Abstract
Purpose
Proposals to return medically actionable secondary genetic findings (SFs) in the clinical and research settings have generated controversy regarding whether to solicit individuals’ preferences about their “right not to know” genetic information. This study contributes to the debate by surveying research participants who have actively decided whether to accept or refuse SFs.
Methods
Participants were drawn from a large NIH environmental health study. Participants who had accepted SFs (n=148) or refused SFs (n=83) were given more detailed information about the types of SFs researchers could return and were given an opportunity to revise their original decision.
Results
41/83 initial refusers (49.4%) opted to receive SFs following the informational intervention. Nearly 75% of these “reversible refusers” thought they had originally accepted SFs. The 50.6% of initial refusers who continued to refuse (“persistent refusers”) demonstrated high levels of understanding of which SFs would be returned post-intervention. The most prominent reason for refusing was concern about becoming worried or sad (43.8%).
Conclusion
This study demonstrates the need for a more robust informed consent process when soliciting research participants’ preferences about receiving SFs. We also suggest that our data support implementing a default practice of returning SFs without actively soliciting preferences.
Keywords: secondary findings, right not to know, research ethics, informed consent
Introduction
With the increasing adoption of whole genome and exome sequencing, researchers and clinicians have had to grapple with how to address the potential for uncovering findings unrelated to the primary sequencing objective (“secondary findings,” or “SFs” which have been distinguished from the broader category of “incidental findings” as information that is still unrelated to the research or clinical aim but are actively sought).1 In 2013, the American College of Medical Genetics and Genomics (“ACMG”) promulgated a list of genes in which disease causing variants should be returned to clinical genomic sequencing recipients without first soliciting their preferences about receiving this information.2,3 This proposal generated much debate around the so-called “right not to know” (“RNTK”) genetic information about oneself.4,5,6,7,8
Reflecting the dominant view, RNTK proponents argued that returning information to patients without soliciting their preferences is a violation of their autonomy.4-7,9,10,11 Even when this information is potentially life-saving, some argued, respecting autonomy ought to trump concerns of beneficence.4,5 In contrast, the ACMG argued that “clinicians have a fiduciary duty to warn patients about high-risk variants” and that it would not be “appropriate to give patients a choice not to learn about” these findings.3 This position has been extended to argue that the potential benefits of learning medically actionable genetic information far outweigh the harms, that support for the RNTK is inconsistent and malleable, and that the extremely small number of patients or research participants who choose to invoke the RNTK should not drive broad policies.3,12 Nonetheless, overwhelming criticism prompted the ACMG to revise their recommendations to allow patients “to opt out of the analysis of medically actionable genes” before their samples are sent for testing.13
Although distinct in some ways, a related debate is occurring among researchers who employ genomic sequencing regarding whether to solicit the informational preferences of study participants. In fact, resolving this controvery is perhaps more urgent in the research setting, where genomic sequencing has become exceedingly common. While different terms, such as “medically important genomic results,” have been proposed to distinguish actionable genetic findings that may surface in the research context from those in the clinical context, this study, which concerns the former, uses the term secondary findings.1
There are two areas where empirical research can inform this debate within the research context. First, proponents of soliciting research participants’ preferences assume that these preferences are strong and stable, and that they can reliably be captured during the consent process. However, studies on the quality of informed consent cast doubt on this assumption; there is significant variation in how people understand consent documents, and the way information is framed can have a significant impact on their understanding and answers.14,15 Therefore, it is critical to determine how strongly and consistently participants endorse the RNTK and how accurately preferences are captured during the consent process. Second, it is unclear why some participants refuse SFs. While some studies have explored refusers’ motivations,16,17,18,19 the data are largely qualitative and have sought respondents’ preferences and views in hypothetical scenarios, in part because the population of people not wanting to receive this information is so small.20 Thus, there has been a lack of generalizable data on the views and characteristics of people who have actively made the decision to refuse medically actionable genetic information.
Through an opportunity to survey a large group of research participants who made the decision not to receive SFs, this study aims to address these questions. Specifically, the study attempts to: (1) gauge the strength and stability of participants’ original decisions; (2) determine how informed participants were when they made their original decision; (3) ascertain whether more detailed information changes participants’ desires to receive SFs; (4) explore the reasoning employed by participants when making their choice about SFs; and (5) identify potential differences in reasons for refusal between racial and ethnic groups.
Materials and Methods
Participants were drawn from the Environmental Polymorphisms Registry (EPR),21 an NIH study of 19,658 participants designed to examine how genetic and environmental factors influence human health, conducted by intramural investigators in the National Institute of Environmental Health Sciences (“NIEHS”; see [21] for more information on EPR’s recruitment strategies and the demographic characteristics of its participants). The consent form used language that has regularly been approved by IRBs, specifically stating that investigators “may find gene changes that are not related to this study” and that, with the participant’s consent, they would return findings when there is “a gene change that is important to you or your family’s health” (see “Original EPR Consent Language” in the supplementary materials). The EPR contacted participants in 2016 by email, mail and phone to get explicit permission for genomic sequencing and to solicit their preferences about whether or not to receive SFs. Non-responders were re-contacted in subsequent years using the same methods.
Of the participants who already had consented to genomic sequencing in the EPR at the time of our sub-study (which was open from November 18, 2019 to January 31, 2020), 8,678 elected to receive SFs, and 165 elected not to receive them. Our sub-study invited all 165 SF “refusers” as well as a random sample of 330 SF “acceptors”. The rate of SF refusal (the number of participants who refused SFs out of the total 8,843 participants who agreed to be sequenced and expressed a preference about knowing or not know) was significantly higher among Black participants (3.2%) compared to white participants (1.6%; odds ratio 2.07, 95% confidence interval 1.44 to 2.94, p=0.00006). To explore factors that may account for this higher rate of refusal (e.g., historical research abuses and subsequent broad distrust of the medical/research establishment) Black participants were oversampled in the acceptors cohort. Other than oversampling by race, acceptors were chosen at random from the EPR acceptors cohort. Participants were contacted about the online survey by text and email, with periodic reminders for initial non-responders.
Arguments in the literature for and against receiving genetic information about oneself were used to develop preliminary survey questions. The survey was piloted using 12 cognitive interviews with EPR participants, conducted by the social science research firm that assists the EPR with their umbrella study. The survey was structured into three parts. In the first part, participants were asked questions to gauge the strength of their original decision and their recall of details about the kinds of SFs that might be returned as described in the original consent form. In the second part (intervention), participants were provided more detailed information about the types of SFs researchers may return to participants, namely that SFs would only be returned if they were associated with serious disease, treatable, and had a strong evidence base (see “Intervention Text” in the supplementary materials). Participants were then given an opportunity to make a revised, binding decision about whether to receive SFs. In the third part, participants were asked questions about their new decision.
Data were analyzed using R software (version 3.6.2). Descriptive statistics were generated for the sample’s demographic characteristics (e.g., race, age, and gender) as well as participants’ responses to many of the survey’s questions, including those testing the strength and stability of their original decisions, their understanding of SFs, and their reasons for making their decisions. Bivariate analyses, including Pearson’s chi-squared test, McNemar’s test, Fisher’s exact test, and t test statistics, were subsequently used to explore differences in responses between acceptors and refusers, differences in responses before and after the survey’s intervention, and differences in responses between racial groups.
Results
There were 231 responses out of a total 495 participants contacted, for a response rate of 46.7 percent. The response rate among refusers was slightly higher (83/165, 50.3 percent) than that of acceptors (148/330, 44.4 percent).
Characteristics of Respondents’ Original Decision
Respondents characterized the strength and stability of their original decision (Table 1). When asked how well they remembered making their decision, 43.3 percent of respondents reported not remembering being asked the question at all or vaguely remembering being asked the question. Refusers may have been more likely not to have remembered making the decision (X2 [2, N = 231] = 5.467, p = 0.065). When asked what they thought their original decision had been, 45.8 percent of refusers thought they had accepted, while 6.8 percent of acceptors thought they had refused—meaning, when comparing one’s actual decision with one’s later reported recollection of that decision, concordance for refusers was significantly lower than concordance for acceptors (Fisher’s exact, p < 0.001). Most participants (80.1 percent) thought they had enough information to make their original decision, and a similar number (80.5 percent) had not thought about changing their decision since making it.
Table 1.
Summary Questions Related to Strength and Stability of Decision (Pre-Intervention)
| How well do you remember being asked whether you wanted to receive SFs? | |||
|---|---|---|---|
| Answer Choice | Accepted (%) | Refused (%) | Total (%) |
| Definitely or mostly remember | 59 (39.9) | 21 (25.3) | 80 (34.6) |
| Somewhat remember | 32 (21.6) | 19 (22.9) | 51 (22.1) |
| Don’t or vaguely remember | 57 (38.5) | 43 (51.8) | 100 (43.3) |
| X2 (2, N = 231) = 5.467, p = 0.065 | |||
| How do you think you answered that question? | |||
| Accepted (%) | Refused (%) | Total (%) | |
| Respondent remembered choice correctly | 138 (93.2) | 45 (54.2) | 183 (79.2) |
| Respondent remembered choice incorrectly | 10 (6.8) | 38 (45.8) | 48 (20.8) |
| Fisher’s exact, p < 0.001 | |||
| Did you feel like you had enough information to make the decision? | |||
| Answer Choice | Accepted (%) | Refused (%) | Total |
| Yes | 122 (82.4) | 63 (75.9) | 185 (80.1) |
| No | 26 (17.6) | 20 (24.1) | 46 (19.9) |
| X2 (1, N = 231) = 1.041, p = 0.308 | |||
| Have you thought about changing your decision since? | |||
| Answer Choice | Accepted (%) | Refused (%) | Total |
| Yes | 26 (17.6) | 19 (22.9) | 45 (19.5) |
| No | 122 (82.4) | 64 (77.1) | 186 (80.5) |
| X2 (1, N = 231) = 0.652, p = 0.420 | |||
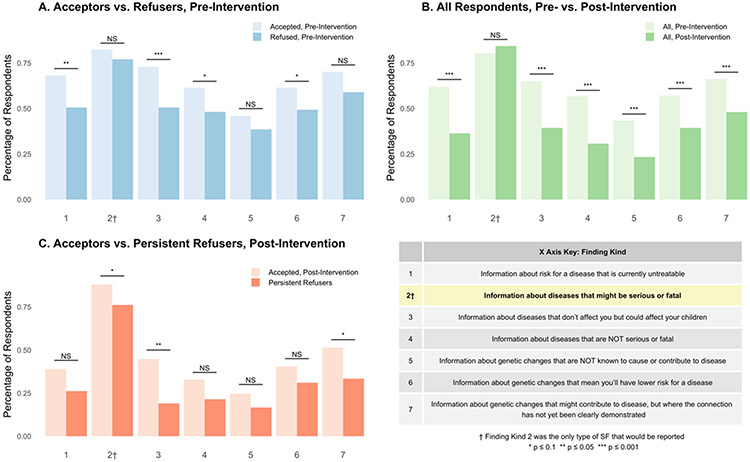
Given a range of options from which to choose, respondents were asked what types of genetic information they thought would have been reported back to them as SFs to gauge how informed their decision had been (Figure 1A). Most respondents (80.5 percent) correctly identified that SFs would only include information about actionable diseases that might be serious or fatal (column 2). However, every other kind of genetic finding presented, except one, was chosen by a majority of respondents as well. When compared with refusers, acceptors were significantly more likely to think SFs included information on diseases that are currently untreatable (column 1, X2 [1, N = 231] = 6.289, p = 0.012) and on diseases that don’t affect them but could affect their children (column 3, X2 [1, N = 231] = 10.726, p = 0.001). Though a significant difference was not found between the percentages of acceptors and refusers choosing other kinds of SFs, it is worth noting that each category of finding was chosen by a higher percentage of acceptors than refusers.
Figure 1. Beliefs about the Kinds of Genetic SFs that Could be Returned.
Notes: Pearson’s Chi-squared tests were conducted for A and C. McNemar’s tests were conducted for B. C does not include the 6 respondents who switched from accepting to refusing. “Persistent Refusers” in C are the 42 participants who refused at both timepoints
When asked what their reasons were for accepting SFs (see Table D: Reasons for Accepting in supplemental materials) 83.1 percent of acceptors thought knowledge of the findings would improve their health, and 60.8 percent reported that this was their most important reason for accepting. Refusers endorsed a variety of reasons for their decision not to receive SFs (Table 2). The most common reason (51.8 percent) was the perception that the information would make the respondent worried or sad. Approximately one-third (32.5 percent) of respondents refused in part because they were not curious about the information. Of refusers, 19.3 percent of refusers would not have done anything with the information, and 13.3 percent refused because they did not think the information was relevant to their health. Another 13.3 percent refused because of concerns that their health insurance would use the information against them. Only 4.8 percent of respondents refused due to concerns of stigma—the worry that their friends or family would treat them differently.
Table 2.
Reasons for Refusing, Pre- vs. Post-Intervention, by Race
| Pre-intervention | Post-intervention | |||||
|---|---|---|---|---|---|---|
| Reason | whites & Asian Americans N = 53 (%) |
Black N = 27 (%) |
Total N = 83 (%) |
whites & Asian Americans N = 28 (%) |
Black N = 18 (%) |
Total N = 48 (%) |
| Would make me worried or sad | 28 (52.8) |
14 (51.9) |
43 (51.8) |
11 (39.3) |
10 (55.6) |
21 (43.8) |
| p = 1 | p = 0.367 | |||||
| Not curious about information | 14 (26.4) |
11 (40.7) |
27 (32.5) |
6 (21.4) |
6 (33.3) |
13 (27.1) |
| p = 0.211 | p = 0.495 | |||||
| Would not have done anything with information | 14 (26.4) |
2 (7.4) |
16 (19.3) |
8 (28.6) |
2 (11.1) |
10 (20.8) |
| p = 0.074 | p = 0.274 | |||||
| Did not think information was relevant to health | 10 (18.9) |
1 (3.7) |
11 (13.3) |
3 (10.7) |
1 (5.6) |
5 (10.4) |
| p = 0.088 | p = 1 | |||||
| Worried health insurance would use it against me | 10 (18.9) |
1 (3.7) |
11 (13.3) |
2 (7.1) |
3 (16.7) |
5 (10.4) |
| p = 0.088 | p = 0.366 | |||||
| Worried friends or family would treat me differently | 1 (1.9) |
3 (11.1) |
4 (4.8) |
1 (3.6) |
0 (0) |
1 (2.1) |
| p = 0.109 | p = 1 | |||||
| Did not trust NIH with information | 0 (0) |
3 (11.1) |
3 (3.6) |
0 (0) |
4 (22.2) |
4 (8.3) |
| p = 0.036 | p = 0.019 | |||||
| Could not take action because no access to healthcare | 1 (1.9) |
0 (0) |
1 (1.2) |
1 (3.6) |
0 (0) |
1 (2.1) |
| p = 1 | p = 1 | |||||
| Other | 7 (13.2) |
4 (14.8) |
11 (13.3) |
6 (21.4) |
0 (0) |
6 (12.5) |
| p = 1 | p = 0.068 | |||||
Notes: Respondents could choose more than one reason, so percentages add up to more than 100 percent. All p values are results from Fisher’s exact tests. Respondents who did not report their race are included in the totals.
Differences in Reasons for Refusal Between Racial and Ethnic Groups
Black respondents were significantly more likely to originally refuse to receive SFs than non-Black participants (including whites and Asian Americans)—47.4 percent refused, versus 31.7 percent of whites and Asian Americans (X2 [1, N = 224] = 3.868, p = 0.049) (see Table A: Demographics and Original Decision in supplemental materials). Refusers’ reasons were analyzed by race and found that Black respondents were more likely than non-Black respondents to refuse SFs in part because they did not trust the NIH with their genetic information; 11.1 percent of Black respondents endorsed this reason versus zero percent of whites and Asian Americans (p = 0.036) (Table 2).
Respondents’ Decision Post-Intervention
Following the provision of more detailed information about SFs, there was a significant increase in the number of respondents accepting (McNemar’s test, X2 [1, 231] = 24.596, p < 0.001) (see Table 3). A total of 41 respondents switched from refusing to accepting, while six people switched from accepting to refusing—a net decrease in refusing of 42.2 percent. Of all respondents, 76.6 percent reported they were “completely” or “pretty sure” about their decision, and 93.9 percent reported they “had enough information” to make their decision—a 13.8 percent increase from before more information was provided.
Table 3.
Decision Post-Intervention
| Decision Post-Intervention | |||
|---|---|---|---|
| Pre-intervention accepted |
Pre-intervention refused |
Total (Percent) | |
| Post-intervention accepted | 142 | 41 | 183 (79.2) |
| Post-intervention refused | 6 | 42 | 48 (20.8) |
| McNemar’s test (X2 (1, 231) = 24.596, p < 0.001) | |||
| How sure are you of your decision? | |||
| Answer Choice | Accepted (Percent) | Refused (Percent) | Total (Percent) |
| Completely or pretty | 143 (78.1) | 34 (70.8) | 177 (76.6) |
| Moderately | 21 (11.5) | 6 (12.5) | 27 (11.7) |
| Not very or somewhat | 19 (10.4) | 8 (16.7) | 27 (11.7) |
| Fisher’s exact test, p = 0.449 | |||
| Did you have enough information to make your decision? | |||
| Answer Choice | Accepted (Percent) | Refused (Percent) | Total (Percent) |
| Yes | 171 (93.4) | 46 (95.8) | 217 (93.9) |
| No | 12 (6.6) | 2 (4.2) | 14 (6.1) |
| Fisher’s exact test, p = 0.74 | |||
Post-intervention, respondents were again asked about the types of SFs that would be reported to them (Figure 1B-C). A slightly higher, though not statistically significant proportion of respondents (p = 0.263) correctly identified that findings would include diseases that might be serious, while a statistically significant decrease was seen in all other options (Figure 1B), suggesting an improvement in respondents’ understanding of the limited set of SFs that might be provided. Each option was chosen again by a higher percentage of acceptors than refusers (Figure 1C). The reasons endorsed by acceptors and refusers post-intervention remained largely the same.
Characteristics of Respondents Who Refused Post-Intervention
Of the 48 respondents who refused post-intervention, 42 had originally refused (Table 4). These 42 respondents (“persistent refusers”) who held a consistent preference not to receive SFs were compared with respondents (n = 41) who originally refused and then switched to accepting (“reversible refusers”). There was significantly higher concordance between the initial decision and the later reported recollection of the decision among the persistent refusers compared to the reversible refusers (Fisher’s exact, p < 0.001). In fact, almost 75 percent of the reversible refusers thought they had originally accepted receipt of SFs. There was no significant difference between the persistent and reversible refusers regarding whether they thought they had enough information (pre-intervention), but reversible refusers may have been more likely to have thought about changing their decision since (Fisher’s exact, p = 0.071). The level of understanding about SFs among persistent refusers was high; in fact, this group may have had a slightly better understanding in some respects than respondents who accepted post-intervention (Figure 1C). The six respondents who switched from accepting to refusing post-intervention were not included in this analysis.
Table 4.
Comparing Persistent Refusers with Reversible Refusers
| How do you think you answered the question of whether to receive SFs? (pre-intervention) | ||
|---|---|---|
| Answer Choice | Persistent Refusers (%) | Reversible Refusers (%) |
| I accepted SFs | 8 (19.0) | 30 (73.2) |
| I refused SFs | 34 (81.0) | 11 (26.8) |
| Fisher’s exact, p < 0.001 | ||
| Did you feel like you had enough information to make the decision? (pre-intervention) | ||
| Answer Choice | Persistent Refusers (%) | Reversible Refusers (%) |
| Yes | 31 (73.8) | 32 (78.0) |
| No | 11 (26.2) | 9 (22.0) |
| Fisher’s exact, p = 0.798 | ||
| Have you thought about changing your decision since? (pre-intervention) | ||
| Answer Choice | Persistent Refusers (%) | Reversible Refusers (%) |
| Yes | 6 (14.3) | 13 (31.7) |
| No | 36 (85.7) | 28 (68.3) |
| Fisher’s exact, p = 0.071 | ||
Discussion
This study contributes a number of important findings to the debate within the research setting on the right not to know genetic information about oneself. In turn, these findings point to several implications for policies governing the return of SFs to research participants.
We found that the group of participants in our sample who originally refused to receive SFs was equally divided between “reversible refusers” and “persistent refusers.” Reversible refusers switched their decision to accepting SFs following the intervention, while persistent refusers continued to refuse. Reversible refusers were significantly less likely to have remembered their original decision accurately and more likely to have thought about changing their original decision than persistent refusers. Despite the observation that refusers, on the whole, were less likely to have remembered their original decision than acceptors, persistent refusers not only had a more stable preference than reversible refusers, but they also had a high level of understanding of what SFs would include. In fact, persistent refusers may have had a higher level of understanding of SFs than acceptors, post-intervention.
These findings add nuance to the ethical and policy debates surrounding the RNTK genetic information about oneself. Scholars who have endorsed a more absolute conception of the RNTK argue that some research participants have a genuine preference not to know certain information about themselves, and that this preference ought to be respected. They advocate that these preferences be actively solicited as part of the consent process for genomic sequencing, instead of having a default practice of returning SFs.4 The critical assumption made here is that a participant’s indicated preference on a consent form reflects their true considered view.
On the one hand, our finding that many participants of genetic testing may initially refuse SFs but do not have strong or stable preferences calls this assumption into question. Rather, it suggests that consent form check boxes may not be the most appropriate method to solicit people’s preferences, and that decisions made in that context should not be seen as definitive expressions of preference. This point is emphasized by our finding that almost half of participants who initially refused SFs subsequently accepted them. By soliciting preferences through check boxes after an accurate but limited presentation of information, it is likely that some participants will make a choice that results in forgoing potentially life-saving information that, upon further reflection, they would have wanted to receive. On the other hand, this study found that some participants have strong convictions about not wanting to know certain genetic information about themselves, and moreover, that these “persistent refusers” continue to refuse despite a high level of understanding of SFs. By not soliciting preferences, these persistent refusers would potentially receive information that they genuinely do not want.
In order to minimize the number of people unintentionally refusing SFs while also respecting those who truly do not want to receive SFs, one path forward would be to implement a more robust informed consent process. By providing more detailed information about the types of genetic information that would and would not be returned, the indicated preferences of research participants would likely become more accurate. Another strategy would be to ask for people’s preferences at multiple timepoints. As will be discussed below, it is possible that our survey’s intervention—the provision of more detailed information—was not responsible for the increased uptake of SFs, but rather asking participants a second time was. Given how difficult it is to maintain contact with research participants, however, this strategy would pose a potentially significant logistical burden on research teams.
More controversially, our findings could also lend support for implementing a default practice of attempting to return medically actionable genetic findings to research participants, without actively soliciting preferences. While we found that approximately half of our sample of refusers continued to refuse even after more information was provided and that their preferences were stable and well-informed, it is important to put this finding into perspective. In the overall sample of EPR participants ~98% of the cohort initially agreed to receive SFs; this group of “persistent refusers” represents a very small proportion of the study population (less than 1%) which is consistent with what studies soliciting the hypothetical preferences of patients, participants, and the public have found about low SF refusal rates.15-17,22,23,24,25,26,27 While many bioethicists approach the RNTK debate primarily through an autonomy lens, our data suggest that an equally legitimate normative frame is to consider whether RNTK policies should be constructed to accommodate this very small group at the expense of a different small group of people who refuse SFs unintentionally or through misunderstanding and subsequently forgo potentially life-saving information. In light of our findings we argue that it is ethically acceptable to consider an approach where the explicitly articulated default is to attempt to return a defined set of high-value SFs, with an implicit mechanism for persistent refusers to self-identify as wanting to opt out of receiving SFs.3 The potentially significant harms of participants misreporting their preferences on a consent form and forgoing valuable health information outweighs the harms of not respecting the preferences of a handful of persistent refusers who do not opt-out. That many of the reasons for refusing SFs endorsed by persistent refusers are based on unfounded concerns—not including a historical mistrust of healthcare institutions and medical research (discussed below)—also supports this view.
While this study demonstrated an increase in accepting SFs following the provision of more detailed information about SFs, the relationship between being well-informed and one’s information preferences remains unclear. On the one hand, it appears that improving people’s understanding leads to a higher rate of accepting SFs, as “reversible refusers” demonstrated improved understanding following the provision of additional information and switched their decision from refusing to accepting. This correlation between being well-informed and wanting genetic information is consistent with other studies.28,29 On the other hand, it appears that many people still refuse despite a high level of understanding (the “persistent refusers” as described above), and acceptors don’t necessarily have high levels of understanding. When we compared the understanding of acceptors (pre- and post-intervention) with that of refusers (pre- and post-intervention), acceptors had poorer understanding, in some respects, than refusers at both timepoints. On the whole, acceptors thought incorrectly that findings would include more information than those who refused.
These contradictory findings suggest that being more informed is not the only factor that contributes to accepting SFs. That is, reversible refusers may be switching not only because they have become better informed, but also because they had more time to think about their decision and are being asked a second time.30 Further research is needed to understand how repeated solicitations of preferences about SFs influence decision-making, and whether the act of asking multiple times creates undue pressure on a person to accept SFs or enhances their autonomy by giving them a chance to reconsider. Additionally, acceptors may not be accepting because they are better informed about what SFs will include, but perhaps because this group is generally more information-seeking.27 More research is needed to understand the causal relationship between being better informed and wanting more health information about oneself, as well as the additional factors that make some people accept and some people refuse.
In revealing some of the justifications for why people accept or refuse SFs, this study also points to potential educational strategies researchers may use to further improve the consent process. Specifically, several of the reasons for refusing can potentially be addressed through provision of additional detail and context. The most common reason chosen for refusing was that the information might make the recipient worried or sad. The concern that learning genetic information will lead to psychological harms has been prominent in the bioethics literature,7 but more recent data suggest that people who have received troubling genetic information do not end up being as anxious as they thought they would be.31,32,33 Likewise, about 10 percent of refusers did so because they were worried their health insurance would use the information against them. Yet the Genetic Information Nondiscrimination Act (GINA) prohibits health insurers from doing precisely this, and there is little evidence to suggest that health insurers and employers have ever done this.34,35 About 10 percent of refusers also endorsed the reason that they did not think the information was relevant to their health. For this subset of people, this suggests a continued lack of understanding that the SFs would be medically actionable and evidence-based. Given that refusers may be basing their decision to forgo valuable health-promoting information on these potentially mitigatable concerns, it is worth considering strategies that researchers can use during the decision-making process to convey how resilient people are following information disclosure, the protections afforded by GINA, and the actionability of the information being returned.
Furthermore, a sizeable proportion of refusers made their decisions in part because they would not have done anything with the information (about 21 percent), and that they were not curious about the information (about 27 percent). More research needs to be done to understand why these reasons may have driven people not to receive important health information, and how researchers might address them. A notable finding was that very few refusers made their decision because they did not have access to healthcare, or because they were worried friends or family would treat them differently (i.e., stigma). This finding suggests that the significant attention given to stigma in the bioethics literature as a reason for not wanting to know genetic information may need to be reexamined.7,36 Additionally, it is important to note that six people switched from agreeing to refusing SFs as a result of this study. More research is also needed to understand why some people revoked their consent to receive SFs.
We analyzed reasons for refusing by race in an attempt to understand why a greater proportion of Black participants refused SFs and found that Black respondents were significantly more likely than whites and Asian Americans to endorse the idea that they did not trust NIH with their genetic information, both before and after the intervention. In fact, it was only Black respondents who chose this reason at both timepoints. Importantly, the absolute number of respondents who chose this reason is very small and thus unlikely to fully explain the racial difference in refusal. Nonetheless, decreased trust in healthcare institutions driving less healthcare utilization among Black people is consistent with what other studies have found.37,38,39,40 Higher distrust in the government’s regulation of research, and in researchers themselves, is driven by continued racial discrimination in the United States and historical medical injustices.39 This finding reminds us that it is important to be sensitive to the fact that there might be important and legitimate reasons for refusing that differ among racial groups.
Limitations
There was a gap of more than a year between respondents making their original decision on whether to receive SFs and the administration of our survey. This may have resulted in our survey demonstrating a lower initial understanding of SFs than when respondents originally made their decision. This time gap also made it difficult to measure whether changes in understanding were due to the passage of time, or the provision of more detailed information about SFs. In addition, given the small numbers of participants in the specific racial and ethnic categories, one should be cautious about the statistical inferences about particular groups that can be drawn from the data. When analyzing by race, we compared Black respondants to whites and Asian Americans together. Yet there is little literature on the genetic information preferences or utilization of Asian Americans and thus there is some uncertainty about how to group them. Finally, though Black participants were significantly more likely to refuse SFs, that result was not strong (p = 0.049) and deserves further study.
Conclusion
This study provides valuable data to inform how research institutions design policies related to returning SFs to research participants. Most significantly, our data raise questions about whether a strong conception of the “right not to know” is the right lens when creating policies to govern the return of SFs. We argue that our data support a default practice of attempting to return SFs without soliciting the preferences of research participants, while also providing a clearly-defined (though implicit) opt-out mechanism for people that independently raise concerns about the RNTK. An alternative policy could involve re-contacting refusers to provide them more time to make their decision and a chance to reconsider. The ability to re-query participants, however, would be logistically challenging and resource-intensive for many research teams. Our study shows that, at the very least, more detailed information about SFs should be provided to participants during the informed consent process. While further study is warranted, we believe that either of these policy choices would help decrease the number of research participants who inadvertently refuse important health information because of misunderstanding or indecision.
Supplementary Material
Acknowledgments:
We thank Sara Chandros Hull, Holly Taylor, and Leila Jamal for their review as well as the Department of Bioethics at the NIH Clinical Center; and Fikri Yucel, Social and Scientific Systems, for assistance with the study.
Funding:
This research was supported by the Intramural Research Program of the National Institutes of Health, including funding from the National Human Genome Research Institute, the National Institute of Environmental Health Sciences, and the NIH Clinical Center.
Footnotes
Conflicts of interest statement: The authors have no conflicts of interest to declare.
Disclaimer:
The views expressed are the authors’ own. They do not represent the position or policy of the National Institutes of Health, U.S. Public Health Service, or the Department of Health and Human Services.
Ethics Declaration:
The EPR (Protocol 04-E-0053, Clinicaltrials.gov NCT00341237) and this sub-study were approved by the NIH IRB. Informed consent was obtained from all participants as required by the IRB. Individual level data was de-identified.
Contributor Information
Will Schupmann, NIH Department of Bioethics.
Skye A. Miner, NIH Department of Bioethics.
Haley K. Sullivan, Harvard University.
Jamie R. Glover, Social and Scientific Systems.
Janet E. Hall, NIEHS Clinical Research Branch, NIEHS, NIH.
Shepherd H. Schurman, NIEHS Clinical Research Branch, NIEHS, NIH.
Benjamin E. Berkman, NIH Department of Bioethics, NHGRI.
Data Availability:
Data is available upon individual request.
References
- 1.Presidential Commission for the Study of Bioethical Issues. Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct-to-Consumer Contexts. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DT, Lee K, Chung WK, et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Berkman BE. Refuting the Right Not to Know. J Health Care Law Policy. 2017;19(1):1–75. [PMC free article] [PubMed] [Google Scholar]
- 5.Burke W, Matheny Antommaria AH, Bennett R, et al. Recommendations for returning genomic incidental findings? We need to talk! Genet Med. 2013;15(11):854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf SM, Annas GJ, Elias S. Patient Autonomy and Incidental Findings in Clinical Genomics. Science. 2013;340(6136):1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross LF, Rothstein MA, Clayton EW. Mandatory Extended Searches in All Genome Sequencing: “Incidental Findings,” Patient Autonomy, and Shared Decision Making. JAMA. 2013;310(4):367. [DOI] [PubMed] [Google Scholar]
- 8.Klitzman R, Appelbaum PS, Chung W. Return of Secondary Genomic Findings vs Patient Autonomy: Implications for Medical Care. JAMA. 2013;310(4):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtzman NA. ACMG recommendations on incidental findings are flawed scientifically and ethically. Genet Med. 2013;15(9):750–751. [DOI] [PubMed] [Google Scholar]
- 10.Allyse M, Michie M. Not-so-incidental findings: the ACMG recommendations on the reporting of incidental findings in clinical whole genome and whole exome sequencing. Trends Biotechnol. 2013;31(8):439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend A, Adam S, Birch PH, Friedman JM. Paternalism and the ACMG recommendations on genomic incidental findings: patients seen but not heard. Genet Med. 2013;15(9):751–752. [DOI] [PubMed] [Google Scholar]
- 12.Gliwa C, Yurkiewicz IR, Lehmann LS, Hull SC, Jones N, Berkman BE. Institutional review board perspectives on obligations to disclose genetic incidental findings to research participants. Genet Med. 2016;18(7):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACMG Updates Recommendation on “Opt Out” for Genome Sequencing Return of Results. American College of Medical Genetics & Genomics. https://www.acmg.net/docs/Release_ACMGUpdatesRecommendations_final.pdf. Published April 1, 2014. [Google Scholar]
- 14.Mandava A, Pace C, Campbell B, Emanuel E, Grady C. The quality of informed consent: mapping the landscape. A review of empirical data from developing and developed countries. J Med Ethics. 2012;38(6):356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong J, Zhang Y, Yang Z, Huang Y, Feng J, Zhang W. The framing effect in medical decision-making: a review of the literature. Psychol Health Med. 2013;18(6):645–653. [DOI] [PubMed] [Google Scholar]
- 16.Jamal L, Robinson JO, Christensen KD, et al. When bins blur: Patient perspectives on categories of results from clinical whole genome sequencing. AJOB Empir Bioeth. 2017;8(2):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn J, Martinez J, Duong J, et al. Research Participants’ Preferences for Hypothetical Secondary Results from Genomic Research. J Genet Counsel. 2017;26(4):841–851. [DOI] [PubMed] [Google Scholar]
- 18.Bennette CS, Trinidad SB, Fullerton SM, et al. Return of incidental findings in genomic medicine: measuring what patients value—development of an instrument to measure preferences for information from next-generation testing (IMPRINT). Genet Med. 2013;15(11):873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vornanen M, Aktan-Collan K, Hallowell N, Konttinen H, Haukkala A. Lay Perspectives on Receiving Different Types of Genomic Secondary Findings: a Qualitative Vignette Study. J Genet Couns. 2019;28(2):343–354. [DOI] [PubMed] [Google Scholar]
- 20.Hoell C, Wynn J, Rasmussen LV, et al. Participant choices for return of genomic results in the eMERGE Network. Genet Med. 2020;22(11):1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chulada PC, Vahdat HL, Sharp RR, et al. The Environmental Polymorphism Registry: A DNA Resource to Study Genetic Susceptibility Loci. Hum Genet. 2008;123(2):207–214. [DOI] [PubMed] [Google Scholar]
- 22.Middleton A, Bragin E, Firth HV, Hurles ME, Wright CF, Parker M. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the return of incidental results from sequencing research. Eur J Hum Genet. 2016;24(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright MF, Lewis KL, Fisher TC, et al. Preferences for results delivery from exome sequencing/genome sequencing. Genet Med. 2014;16(6):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10(11):831–839. [DOI] [PubMed] [Google Scholar]
- 25.Bollinger JM, Scott J, Dvoskin R, Kaufman D. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med. 2012;14(4):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahmirzadi L, Chao EC, Palmaer E, Parra MC, Tang S, Gonzalez KDF. Patient decisions for disclosure of secondary findings among the first 200 individuals undergoing clinical diagnostic exome sequencing. Genet Med. 2014;16(5):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facio FM, Eidem H, Fisher T, et al. Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study. Eur J Hum Genet. 2013;21(3):261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morren M, Rijken M, Baanders AN, Bensing J. Perceived genetic knowledge, attitudes towards genetic testing, and the relationship between these among patients with a chronic disease. Patient Educ Couns. 2007;65(2):197–204. [DOI] [PubMed] [Google Scholar]
- 29.Henneman L, Timmermans DRM, Wal GVD. Public Attitudes Toward Genetic Testing: Perceived Benefits and Objections. Genet Test. 2006;10(2):139–145. [DOI] [PubMed] [Google Scholar]
- 30.Halverson CME, Wessinger BC, Clayton EW, Wiesner GL. Patients’ willingness to reconsider cancer genetic testing after initially declining: Mention it again. J Genet Couns. 2020;29(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parens E, Appelbaum PS. On What We Have Learned and Still Need to Learn about the Psychosocial Impacts of Genetic Testing. Hastings Center Report. 2019;49:S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson TD, Gilbert DT. Affective Forecasting: Knowing What to Want. Curr Dir Psychol Sci. 2005;14(3):131–134. [Google Scholar]
- 33.Prince AE, Berkman BE. Reconceptualizing harms and benefits in the genomic age. Per Med. 2018;15(5):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genetic Discrimination. Accessed June 3, 2020. https://www.genome.gov/about-genomics/policy-issues/Genetic-Discrimination [Google Scholar]
- 35.Rothstein MA. Putting the Genetic Information Nondiscrimination Act in context. Genet Med. 2008;10(9):655–656. [DOI] [PubMed] [Google Scholar]
- 36.Bortolotti L, Widdows H. The right not to know: the case of psychiatric disorders. JMed Ethics. 2011;37(11):673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong K Racial Differences in the Use of BRCA1/2 Testing Among Women with a Family History of Breast or Ovarian Cancer. JAMA. 2005;293(14):1729. [DOI] [PubMed] [Google Scholar]
- 38.Moorman PG, Skinner C, Evans JP, Newman B, Sorenson JR. Racial Differences in Enrolment in a Cancer Genetics Registry. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1349–1354. [PubMed] [Google Scholar]
- 39.Peters N, Rose A, Armstrong K. The Association Between Race and Attitudes About Predictive Genetic Testing. Cancer Epidemiol Biomarkers Prev. 2004;13(3):361–365. [PubMed] [Google Scholar]
- 40.Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon individual request.