Abstract
Background:
Low-dose glucocorticoids are commonly used in the treatment of rheumatoid arthritis (RA). Observational studies have found increased risk of serious infection associated with low-dose glucocorticoids, but concerns about residual confounding remain.
Methods:
We identified adults with RA on stable immunomodulatory therapy for >6 months receiving no glucocorticoids or ≤5mg/day using Medicare data from 2006–2015. We used provider preference for glucocorticoids as an instrumental variable (IV) to assess associations between low-dose glucocorticoid use and risk of infection requiring hospitalization using a cause-specific proportional hazards model.
Results:
We identified 163,603 qualifying treatment episodes among 120,656 patients. Glucocorticoids ≤5mg/day were used by 25,373/81,802 (31.0%) of patients seen by a rheumatologist with low provider preference for glucocorticoids and by 36,087/81,801 (44.1%) of patients seen by a rheumatologist with high provider preference for glucocorticoids (adjusted OR 1.81, 95% CI 1.77–1.84 for association between provider preference and glucocorticoids). Chronic obstructive pulmonary disease, opioids, antibiotics, previous emergency department visits, hospitalizations, and infections requiring hospitalization infections were unbalanced with regard to exposure but not to the IV. The incidence of infection requiring hospitalization was 8.0/100 person–years among patients unexposed to glucocorticoids versus 11.7/100 person–years among those exposed. The association between glucocorticoids and infection requiring hospitalization from IV analysis [HR 1.26 (1.02–1.56)] was similar to results from a standard multivariable model [HR 1.24 (1.21–1.28)].
Conclusions:
Among patients with RA on stable immunomodulatory therapy, IV analysis based on provider preference demonstrated an increased risk of infection requiring hospitalization associated with low-dose glucocorticoids, similar to a traditional analysis.
Introduction
Glucocorticoids have long been recognized as effective treatments for rheumatoid arthritis (RA) and remain a common adjunctive treatment despite a increasing array of disease modifying anti-rheumatic drugs (DMARDs) such as methotrexate and biologics. (1–3) Because of their rapid onset of action but also toxicity concerns, guidelines recommend limiting glucocorticoid use to short-term bridging therapy when initiating or changing DMARDs. (4,5) Yet 30–60% of patients with RA remain on glucocorticoids long-term, typically at low doses. (6–8)
Doses of glucocorticoids >10mg per day are associated with more than twice the risk for infection, (9–14) but the risk with lower dose glucocorticoids (e.g. ≤5mg/day) remains controversial. (15) Randomized trials have not been powered to evaluate low-dose glucocorticoid safety, and although several observational studies have found associations between low-dose glucocorticoids and infection risk, concerns remain about residual confounding from disease activity and severity. (6,9,10,14,16,17) Consequently, there is substantial variability among rheumatologists in the long-term use of low-dose glucocorticoids. (18)
This variability in how frequently rheumatologists prescribe low-dose glucocorticoids for RA provides an opportunity to assess infection risk with alternative methods intended to avoid confounding using instrumental variable (IV) analysis. An IV should influence treatment assignment, should not directly affect outcomes, and should not be associated with unmeasured confounders. (19) Provider preference has previously been used as an IV in other contexts (20,21), and our previous study demonstrated that a measure of provider preference is predictive of long-term glucocorticoid use in patients with RA. (18) In this study we aimed to use provider preference as an IV to study the association between glucocorticoids and the risk for serious infection.
Methods
Data source
We used Medicare claims data 2006–2015, evaluating patients with Medicare Part A (in-hospital care), Part B (physician and outpatient services), and Part D (pharmaceuticals). Medicare is a public health plan covering >95% of US adults age ≥65. Younger individuals with disabilities such as RA may also be covered. (22)
Cohort identification
We identified patients ≥18 years-old with RA by a validated algorithm requiring 2 physician ICD-9 codes for RA (714.xx) ≥7 days apart and DMARD use. (23) We were interested in the effects of long-term glucocorticoid use rather than short-term use as bridging therapy during DMARD initiation; for this reason we identified patients with a stable DMARD course for ≥6 months (180 days) and compared patients receiving glucocorticoids 91–180 days after starting the DMARD course (beyond when bridging therapy would typically end) to those not receiving glucocorticoids. This prevalent rather than new-user design was intended to mirror a randomized trial in which patients who responded well to DMARD therapy (here proxied by continuing a stable DMARD course) are randomized to stop versus continue glucocorticoids. (24) Additionally, this design avoids the time periods immediately after DMARD initiation during which fluctuations in disease activity and glucocorticoid dosing are more substantial. A stable DMARD course was defined as either a) continuous use of methotrexate with no biologic or janus kinase inhibitor (JAKi) (i.e. tofacitinib) or b) continuous use of a biologic or JAKi, with or without methotrexate. Continuous use was defined as no gaps in treatment >90 days based on the days’ supply from prescription fills or expected infusion intervals for infused therapies. (25) Rituximab courses were required to be ≥210 days to exclude patients receiving two initial infusions with no subsequent infusions at 6 months.
To ensure stable DMARD use at cohort entry, the index date anchoring the start of follow-up was 6 months after the start date of the DMARD course (eFigure 1). Included patients had an index date between January 1st, 2007 and August 31st, 2015. We required ≥6 months data available prior to the DMARD course start date; this 6-month period and the first 6 months of the DMARD course comprised a 1-year baseline period. Patients with diagnoses of psoriatic arthritis, ankylosing spondylitis, inflammatory bowel disease, systemic lupus erythematosus, malignancy, or HIV during the baseline period were excluded (codes in eTable 1–2). We required that patients have an identifiable rheumatologist who saw ≥10 patients with RA within that calendar year to allow accurate estimation of provider preference for glucocorticoids (see below). Patients could contribute multiple observations if they had multiple distinct stable DMARD courses.
Exposure
We assessed average daily glucocorticoid dose in the 90 days prior to the index date based on oral prescriptions for prednisone, prednisolone, and methylprednisolone as previously described. (6) Prescribed dose in prednisone equivalents (methylprednisolone 1mg equivalent to 1.25mg prednisone) and days’ supply were used to calculate a dose for each day, truncating prescriptions if a new prescription was filled before the prescription end date. Because prescriptions may last longer than the prescribed days’ supply (leading to gaps between filled prescriptions), the daily doses for every day in the 90 days prior to the index date were averaged to obtain the average daily glucocorticoid dose. Because the primary interest was the effect of low-dose glucocorticoids, we restricted the cohort to patients receiving no glucocorticoids or glucocorticoids ≤5mg/day in the 90 days prior to the index date, and dichotomized exposure as none versus ≤5mg/day. Unlike in our previous study, glucocorticoid dose was not updated after the index date because associations between the proposed IV and glucocorticoid use were only evaluated with glucocorticoid use at baseline. (6)
Outcome and Follow-up
The outcome of interest was the time to a first infection requiring hospitalization. The primary outcome definition was an ICD-9 diagnosis in any position of the discharge diagnoses from an acute care hospitalization (positive predictive value 85–90%). (12,26) In secondary analyses, we evaluated an alternative definition requiring a principal discharge diagnosis of infection.
The start of follow-up began at the index date, and patients were followed to the soonest of end of enrollment in the health plan, September 30th 2015, end of the stable DMARD course, or death.
Covariates
Covariates measured during the baseline period included age, sex, race, region, calendar year, disability, dual Medicare and Medicaid eligibility, quintiles of median household income based on ZIP code from the American Community Survey 2009–2013 (27), comorbidities, Charlson comorbidity index (28), healthcare utilization (outpatient visits, rheumatologist outpatient visits, emergency department visits, hospitalizations), previous infection requiring hospitalization, influenza vaccination, skilled nursing facility stay, and durable medical equipment. In addition to including the DMARD defining the stable DMARD treatment course, we included presence of a prescription fill for non-steroidal anti-inflammatory drugs, opioids, methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, proton pump inhibitors, and antibiotics in the 90 days prior to the index date. We used all available data prior to the index date to assess pneumococcal and zoster vaccination, cancer screening, and number of previous biologics.
Instrumental variable definition
For each patient, we identified the treating rheumatologist using the National Provider Index (NPI) number from the most recent rheumatology outpatient visit (Medicare provider type 66) that included a diagnosis of RA and occurred before the index date.
Measurement of provider preference for glucocorticoids in this dataset has been previously described in detail (18), and specifics can be found in the eAppendix. We measured provider preference for glucocorticoids for each rheumatologist based on how commonly the rheumatologist’s patients with RA received glucocorticoids using a larger cohort of patients with RA in the same dataset who were new or prevalent DMARD users (with no stable DMARD requirement). To allow preference to change over time, we evaluated provider preference separately in each calendar year.
We evaluated two different methods of defining provider preference: 1) the proportion of a provider’s patients receiving glucocorticoids, and 2) an observed/expected measure of provider preference created by dividing the number of a rheumatologist’s patients actually receiving glucocorticoids by the number of patients expected to receive glucocorticoids (based on a model predicting glucocorticoid use which included calendar year, patient demographics, current DMARDs, and comorbidities). This observed/expected measure sought to more accurately identify provider preference by accounting for differences in case mix. Additionally, for each of these two definitions we assessed four different potential definitions of glucocorticoid use based on different exposure windows and thresholds for defining glucocorticoid exposure, resulting in eight pre-specified potential IV candidates (see eAppendix for details). We then applied these measures of provider preference for glucocorticoids to each patient in the smaller analytic cohort of patients on stable DMARD therapy, linked by provider and calendar date.
To select an IV, we assessed univariate associations between each of the eight potential IVs (as continuous measures) and glucocorticoid use at baseline in our cohort of patients on stable DMARD therapy using ANOVA, comparing F-statistics and r2 values. (19) The strongest IV was an observed/expected measure of provider preference with glucocorticoid use defined as ≥30 days of glucocorticoids within 90 days of the first methotrexate or biologic or JAKi prescription fill or infusion of the calendar year (eTable 3). This measure was dichotomized (top 50th versus bottom 50th percentile) and selected as the proposed IV for subsequent analysis.
Instrumental variable analysis assumptions
We assessed associations between the dichotomized proposed IV and glucocorticoid exposure, measuring IV strength using the complier rate (difference in the frequency of glucocorticoid use by IV status, assuming no defiers) and by calculating the partial F using ANOVA, with glucocorticoid use as the dependent variable and the proposed IV and all covariates as independent variables (F-statistics <10 are typically considered weak IVs). (19) Similar analysis with a logistic regression model estimated the odds ratio.
To assess whether the proposed IV was likely to be associated with unmeasured confounders, we assessed associations with all measured covariates. Covariate balance across the proposed IV and across glucocorticoid exposure (none versus ≤5mg/day) was assessed using standardized differences. Standardized differences >0.1 were considered potentially important differences across glucocorticoid exposure. Because weak IVs can amplify bias, potentially important differences across levels of the proposed IV were defined more conservatively as the complier rate multiplied by 0.2 (calculated as 0.026). (19)
To be a valid IV, provider preference cannot affect a patient’s risk of infection requiring hospitalization other than through differences in glucocorticoid use (exclusion restriction). Although DMARD use may differ across providers, stable DMARD use was required for cohort entry and we assessed differences in types of DMARDs used across the proposed IV. To further assess possible violations of exclusion restriction, we assessed differences in vaccination rates and preventative healthcare (e.g. cancer screening) across the proposed IV.
This proposed IV should also satisfy the stable unit treatment value assumption: one patient’s glucocorticoid use is not expected to affect a different patient, and we avoided including different versions of treatment by restricting glucocorticoid dose to ≤5mg/day. Additionally, monotonicity is expected since it is unlikely that there are defiers who would not be prescribed glucocorticoids if seen by a physician who prefers glucocorticoids but would be prescribed glucocorticoids if seen by a physician who does not prefer glucocorticoids.
Statistical Analysis
We conducted IV analyses with cause-specific proportional hazards models using the ivcoxph package in R with time to first infection requiring hospitalization as the outcome, treating competing events (deaths) as censoring, glucocorticoid use as the exposure, and dichotomized provider preference as the IV. (29–31) Covariates with standardized differences >0.026 across the proposed IV (complier rate multiplied by 0.2) were included as covariates in models for both the instrument and outcome. Age, age2, and sex were prespecified to be included in the models. Additionally, number of emergency department visits and of infections requiring hospitalization in the past year were included as categorical covariates in both stages of the models because they were strongly associated with the outcome, although they were not imbalanced across levels of the proposed IV. Under the assumption of monotonicity the results estimate the complier average causal effect: the average causal effect among patients who would receive glucocorticoids if treated by a rheumatologist with a higher value of the proposed IV but would not receive glucocorticoids if treated by a rheumatologist with a lower value.
For comparison, we used a standard cause-specific proportional hazards model to evaluate the association between glucocorticoid use and infection requiring hospitalization in the same cohort, including all covariates of interest (not limited to imbalanced covariates) and using cluster robust standard errors to account for patients contributing multiple observations.
We created the dataset with SAS 9.4 (SAS Institute) and performed analysis using Stata 15.1 (StataCorp, College Station, TX), and IV analysis using R version 4.0.3 (Vienna, Austria). The protocol was approved by the institutional review boards of the University of Pennsylvania and the University of Alabama at Birmingham.
Results
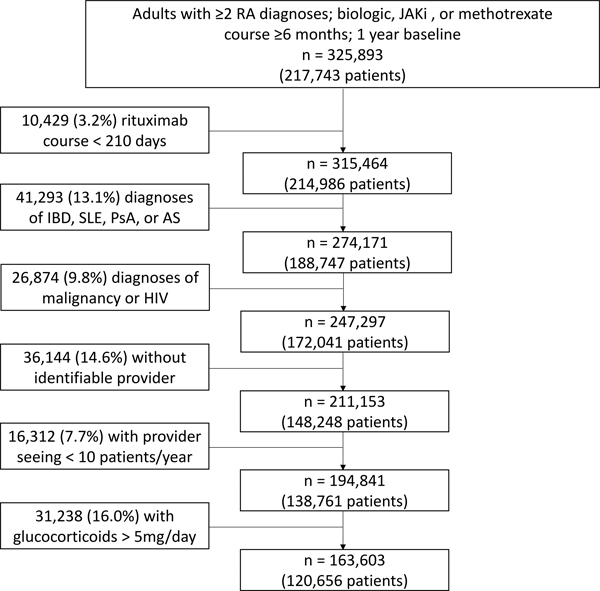
We identified 247,297 qualifying medication courses among 172,041 patients as previously described (Figure 1). (6) Limiting to patients with an identifiable treating rheumatologist who saw ≥10 RA patients in the same calendar year and excluding patients receiving >5mg/day of glucocorticoids left 163,603 medication courses among 120,656 patients.
Figure 1: Cohort identification.
JAKi = janus kinase inhibitor; IBD = inflammatory bowel disease; SLE = systemic lupus erythematosus; PsA = psoriatic arthritis; AS = ankylosing spondylitis
Glucocorticoids ≤5mg were used by 61,460 (37.6%) of the cohort, including 25,373/81,802 (31.0%) patients seen by a rheumatologist with low provider preference for glucocorticoids and 36,087/81,801 (44.1%) seen by a rheumatologist with high provider preference for glucocorticoids, for a complier rate of 13.1%. In a multivariable analysis of variance (ANOVA) model assessing associations between the proposed IV and glucocorticoid use and including all covariates of interest, the partial-F for the IV was 3,191. In a similar logistic regression model, the odds ratio (OR) for the association between the proposed IV and glucocorticoid use was 1.81 (95% CI 1.77–1.84) with predicted probabilities of glucocorticoid use 44.1% vs. 30.4% (complier rate 13.7% accounting for covariates).
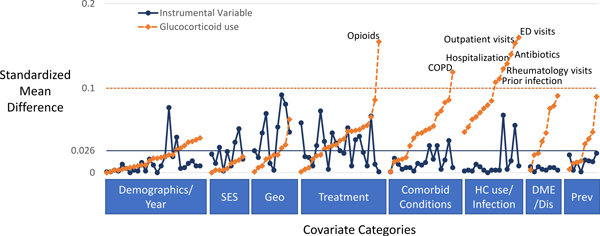
Cohort characteristics are shown in Table 1 and eTable 4, with imbalance in covariates across the exposure (glucocorticoid use) and across the proposed IV shown graphically in Figure 2. Patients receiving glucocorticoids were more likely to be treated with opioids, have chronic obstructive pulmonary disorder (COPD), recent antibiotic use, and emergency department visits, hospitalizations, and infections requiring hospitalization in the previous year. In contrast, there were no important differences in these characteristics across the proposed IV, even with a more stringent cut-off of 0.026 for standardized differences (Table 1, Supplemental Table, Supplemental Figure). Patients receiving glucocorticoids also had more outpatient and rheumatology visits in the past year. There were small differences (standardized differences <0.1 but >0.026) in the frequency of these visits across the proposed IV as well as small differences in race, region, urban versus rural, median household income, dual Medicare and Medicaid eligibility, Charlson comorbidity score, asthma, current DMARDs, and number of previous biologics.
Table 1:
Select cohort characteristics based on exposure (glucocorticoid use) or based on instrumental variable status (observed/expected provider preference for glucocorticoids high versus low)
| Comparisons by Exposure |
Comparisons by Instrumental Variable |
|||||
|---|---|---|---|---|---|---|
| No Glucocorticoids | Glucocorticoids ≤5mg/day | SMD | Low IV | High IV | SMD | |
|
|
||||||
| N | 102,143 | 61,460 | 81802 | 81801 | ||
| Age, years mean (SD)a | 68.6 (12) | 69.3 (11) | 0.068 | 68.9 (12) | 68.7 (12) | −0.018 |
| Female, N(%) | 83,599 (82) | 49,896 (81) | −0.017 | 66,754 (82) | 66,741 (82) | 0.000 |
| White, N(%) | 73,289 (72) | 44,852 (73) | 0.027 | 58,293 (71) | 59,848 (73) | 0.042 |
| Year 2011–2015 (vs. 2007–2010), N(%)a | 64,832 (63) | 38,803 (63) | −0.007 | 51,979 (64) | 51,656 (63) | −0.008 |
| Urban, N(%) | 72,776 (71) | 42,984 (70) | −0.029 | 59,589 (73) | 56,171 (69) | −0.092 |
| Treatments | ||||||
| DMARD | ||||||
| MTX (no biologic/JAKi), N(%) | 51,459 (50) | 31,618 (51) | 0.021 | 40,052 (49) | 43,025 (53) | 0.073 |
| TNF inhibitor, N(%)a | 33,724 (33) | 18,221 (30) | −0.073 | 26,357 (32) | 25,588 (31) | −0.020 |
| Abatacept, N(%) | 9,431 (9) | 6,261 (10) | 0.032 | 8,417 (10) | 7,275 (9) | −0.047 |
| Rituximab, N(%) | 3,800 (4) | 2,683 (4) | 0.033 | 3,523 (4) | 2,960 (4) | −0.035 |
| Tocilizumab, N(%) | 2,713 (3) | 2,029 (3) | 0.038 | 2,548 (3) | 2,194 (3) | −0.026 |
| Tofacitinib, N(%) | 1,016 (1) | 648 (1) | 0.006 | 905 (1) | 759 (1) | −0.018 |
| Prior biologics | ||||||
| None, N(%) | 66,946 (66) | 38,330 (62) | −0.066 | 51,336 (63) | 53,940 (66) | 0.067 |
| 1, N(%) | 24,691 (24) | 15,399 (25) | 0.020 | 20,601 (25) | 19,489 (24) | −0.032 |
| 2, N(%) | 7,439 (7) | 5,311 (9) | 0.050 | 6,803 (8) | 5,947 (7) | −0.039 |
| ≥3, N(%) | 3,067 (3) | 2,420 (4) | 0.051 | 3,062 (4) | 2,425 (3) | −0.043 |
| Opioids, N(%) | 43,114 (42) | 30,690 (50) | 0.155 | 36,918 (45) | 36,886 (45) | −0.001 |
| Comorbidities | ||||||
| Charlson, median[IQR] | 2 [0–3] | 2 [0–4] | 0.086 | 2 [0–4] | 2 [0–3] | −0.038 |
| Diabetes, N(%) | 22,745 (22) | 12,885 (21) | −0.032 | 17,720 (22) | 17,910 (22) | 0.006 |
| COPD, N(%) | 10,474 (10) | 8,686 (14) | 0.119 | 9,661 (12) | 9,499 (12) | −0.006 |
| Asthma, N(%) | 7,212 (7) | 5,487 (9) | 0.069 | 6,698 (8) | 6,001 (7) | −0.032 |
| CHF, N(%) | 7,822 (8) | 5,967 (10) | 0.073 | 6,935 (8) | 6,854 (8) | −0.004 |
| CAD, N(%) | 19,523 (19) | 12,973 (21) | 0.050 | 16,446 (20) | 16,050 (20) | −0.012 |
| Extra-articular RA, N(%) | 2,374 (2) | 1,837 (3) | 0.041 | 2,092 (3) | 2,119 (3) | 0.002 |
| Healthcare utilization past year | ||||||
| Outpatient visits, median[IQR] | 13 [8–18] | 14 [9–20] | 0.153 | 13 [9–19] | 13 [9–19] | −0.056 |
| Rheumatology visits, median[IQR] | 4 [3–6] | 4 [3–6] | 0.123 | 4 [3–6] | 4 [3–6] | −0.068 |
| ED visits | ||||||
| None, N(%) | 67,144 (66) | 35,632 (58) | −0.160 | 51,550 (63) | 51,226 (63) | −0.008 |
| 1, N(%) | 20,457 (20) | 13,970 (23) | 0.066 | 17,044 (21) | 17,383 (21) | 0.010 |
| 2, N(%) | 7,614 (7) | 5,884 (10) | 0.076 | 6,719 (8) | 6,779 (8) | 0.003 |
| ≥3, N(%) | 6,928 (7) | 5,974 (10) | 0.107 | 6,489 (8) | 6,413 (8) | −0.003 |
| Hospitalizations | ||||||
| None, N(%) | 81,201 (79) | 45,515 (74) | −0.129 | 63,429 (78) | 63,287 (77) | −0.004 |
| 1, N(%) | 14,023 (14) | 10,194 (17) | 0.08 | 12,109 (15) | 12,108 (15) | 0.000 |
| 2, N(%) | 4,471 (4) | 3,533 (6) | 0.063 | 3,981 (5) | 4,023 (5) | 0.002 |
| ≥3, N(%) | 2,448 (2) | 2,218 (4) | 0.071 | 2,283 (3) | 2,383 (3) | 0.007 |
| Infection requiring hospitalization | ||||||
| None, N(%) | 94,331 (92) | 54,780 (89) | −0.111 | 74,518 (91) | 74,593 (91) | 0.003 |
| 1, N(%) | 6,081 (6) | 4,986 (8) | 0.085 | 5,564 (7) | 5,503 (7) | −0.003 |
| 2, N(%) | 1,237 (1) | 1,152 (2) | 0.054 | 1,210 (1) | 1,179 (1) | −0.003 |
| ≥3, N(%) | 494 (0) | 542 (1) | 0.048 | 510 (1) | 526 (1) | 0.002 |
| Antibiotics, N(%) | 30,739 (30) | 22,553 (37) | 0.140 | 26,914 (33) | 26,378 (32) | −0.014 |
| Durable medical equipment, N(%) | 12,876 (13) | 9,706 (16) | 0.091 | 11,340 (14) | 11,242 (14) | −0.003 |
| Immunizations | ||||||
| Influenza vaccine (past year), N(%) | 56,829 (56) | 34,612 (56) | 0.014 | 45,735 (56) | 45,706 (56) | −0.001 |
| Pneumococcal vaccine (ever), N(%) | 29,978 (29) | 18,380 (30) | 0.012 | 24,403 (30) | 23,955 (29) | −0.012 |
| Herpes zoster vaccine (ever), N(%) | 7,456 (7) | 3,956 (6) | −0.034 | 5,860 (7) | 5,552 (7) | −0.015 |
For assessing covariate balance and including covariates in models, age was categorized in 5 year increments (vs. <50 years old), each year 2007 categorized, and each TNF inhibitor was considered separately – these are shown along with full list of covariates assessed in Appendix Table 4.
Standardized mean differences (SMD) were considered meaningful across the exposure (glucocorticoids) if > 0.1 and across the IV if > 0.026 (the complier rate times 0.2) – these values are bolded.
DMARD = disease-modifying anti-rheumatic drug; MTX = methotrexate; JAKi = janus kinase inhibitor; TNF = tumor necrosis factor; IQR = interquartile range; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure; CAD = coronary artery disease; ED = emergency department
Figure 2: Standardized differences for covariates across the proposed IV (blue) or across the exposure (glucocorticoids, orange).
Each dot represents a dichotomous variable or category of a categorical variable (for specific values see eTable 4). Horizontal lines represent cut-offs for potentially importance standardized differences (0.1 for differences across the exposure and a more stringent 0.026 for differences across the proposed instrumental variable based on the complier rate times 0.2). SES = socioeconomic status, Geo = geography, HC use = healthcare use, DME/Dis = durable medical equipment/disability; Prev = preventive treatment (screening, immunizations)
Median follow-up time after the index date was 294 days (interquartile range [IQR] 112 to 665) in patients not receiving glucocorticoids and 270 (IQR 104–619) in patients receiving glucocorticoids. Censoring due to death was infrequent, occurring in 0.9% versus 1.3% of patients not receiving versus receiving glucocorticoids and 1.0% versus 1.1% across the proposed IV.
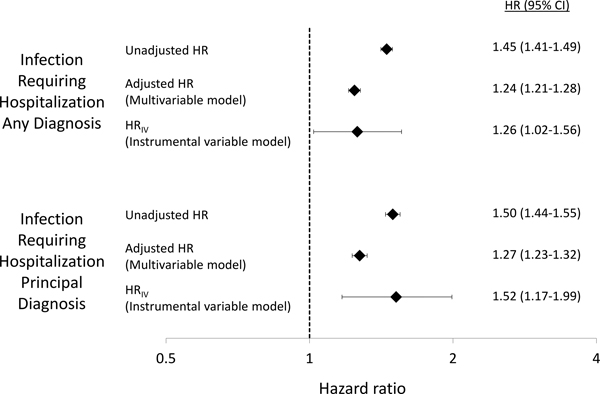
As shown in Table 2, there were 11,167 (8.0/100 person–years) infections requiring hospitalization among patients not receiving glucocorticoids and 9,204 (11.7/100 person–years) in patients receiving glucocorticoids using any discharge diagnosis, unadjusted HR 1.45 (95% CI 1.41–1.49). Rates were lower [6,755 (4.7/100 person–years) and 5,782 (7.1/100 person–years)] using a principal discharge diagnosis, unadjusted HR 1.50 (95% CI 1.44–1.55) (Table 2, Figure 3). The most common infections were urinary infection, pneumonia, bacteremia or septicemia, and skin or soft-tissue infections.
Table 2:
Frequency of outcomes by glucocorticoids and by provider preference for glucocorticoids
| N | Person–years | Infections (Incidence per 100 person–years) | |
|---|---|---|---|
|
|
|||
| Infection requiring hospitalization any discharge diagnosis | |||
| No glucocorticoids | 102143 | 138901 | 11167 (8.0) |
| ≤5mg/day | 61460 | 78406 | 9204 (11.7) |
| Low provider preference for GC | 81802 | 106921 | 9971 (9.3) |
| High provider preference for GC | 81801 | 110385 | 10400 (9.4) |
| Infection requiring hospitalization principal discharge diagnosis | |||
| No glucocorticoids | 102143 | 143589 | 6755 (4.7) |
| ≤5mg/day | 61460 | 81921 | 5782 (7.1) |
| Low provider preference for GC | 81802 | 111054 | 6050 (5.4) |
| High provider preference for GC | 81801 | 114456 | 6487 (5.7) |
GC = glucocorticoids
Figure 3: Results of a traditional multivariable model compared to instrumental variable (IV) analysis.
Unadjusted hazard ratios (HR) are from univariate cause-specifics hazards models (unadjusted hazard ratio). Adjusted hazard ratios are from multivariable cause-specific hazards models including all covariates of interest. HRIV are from instrumental variable analyses (IV) performed using Cox models. IV analyses included covariates with SMD > 0.026 (race, urban versus rural, region, dual Medicare/Medicaid eligibility, median household income quintiles, sulfasalazine, methotrexate, current disease-modifying drug use, number of previous biologics, chronic pain, asthma, number of outpatient visits and number of rheumatology visits in the past year), along with age, age2, sex, and covariates strongly associated with the outcome (emergency department visits, prior infection requiring hospitalization).
Results of the IV analysis showed an association between glucocorticoid use ≤5mg/day and infection requiring hospitalization with HR 1.26 (1.02–1.56) for any discharge diagnosis of infection and HR 1.52 (1.17–1.99) for principal diagnosis of infection from an acute care hospitalization (Table 2, Figure 3). A standard multivariable model with all covariates of interest included showed similar results with HR 1.24 (1.21–1.28) for any discharge diagnosis of infection and 1.27 (1.23–1.32) for principal diagnosis of infection.
Discussion
In this cohort study in Medicare claims data, we found that low-dose glucocorticoid use ≤5mg/day was associated with an increased risk for infection requiring hospitalization in patients with RA on stable DMARD therapy, using the natural variability in glucocorticoid prescribing between rheumatologists (provider preference for glucocorticoids) as an IV. Results were very similar to a standard multivariable analysis and were consistent across both definitions of the outcome. These results are also similar to our previous inverse probability weighted analysis, which found a HR of 1.29 (1.25–1.34) for any diagnosis code of infection and a HR of 1.34 (1.29–1.40) for a principal diagnosis code of infection in a very similar Medicare cohort. (6) We previously found that this magnitude of association represents a clinically meaningful increase of approximately 1–2 additional infections requiring hospitalization among 100 patients treated for 1 year, (6) similar to the magnitude of risk with biologic therapies. (32)
IV analyses can serve as a useful complement to traditional analyses, with each having different assumptions and limitations. IV analyses may be particularly helpful when there are concerns about residual unmeasured confounding in traditional analyses, but large sample sizes are needed because of the reduced power in IV analyses, particularly when IV strength is modest (as expected in preference-based IVs). Under the assumption of monotonicity our results reflect the complier average causal effect and specifically apply to the approximately 13% of the population identified as compliers. (33) Effects may be similar in other patients – substantial effect heterogeneity is not expected and we previously found similar associations regardless of age, biologic use, and insurance (Medicare versus commercial insurance). (6) In this context, it is notable that IV analysis results were quite similar to a traditional multivariable analysis, our previous inverse probability weighted analysis in this same dataset (6), and observational studies in other datasets. (9,10,14,16,17) These similarities might suggest that residual confounding in these previous analyses was not substantial, despite the lack of disease activity measures in most studies. Indeed, we previously found that patients receiving ≤5mg/day of glucocorticoids were quite similar to those not receiving glucocorticoids (including C-reactive protein levels), with greater differences in patients receiving glucocorticoids at higher doses. (6) The similar results in this study using different pharmacoepidemiologic methods provides further evidence about the risk of low-dose glucocorticoids. These results are important since randomized trials large enough to evaluate serious infection risk are not likely to be performed.
As also done in previous studies using provider preference as an IV (20,21), we created a measure of provider preference that was associated with treatment assignment, taking advantage of the substantial variability across rheumatologists in the use of glucocorticoids for the treatment of RA. (18) In part because of the size of our dataset, the partial-F for our instrumental variable was large (3191), although the complier rate was more modest (13.1%). This modest effect on glucocorticoid use is expected, since other factors such as sex, disease severity, and comorbidities also influence the decision to use glucocorticoids. (18)
Although it is not possible to prove that an IV is not associated with unmeasured confounders, several aspects of our analysis are reassuring. Associations between the proposed IV and measured confounders were small. Notably, there were minimal differences across the proposed IV for factors such as prior emergency department visits, infections, hospitalizations, opioid use, antibiotic use, and COPD, and the proposed IV was associated with lower Charlson comorbidity scores; these factors are strongly associated with the subsequent risk of serious infection are also associated with glucocorticoid use. Domains in which there were differences across the proposed IV were quite different from those in which there were differences across the exposure. Factors that were different across the IV, especially measures of geography, race, socioeconomic status, and the frequency of outpatient visits were not strongly associated with outcomes in our previous analyses. (6) These results suggesting that if unmeasured confounding exists in the traditional analysis, the IV analysis may not have these same unmeasured confounders. Weak IVs may amplify bias, and so we used a more stringent criteria to define important standardized differences across the IV based on the complier rate. Alternative methods to compare bias in IV versus traditional analyses have also been described. (34)
Disease activity and disease severity are the factors that may pose the greatest potential threat to confound an association between glucocorticoids and serious infection, and claims data do not include direct measures of disease severity or activity. It is conceivable that certain providers may see a patient population with more severe disease and so prescribe more glucocorticoids, but several pieces of evidence argue against this possibility. Opioid use would be expected to be associated with more severe disease, (35) but was not different across the proposed IV (although differed across the exposure). Biologic and JAK inhibitor use was less common in patients seen by providers with greater preference for glucocorticoids, also arguing against the likelihood that these patients had more severe disease requiring aggressive therapies. Additionally, rheumatologists specializing in the treatment of patients with RA might be expected to both see more patients with RA and see patients with more severe disease, but we previously found that providers who saw a larger number of patients with RA tended to use less glucocorticoids. (18)
We expect that our measure of provider preference for glucocorticoids should only affect the outcome through its influence on the exposure (exclusion restriction). Although providers who prescribe more glucocorticoids might have differences in their practices, few of these differences are expected to directly affect rates of infection. Providers who use glucocorticoids differently may prescribe DMARDs differently, but we accounted for current and previous DMARD use in our analysis and required that patients be on stable DMARD treatment throughout follow-up. We did not observe any differences in rates of vaccinations or preventive healthcare (e.g. cancer screening) across the proposed IV, supporting similar overall healthcare in patients seeing providers with higher versus lower preference for glucocorticoids.
We sought to emulate previous randomized trials of glucocorticoid discontinuation, (24) but our design necessarily differs from the ideal trial in two ways. We did not have sufficient power to only compare patients discontinuing versus continuing glucocorticoids (requiring glucocorticoid use at DMARD initiation), but in our previous analysis we found similar results in a sensitivity analysis using this design. Additionally, because glucocorticoid dose was calculated by averaging prescriptions over the previous 90 days, our exposure definition at the index date in fact may reflect treatment decisions made 90 days previously, with time-zero misaligned from the target trial. (36) Selection bias from this misalignment is expected to be small and towards the null but could affect IV and non-IV analyses.
Several additional limitations should be noted. Weak IVs may amplify bias, and although stringent criteria were used to define important standardized differences across the proposed IV small differences in unmeasured confounders could influence results. The proposed IV was dichotomized to allow better interpretation of results and evaluation of IV characteristics, but at the loss of discrimination. We could not assess possible monotonicity violations, which may occur with preference-based IVs and would complicate the identification of compliers. (37) Glucocorticoids may be prescribed by a non-rheumatology provider or for reasons other than RA – while such scenarios may weaken the proposed IV, they do not impact its validity. Patients were not censored if they changed glucocorticoid dose, but because patients tend to reduce glucocorticoids over time this lack of censoring is expected to bias towards the null. (6) The associations found do not confirm causation, and hazard ratios have limitations as causal parameters. (38) We focused specifically on patients on stable DMARD therapy; results may not be generalizable to patients receiving low-dose glucocorticoids as short-term bridging therapy or for other indications.
In conclusion, results of an IV analysis based on provider preference for glucocorticoids found an association between low-dose glucocorticoid use ≤5mg/day and the risk for infection requiring hospitalization among patients on stable csDMARD, biologic, or JAKi therapy, with similar results to standard analyses and to previous studies using different methods. Glucocorticoids can provide substantial symptomatic benefit for patients with RA, and some patients may continue to require low-dose therapy even after DMARD therapy is optimized, but results of this study provides further evidence that infection risk exists even with low-dose glucocorticoid therapy (≤5mg/day). Although the risk is modest, a difference of one to two serious infections in 100 patients treated for a year is clinically important, especially given the frequency of glucocorticoid use in RA. Recognizing the risks of low-dose glucocorticoid therapy can allow clinicians and patients to weigh the risks and benefits of different treatment approaches.
Supplementary Material
Sources of funding:
Michael George is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases 1K23AR073931-01
Conflicts of Interest: Michael George has received research support from Bristol-Myers Squibb. Sean Hennessy has received consulting fees from Mallinckrodt Pharmaceuticals, Sage Therapeutics, Medullary Thyroid Cancer Consortium (Novo Nordisk In, AstraZeneca Pharaceuticals LP, GlaxoSmithKline LLC, Eli Lilly and Company), Merck Research Labs, Nektar Therapeutics, Pfizer Inc, and Estave Pharmaceuticals. Lang Chen and Fenglong Xie have no conflicts of interest to report. Jeffrey Curtis has received consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB and research support from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB.
Data/Statistical Code Availability:
Medicare data use is governed by a data use agreement and is available through the Centers for Medicare & Medicaid Services. Statistical code is available in the supplementary material.
References
- 1.Bakker MF, Jacobs JWG, Welsing PMJ, Verstappen SMM, Tekstra J, Ton E, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329–339. [DOI] [PubMed] [Google Scholar]
- 2.Svensson B, Boonen A, Albertsson K, Heijde D van der, Keller C, Hafström I. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: A two-year randomized trial. Arthritis Rheum 2005;52:3360–3370. [DOI] [PubMed] [Google Scholar]
- 3.Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, et al. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis 2013;72:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol Hoboken NJ 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–699. [DOI] [PubMed] [Google Scholar]
- 6.George MD, Baker JF, Winthrop K, Hsu JY, Wu Q, Chen L, et al. Risk for Serious Infection With Low-Dose Glucocorticoids in Patients With Rheumatoid Arthritis : A Cohort Study. Ann Intern Med 2020;173:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace BI, Lin P, Kamdar N, Noureldin M, Hayward R, Fox DA, et al. Patterns of glucocorticoid prescribing and provider-level variation in a commercially insured incident rheumatoid arthritis population: A retrospective cohort study. Semin Arthritis Rheum 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makol A, Davis JM, Crowson CS, Therneau TM, Gabriel SE, Matteson EL. Time trends in glucocorticoid use in rheumatoid arthritis: results from a population-based inception cohort, 1980–1994 versus 1995–2007. Arthritis Care Res 2014;66:1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:785–791. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006;54:628–634. [DOI] [PubMed] [Google Scholar]
- 11.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum 2007;56:1125–1133. [DOI] [PubMed] [Google Scholar]
- 13.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008;35:387–393. [PubMed] [Google Scholar]
- 14.Dixon WG, Abrahamowicz M, Beauchamp M-E, Ray DW, Bernatsky S, Suissa S, et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012;71:1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmowski Y, Buttgereit T, Dejaco C, Bijlsma JW, Matteson EL, Voshaar M, et al. “Official View” on Glucocorticoids in Rheumatoid Arthritis: A Systematic Review of International Guidelines and Consensus Statements. Arthritis Care Res 2017;69:1134–1141. [DOI] [PubMed] [Google Scholar]
- 16.Grijalva CG, Chung CP, Stein CM, Gideon PS, Dyer SM, Mitchel EF, et al. Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Saf 2008;17:890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson JC, Sarsour K, Gale S, Pethö-Schramm A, Jick SS, Meier CR. Incidence and Risk of Glucocorticoid-Associated Adverse Effects in Patients With Rheumatoid Arthritis. Arthritis Care Res 2019;71:498–511. [DOI] [PubMed] [Google Scholar]
- 18.George MD, Baker JF, Wallace B, Chen L, Wu Q, Xie F, et al. Variability in glucocorticoid prescribing for rheumatoid arthritis and the influence of provider preference on long-term use. Arthritis Care Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ertefaie A, Small DS, Flory JH, Hennessy S. A tutorial on the use of instrumental variables in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:357–367. [DOI] [PubMed] [Google Scholar]
- 20.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiol Camb Mass 2006;17:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf 2010;19:537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medicare C for, Baltimore MS 7500 SB, Usa M. SummaryMedicareMedicaid. 2015. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/SummaryMedicareMedicaid.html. Accessed May 1, 2015.
- 23.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burmester GR, Buttgereit F, Bernasconi C, Álvaro-Gracia JM, Castro N, Dougados M, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet Lond Engl 2020;396:267–276. [DOI] [PubMed] [Google Scholar]
- 25.Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res 2011;63:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 2007;60:397–409. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Census Bureau, 2009–2013 5-Year American Community Survey. Available at: https://www.census.gov/programs-surveys/acs. Accessed October 3, 2015.
- 28.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjolander A, Martinussen T. Instrumental Variable Estimation with the R Package ivtools. Epidemiol Methods 2019;8. Available at: https://www.degruyter.com/view/journals/em/8/1/article-20180024.xml. Accessed January 11, 2021.
- 30.Martinussen T, Nørbo Sørensen D, Vansteelandt S. Instrumental variables estimation under a structural Cox model. Biostat Oxf Engl 2019;20:65–79. [DOI] [PubMed] [Google Scholar]
- 31.Tchetgen Tchetgen EJ, Walter S, Vansteelandt S, Martinussen T, Glymour M. Instrumental variable estimation in a survival context. Epidemiol Camb Mass 2015;26:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet Lond Engl 2015;386:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanson SA, Hernán MA. Think globally, act globally: An epidemiologist’s perspective on instrumental variable estimation. Stat Sci Rev J Inst Math Stat 2014;29:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson JW, Swanson SA. Toward a Clearer Portrayal of Confounding Bias in Instrumental Variable Applications. Epidemiol Camb Mass 2015;26:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YC, Kremer J, Guan H, Greenberg J, Solomon DH. Chronic Opioid Use in Rheumatoid Arthritis: Prevalence and Predictors. Arthritis Rheumatol Hoboken NJ 2019;71:670–677. [DOI] [PubMed] [Google Scholar]
- 36.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016;79:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson SA, Miller M, Robins JM, Hernán MA. Definition and Evaluation of the Monotonicity Condition for Preference-Based Instruments. Epidemiol Camb Mass 2015;26:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernán MA. The Hazards of Hazard Ratios. Epidemiol Camb Mass 2010;21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Medicare data use is governed by a data use agreement and is available through the Centers for Medicare & Medicaid Services. Statistical code is available in the supplementary material.