for the study of eGC composition and allow experiments to analyse modification as a function of different signalling cascades.
Visualization of the endothelial glycocalyx at the bedside
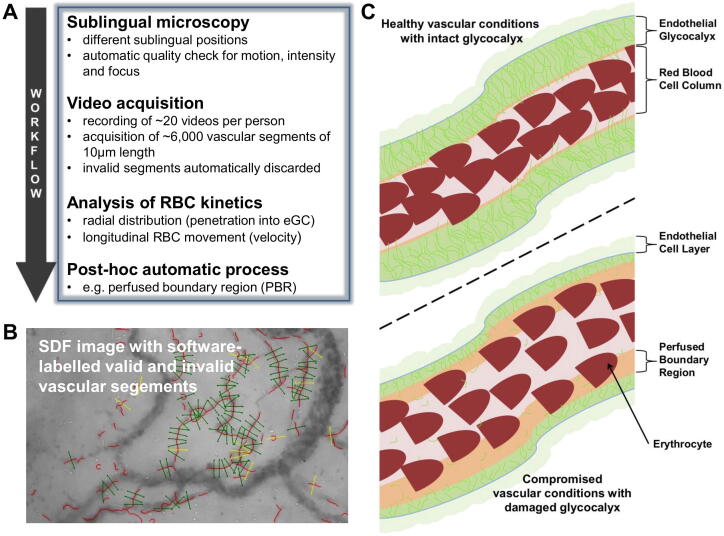
A completely new technique, the Glycocheck™ system, now offers the possibility to non-invasively estimate the eGC in vivo (Fig. 3). In brief, a side-stream dark field camera visualizes passing red blood cells (RBC) by emitting stroboscopic green light in the sublingual microcirculation. The dedicated Glycocheck™ software then estimates the dynamic lateral RBC movement into the glycocalyx, which is expressed as the perfused boundary region (PBR; in µm) [41]. An altered or degraded glycocalyx allows more RBCs to penetrate deeply toward the endothelial surface, with a consequent increase in the PBR. In our hands, the method showed not only a good intra- and inter-observer reproducibility, but also a great accuracy to estimate eGC thickness [42], [43]. Recent data suggest, that adjustment of PBR to feed vessel (diameter ≥10 µm) red blood cell (RBC) velocity can further improve discrimination between sepsis patients and healthy controls by about 50% [44]. Overall, PBR measurement is a very promising, non-invasive method for analysing eGC in future preclinical and clinical trials [45].
Fig. 3.
Analysis of the sublingual microvasculature and illustration of PBR measurement: (A) Overview on the workflow of performing sublingual microscopy in humans. Parameters such as the perfused boundary region (PBR), an inverse estimate of the eGC thickness, are calculated by the software post-hoc. Image adapted from Rovas et al. [44], modified. (B) Representative image acquired with the GlycoCheckTM system using side-stream darkfield imaging. The software automatically discards invalid segments (yellow lines). Image adapted from Rovas et al. [42], modified. (C) Schematic illustration of the PBR in healthy vascular conditions with intact eGC (low PBR) in contrast to compromised vasculature with damaged eGC (high PBR). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Evidence of eGC damage in bacterial sepsis
Observational studies in critically ill patients have shown that the severity of eGC damage – measured by shed eGC constituents in blood or urine – correlates with disease activity and predicts patient outcomes [28], [46]. In 2008 Nelson et al. detected increased levels of GAGs in plasma of sepsis patients with a maximum increase in non-survivors, while elevated levels of syndecan-1 correlated well with clinical sequential organ failure assessment (SOFA) score [29]. Similarly, Anand et al. observed increased levels of syndecan-1 and hyaluronan dependent on sepsis severity indicating prognostic value of these parameters in prediction of morbidity and survival in sepsis disease [30]. Further, in a post hoc analysis of 184 sepsis/septic shock patients admitted to two Danish intensive care units, Ostrowski et al. found that elevated blood levels of syndecan-1, a transmembrane heparan sulfate proteoglycan, were independently correlated with thrombelastography findings and functional fibrinogen levels, indicating a strong association between eGC damage and coagulopathy in sepsis [47]. However, because of the post hoc study design, validation in further studies is essential. Nonetheless, supportive data comes from Ikeda et al. who reported that syndecan-1 levels had strong discriminative power for the prediction of both disseminated intravascular coagulation (DIC) development and subsequent mortality in sepsis [48]. Beurskens et al. reported an increase in PBR within 24 h after ICU admission that correlated with endothelial injury markers and was further associated with mortality in sepsis [49].
With regard to the recently emerging coronavirus disease 2019 (COVID-19) pandemic, Queisser et al. observed significantly elevated activity of eGC degrading hyaluronidase and heparanase as well as elevated levels of circulating hyaluronan and heparan sulfate in COVID-19 and bacterial sepsis patients [50]. Consistently, Fraser et al. observed elevated markers of eGC shedding (especially syndecan-1 and hyaluronic acid) in a small COVID-19 ICU cohort compared to PCR-negative, age- and sex-matched ICU patients [51]. Of note, disease severity might be a relevant confounder in this small study as 100% of the COVID-19 positive group were diagnosed with sepsis in concordance with the Sepsis-3 definition [52] while this was the case for only 20% in the COVID-19 negative group. Comparison of eGC markers of the COVID-19 negative group versus healthy controls was not provided.
It should be noted that levels of shed eGC components seem to increase in both, acute kidney injury (AKI) and chronic kidney disease (CKD) [53]. However, it is completely unclear whether this effect is due rather to reduced renal clearance or to the severity of the underlying disease. Until this relationship is better understood, renal function should be considered as a potential confounder of circulating eGC fragments [54].
Stahl et al. also detected elevated syndecan-1 levels in a cohort of 19 ICU patients with severe COVID-19 on mechanical ventilation [55]. This indirect method of detecting eGC damage was complemented with sublingual estimation of eGC dimensions (as explained above), where increased PBR values in the COVID-19 group indicated significant damage of the endothelial glycocalyx compared to healthy controls.
In a further COVID-19 cohort study conducted by our lab, patients on mechanical ventilation showed severe glycocalyx damage as indicated by higher PBR values and increased blood levels of shed glycocalyx constituents. Of note, among several altered markers of endothelial dysfunction, PBR showed the best discriminatory ability to predict 60-day in-hospital mortality [45]. The new technique of in vivo visualization is one development that offers the possibility to intensify the investigation of eGC properties and its dynamic alterations in disease course [44].
Mechanisms of eGC damage, protection, and rebuilding
In vitro studies have shown, that eGC damage can occur upon exposure to various sepsis mediators, such as endotoxin [38], [56], TNFα [33], [40], [57], Angiopoietin-2 [19] or thrombin [38], [58] (Fig. 1, middle panel). Since all of these mechanisms play more or less an important role in sepsis, one assumes a multifactorial mode of injury to the eGC. However, even though the underlying molecular mechanisms here are still largely unknown, the final pathway of eGC impairment in sepsis seems to be fairly uniform. Therefore, the prevention or mitigation of eGC damage in systemic inflammation, and particularly in sepsis, appears to be a promising target for therapeutic interventions. Schematic illustration of the following approaches is represented in Fig. 1, right panel and a tabular summary is provided in Table 1.
Table 1.
Overview on mechanistic approaches to prevent or counteract damage of the endothelial glycocalyx.
| First Author | Year | Model | Methods | Protection/Rescue strategy |
|---|---|---|---|---|
| X. Du [6] | 2021 | - cell culture | - immunofluorescence - FITC-dextran permeability assay |
Anisodamine Hydrobromide prevents LPS-induced loss of HS and reduces cell barrier permeability. |
| A. Lukasz [19] | 2019 | - C57BL/6J mice - cell culture |
- IVM (SDF, back skin) - Miles assay - AFM - immunofluorescence - heparanase activity |
Angpt-2 leads to PBR increase in vivo and causes heparanase-dependent eGC decline in vitro. NAH reduces Angpt-2 induced leakage. Angpt-1, L1-10 (Angpt-2 inhibition) and NAH prevent Angpt-2 induced eGC decline. |
| A.A. Constantinescu [24] | 2011 | - male C57BL/6J mice - transgenic ApoE3-Leiden mice |
- IVM (M. cremaster) | High-fat cholesterol-enriched diets lead to subendothelial lipid deposits and decrease eGC in these areas. |
| H. Vink [26] | 2000 | - male golden hamster | - IVM (M. cremaster) | Bolus of oxidized LDL leads to transient reduction of eGC and increased platelet adhesion. Super-oxide dismutase + catalase treatment prevents eGC reduction and platelet adhesion. |
| E.P. Schmidt [31] | 2012 | - male C57BL/6 mice - Hpse−/− mice - Tnfrsf1atm1Imx mice - cell culture |
- IVM (FITC-dextran 150kDa, lung, M. cremaster) - HS degradation activity - Western blot |
In wild type mice LPS-induced eGC loss is heparanase-dependent and can be prevented with NAH or heparin administration. Neutrophil adhesion is attenuated and survival improved in Hpse−/− mice. TNFR1-knockout and Hpse-/- mice show no loss of eGC upon treatment with LPS. |
| X. Yang [32] | 2018 | - ADAM15-/- mice - wild-type mice - human lungs ex vivo |
- IVM (FITC-albumin, -dextran 150kDa, cremaster, mesentery) - electron microscopy - ELISA |
LPS-induced eGC damage and vascular leakage is significantly reduced in ADAM15-/- mice. In CLP ADAM15-/- mice show less increase of soluble CD44, mechanistically suggesting ADAM15-dependent shedding of CD44. |
| C.B.S. Henry [33] | 2000 | - male Syrian golden hamsters | - IVM (FITC-dextran 40, 70, 580, or 2,000, FITC-albumin, M. cremaster) | Fucoidan leads to reduction of TNFα-induced leukocyte adherence but does not change FITC penetration to the eGC. |
| R. Ramnath [34] | 2014 | - cell culture | - immunofluorescence - Alcian blue dye binding assay - TaqMan low-density array |
TNFα induces shedding of HS and SDC-4 which can be counteracted by pretreatment with batimastat (MMP inhibitor) or MMP9 siRNA. |
| Y. Zeng [35] | 2014 | - cell culture | - immunofluorescence - DMMB assay |
S1P prevents loss of CS, HS and SDC-1 coverage in FBS and BSA free medium and reduces MMP activity (which can be counteracted by S1P inhibitor W146). Specific inhibition of MMP-9 and MMP13 prevents loss of CS. |
| A. Wiesinger [38] | 2013 | - Lewis-Brown Norway rats - male C57BL/6 mice - cell culture |
- AFM, (cells, aorta ex vivo) - electron microscopy |
TNFα, LPS, thrombin or heparinase I induce eGC decline. Heparin preserves eGC upon treatment with heparinase I. |
| D. Chappell [40] | 2009 | - isolated guinea pig hearts | - ELISA - electron microscopy |
Hydrocortisone and antithrombin III prevent TNFα-induced eGC decline. Both prevent increase of shed SDC-1 and HS (for HS stronger effect of hydrocortisone). |
| K. Stahl [55] | 2020 | - cell culture | - immunofluorescence | COVID-19 serum induces eGC damage in vitro but not in case of transgenic overexpression of heparanase 2. |
| N. Cui [56] | 2015 | - male Sprague-Dawley rats | - gelatin zymogram -immunohistochemistry - ELISA |
Dexamethasone and doxycycline both significantly suppress LPS-induced MMP-2 and -9 expression and both partially inhibit decline of SDC-1. |
| M. Nieuwdorp [57] | 2009 | - human male volunteers | - IVM (orthogonal polarization spectroscopy imaging, sublingual) - ELISA |
Etanercept reduces LPS-induced decline in eGC thickness and attenuates increase in hyaluronan. |
| C.C. Drost [59] | 2019 | - cell culture | - AFM - immunofluorescence |
Sepsis serum induces eGC decline which can be counteracted with administration of NAH, VT (Tie2 activation), or L1-10 (Angpt-2 inhibition). VT restores eGC damage in case of delayed administration and reduces heparanase activity. Delayed VT and Angpt-1 administration attenuate heparinase I induced eGC damage. |
| S. Chen [60] | 2017 | - male C57BL/6 mice | - immunohistochemistry - ELISA - Western blot |
NAH attenuates CLP-induced eGC damage, inhibits leukocyte infiltration and reduces heparanase serum levels, heparanase expression and loss of SDC-1, potentially via MMP-9 and uPA reduction. NAH further suppresses production of inflammatory cytokines. |
| X. Huang [61] | 2019 | - male Sprague-Dawley rats | - ELISA - heparanase activity assay - immunofluorescence - wet/dry ratio - histologic examination |
UFH and NAH similarly prevent LPS-induced increase of HS, SDC-1 and heparanase activity. Both attenuate lung tissue injury and reduce production of inflammatory cytokines. |
| S. Yini [63] | 2015 | - adult beagle dogs | - ELISA | UFH significantly prevents decline of SDC-1 and HS upon infusion of E. coli compared to basic treatment (ceftriaxone + fluid resuscitation). Survival between both groups was not significantly different. |
| L. Wang [65] | 2016 | - C57BL/6 mice - cell culture |
- ELISA - heparanase activity assay - immunofluorescence - Evans blue dye extravasation technique |
Ulinastatin prevents LPS-induced HS increase, reduces heparanase activity and expression and attenuates vascular permeability. |
| V. Masola [69] | 2012 | - cell culture | - heparanase activity assay - mRNA expression analysis |
Sulodexide reduces FGF-2-mediated heparanase overexpression and SDC-1 downregulation. |
| J.W. Song [70] | 2017 | - C57/BL6 mice - cell culture |
- distribution of FITC-dextran 40, 70, 500kDa - Evans blue dye extravasation technique - immunofluorescence - AFM |
Sulodexide restores FIP- (feces-induced peritonitis) or LPS-induced loss of eGC volume, reduces vascular permeability and improves survival. |
| T. Li [71] | 2017 | - male Sprague-Dawley rats | - electron microscopy - immunohistochemistry - immunofluorescence |
Sulodexide leads to recovery of eGC and normal eNOS levels, reduces CD31 and ICAM-1 and decreases inflammatory cell infiltration after carotid artery balloon-injury. |
| D. Chappell [75] | 2007 | - isolated guinea pig hearts | - electron microscopy - ELISA |
Hydrocortisone preserves eGC with less tissue edema and reduces HS, hyaluronan and SDC-1 shedding in an ischemia-reperfusion model. |
| S.L. Gao [76] | 2015 | - male Sprague-Dawley rats | - electron microscopy - ELISA |
Hydrocortisone stabilizes eGC and reduces HS and SDC-1 levels and improves intestinal perfusion in severe acute pancreatitis. |
| T. Iba [79] | 2021 | - rats (Wistar) | - IVM (leukocyte adhesion, SDF) - ELISA |
AT-γ ameliorates LPS-induced impairment of microcirculation with decrease of PBR and reduction of SDC-1 and hyaluronan levels. |
| S. Han [87] | 2016 | - C57BL/6J mice - Ang1flox/flox mice - Ang2flox/flox mice - Tie2flox/flox mice - cell culture |
- IVM (FITC-dextran 40kDa) - immunofluorescence - Evans blue dye extravasation technique |
Administration of ABTAA (Angpt-2 binding and Tie2 activating antibody) improves survival in LPS, CLP and S. aureus sepsis model, reduces leakage and production of inflammatory cytokines, prevents shedding of HS and perlecan and reduces heparanase density in lung and kidney tissue. Thickness of the eGC improves upon ABTAA treatment with reduced leukocyte adhesion and improved vascular perfusion. |
| A.H.J. Salmon [88] | 2009 | - adult frog - rats (Wistar) - cell culture |
- perfusion (Landis–Michel technique, oncopressive permeability technique) - electron microscopy - Alcian Blue assay |
Angpt-1 restores direct contact of eGC to plasmalemma after separation upon pronase perfusion. Angpt-1 decreases water permeability of the endothelium and increases depth of eGC as well as GAG turnover in healthy vessels. |
Abbreviations: ADAM (a disintegrin and metalloproteinase), AFM (atomic force microscopy), Angpt-1 (angiopoietin-1), Angpt-2 (angiopoietin-2), AT-γ (newly developed recombinant non-fucosylated antithrombin), BSA (bovine serum albumin), CLP (cecal ligation and puncture), CS (chondroitin sulfate), E. coli (Escherichia coli), eGC (endothelial glycocalyx), ELISA (enzyme-linked immunosorbent assay), eNOS (endothelial nitric oxide synthase), FBS (fetal bovine serum), FGF-2 (fibroblastic growth factor 2), FIP (feces-induced peritonitis), FITC (fluorescein isothiocyanate), GAG (glycosaminoglycan), HS (heparan sulfate), Hpse (heparanase), ICAM-1 (intercellular adhesion molecule 1), IF (immunofluorescence), IVM (intravital microscopy), LDL (low-density lipoprotein), LPS (lipopolysaccharide), MMP (matrix metalloproteinase), NAH (N-desulfated/re-N-acetylated heparin), PBR (perfused boundary region), S. aureus (Staphylococcus aureus), SDC (syndecan), SDF (side-stream darkfield), siRNA (short interfering ribonucleic acid), S1P (sphingosine-1-phosphate), TNFα (tumor necrosis factor α), UFH (unfractionated heparin), uPA (urokinase-type plasminogen activator), VT (Vasculotide™)
Inhibition of the heparan sulfate (HS) degrading enzyme heparanase
The molecule that has been identified as playing a key role in sepsis-induced eGC degradation is heparanase, the heparan sulfate (HS) degrading enzyme. Schmidt et al. could show that lipopolysaccharide (LPS)-induced pulmonary glycocalyx degradation in mice was strongly dependent on TNFα-induced activation of endothelial heparanase, as pre-treatment with heparin or the non-anticoagulant heparanase inhibitor N-desulfated/re-N-acetylated heparin (NAH) was able to prevent LPS-induced eGC loss [31]. The LPS-induced adhesion and extravasation of neutrophils as well as pulmonary hyperpermeability were also significantly reduced by delayed inhibition of heparanase (administered 3 h after LPS). Delayed heparanase inhibition by a single dose of heparin (administered 24 h after CLP) reduced pulmonary endothelial hyperpermeability and improved survival in mice undergoing cecal ligation and puncture (CLP), a clinically relevant model of polymicrobial sepsis. Using AFM we could show earlier, that unfractionated heparin (UFH) completely abolished the decline of eGC thickness in freshly isolated rat aorta [38]. AFM also revealed that the addition of serum from sepsis patients, but not serum from healthy controls, induced thinning of the eGC on human ECs in vitro, which was prevented by NAH [59].
However, prophylactic administration of heparin or NAH seems not only to protect the eGC, but also to reduce systemic inflammation and further heparanase release. In a CLP mouse model, Chen et al. observed a suppressed heparanase expression and syndecan-1 shedding upon NAH pre-treatment [60]. Neutrophil infiltration and production of pro-inflammatory cytokines such as TNFα, IL-1ß or IL-6 were reduced and systemic inflammation and septic intestinal injury were attenuated. These findings were essentially reproducible in LPS-treated rats that simultaneously received UFH or NAH [61]. Similar to the results of Schmidt et al. no significant difference between UFH and the non-anticoagulant NAH was observed. Mortality and LPS-induced organ dysfunction were significantly attenuated in endotoxemic mice when treated with UFH [62]. Further cross-species evidence for the protective effect of UFH or NAH comes from Yini et al., who observed an UFH-dependent reduction of HS and syndecan-1 levels upon LPS administration in beagle dogs [63].
Another interesting approach of inhibiting heparanase is the use of the urinary protease inhibitor Ulinastatin. Several clinical trials already suggest reduced mortality and length of hospital stay in patients with acute respiratory distress syndrome (ARDS) treated with Ulinastatin, although further clinical studies are certainly needed [64]. Briefly, Ulinastatin was shown to inhibit LPS-induced heparanase expression and activity and to suppress vascular permeability and HS degradation in a murine ARDS model. The latter result was confirmed mechanistically in HUVECs treated with LPS with or without Ulinastatin [65].
In summary, endothelial heparanase and its inhibition seem to represent a promising target in eGC protection and rebuilding upon inflammatory diseases. Heparanase inihibitors, developed for inhibition of metastasis, already entered clinical trials [66]. Evaluation of these inhibitors to ameliorate vascular hyperpermability and subsequent organ-damage in sepsis might also be justified. Even more suggestive to examine is the use of heparin [67]. Heparins are relatively safe and well established drugs in clinical routine, that appear as promising candidates to inhibit heparanase-mediated eGC damage in sepsis. As outlined above, several other off-target effects of heparins might reduce stress on the vascular barrier. In a recent meta-analysis of 10 randomized controlled trails in adult sepsis patients, low-molecular-weight heparin (LMWH), which has several advantages over UFH, reduced inflammatory reaction, the incidence of multiple organ dysfunction syndrome (MODS) and 28-day mortality compared to standard treatment [68].
Even though the results of this meta-analysis and the above mentioned preclinical studies provide a consistent picture of the protective effect of UFH, NAH or Ulinastatin on the eGC, further studies, preferably using PBR measurement, are necessary to quantify and monitor the specific therapy effect on the eGC and to derive appropriate dosage regimens.
Substitution of eGC components or precursors
Several studies have investigated the concept of endogenous sealing of a damaged glycocalyx using GAGs or its precursors. From a pharmacological point of view, the studies on Sulodexide, a HS-like compound resistant to degradation by heparanase are particularly interesting. Of note, besides providing eGC precursor constituents, Sulodexide was shown to comprise the ability of not only being resistant to, but also inhibiting the eGC degrading enzyme heparanase [69]. The delayed subcutaneous administration of Sulodexide, two hours after sepsis induction, reduced pulmonary permeability and significantly improved survival rates in mice with feces-induced peritonitis (FIP). Staining of eGC components on mouse aortas and measurement of glycocalyx (plasma) volume revealed strong preservation of the eGC by Sulodexide [70]. Further evidence for the restorative properties of Sulodexide comes from a balloon-injury rat carotid artery model, where Sulodexide (administered for 7 days after injury) normalized eGC remodelling and endothelial function [71].
Similar protective effects were also observed after administration of the nonsulfated GAG hyaluronan upon binding to the CD44 receptor. Specifically, the administration of high molecular weight hyaluronan (HMW-HA) blocked pulmonary vascular leakage in the LPS murine model [72] and significantly improved blood oxygenation, lung histology and survival in CLP mice [73]. Mechanistically, the results of Queisser et al. provide complementary insight into the complex and fine adjustment of CD44 interaction with hyaluronan as low molecular weight hyaluronan fragments (LMW-HA) isolated from plasma of COVID-19 patients induce CD44 dependent disruption of the endothelial barrier in lung microvascular endothelial cells [50]. Unfortunately, the eGC was not investigated in either study, so the effects on the reconstitution of the eGC itself remain unclear.
The fact that CD44 apparently plays an interesting role is also shown by the data from Yang et al., who observed an upregulated expression of a disintegrin and metalloproteinase 15 (ADAM15), which cleaves the hyaluronan receptor CD44, in LPS-treated human lung tissue [32]. Along with a preserved eGC in cremasteric and pulmonary microvessels, vascular leakage was greatly attenuated in ADAM15-/- mice upon LPS administration, again underlining a relevant contribution of hyaluronan to the endothelial barrier function. Of note, cleaved ectodomain of CD44 was additionally shown to cause disorganisation of cell–cell adherens junctions - an obvious hint that eGC and endothelial cells represent a deeply interlaced unit regulating vascular barrier function [32].
Administration of corticosteroids
Another interesting therapeutic approach might be the administration of corticosteroids. Interestingly, the current sepsis guidelines recommend hydrocortisone administration as therapeutic option in patients with refractory septic shock [74]. In 2007 Chappell et al. could show for the first time protective effects of hydrocortisone on the vascular endothelial glycocalyx in ischemia–reperfusion injury of isolated guinea pig hearts [75]. Administration of hydrocortisone led to reduced eGC shedding indicated by decreased levels of syndecan-1, HS and hyaluronan and a markedly reduction of tissue edema. These protective effects could be reproduced in context of inflammatory eGC degradation induced by TNFα. Vascular leak was prevented upon perfusion of guinea pig hearts with hydrocortisone followed by TNFα [40]. Corroborative results come from a rodent model of severe acute pancreatitis by Gao et al. who detected preserved eGC in electron microscopy in case of hydrocortisone treatment in contrast to sham treatment, where significant eGC damage was observed [76]. Mechanistically, Cui et al. determined a reduced activity of the matrix metalloproteinases 2 and 9 (MMPs) in a rat model when dexamethasone was applied prior to sepsis induction with LPS [56]. LPS-induced decline of syndecan-1 expression in aortic tissue was partially, but significantly, counteracted by dexamethasone. A similar effect could be observed for the expression of ZO-1, a structural protein of cellular tight junctions, indicating also an impact on the cellular part of the vascular barrier in case of dexamethasone. Nevertheless, clinical observations concerning the interplay of corticosteroids and glycocalyx properties are rare. Brettner et al. could show that pre-OP administration of hydrocortisone led to attenuated increase of heparan sulfate and syndecan-1 after cardiac surgery, although no relevant impact on clinical course or outcome was observed [77].
Recombinant antithrombin
As regulation of blood coagulation is also known to be tightly dependent on eGC interaction, there are also components of the coagulation cascade coming to interest when discussing protection of eGC. In sepsis, the development of DIC represents the fulminant stage of dysregulated coagulation. Chappell et al. could show that antithrombin preserved the eGC and reduced vascular leakage in TNFα-exposed isolated guinea pig hearts [40]. However, real-world data of 30 randomized controlled trials (RCTs) focusing on the administration of antithrombin III in critically ill patients did not reveal a survival benefit [78]. Nonetheless, further studies are needed to decode the complex interplay between coagulation and the eGC. For example, Iba et al. could recently observe less syndecan-1 and hyaluronan shedding, as well as preservation of glycocalyx thickness (PBR method) after administration of recombinant antithrombin in LPS-treated rats [79].
The Angiopoietin/Tie2 ligand-receptor axis
The endothelial specific angiopoietin (Angpt)/Tie2 system was already shown to control gap formation and the development of vascular leakage in human sepsis [80]. Under physiological conditions, Tie2 is tonically activated by Angpt-1, a vasculoprotective protein secreted by peri-endothelial cells and platelets [81]. In human sepsis, its intrinsic antagonist called Angpt-2 is rapidly released from activated endothelium, competitively inhibits Tie2 and predicts mortality as a biomarker [80], [82], [83]. Using AFM and conventional intravital microscopy, we could show that Angpt-2 mediates breakdown of the eGC as well [19]. Mechanistically, Angpt-2 caused heparanase secretion from distinctive cellular storage pools with consecutive enzymatic degradation of the eGC in vitro. Corresponding in vivo experiments indicated that heparanase-dependent eGC breakdown, induced by exogenous Angpt-2, contributes to plasma leakage. These results extend our understanding of the Angpt/Tie2 system as being a shared and non-redundant regulator of both layers of the vascular double barrier: the endothelial cell and the endothelial glycocalyx. Therefore, it is quite possible that the protection against plasma leakage conferred by either exogenous Tie2 stimulation, Angpt-2 scavenging, or genetic deletion of Angpt-2 in different models of sepsis is partly due to the preservation of the eGC [84], [85], [86]. Unfortunately, very few of these studies have investigated glycocalyx properties. After delayed administration of an Angpt-2 binding and Tie2-activating antibody (ABTAA), Han et al. observed reduced vascular leakage, a dampened cytokine storm and improved survival in CLP mice [87]. ABTAA treatment prevented upregulation of heparanase and subsequent enzymatic degradation of HS in pulmonary capillaries. Intravital microscopy revealed that pulmonary eGC was well preserved in ABTAA-treated, but markedly reduced in untreated septic mice (exclusion zone method). Of note, delayed Angpt-2 inhibition alone (without additional Tie2 activation) had no protective effect. Further corroborative in vitro studies from our lab confirm, that preventative, but not delayed Angpt-2 inhibition abolished heparanase-induced glycocalyx loss in response to (Angpt-2 containing) sepsis serum [59]. Of note, Tie2 activation by either recombinant Angpt-1 or the Tie2-activating drug Vasculotide™, led to immediate rebuilding of a severely damaged eGC within only 30 min [59], while no recovery was seen within 24 h after vehicle treatment (unpublished results). Consistent with this observation, Tie2 activation enhanced the sulfated GAG content in supernatant of ECs, indicating that Tie2 activation accelerates glycocalyx refurbishment through enhanced GAG supply. This working hypothesis is supported by early studies conducted by Salmon et al. who were able to show that the Angpt-1-induced recovery of the eGC and subsequent reduction in permeability are inhibited by the intracellular transport inhibitor Brefeldin [88].
Conclusion and outlook
The above presented studies provide clear evidence that the eGC plays a central role in the pathophysiology of sepsis and development of vascular leakiness with subsequent organ dysfunction. One might even suggest a “gatekeeper” function, as cytokine production, leukocyte adhesion and cellular signalling are shown to be strongly interlaced with eGC constitution. Consequently, approaches to protect this highly delicate structure appear important to ameliorate sepsis-induced vascular leakiness early on. Targets such as the Tie2 system seem promising, given its profound effect on the whole vascular barrier. Nevertheless, effort on the evaluation of (non-anticoagulant) heparins or corticosteroids in clinical studies might also be of great value. Although it is unclear whether or not they have a restorative effect, the safety and years of clinical experience with these agents are undisputed advantages over experimental drug candidates. The development of new in vivo visualization techniques allows assessment of eGC changes in vivo, facilitating the integration of eGC bedside monitoring in future clinical studies.
Indeed, over the last years, the endothelial glycocalyx has moved from the fringes of research to the centre of attention in sepsis pathophysiology. To date, targeted eGC protection and rebuilding in clinical routine is still fiction. However, with regard to the impressive progress in eGC research, the scientific fundamentals are in place allowing this promising approach to become a reality in the near future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by the German Research Foundation (KFO342), to CD (ZA 428/18-1), AR (ZA 428/18-1) and PK (KU 2873/3-1). The funding sources of the study had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors’ contributions
CD did literature research, drafted the manuscript and designed the graphical illustrations. AR and PK did literature research, proofread and complemented the manuscript.
Acknowledgments
We would like to acknowledge the support of the Open Access Publication Fund of the University of Münster.
Contributor Information
Carolin Christina Drost, Email: carolinchristina.drost@ukmuenster.de.
Alexandros Rovas, Email: alexandros.rovas@ukmuenster.de.
Philipp Kümpers, Email: philipp.kuempers@ukmuenster.de.
References
- 1.Fleischmann C., Scherag A., Adhikari N.K.J., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.PRISM Investigators K.M., Rowan D.C., Angus M., Bailey A.E., Barnato R., Bellomo R.R., Canter T.J., Coats A., Delaney E., Gimbel R.D., Grieve D.A., Harrison A.M., Higgins B., Howe D.T., Huang J.A., Kellum P.R., Mouncey E., Music S.L., Peake F., Pike M.C., Reade M.Z., Sadique M., Singer D.M. Yealy, early, goal-directed therapy for septic shock — a patient-level meta-analysis. N. Engl. J. Med. 2017;376:2223–2234. doi: 10.1056/nejmoa1701380. [DOI] [PubMed] [Google Scholar]
- 3.Marik P., Bellomo R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth. 2016;116(3):339–349. doi: 10.1093/bja/aev349. [DOI] [PubMed] [Google Scholar]
- 4.Clajus C., Lukasz A., David S., Hertel B., Lichtinghagen R., Parikh S.M., Simon A., Ismail I., Haller H., Kümpers P. Angiopoietin-2 is a potential mediator of endothelial barrier dysfunction following cardiopulmonary bypass. Cytokine. 2012;60(2):352–359. doi: 10.1016/j.cyto.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae J.-S., Lee W., Son H.-N., Lee Y.-M., Kim I.-S. Anti-transforming growth factor β-induced protein antibody ameliorates vascular barrier dysfunction and improves survival in sepsis. Acta Physiol. 2014;212(4):306–315. doi: 10.1111/apha.12398. [DOI] [PubMed] [Google Scholar]
- 6.Du X., Liu H., Yue Y., Wu Q., Jiang W., Qiu Y., Zeng Y. Anisodamine hydrobromide protects glycocalyx and against the lipopolysaccharide-induced increases in microvascular endothelial layer permeability and nitric oxide production. Cardiovasc. Eng. Technol. 2021;12(1):91–100. doi: 10.1007/s13239-020-00486-8. [DOI] [PubMed] [Google Scholar]
- 7.Hakanpaa L., Sipila T., Leppanen V., Gautam P., Nurmi H., Jacquemet G., Eklund L., Ivaska J., Alitalo K., Saharinen P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luft J.H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 1966;25:1773–1783. [PubMed] [Google Scholar]
- 9.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A.M.J., oude Egbrink M.G.A. The endothelial glycocalyx: composition, functions, and visualization. Pflügers Arch. – Eur. J. Physiol. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushiyama A., Kataoka H., Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensive Care. 2016:1–11. doi: 10.1186/s40560-016-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipowsky H.H., Gao L., Lescanic A. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am. J. Physiol. – Hear. Circ. Physiol. 2011;301:2235–2245. doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell D., Jacob M., Paul O., Rehm M., Welsch U., Stoeckelhuber M., Conzen P., Becker B.F. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ. Res. 2009;104(11):1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwdorp M., Meuwese M.C., Vink H., Hoekstra J.B.L., Kastelein J.J.P., Stroes E.S.G. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 14.Yen W., Cai B., Zeng M., Tarbell J.M., Fu B.M. Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc. Res. 2012;83:337–346. doi: 10.1016/j.mvr.2012.02.005.Quantification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry F.E., Adamson R.H. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012;40:828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levick J.R., Michel C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berg B.M., Vink H., Spaan J.A.E. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 18.Rehm M., Haller M., Orth V., Kreimeier U., Jacob M., Dressel H., Mayer S., Brechtelsbauer H., Finsterer U. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95:849–856. doi: 10.1097/00000542-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lukasz A., Hillgruber C., Oberleithner H., Kusche-Vihrog K., Pavenstädt H., Rovas A., Hesse B., Goerge T., Kümpers P. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc. Res. 2017;9:698–704. doi: 10.1093/cvr/cvx023. [DOI] [PubMed] [Google Scholar]
- 20.Starling E. On the absorption of fluids from the connective tissue spaces. J. Physiol. 1894 doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler M.J., Down C.J., Foster R.R., Satchell S.C. The pathological relevance of increased endothelial glycocalyx permeability. Am. J. Pathol. 2020;190:742–751. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Sarmiento J., Salazar-Pelaéz L.M., Carcillo J.A. The endothelial glycocalyx: a fundamental determinant of vascular permeability in sepsis. Pediatr. Crit. Care Med. 2020;21(5):E291–E300. doi: 10.1097/PCC.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulivor A.W., Lipowsky H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. – Hear. Circ. Physiol. 2002;283:1282–1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 24.Constantinescu A.A., Spaan J.A.E., Arkenbout E.K., Vink H., van Teeffelen J.W.G.E. Degradation of the endothelial glycocalyx is associated with chylomicron leakage in mouse cremaster muscle microcirculation. Thromb. Haemost. 2011;105(05):790–801. doi: 10.1160/TH10-08-0560. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu A.A., Vink H., Spaan J.A.E. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler. Thromb. Vasc. Biol. 2003;23(9):1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 26.Vink H., Constantinescu A.A., Spaan J.A.E. Oxidized lipoproteins degrade the endothelial surface layer implications for platelet-endothelial cell adhesion. Circulation. 2000:3075–3078. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 27.Lipowsky H.H. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 28.Luker J.N., Vigiola Cruz M., Carney B.C., Day A., Moffatt L.T., Johnson L.S., Shupp J.W. Shedding of the endothelial glycocalyx is quantitatively proportional to burn injury severity. Ann. Burns Fire Disasters. 2018;31:17–22. [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson A., Berkestedt I., Schmidtchen A., Ljunggren L., Bodelsson M. Increased levels of glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 30.Anand D., Ray S., Srivastava L.M., Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016;49:768–776. doi: 10.1016/j.clinbiochem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., Lynelle P., Cheng S.S., Overdier K.H., Thompson K.R., Geraci M.W., Ivor S. The pulmonary endothelial glycalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012;18:1–20. doi: 10.1038/nm.2843.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Meegan J.E., Jannaway M., Coleman D.C., Yuan S.Y. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc. Res. 2018;114:1752–1763. doi: 10.1093/cvr/cvy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry C.B.S., Duling B.R. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. – Hear. Circ. Physiol. 2000;279:2815–2823. doi: 10.1152/ajpheart.2000.279.6.h2815. [DOI] [PubMed] [Google Scholar]
- 34.Ramnath R., Foster R.R., Qiu Y., Cope G., Butler M.J., Salmon A.H., Mathieson P.W., Coward R.J., Welsh G.I., Satchell S.C. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor α: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28(11):4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y., Adamson R.H., Curry F.R.E., Tarbell J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. – Hear. Circ. Physiol. 2014;306(3):H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Callaghan R., Job K.M., Dull R.O., Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Am. J. Physiol. – Lung Cell. Mol. Physiol. 2011;301(3):L353–L360. doi: 10.1152/ajplung.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai K., Wang W. Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J. R. Soc. Interface. 2012;9(74):2290–2298. doi: 10.1098/rsif.2011.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiesinger A., Peters W., Chappell D., Kentrup D., Reuter S., Pavenstädt H., Oberleithner H., Kümpers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8:e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao L., Lipowsky H.H. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;2010(80):394–401. doi: 10.1016/j.mvr.2010.06.005.Composition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chappell D., Hofmann-Kiefer K., Jacob M., Rehm M., Briegel J., Welsch U., Conzen P., Becker B.F. TNF-α induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009;104(1):78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee D.H., Dane M.J.C., Van Den Berg B.M., Boels M.G.S., Van Teeffelen J.W., De Mutsert R., Den Heijer M., Rosendaal F.R., Van Der Vlag J., Van Zonneveld A.J., Vink H., Rabelink T.J. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0096477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rovas A., Lukasz A., Vink H., Urban M., Sackarnd J., Pavenstädt H. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit – the GlycoNurse study. Scand. J. Trauma. Resusc. Emerg. Med. 2018;26(1) doi: 10.1186/s13049-018-0483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rovas A., Seidel L.M., Vink H., Pohlkötter T., Pavenstädt H., Ertmer C., Hessler M., Kümpers P. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit. Care. 2019;23:260. doi: 10.1186/s13054-019-2542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rovas A., Sackarnd J., Rossaint J., Kampmeier S., Pavenstädt H., Vink H., Kümpers P. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: the NOSTRADAMUS study. Crit. Care. 2021;25:1–14. doi: 10.1186/s13054-021-03520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rovas A., Osiaevi I., Buscher K., Sackarnd J., Tepasse P.R., Fobker M., Kühn J., Braune S., Göbel U., Thölking G., Gröschel A., Pavenstädt H., Vink H., Kümpers P. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt E.P., Overdier K.H., Sun X., Lin L., Liu X., Yang Y., Ammons L.A., Hiller T.D., Suflita M.A., Yu Y., Chen Y., Zhang F., Burlew C.C., Edelstein C.L., Douglas I.S., Linhardt R.J. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrowski S.R., Haase N., Müller R.B., Møller M.H., Pott F.C., Perner A., Johansson P.I. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit. Care. 2015:1–10. doi: 10.1186/s13054-015-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda M., Matsumoto H., Ogura H., Hirose T., Shimizu K., Yamamoto K., Maruyama I., Shimazu T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care. 2018;43:48–53. doi: 10.1016/j.jcrc.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Beurskens D.M.H., Bol M.E., Delhaas T., van de Poll M.C.G., Reutelingsperger C.P.M., Nicolaes G.A.F., Sels J.W.E. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth. Intensive Care. 2020;48:221–228. doi: 10.1177/0310057X20916471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Queisser K.A., Mellema R.A., Middleton E.A., Portier I., Manne B.K., Denorme F., Beswick E.J., Rondina M.T., Campbell R.A., Petrey A.C. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight. 2021;6 doi: 10.1172/jci.insight.147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraser D.D., Patterson E.K., Slessarev M., Gill S.E., Martin C., Daley M., Miller M.R., Patel M.A., dos Santos C.C., Bosma K.J., O’Gorman D.B., Cepinskas G. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit. Care Explor. 2020;2 doi: 10.1097/cce.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer M., Deutschmann C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padberg J., Wiesinger A., Seno G., Reuter S., Grabner A., Kentrup D., Lukasz A., Oberleithner H., Pavenstädt H., Brand M., Kümpers P. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Hahn R.G., Hasselgren E., Björne H., Zdolsek M., Zdolsek J. Biomarkers of endothelial injury in plasma are dependent on kidney function. Clin. Hemorheol. Microcirc. 2019;72(2):161–168. doi: 10.3233/CH-180444. [DOI] [PubMed] [Google Scholar]
- 55.Stahl K., Gronski P.A., Kiyan Y., Seeliger B., Bertram A., Pape T., Welte T., Hoeper M.M., Haller H., David S. Injury to the endothelial glycocalyx in critically Ill patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020;202(8):1178–1181. doi: 10.1164/rccm.202007-2676LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui N., Wang H., Long Y., Su L., Liu D. Dexamethasone suppressed LPS-induced matrix metalloproteinase and its effect on endothelial glycocalyx shedding. Mediators Inflamm. 2015;2015:1–8. doi: 10.1155/2015/912726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nieuwdorp M., Meuwese M.C., Mooij H.L., van Lieshout M.H.P., Hayden A., Levi M., Meijers J.C.M., Ince C., Kastelein J.J.P., Vink H., Stroes E.S.G. Tumor necrosis factor-α inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202(1):296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Jannaway M., Yang X., Meegan J.E., Coleman D.C., Yuan S.Y. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PLoS One. 2019;14(5):1–26. doi: 10.1371/journal.pone.0214737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drost C.C., Rovas A., Kusche-Vihrog K., Van Slyke P., Kim H., Hoang V.C., Maynes J.T., Wennmann D.O., Pavenstädt H., Linke W., Lukasz A., Hesse B., Kümpers P. Tie2 activation promotes protection and reconstitution of the endothelial glycocalyx in human sepsis. Thromb. Haemost. 2019;119(11):1827–1838. doi: 10.1055/s-0039-1695768. [DOI] [PubMed] [Google Scholar]
- 60.Chen S., He Y., Hu Z., Lu S., Yin X., Ma X., Lv C., Jin G. Heparanase mediates intestinal inflammation and injury in a mouse model of sepsis. J. Histochem. Cytochem. 2017;65(4):241–249. doi: 10.1369/0022155417692536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X., Han S., Liu X., Wang T., Xu H., Xia B., Kong G., Li J., Zhu W., Hu H., Hao D., Wang X. Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPS-induced sepsis. Exp. Ther. Med. 2019:913–922. doi: 10.3892/etm.2019.8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding R., Zhao D., Guo R., Zhang Z., Ma X. Treatment with unfractionated heparin attenuates coagulation and inflammation in endotoxemic mice. Thromb. Res. 2011;128(6):e160–e165. doi: 10.1016/j.thromres.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 63.Yini S., Heng Z., Xin A., Xiaochun M. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015;59(2):160–169. doi: 10.1111/aas.12418. [DOI] [PubMed] [Google Scholar]
- 64.Linder A., Russell J.A. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med. 2014;40(8):1164–1167. doi: 10.1007/s00134-014-3366-9. [DOI] [PubMed] [Google Scholar]
- 65.Wang L., Huang X., Kong G., Xu H., Li J., Hao D., Wang T., Han S., Han C., Sun Y., Liu X., Wang X. Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS. Biochem. Biophys. Res. Commun. 2016;478(2):669–675. doi: 10.1016/j.bbrc.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 66.I. Vlodavsky, N. Ilan, R.D. Sanderson, Forty years of basic and translational heparanase research, in: Adv. Exp. Med. Biol., 2020: pp. 3–59. doi: 10.1007/978-3-030-34521-1_1. [DOI] [PMC free article] [PubMed]
- 67.Meziani F., Gando S., Vincent J.L. Should all patients with sepsis receive anticoagulation? Yes. Intensive Care Med. 2017;43(3):452–454. doi: 10.1007/s00134-016-4621-z. [DOI] [PubMed] [Google Scholar]
- 68.Li X., Liu Z., Luo M., Xi Y., Li C., Wang S., Yang R. Therapeutic effect of low-molecular-weight heparin on adult sepsis: a meta-analysis. Ann. Palliat. Med. 2021;10:3115–3127. doi: 10.21037/apm-21-169. [DOI] [PubMed] [Google Scholar]
- 69.Masola V., Onisto M., Zaza G., Lupo A., Gambaro G. A new mechanism of action of sulodexide in diabetic nephropathy: Inhibits heparanase-1 and prevents FGF-2-induced renal epithelial-mesenchymal transition. J. Transl. Med. 2012;10:1. doi: 10.1186/1479-5876-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song J.W., Zullo J.A., Liveris D., Dragovich M., Zhang X.F., Goligorsky M.S. Therapeutic restoration of endothelial glycocalyx in sepsis. J. Pharmacol. Exp. Ther. 2017;361(1):115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T., Liu X., Zhao Z., Ni L., Liu C. Sulodexide recovers endothelial function through reconstructing glycocalyx in the balloon-injury rat carotid artery model. Oncotarget. 2017;8:91350–91361. doi: 10.18632/oncotarget.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singleton P.A., Mirzapoiazova T., Guo Y., Sammani S., Mambetsariev N., Lennon F.E., Moreno-Vinasco L., Garcia J.G.N. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am. J. Physiol. – Lung Cell. Mol. Physiol. 2010;299:639–651. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J.H., Liu A., Park J.H., Kato H., Hao Q., Zhang X., Zhou L., Lee J.W. Therapeutic effects of hyaluronic acid in peritonitis-induced sepsis in mice. Shock. 2020;54:488–497. doi: 10.1097/SHK.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E., Rochwerg B., Rubenfeld G.D., Angus D.C., Annane D., Beale R.J., Bellinghan G.J., Bernard G.R., Chiche J.D., Coopersmith C., De Backer D.P., French C.J., Fujishima S., Gerlach H., Hidalgo J.L., Hollenberg S.M., Jones A.E., Karnad D.R., Kleinpell R.M., Koh Y., Lisboa T.C., Machado F.R., Marini J.J., Marshall J.C., Mazuski J.E., McIntyre L.A., McLean A.S., Mehta S., Moreno R.P., Myburgh J., Navalesi P., Nishida O., Osborn T.M., Perner A., Plunkett C.M., Ranieri M., Schorr C.A., Seckel M.A., Seymour C.W., Shieh L., Shukri K.A., Simpson S.Q., Singer M., Thompson B.T., Townsend S.R., Van der Poll T., Vincent J.L., Wiersinga W.J., Zimmerman J.L., Dellinger R.P. Springer Berlin Heidelberg; 2017. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. doi: 10.1007/s00134-017-4683-6. [Google Scholar]
- 75.Chappell D., Jacob M., Hofmann-Kiefer K., Bruegger D., Rehm M., Conzen P., Welsch U., Becker B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 76.Gao S.L., Zhang Y., Zhang S.Y., Liang Z.Y., Yu W.Q., Liang T.B. The hydrocortisone protection of glycocalyx on the intestinal capillary endothelium during severe acute pancreatitis. Shock. 2015;43:512–517. doi: 10.1097/SHK.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 77.Brettner F., Chappell D., Nebelsiek T., Hauer D., Schelling G., Becker B.F., Rehm M., Weis F. Preinterventional hydrocortisone sustains the endothelial glycocalyx in cardiac surgery. Clin. Hemorheol. Microcirc. 2019;71(1):59–70. doi: 10.3233/CH-180384. [DOI] [PubMed] [Google Scholar]
- 78.Allingstrup M., Wetterslev J., Ravn F.B., Møller A.M., Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42(4):505–520. doi: 10.1007/s00134-016-4225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iba T., Levy J.H., Aihara K., Kadota K., Tanaka H., Sato K., Nagaoka I. Newly developed recombinant antithrombin protects the endothelial glycocalyx in an endotoxin-induced rat model of sepsis. Int. J. Mol. Sci. 2021;22:1–9. doi: 10.3390/ijms22010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parikh S.M., Mammoto T., Schultz A., Yuan H.T., Christiani D., Karumanchi S.A., Sukhatme V.P. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J.J., Huang Y.Q., Basch R., Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb. Haemost. 2001;85(02):204–206. [PubMed] [Google Scholar]
- 82.Clark D.V., Banura P., Bandeen-Roche K., Conrad Liles W., Kain K.C., Michael Scheld W., Moss W.J., Jacob S.T. Biomarkers of endothelial activation/dysfunction distinguish subgroups of Ugandan patients with sepsis and differing mortality risks. JCI Insight. 2019;4:1–12. doi: 10.1172/jci.insight.127623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pierce R.W., Shabanova V., Canarie M., Pinto M., Da Silva Y.S., Bhandari V., Giuliano J.S. Angiopoietin level trajectories in toddlers with severe sepsis and septic shock and their effect on capillary endothelium. Shock. 2019;51:298–305. doi: 10.1097/SHK.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benest A.V., Kruse K., Savant S., Thomas M., Laib A.M., Loos E.K., Fiedler U., Augustin H.G. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., Gale N.W., Witzenrath M., Rosseau S., Suttorp N., Sobke A., Herrmann M., Preissner K.T., Vajkoczy P., Augustin H.G. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat. Med. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 86.Kümpers P., Gueler F., David S., Van Slyke P., Dumont D.J., Park J.K., Bockmeyer C.L., Parikh S.M., Pavenstädt H., Haller H., Shushakova N. The synthetic Tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit. Care. 2011;15(5):R261. doi: 10.1186/cc10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han S., Lee S., Kim K.E., Lee H.S., Oh N., Park I., Ko E., Oh S.J., Lee Y., Kim D., Lee S., Lee D.H., Lee K., Chae S.Y., Lee J., Kim S., Kim H., Kim S., Kim S.H., Kim C., Nakaoka Y., He Y., Augustin H.G., Hu J., Song P.H., Kim Y., Kim P., Kim I., Koh G.Y. Amelioration of sepsis by TIE2 activation – induced vascular protection. Sci. Transl. Med. 2016;8:1–12. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- 88.Salmon A.H.J., Neal C.R., Sage L.M., Glass C.A., Harper S.J., Bates D.O. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc. Res. 2009;83:24–33. doi: 10.1093/cvr/cvp093. [DOI] [PMC free article] [PubMed] [Google Scholar]