Abstract
Purpose:
The increased availability of clinical pharmacogenetic (PGx) guidelines and decreasing costs for genetic testing have slowly led to increased utilization of PGx testing in clinical practice. Preemptive PGx testing, where testing is performed in advance of drug prescribing, is one means to ensure results are available at the time of prescribing decisions. However, the most efficient and effective methods to clinically implement this strategy remain unclear.
Methods:
In this report, we compare and contrast implementation strategies for preemptive PGx testing by 15 early-adopter institutions. We surveyed these groups, collecting data on testing approaches, team composition, and workflow dynamics, in addition to estimated third-party reimbursement rates.
Results:
We found that while preemptive PGx testing models varied across sites, institutions shared several commonalities, including methods to identify patients eligible for testing, involvement of a precision medicine clinical team in program leadership, and the implementation of pharmacogenes with Clinical Pharmacogenetics Implementation Consortium guidelines available. Lastly, while reimbursement rate data were difficult to obtain, the data available suggested that reimbursement rates for preemptive PGx testing remain low.
Conclusion:
These findings should inform the establishment of future implementation efforts at institutions considering a preemptive PGx testing program.
INTRODUCTION
Pharmacogenetic (PGx) testing is increasingly used in clinical practice as one approach to increase the implementation of precision medicine. As cost-efficiency of genotyping technology has improved and the availability of clinical guidelines to inform the use of PGx test results have become available, so has clinical PGx testing become increasingly utilized.1,2 When testing for PGx variants, genotyping can follow a reactive or preemptive testing strategy. Reactive PGx testing refers to genotyping performed after a decision is made to prescribe a medication or in response to suspected drug-induced adverse effects or poor pharmacotherapy response.3 This requires the prescriber to either wait for the test results before prescribing a medication or prescribe without PGx information and then potentially change the prescription once results are available. Preemptive PGx testing, on the other hand, occurs when the testing is performed, and results are made available, prior to any medication decisions being made. The goal of preemptive testing is for PGx information to be readily available at the time pharmacotherapy decisions are being made in order to guide initial medication selection and dosing. Preemptive testing can include a variety of strategies, including PGx testing of large, unselected populations of individuals, testing of selected populations likely to be treated with a relevant drug, or panel-based testing where the initial use of the test is reactive, but results relevant to future prescriptions are available preemptively as discussed below.
PGx test results are relevant well beyond their initial use and are applicable to guide future prescribing decisions.4 This is especially true given that multiple medications may be impacted by a single pharmacogene (e.g., CYP2D6 or CYP2C19). In addition, medications addressed in PGx guidelines are commonly used in clinical practice, further supporting the value of panel-based PGx test results. Of the top 300 prescribed medications in 2020, 34% are moderately to strongly associated with genetic information that could be used to guide their prescribing.5 Moreover, multiple reports have found that approximately two-thirds of the general patient population will be prescribed a pharmacogenetically-actionable medication within five years.6 While the exact population for preemptive testing has not been widely established or accepted, these data indicate that a high percentage of the population will receive a medication impacted by PGx variants at some point in their lifetime.
The strategies employed for preemptive PGx testing can be separated into three categories. First, fully preemptive testing involves genotyping patients (often using a multi-gene panel), before any specific prescription associated with potential genetic guidance was written. Next, partially preemptive testing entails genotyping using a multi-gene panel in response to a specific prescription where genetic guidance was sought (a reactive approach), but with the inclusion of additional genes providing preemptive data for subsequent prescribing. Finally, reactive testing with planned reuse involves ordering PGx testing in response to a specific prescription where genetic guidance was sought, and only testing for the gene(s) associated with that prescription (a reactive approach). However, a method is implemented (usually an automated clinical decision support tool), allowing use of the genotype data to inform future therapy with other drugs. An example of reactive testing with planned reuse would be a patient tested for CYP2C19 to inform an antidepressant prescription but then having those data stored in the EHR with automated clinical decision support tools built to alert prescribers of these genotype results and relevant CYP2C19-guided antiplatelet recommendations if the patient were to undergo percutaneous coronary intervention in the future.
Historically, fully preemptive PGx testing (in the absence of an immediate need for test results) has only been implemented at a few institutions, often focused in the cancer setting.1,7,8 However, multiple programs have recently implemented mixed models of reactive and preemptive testing. The objective of this project was to characterize institutions implementing preemptive PGx testing within the NHGRI-funded Implementing GeNomics In pracTicE (IGNITE) Network Pharmacogenetics Working Group as well as to compare and contrast the strategies used in order to inform future preemptive PGx implementation efforts.
MATERIALS AND METHODS
Members of the IGNITE Pharmacogenetics Working Group who represented institutions that were either currently offering, planning to offer, or previously offered preemptive PGx testing were invited to participate in an online survey. Briefly, the PGx Working Group includes both funded and affiliate members of the IGNITE Network (https://www.genome.gov/Funded-Programs-Projects/Implementing-Genomics-in-Practice-IGNITE). Application for affiliate membership is open to institutions with an interest in clinical genomic or pharmacogenomic testing. The IGNITE PGx Working Group was formed in 2015 with the goal of broadly engaging institutions that had implemented PGx in practice to share experiences with implementation and collectively disseminate implementation strategies. Implementation at each site occurred independently and in many cases began prior to Working Group activities. Thus, the resources and capacities of the institutions included in the Working Group, and the unfunded affiliate member sites in particular, are expected to be similar to those at other institutions that have implemented or are interested in implementing PGx testing.9
The survey was administered using a Research Electronic Data Capture (REDCap) instance hosted at the University of Florida.10 A copy of the survey questions is provided in the supplemental materials. The study was approved by University of Florida Institutional Review Board as exempt research. Survey questions were designed to assess implementation strategies, priorities, and challenges encountered. The survey captured general characteristics of the institutions (e.g., practice settings, stage in the implementation process, research versus clinical approach to implementation) and specific strategies for implementing preemptive PGx (e.g., how results were stored, types of providers ordering testing, clinical decision support). The survey consisted of 34 questions and was estimated to take the participants approximately 30–60 minutes to complete.
Responses were exported from REDCap and analyzed using R version 3.6.3. For some questions, it was possible for each site to select multiple responses and/or select “other” and enter a free-text response. Data were summarized by calculating the proportion of sites that selected each response. Free-text responses were either recoded as a similar response from the survey or as a new response variable. A consensus between two members of the study team was required before recoding. Any disagreements were decided by a third study team member.
RESULTS
Representatives from 15 institutions completed the survey (Table 1), representing over 60% of all affiliated institutions within the IGNITE PGx Working Group. Most sites represented in the survey were academic health centers, but non-profit and for-profit health systems were also represented. While two sites were still in the planning stages, the rest of the sites had implemented some form of preemptive PGx testing. Three sites had previously implemented preemptive testing but were no longer offering this type of testing at the time of the survey. Two of these institutions ended preemptive testing because their programs were primarily funded as part of a research effort that ended. Another institution stopped preemptive PGx testing for multiple reasons including poor third-party payer reimbursement and a perceived lack of sufficient data supporting every gene-drug pair being tested for.
Table 1.
Institutions implementing preemptive PGx testing within the IGNITE PGx Working Group
| Institution Name | Institution Type | Implementation status | Launch Year | Clinical or Research | Where testing was done | Number of genes tested in panel | Preemptive testing strategy primarily employed |
|---|---|---|---|---|---|---|---|
| Cincinnati Children’s Hospital Medical Center | Academic hospital | Active | 2005 | Clinical | In-house | 6 | Fully |
| Icahn School of Medicine at Mount Sinai / The Mount Sinai Hospital | Academic hospital | Halted | 2013 | Both | In-house; Non-profit lab | 5 | Fully |
| Indiana University School of Medicine | Academic hospital | Halted | 2014 | Both | In-house | 12 | Partially |
| MedStar Health | Nonprofit hospital; nonprofit ambulatory care clinic | Halted | 2017 | Clinical | Commercial | 41 | Fully |
| Michigan Medicine | Academic hospital; academic ambulatory care clinic | Planning | N/A | Clinical | Commercial | 12 | Partially |
| Mission Health | For profit hospital | Active | 2016 | Clinical | Commercial | 27 | Partially |
| Moffitt Cancer Center | Academic hospital | Active | 2014 | Both | In-house; Commercial | 27 | Reactive- planned reuse |
| Nemours Children’s Health System | Nonprofit hospital | Active | 2020 | Clinical | In-house | 9 | Partially |
| Sanford Health | Nonprofit hospital | Active | 2018 | Clinical | In-house | 11 | Fully |
| University of Colorado/UC Health | Academic hospital | Active | 2019 | Clinical | In-house | 12 | Fully |
| University of Florida | Academic hospital; academic ambulatory care clinic | Active | 2012 | Both | In-house; Commercial | 1–7 | Fully |
| University of Maryland School of Medicine/UMMC | Academic hospital | Active | 2013 | Clinical | In-house | 1 | Fully |
| University of Pittsburgh/UPMC | Academic hospital | Active | 2018 | Both | In-house | 14 | Fully |
| University of Pennsylvania | Academic hospital | Planning | N/A | Both | In-house | 2 | Reactive- planned reuse |
| Vanderbilt University Medical Center | Academic hospital; academic ambulatory care clinic | Active | 2010 | Clinical | In-house | 10 | Partially |
N/A – not applicable; Preemptive testing strategy primarily employed – the strategy employed most often at that institution
Implementation Strategies Used
The preemptive PGx testing model being utilized varied among institutions, with approximately half implementing fully preemptive testing (Table 1). In most cases, this involved testing with a multi-gene panel. However, in some cases it involved testing for a single gene to assist with drug prescribing in a population selected for a high likelihood of being prescribed the target drug(s), such as CYP2D6 genotyping prior to surgery to assist with post-operative pain management or CYP2C19 testing at the time of cardiac catheterization in the event the patient proceeded to percutaneous coronary intervention and required antiplatelet therapy. Another 33% of sites implemented partially preemptive testing, and the remaining 33% implemented reactive testing with planned reuse only. All sites reported that in addition to preemptive PGx testing, reactive PGx genotyping was also offered at their institutions. Where applicable, figures are presented with testing strategy color-coded. Institutions implementing fully or partially preemptive testing were combined to reflect their more preemptive nature. The remaining institutions fell in the “reactive with planned reuse” category and were labeled as such.
Implementation Characteristics
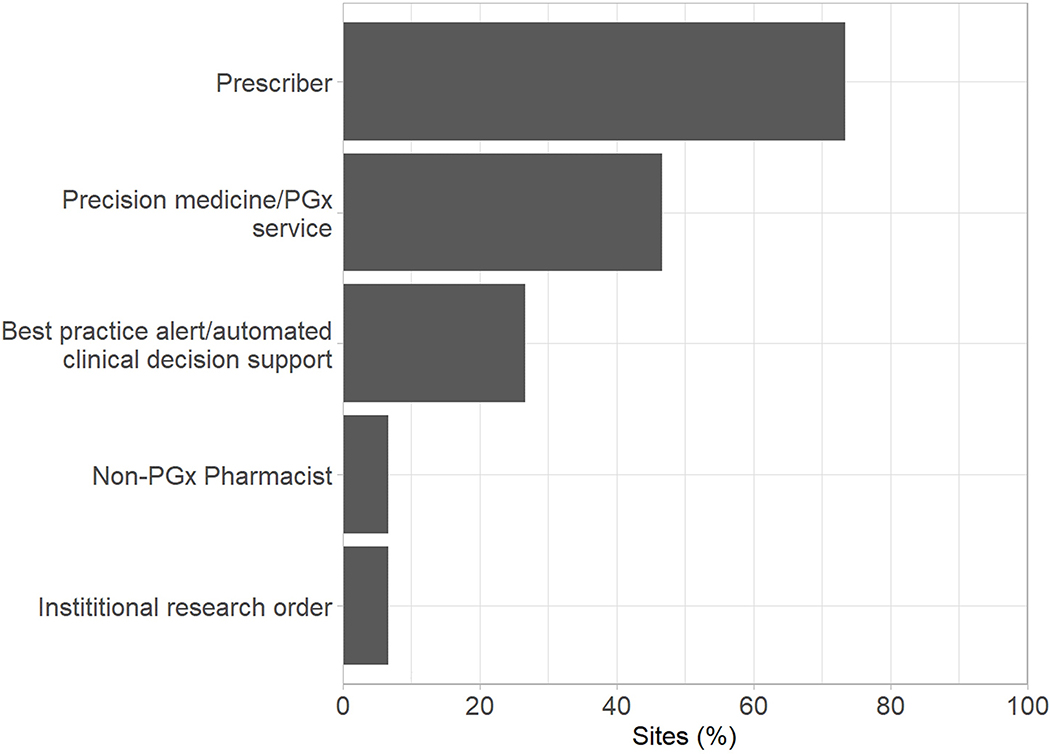
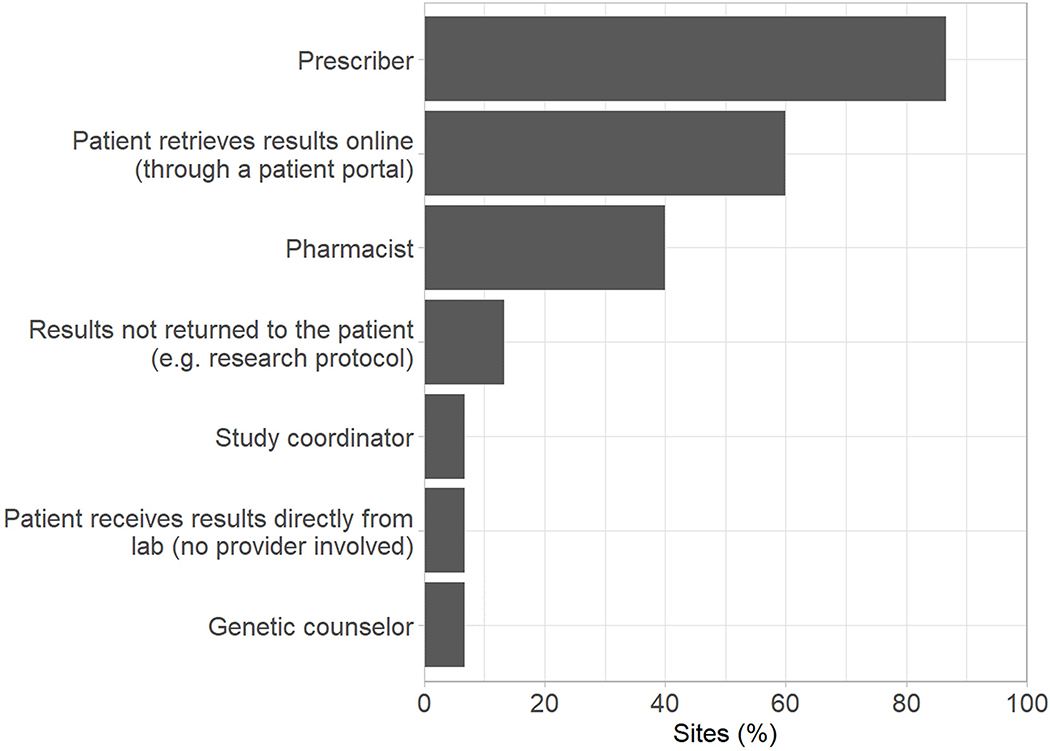
All institutions reported that preemptive PGx testing efforts were, at least in part, led by a precision medicine/PGx service (Supplementary Figure 1A). This team also shared responsibility at all sites for providing guidance on test interpretation (Supplementary Figure 1B). Pharmacy service departments were the next most common organizational units responsible for leading testing efforts and interpreting results. In a majority of institutions, the medication prescriber was most often responsible for identifying patients for testing, ordering the PGx test, and communicating results to the patient (Figure 1). Along with the medication prescriber, the patient (by self-referral) also initiated testing in over 50% of institutions. When it came to actually ordering the test, the PGx/precision medicine team shared responsibility at nearly 50% of institutions.
Figure 1.
Personnel responsible for: ordering preemptive PGx tests (A) and communicating PGx test results to patients (B).
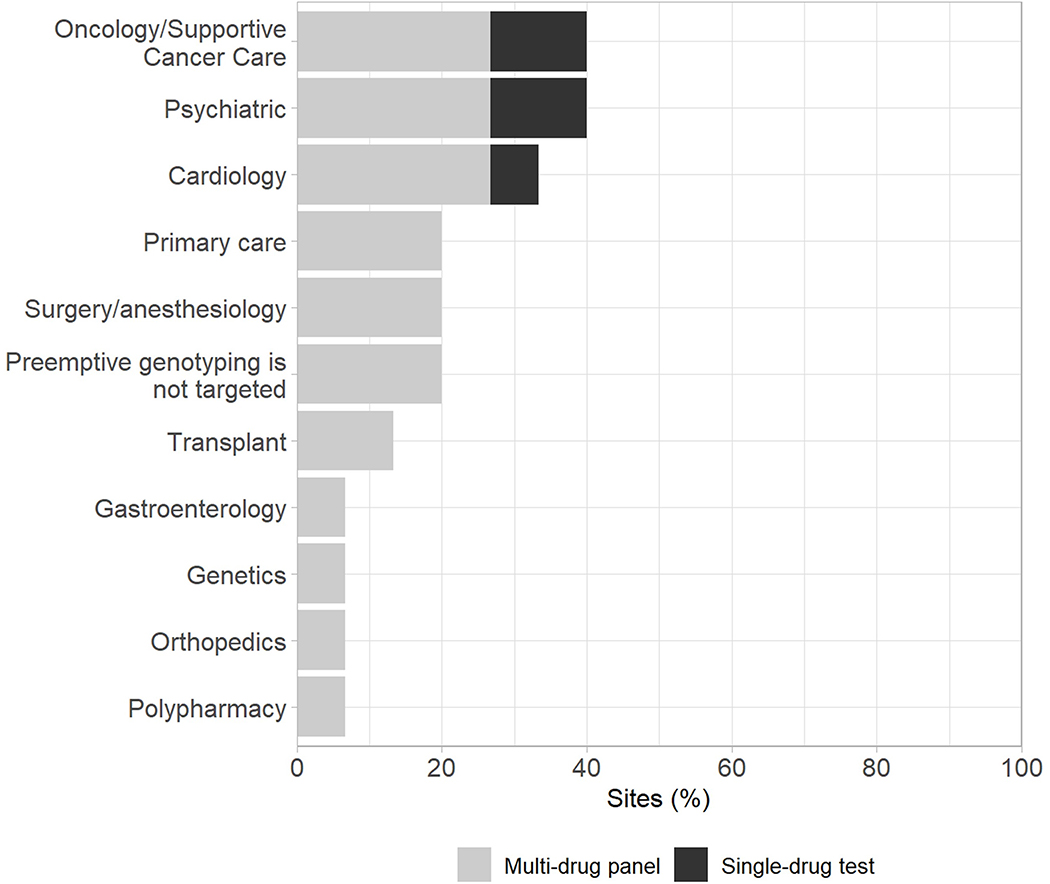
Institutions were almost evenly split between targeting only adults for preemptive testing (47%) and having no specific age target (53%). Similarly, institutions were nearly evenly-distributed between a solely outpatient-focused program (47%) versus having no setting restrictions (47%), with only 6% focusing exclusively on inpatients. Psychiatric and oncology patients were the most commonly targeted patient populations, with 40% of institutions targeting these patients for preemptive PGx testing (Figure 2).
Figure 2.
Targeting of preemptive PGx testing at institutions by medical service. Shades represent preemptive strategy utilized within each category.
Once PGx results were obtained, nearly 70% of institutions stored results within the laboratory results section and just over 50% used a specific PGx section within the EHR (Supplementary Figure 2A). PGx results were most often communicated to the prescriber via either a laboratory result or through a clinical consultation note within the EHR (Supplementary Figure 2B).
Implemented Tests
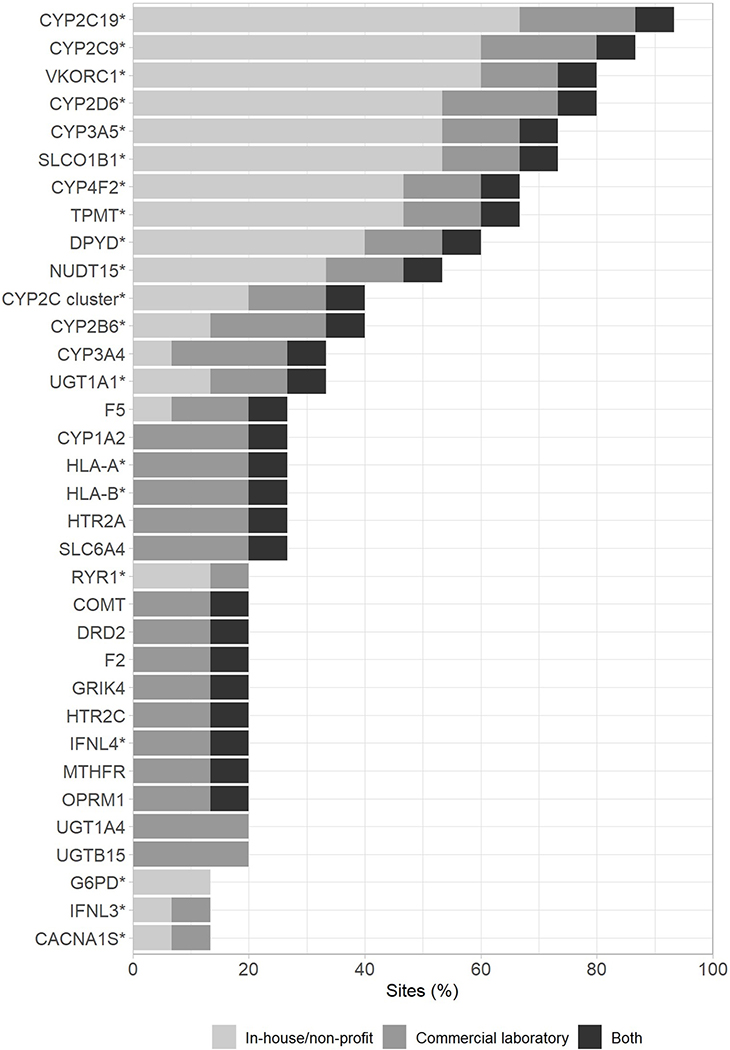
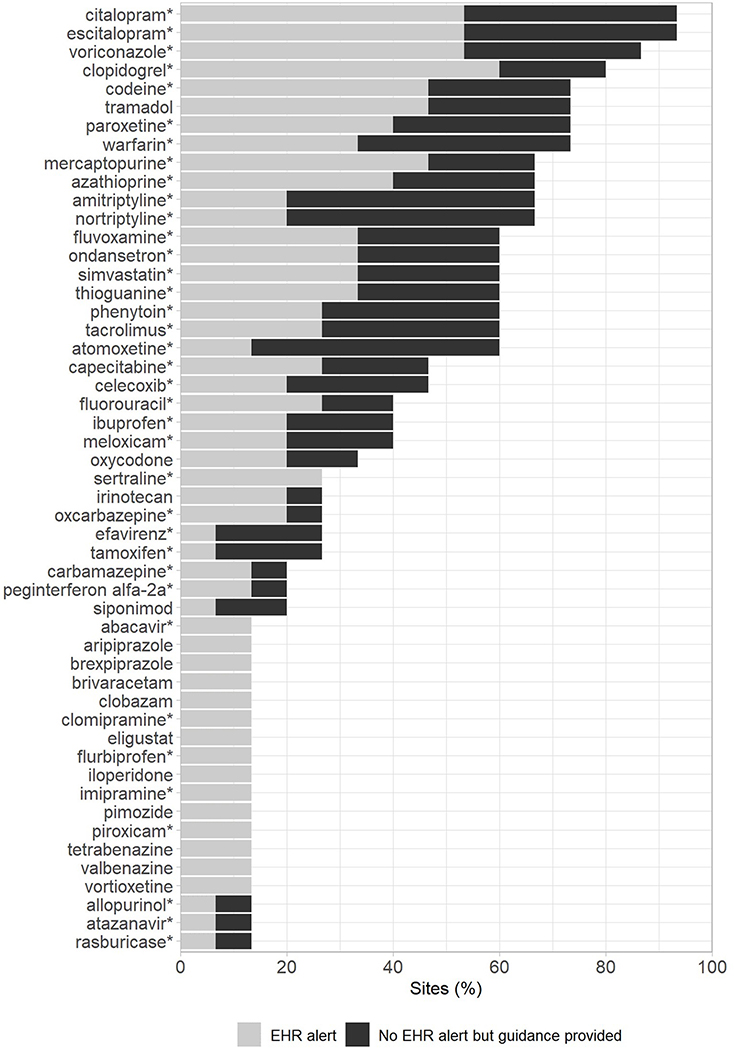
The genes preemptively tested varied among sites, but CYP2C19, CYP2C9, VKORC1, and CYP2D6 were tested at nearly all institutions (Figure 3A). Reflecting the popularity of testing these genes, selective serotonin reuptake inhibitors (SSRIs), voriconazole, clopidogrel, opioids, and warfarin were the drugs for which PGx recommendations or guidance were most commonly provided (Figure 3B). Along with the SSRIs, amitriptyline, nortriptyline, and atomoxetine had some of the highest rates of automated clinical decision support tools built within the EHR to provide PGx recommendations. Within institutions not providing EHR alerts, all implementations not part of a research project reported providing a consult note within the medical record as an alternative method of providing recommendations. Nearly all medications that were guided by preemptive PGx data at more than one institution had Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines available to guide therapy.
Figure 3.
Genes included in preemptive PGx tests (A) and the drugs informed by preemptive PGx testing at > 1 institution with or without EHR clinical decision support alert/tool (B). * indicates availability of a Clinical PGx Implementation Consortium (CPIC) guideline to guide prescribing. Shades represent test characteristics within each category.
Test Reimbursement
Within the subset of 9 institutions that billed for any type of preemptive PGx testing at the time of the survey, respondents reported an estimated maximum patient out-of-pocket costs of $500 or less. Approximately 55% reported a maximum out-of-pocket cost between $251–500, with the other 45% reporting a cost of $250 or less (Supplementary Figure 3A). The estimated cost did not appear to differ between preemptive or reactive with planned reuse strategies. Only 5 sites were able to provide estimates of third-party reimbursement rates for preemptive testing. Of the sites that responded, most estimated that reimbursement (of any amount) was received for less than 25% of the tests completed (Supplementary Figure 3B).
DISCUSSION
The results of our survey reflect a relatively even distribution of preemptive PGx testing models in clinical practice. Despite this, we observed many common elements to each implementation, such as leadership by a PGx or precision medicine team, prescribers being primarily responsible for identifying which patients to test, implementation of testing within the outpatient setting, and inclusion of gene-drug pairs supported by CPIC guidelines. These commonalties suggest that while a consensus may not yet exist regarding the most effective overall preemptive testing strategy to implement, there appears to be notable agreement regarding many of the implementation logistics. The most common overall strategy for preemptive testing was a fully preemptive testing design (often in select populations with high likelihood of exposure to relevant drugs) with many institutions also implementing a partially preemptive strategy where testing is completed reactively for a specific drug, but additional genes are also tested for preemptively. The high rate of partial preemptive testing may be because reimbursement for fully preemptive testing with a panel (in absence of an immediate use of test results) is currently a major challenge that likely limits broad use of this approach. On the other hand, a model that includes use of some panel results immediately to guide prescribing may allow for reimbursement for at least the gene relevant for immediate prescribing decisions.
PGx implementation efforts and test interpretation services were primarily led by PGx/precision medicine teams. While this could be due to a desire to consolidate PGx expertise into a single group, the lack of experience and comfort level in interpreting PGx results by many healthcare providers might also play a role.11,12 The most common uses of panel-based testing were for cancer-related supportive care as well as the management of psychiatric disorders and cardiovascular disease. The number of genes included on testing panels ranged from as few as 2 to over 40. Four pharmacogenes (CYP2C9, CYP2C19, VKORC1, and CYP2D6) were consistently tested by ≥ 75% of institutions, suggesting a consensus regarding the importance of preemptively testing for those genes. Nearly all sites reported using preemptive PGx testing to inform prescribing of SSRIs, voriconazole, or clopidogrel. All of these medications, as well as a majority of those reported by more than one institution, were associated with a CPIC guideline to guide prescribing. However, other medications like oxycodone and siponimod were informed by PGx at multiple institutions, but did not have CPIC recommendations available at the time of the survey. Additionally, many of the medications reported by only a few institutions do not have CPIC recommendations available. This likely reflects institution-specific decision making processes that are used to determine which medications have sufficient evidence to be informed by PGx data. It could also reflect the use of commercial testing panels that often include a larger number of pharmacogenes.
Our survey results also indicate that reactive testing with planned reuse is associated with a similar out-of-pocket cost for patients as more comprehensive panel based testing. This might seem counter-intuitive since smaller panels test for far fewer variants than larger panels, but a majority of the costs associated with PGx testing are fixed, such as labor, equipment, and overhead. The ability to batch samples for truly preemptive panel-based testing, where there is no immediate indication for the results, significantly reduces the labor cost compared to reactive PGx tests in which only a few samples and, in some cases, only a single sample may be run at a time. Despite the improved cost efficiency associated with panel-based preemptive testing, the fact that most institutions reported little to no reimbursement from third-party payers remains a major barrier to broad scale preemptive PGx testing across the nation. Indeed, only two institutions reported receiving reimbursement for at least 50% of patients tested. Reimbursement amounts vary greatly by gene-drug pair, as do reimbursement success rates.13 Even when reimbursement occurs, anecdotal reports from our Working Group suggest that the amount collected commonly does not cover the cost of testing for the institution. Third-party payers may be seeking additional research to provide prospective outcome measures, defined target populations, predictive economic models, and randomized trial data to support new reimbursement policies.3 However, requiring this magnitude of data, particularly randomized trial data, prior to reimbursement has been proposed to hold PGx testing to a higher standard than most other laboratory tests used to guide pharmacotherapy.14 Movement toward healthcare supporting value-based care and preemptive health service may drive demand for preemptive PGx coverage.3 In fact, there are signs that some payers are beginning to embrace preemptive PGx testing with some Medicare administrative contractors now providing reimbursement for multi-gene PGx tests.15
To optimize the value of preemptive testing, it is critical to store the results as discrete data that are easily accessible and build a mechanism to alert the prescriber to the availability of the results and the appropriate action based on results. Ideally, results should be placed in a section of the EHR for lifetime results. Almost every site in this study reported storing PGx results as discrete data somewhere within the EHR. This allows for electronic clinical decision support to alert the prescriber at the time a medication is being ordered if the patient has a genotype associated with risk for reduced drug effectiveness or increased toxicity. Automated computer decision support (CDS) is crucial to sustainable preemptive PGx implementation because as the number of patients being tested and drugs being informed by the results increase, it becomes less feasible for a clinician specializing in PGx to manually provide a recommendation each time. A commonly used alternative to CDS, consult notes placed in the EHR, provide decision support at the time they are written, but they are unable to provide point of care decision support each time a drug impacted by an actionable genotype is prescribed.
In addition to the institutions surveyed as part of this project, other institutions outside of the IGNITE Pharmacogenetics Working Group have reported their approaches to implementation of preemptive PGx testing. St. Jude Children’s Research Hospital launched the PG4KDS protocol in 2011, which uses fully preemptive array-based PGx testing as part of routine clinical practice and systematically introduces new gene/drug pairs as the evidence evolves.16 Mayo Clinic also offers fully preemptive PGx testing through their RIGHT protocol, storing PGx data into the EHR for clinical use, as well as using the data for research.17,18 Outside of the United States, the Ubiquitous Pharmacogenomics Consortium developed a standardized preemptive panel called the “PGx-Passport” that is based on the Dutch Pharmacogenetics Working Group (DPWG) guidelines and consists of 58 germline variant alleles within 13 pharmacogenes.19 The PGx-Passport is being used in their PREemptive Pharmacogenomic testing for Preventing Adverse Drug REaction (PREPARE) study, a prospective, randomized, controlled clinical trial in which they are enrolling 8,100 patients from seven European countries for partially preemptive PGx testing and quantifying the clinical utility and cost-effectiveness of this panel-based approach to guide dose and drug selection across multiple actionable gene-drug pairs.20 Several pharmacogenes are clinically tested for across these additional sites, including: CYP2C9, CYP2C19, CYP2D6, CYP3A5, TPMT, NUDT15, SLCO1B1, DPYD, UGT1A1. This list is nearly identical to the most-tested genes in our survey, further indicating a consensus regarding pharmacogenes that are ready for clinical implementation.
When designing a preemptive PGx testing strategy, there are several factors to consider. One important factor is what genes should be included in the panel. As discussed above, the genes most often included in preemptive PGx testing (both within and outside of the IGNITE PGx Working Group) all have CPIC guidelines associated with them. CPIC is an international consortium whose goal is to reduce barriers to clinical implementation of PGx testing by providing evidence-based guidelines for the clinical use of PGx information.2,21 As of May 2021, they have published 25 guidelines, many encompassing multiple pharmacogenes and drugs. CPIC guidelines do not provide recommendations on whether or not to test, but do provide guidance on how to use PGx results if they are available. Thus, once institutions make a decision on what pharmacogenes to include for testing, CPIC guidelines can provide a valuable resource informing best practices for how to clinically use the results. Another consideration when designing a preemptive strategy is cost. As discussed above, reimbursement experiences for clinical testing vary significantly, so narrowing testing platforms to include fewer genes with better reimbursement histories is a potential approach. However, as also discussed, including more genes in a panel increases clinical utility while generally incurring a similar cost. As PGx panel reimbursement becomes more commonplace, we expect these dynamics will change.
To our knowledge, this is the largest survey of institutions that have implemented preemptive PGx testing into clinical practice. The results of this survey provide important insights into how institutions across the United States have implemented preemptive PGx testing, the genes included for testing, and methods for disseminating the results. As discussed above, many similarities exist in how preemptive PGx testing is implemented among institutions. However, some components are tailored to the environment of the institution, and this work identified different approaches for implementing preemptive PGx testing (e.g. location in EHR where preemptive PGx test results are stored for future use, methods used for identifying patients for preemptive PGx testing). These data should help inform efforts at institutions considering implementation of preemptive PGx testing by providing data on aspects where wide agreement exists and where institution-specific methods should potentially be explored.
This study also had some limitations. The majority of survey respondents represented academic hospitals, which may limit the applicability to community ambulatory settings. Furthermore, specific genotype-guided recommendations were not captured in this survey, nor did we capture the medication indications for which PGx testing was provided. Although most sites were able to report their estimated maximum out-of-pocket test costs, only a few were able to provide estimates on the percentage of tests reimbursed by third-party payers. While a comprehensive analysis of reimbursement rates and financial impact on health systems was beyond the scope of this study, this study highlights the difficulty of obtaining these data in the current healthcare environment in the U.S.
Preemptive PGx testing models varied among sites (e.g., fully, preemptive, partially preemptive and reactive testing with planned reuse). However, institutions shared several commonalities (e.g., prescribers identify candidates for testing, involvement of a precision medicine or PGx service). More research is needed on the clinical utility, clinical outcomes, and cost-effectiveness of these programs. Future research should also explore mechanisms for reporting preemptive PGx panel results and potentially billing for preemptive test results when they are needed in the future.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge the following funding sources: NIH grants U01 HG007269, T32 HG008958, UL1 TR001427, U01 HG010232, U01 HG007775, and U01 HG008701. The authors would also like to thank Dr. Jeffery Bishop with his assistance in formulating survey questions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Disclosure: The authors declare no conflicts of interest.
Data Availability:
Data is available upon individual request.
Ethics Declaration:
This study was approved as exempt by the University of Florida IRB. Informed consent was provided by all survey participants. No individual patient data were used.
REFERENCES
- 1.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relling MV, Klein TE, Gammal RS, Whirl-Carrillo M, Hoffman JM, Caudle KE. The Clinical Pharmacogenetics Implementation Consortium: 10 Years Later. Clin Pharmacol Ther. 2020;107(1):171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeling NJ, Rosenthal MM, West-Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2019;21(5):1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitelshees AL, Stevenson JM, El Rouby N, et al. Evaluating the extent of reusability of CYP2C19 genotype data among patients genotyped for antiplatelet therapy selection. Genet Med. 2020;22(11):1898–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medical Expenditure Panel Survey (MEPS) 2008–2018. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ). [PubMed] [Google Scholar]
- 6.Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasi PM, Koep T, Schnettler E, et al. Feasibility of Integrating Panel-Based Pharmacogenomics Testing for Chemotherapy and Supportive Care in Patients With Colorectal Cancer. Technol Cancer Res Treat. 2019;18:1533033819873924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero RJ, Cicali EJ, Arwood MJ, et al. How to Transition from Single-Gene Pharmacogenetic Testing to Preemptive Panel-Based Testing: A Tutorial. Clin Pharmacol Ther. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavallari LH, Beitelshees AL, Blake KV, et al. The IGNITE Pharmacogenetics Working Group: An Opportunity for Building Evidence with Pharmacogenetic Implementation in a Real-World Setting. Clin Transl Sci. 2017;10(3):143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owusu Obeng A, Fei K, Levy KD, et al. Physician-Reported Benefits and Barriers to Clinical Implementation of Genomic Medicine: A Multi-Site IGNITE-Network Survey. J Pers Med. 2018;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DM, Namvar T, Brown RP, et al. Assessment of primary care practitioners’ attitudes and interest in pharmacogenomic testing. Pharmacogenomics. 2020;21(15):1085–1094. [DOI] [PubMed] [Google Scholar]
- 13.Services USCfMM, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/PAMA-Regulations. Accessed 11/13/2020 2020.
- 14.Huddart R, Sangkuhl K, Whirl-Carrillo M, Klein TE. Are Randomized Controlled Trials Necessary to Establish the Value of Implementing Pharmacogenomics in the Clinic? Clin Pharmacol Ther. 2019;106(2):284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Services USCfMM, 2020. https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=38294&ver=16&Cntrctr=All&UpdatePeriod=889&bc=AAAACAAAAAAA&. Accessed 11/13/2020.
- 16.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielinski SJ, St Sauver JL, Olson JE, et al. Cohort Profile: The Right Drug, Right Dose, Right Time: Using Genomic Data to Individualize Treatment Protocol (RIGHT Protocol). Int J Epidemiol. 2020;49(1):23–24k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Wouden CH, van Rhenen MH, Jama WOM, et al. Development of the PGx-Passport: A Panel of Actionable Germline Genetic Variants for Pre-Emptive Pharmacogenetic Testing. Clin Pharmacol Ther. 2019;106(4):866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Wouden CH, Bohringer S, Cecchin E, et al. Generating evidence for precision medicine: considerations made by the Ubiquitous Pharmacogenomics Consortium when designing and operationalizing the PREPARE study. Pharmacogenet Genomics. 2020;30(6):131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.