Abstract
Background:
Very low-birth-weight (VLBW) infants are disproportionately affected by inflammatory morbidities including necrotizing enterocolitis. Despite the influence of social and demographic factors on infant health outcomes, their relationship with intestinal inflammation is unknown.
Purpose:
To explore the influence of maternal race, maternal socioeconomic status and infant sex on intestinal inflammation in VLBW infants.
Methods:
This was a secondary analysis of existing data from a randomized controlled trial of 143 infants ≤ 32 weeks gestation and ≤ 1250g. In the previous study, fecal calprotectin and S100A12 were collected at weeks 3 and 6. The infant sample was determined based on the availability of these results which served as intestinal inflammation biomarkers for the present study. General linear mixed models assessed the relationship between biomarkers and social and demographic factors. Gestational age, antibiotic exposure, mother’s own milk feeding, acuity, and week of sample collection were used as control variables.
Findings/Results:
The sample included 124 infants. Fifty-two (42%) were African American, 86 (69%) had Medicaid coverage and 65 (53%) were male. Fecal calprotectin levels were higher in African American infants (P=.02) and infants with private insurance coverage (P=.009), no difference was found between sexes. There was no association between S100A12 levels and infant sex, maternal race, or socioeconomic status.
Implications for Practice and Research:
Consideration of social and demographic factors may be important when caring for VLBW infants. Further exploration of factors contributing to associations between social or demographic factors and intestinal inflammation is needed.
Keywords: Infant, Very Low Birth Weight; Leukocyte L1 Antigen Complex; S100A12 Protein; Epidemiologic Factors; Intensive Care Units, Neonatal
BACKGROUND AND SIGNIFICANCE
Infants who are born prematurely constitute a physiologically and immunologically vulnerable population.1 Very low-birth-weight infants (VLBW; born < 1500g) are at high risk for serious intestinal inflammatory injuries such as necrotizing enterocolitis (NEC).2 Approximately 9% of VLBW infants are affected by NEC and nearly 28% of diagnosed cases result in fatality.2 While the pathogenesis of NEC is multifactorial, intestinal inflammation is a hallmark of the disease and contributes to intestinal injury and necrosis. Survivors of NEC often require long-term treatment for NEC-associated morbidities including short bowel syndrome, intestinal failure and neurodevelopmental delay.3 Due to the negative impact of severe intestinal inflammation and NEC on VLBW infant survival and long-term health, identifying factors that may contribute to intestinal inflammation is a research priority. Research describing determinants of intestinal inflammation serves to inform and guide healthcare practice which may ultimately lead to improvements in gastrointestinal health outcomes of VLBW infants.
Previous research has linked social and demographic factors including race, sex, and socioeconomic status to morbidity and mortality, suggesting that these factors have the potential to influence the health outcomes of infants hospitalized in neonatal intensive care units (NICUs). African American mothers have been found to have higher systemic inflammation during the second trimester of pregnancy and are more likely to deliver infants prematurely.4–6 Increased rates of premature delivery result in a higher number of VLBW infants born to African American mothers.5,6 African American and Hispanic infants are also more likely to suffer from NEC and experience higher odds of NEC-related death, even when controlling for gestational age and birthweight.7 Female infants born prematurely also have a survival advantage over males as high as 20%.8–10 In addition to race and sex, higher socioeconomic status as indicated by private insurance coverage and maternal education >12 years have been associated with increased survival post-resuscitation.9 These findings suggest that social and demographic factors are relevant to the health and wellbeing of premature infants yet there has been limited research dedicated to understanding whether social and demographic factors influence intestinal inflammatory processes despite the impact of inflammatory morbidities, including NEC, on the preterm infant population.11–13 Investigation of race, sex, and socioeconomic status as determinants of intestinal inflammation is needed to further our understanding of preterm infant intestinal morbidity.
Biomarkers are measurable physiologic indicators used to study and diagnose states of health and disease. Calprotectin and S100A12 are proteins found in neutrophils and other immunity-linked cells and are biomarkers of intestinal inflammation that can be measured in stool.11,14 Elevated fecal calprotectin and S100A12 levels have been associated with signs of gastrointestinal distress including bleeding, diarrhea, abdominal distention, bowel perforation, feeding intolerance and NEC in preterm infants.11,12,15–18 Fecal calprotectin levels have been shown to increase at NEC onset,14 and S100A12 levels show patterns of elevation several days prior to NEC onset.11
Despite these associations, an increase in fecal calprotectin or S100A12 does not always indicate disease. Previous studies suggest mild increases in fecal calprotectin may reflect expected development and maturation of the infant’s immune and gastrointestinal systems.19,20 One proposed threshold for delineation between normal and mild or severe intestinal inflammation is a fecal calprotectin level of 363 μg/g for mild and 636 μg/g for severe inflammation.21 S100A12 levels as high as 210 ng/g may indicate NEC.11 These proposed thresholds provide context for interpreting mild inflammation that may be related to developmental maturation versus excessive and potentially pathogenic levels of intestinal inflammation.
Evidence suggests that social and demographic factors influence premature infant health outcomes, including NEC, yet whether they determine patterns of intestinal inflammation is not known. To elucidate factors that may predispose infants to intestinal inflammation and its associated adverse health outcomes, investigation of social and demographic factors using these previously validated biomarkers of intestinal inflammation is needed. To address this gap in knowledge, the purpose of this study was to explore the relationship between race, socioeconomic status, and sex and fecal calprotectin and S100A12 in a cohort of VLBW infants.
METHODS
Setting and Sample
The present study is a secondary analysis of data from a previous study exploring whether social and demographic factors are related to biomarkers of intestinal inflammation including fecal calprotectin and S100A12 in VLBW infants at weeks 3 and 6 of life. Infants were included in the secondary analysis if at least 1 calprotectin or S100A12 value was available for analysis.
The Parent Study
The parent study was a randomized controlled trial of 143 infants designed to determine the risks and benefits of routine gastric residual evaluation.22 Approval of the parent study was granted by the University of Florida institutional review board. Infants admitted to the NICU during the enrollment period were screened for study eligibility. Infants were recruited from a level IV NICU and enrolled in the parent study from October 2013 to October 2016. Parental consent was obtained by a trained member of the research team prior to subject enrollment. Inclusion criteria for the parent study specified that infants must be <72 hours old and receiving enteral feedings. Additionally, infants must have been born ≤32 weeks gestational age with a birthweight of ≤1250g. Those diagnosed with congenital or chromosomal abnormalities were excluded from the parent study. Infants that developed stage II or greater NEC or spontaneous intestinal perforation were withdrawn. All were exclusively fed human milk, either mother’s own milk (MOM) or donor human milk. Initiation, advancement, and fortification of feedings followed a standardized NICU feeding protocol and none of the infants in this study received probiotic supplementation. Demographic and clinical data was collected from the infant’s electronic medical record and stored in a secure, online data management system at study enrollment. Stool samples were collected by trained research staff at weeks 3 and 6 of life and immediately frozen to −80°C. Fecal calprotectin and S100A12 concentrations were determined by ELISA assay using the fCal ELISA kit from BUHLMANN Laboratories AG (Schonen-buch, Switzerland) and the S100A12 ELISA kit from Cloun-Clone Corp (Houston, Texas) according to manufacturer instructions. Missing biomarker levels resulted if the infant did not have stool available or if the stool quantity was insufficient. Of the 124 infants who met inclusion criterion, 1 infant was missing a calprotectin value at week 3 and 8 infants were missing calprotectin values at week 6. Additionally, 39 infants were missing S100A12 values at week 3 and 45 were missing S100A12 values at week 6.
Measures
Fecal Calprotectin and S100A12 Levels
Fecal calprotectin and S100A12 levels were utilized as they were recorded for the parent study as a continuous numeric result determined by ELISA assay. Fecal calprotectin was measured in μg/g and S100A12 was measured in ng/g.
Social and Demographic Factors
Race, socioeconomic status and sex were chosen based on previous literature that suggests they are related to health outcomes in premature infant populations.7,9,10 Infant race and Medicaid coverage were determined by self-reported maternal race and insurance status. Infant race was defined as African American or non-African American secondary to sample size restrictions preventing analysis of additional racial or ethnic groups. Insurance status (a proxy measure for socioeconomic status) was defined as Medicaid if the mother reported coverage by Medicaid or by both Medicaid and private insurance. Infant sex was recorded as male or female. All social and demographic factors were recorded as categorical.
Clinical Factors
Mortality risk was measured through SNAPPE-II Score, which has demonstrated validity in premature infant populations.23 The SNAPPE-II mortality risk score considers six physiologic items in addition to birth weight, Apgar score and small for gestational age status.23 Scores are intended to reflect infant status within the first 12 hours of life and are recorded once, with higher SNAPPE-II scores indicating a higher mortality risk. Feeding of MOM was determined through calculation of median percent MOM consumed during the six-week study. Additionally, antibiotic exposure was assessed as dichotomous at weeks 3 and 6 based on whether the infant received antibiotics during the week of sample collection. Gestational age and SNAPPE-II score were recorded as continuous numeric variables.
Procedures
Data regarding social, demographic, and clinical factors including race, insurance coverage, sex, mortality risk, MOM feeding, antibiotic exposure and gestational age were collected from the secure, online data management system created and managed by the parent study.
Statistical Analysis
General linear mixed models were used to examine the relationship between calprotectin or S100A12 and social and demographic factors while controlling for clinical covariates. First, clinical and demographic characteristics of the population along with the calprotectin and S100A12 levels resulting from the ELISA assay were evaluated using descriptive statistics. The calprotectin and S100A12 levels were found to follow non-normal data distributions. Next, natural log transformations were applied to levels of calprotectin and S100A12 to remediate the violation of normality assumption for the general linear mixed model (GLMM) residuals.25 Accordingly, calprotectin and S100A12 values used in the analyses are natural logs of the raw values. Higher mean log transformed values correspond to higher values of the raw variable. Following log-transformation, separate GLMMs were run for natural log transformed values of the dependent variables, calprotectin and S100A12. Each model contained race, insurance coverage, and sex, which served as independent variables, as well as pre-determined covariates of week of sample collection, antibiotic exposure, gestational age, SNAPPE-II neonatal mortality risk score23 and MOM feeding. The models tested the dependent variables of calprotectin and S100A12 separately and provided model comparisons of mean values for either calprotectin or S100A12 that are reported as least square means. Inclusion of covariates in the models also yielded information regarding the covariate’s relationship to fecal calprotectin and S100A12. Finally, each GLMM was also run using raw (non-log transformed) values to provide clinically interpretable values. These values are intended to provide approximation of the values used in clinical practice and must be interpreted as estimations given that the non-normal distribution of residuals violates statistical assumptions of the general linear mixed model analysis. As assessed using the Bayes Information Criterion, a compound symmetry covariance structure was used for the S100A12 model and a variance components covariance structure was used for the calprotectin model. Missing data was evaluated using Little’s technique and no biasing pattern was identified.24 All statistical analyses were preformed using SAS (v9.4) and R studio (v4.0).
RESULTS
A total of 124 infants originally enrolled in the parent study had inflammatory biomarker levels available for this secondary analysis. The mean gestational age of included infants was 27.35 (±1.96) weeks and the mean birthweight was 931.58g (±177.29). Fifty-two infants (42%) were African American, 86 (69%) of infants’ mothers reported Medicaid coverage and 65 (53%) infants were male. The median fecal calprotectin level at week 3 was 337.95 μg/g (IQR; 320.05) and at week 6 was 421.82 μg/g (IQR; 378.73). The median S100A12 value at week 3 was 55.21 ng/g (IQR; 193.26) and at week 6 was 51.48 ng/g (IQR; 134.55). Description of fecal biomarker levels are provided in medians to facilitate comparison with previously published studies. Demographic and clinical characteristics of the sample are listed in Table 1.
Table 1:
Demographics and Clinical Characteristics of the Sample
| Variable | Frequency, No. (%) or Mean ± Standard Deviation |
|---|---|
| Race* | |
| Insurance | |
| Sex | |
| Gestational Age (weeks) | 27.35 ± 1.96 |
| Birthweight (grams) | 931.58 ± 177.29 |
| MOM* | 53.70 ± 45.55 |
| Antibiotics | |
| SNAPPE-II* | 19.93 ±12.24 |
| Mode of Delivery | |
| Calprotectin (μg/g) | |
| S100A12 (ng/g) |
AA- African American
MOM- Mother’s Own Milk Feeding
SNAPPE-II- Newborn Mortality Risk Score
Median and Interquartile Range (IQR)
Calprotectin Model Results
In the model that analyzed the relationship between fecal calprotectin and social or demographic factors, least square means were higher for African American infants than non-African American infants while controlling for covariates including week of sample collection, antibiotic exposure, gestational age, SNAPPE-II neonatal mortality risk score23 and MOM feeding (5.90 [95% CI, (5.66– 6.14)] vs 5.62 [95% CI, (5.43– 5.82)]; P=.02; Table 2; Figure 1). Additionally, least square mean calprotectin levels were higher in infants whose mothers held private insurance coverage than in infants whose mothers were insured by Medicaid (5.94 [95% CI, (5.68– 6.20)] vs 5.59 [95% CI, (5.40– 5.77)]; P=.009; Table 2; Figure 2). Fecal calprotectin levels of male and female infants were similar.
Table 2:
Calprotectin General Linear Mixed Model Results
| Variablea | P-value | Log-Transformed Estimate (95% CI)b | Raw Value Estimates (95% CI)bc |
|---|---|---|---|
| Race* | .02 | ||
| Sex | .89 | ||
| Insurance | .009 | ||
| Week | .02 | ||
| Antibiotics | .03 | ||
| Gestational Age | .03 | 0.07 (0.008–0.13) | N/A |
| MOM* | .004 | −0.004 (−0.006–−0.001) | N/A |
| SNAPPE-II* | <.001 | −0.02 (−0.03–−0.01) | N/A |
Race, sex, and insurance served as independent variables in the models. Week of sampling, antibiotic exposure, gestational age, MOM feeding and SNAPPE-II score were added as covariates.
Estimates for categorical variables (race, sex, insurance, week and antibiotics) are least square means. Estimates for continuous variables (gestational age, MOM, and SNAPPE-II) are regression weights.
Non-log transformed calprotectin (μg/g) values provided for clinical reference only.
African American (AA); Non-African American (Non-AA); Mother’s Own Milk (MOM); SNAPPE-II (Mortality risk score)
Figure 1:
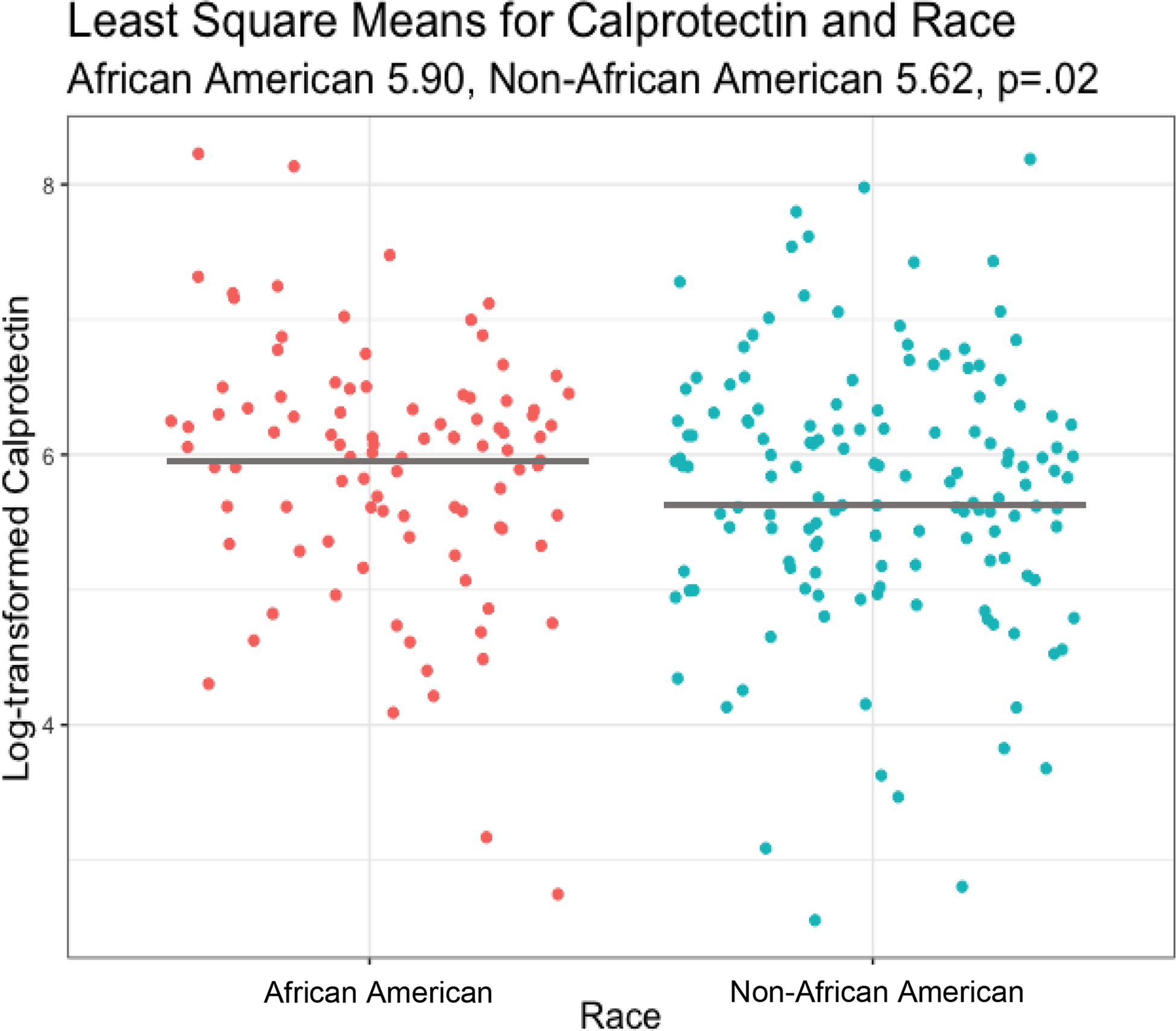
Log-transformed Fecal Calprotectin Level by Race
Figure 2:
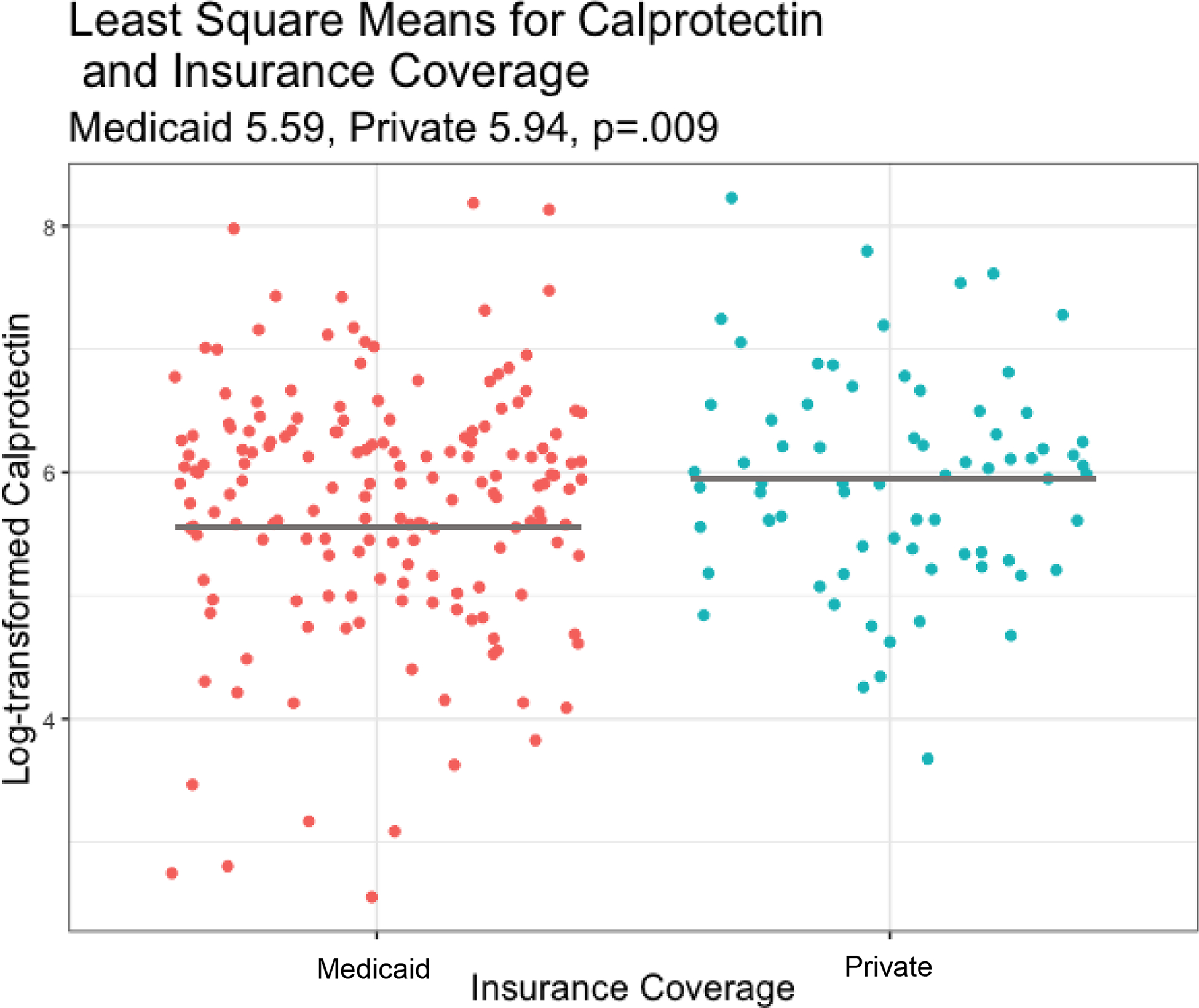
Log-transformed Fecal Calprotectin level by Insurance Coverage
Inclusion of covariates in the calprotectin model indicated that least square mean calprotectin levels were lower on week 3 than week 6 (5.63 [95% CI, (5.43–5.83); 5.90 [95% CI, (5.67–6.13)]; P=.02; Table 2) and higher in infants not exposed to antibiotics during the week of sample collection (5.96 [95% CI, (5.82–6.11)]; 5.56 [95% CI, (5.22–5.91)]; P=.03; Table 2). Results of the GLMM suggest the median percent MOM consumed and the SNAPPE-II neonatal mortality risk score are inversely related to fecal calprotectin levels with calprotectin levels decreasing as median percent MOM consumption (Estimate −0.004 [95% CI, (−0.006–−0.001)]; P=.004; Table 2) and SNAPPE-II score increase (Estimate −0.02 [95% CI, (−0.03–−0.01)]; P=<.001; Table 2). Infants born at higher gestational ages also had higher calprotectin levels while controlling for other factors included in the model (Estimate 0.07 [95% CI, (0.008–0.13)]; P=.03; Table 2). Non-log transformed values from the non-log transformed fecal calprotectin model are reported in Table 2 for clinical interpretability.
S100A12 Model Results
No social, demographic, or clinical factors revealed statistically significant relationships with fecal S100A12 levels (Table 3). Mean S100A12 values were similar between African American and non-African American infants as well as in comparisons between insurance coverage and infant sex. Similarly, none of the included covariates revealed statistically significant relationships including week of sample collection, antibiotic exposure, median percent MOM consumed, SNAPPE-II score, or gestational age. Non-log transformed values are reported in Table 3 for clinical interpretability.
Table 3:
S100A12 General Linear Mixed Model Results
| Variablea | P-value | Log-Transformed Estimate (95% CI)b | Raw Value Estimates (95% CI)bc |
|---|---|---|---|
| Race* | .67 | ||
| Sex | .59 | ||
| Insurance | .32 | ||
| Week | .55 | ||
| Antibiotics | .70 | ||
| Gestational Age | .54 | −0.04 (−0.17–0.09) | N/A |
| MOM* | .14 | 0.004 (−0.001–0.01) | N/A |
| SNAPPE-II* | .09 | −0.02 (−0.04–0.003) | N/A |
Race, sex, and insurance served as independent variables in the models. Week of sampling, antibiotic exposure, gestational age, MOM feeding and SNAPPE-II score were added as covariates.
Estimates for categorical variables (Race, Sex, Insurance, Week and Antibiotics) are least square means. Estimates for continuous variables (Gestational Age, MOM, and SNAPPE-II) are regression weights.
Non-log transformed S100A12 (ng/g) values provided for clinical reference only.
African American (AA); Non-African American (Non-AA); Mother’s Own Milk (MOM); SNAPPE-II (Mortality risk score)
DISCUSSION
Results of this study suggest maternal race and insurance coverage are associated with intestinal inflammation in VLBW infants and highlight the utility of fecal calprotectin levels as a biomarker of intestinal inflammation. Inclusion of clinical factors as covariates also revealed that calprotectin levels increased from week 3 to 6 and with increasing gestational age at birth but decreased with higher SNAPPE-II score, percent MOM consumed and antibiotic exposure. The median fecal calprotectin values reported in this study are higher than those reported by other studies. Median calprotectin levels of 226 μg/g have been reported in preterm infants during weeks of life 1–4,21 which is lower than our report of a median fecal calprotectin level of 337.95 μg/g at week 3 and 421.82 μg/g at week 6. Differences in median values between previously published literature and the current study may result from differences in timing of stool collection and median calculation (weeks 1–4 compared with weeks 3 and 6) or distinction between the sample populations as the current study sampled a cohort of VLBW infants exclusively receiving human milk feedings and <1250g at birth.
This study found no statistically significant relationships between race, sex or socioeconomic status and fecal S100A12 levels. The median fecal S100A12 levels we report in this study are higher than in other studies. For example, S100A12 levels have been previously reported as 89 ng/g during the first week of life and 33 ng/g during the subsequent 3 weeks.11 Our results show median levels of 55.21 ng/g at week 3 and 51.48 ng/g at week 6. A potential explanation for the differences in findings between calprotectin and S100A12 models includes limitations in power of S100A12 models resulting from a higher proportion of missing values.
Associations between fecal calprotectin or S100A12 and social, demographic or clinical factors in the present study may reveal insights into determinants of intestinal inflammation yet are exploratory and do not necessarily suggest pathophysiology. Comparisons revealing higher least square mean fecal calprotectin levels in a particular group may still reside within proposed cutoffs for expected or non-pathological levels. Further studies are needed to confirm our findings and to determine the mechanism of action by which maternal race and insurance coverage are related to intestinal inflammation in VLBW infants.
Race, Socioeconomic Status and Sex
Maternal race may be relevant to understanding patterns of inflammation in the VLBW infant intestine. We found infants of African American mothers had higher least square mean calprotectin levels than infants of non-African American mothers, even when controlling for all covariates in the model. While this study is the first to examine maternal race as a determinant of intestinal inflammation, these differences are consistent with previous research documenting race as a factor relevant to VLBW infant health. Race is strongly related to premature birth, with African American women experiencing preterm birth at rates of 14.13% compared to white women at 9.09%.6 The risk of recurrent preterm birth is also higher in African American mothers.26 African American mothers have higher systemic elevation of the inflammatory markers IL-4 and IL-6 during their second trimester of pregnancy which studies have suggested may contribute to premature delivery.4 Because African American mothers are more likely to deliver prematurely, VLBW infants are disproportionately African American with 2.92% of African American infants born VLBW compared to 1.02% of non-Hispanic white infants.6 Furthermore, infants born to African American mothers suffer increased burdens of morbidity and mortality. Studies suggest that African American infants are more than twice as likely as white infants to die in the first 28 days of life.27 African American infants are also disproportionately affected by NEC and are more likely to die following NEC diagnosis than non-Hispanic white infants.7 Our finding that African American infants in this study displayed higher least square mean fecal calprotectin levels than non-African American infants is in congruence with the extensive body of literature that describes race as a determinant of preterm infant health outcomes. Higher levels of fecal calprotectin in VLBW infants born to African American mothers are consistent with the higher levels of systemic inflammation experienced by African American mothers during pregnancy and the disproportionate effect of NEC on African American preterm infant populations.4,7 Future studies examining biomarkers of systemic inflammation in mothers and their infants may provide additional insights regarding the potential contribution of maternal systemic inflammation to intestinal inflammatory processes in VLBW infants.
Results of this study suggest maternal socioeconomic status may also be related to VLBW infant fecal calprotectin level. Our finding that private insurance coverage is associated with higher least square mean fecal calprotectin levels is contrary to our initial hypothesis yet in accordance with other studies describing socioeconomic status as a factor relevant to preterm infant health. Several studies have established that low socioeconomic status is associated with negative health outcomes in preterm infants. Low maternal education has been linked to increased rates of preterm delivery, admission to a NICU, and longer length of hospital stay.28 Furthermore, infants diagnosed with neonatal sepsis with a maternal history of low household income or self-pay insurance status have a disproportionately high mortality risk compared to infants of higher household income or private insurance coverage.29 Interestingly, maternal socioeconomic disadvantage in childhood has also been linked to negative birth outcomes including preterm delivery, small for gestational age status, NICU admission and longer infant length of stay.30 Maternal systemic inflammation may contribute to negative health outcomes such as preterm delivery considering that maternal socioeconomic disadvantage in childhood was associated with higher levels of a maternal systemic inflammatory cytokine, IL-6.30 The finding that low maternal socioeconomic status in childhood is related to increased systemic levels of IL-6 is interesting because it supports our results that suggest a relationship between socioeconomic status and inflammatory processes yet is contradictory to our findings that show private insurance coverage, a measure of higher socioeconomic status, is associated with higher levels of fecal calprotectin in our cohort. Our findings are surprising considering that measures of low socioeconomic status in childhood have been associated with higher maternal inflammation later in life.30 It is possible that our finding of higher fecal calprotectin levels in infants with private insurance coverage reflects an adaptive establishment of the intestinal microbiota as opposed to pathological inflammation.
A potential explanation for increases in fecal calprotectin level with private insurance coverage is based in transgenerational patterns of epigenetic influence. Recent studies suggest maternal exposures can affect the health of offspring both immediately and throughout the lifespan secondary to epigenetic mechanisms, or inherited patterns of gene expression.31 Maternal diet, for example, has been associated with metabolic syndrome in offspring, a condition which includes morbidities such as insulin resistance, hypertension, and atherosclerosis.31 Because socioeconomic status is intricately entwined with many lifestyle factors, one potential hypothesis is that mothers of infants with similar insurance coverage may also share exposure to environmental conditions that could influence fecal calprotectin level in their offspring. Future studies exploring the relationship between socioeconomic status and biomarkers of intestinal inflammation may consider alternative measures of socioeconomic status, including income and maternal education to confirm our results and further a scientific understanding regarding the role of socioeconomic status in inflammatory processes.
Results of this study suggest that infant sex is not related to fecal calprotectin or S100A12 concentration. This finding is surprising considering the existence of documented disparities in survival outcome and differences in intestinal microbiome composition between male and female infants.10,32 Distinct patterns in the intestinal microbiome of male and female infants may be relevant to intestinal inflammation as colonization of certain microorganisms have been linked to increases in fecal calprotectin.13,33 Previous investigations have also reported findings of no association between infant sex and fecal calprotectin or S100A12 levels.11–13 Together, the evidence strongly suggests that patterns of intestinal inflammation in VLBW infants are similar between sexes and that other determinants of intestinal inflammation should be prioritized in future studies.
Feeding Regime, Antibiotic Exposure, Age and SNAPPE-II Score
Several factors were included in the statistical models as covariates and have provided useful information regarding inflammatory processes in VLBW infants. This study revealed an inverse association between MOM feedings and calprotectin levels, contrasting previous reports that feeding regime is not related to calprotectin level12 and that feeding infants exclusive MOM is associated with higher fecal calprotectin levels.20 Contradictory results reflect the possibility that MOM feeding stimulates a mild, adaptive inflammation20 while reducing severe inflammation. Study design may also contribute to contrasting results as the current study measured MOM feeding using a single median percentage value to reflect feeding during the six weeks of study while another20 included both feeding volume, feeding type and feedings mixed with preterm infant formula in the analysis.
Results of the current study also suggest antibiotic exposure during the week of sample collection is associated with decreased fecal calprotectin levels, a finding that lends support to hypotheses suggesting that elevated fecal calprotectin levels early in life represent response to bacterial establishment in the intestine. Despite conflicting reports in previously published literature,13,15,34,35 our results also suggest higher gestational age at birth is associated with higher least square mean fecal calprotectin levels. Additionally, we found increasing postnatal age was associated with increased fecal calprotectin, a finding that has been controversial and explored in a variety of other studies.13,35,36 Intestinal calprotectin concentration likely varies throughout the first several weeks of life and future longitudinal studies featuring weekly or biweekly sampling may be informative.35 Inclusion of SNAPPE-II mortality risk score as a covariate in the model revealed that mortality risk was inversely related to fecal calprotectin concentration. The finding that infants with a higher mortality risk have lower least square mean fecal calprotectin concentration is unexpected and hypothesis-generating. Based on this finding, it is possible that lower calprotectin levels in infants with high mortality risk scores could result from clinical care practices for critically ill infants that may include intensive antibiotic exposure or interruptions in enteral feeding practice.
There are several limitations of the current study. As a secondary analysis of existing data, a causal relationship cannot be established and the measurement and operationalization of variables such as socioeconomic status were investigated using data collected for the purposes of the parent study. There are several other validated measures of socioeconomic status that may provide insight into the observed relationships and should be pursued in future investigations. Mother’s own milk feeding was measured as a single percentage representing the six-week duration of the study and measurement of feeding volume longitudinally may reveal further insights into the relationship between mother’s own milk feeding and fecal calprotectin levels. While this study included several covariates, there are many potentially relevant variables not included in the models that may be applicable to our findings. Additionally, race could only be defined as African American and non-African American secondary to sample size limitations. Another study has reported a positive association between Hispanic ethnicity and fecal calprotectin level that represents an opportunity for continuing research.33 There are no studies that have been conducted to determine if race is associated with fecal calprotectin or S100A12 levels in other populations such as adults. Future studies should seek to investigate whether this study’s findings can be reproduced in other cohorts and prioritize recruitment of additional racial and ethnic populations. It should also be noted that missing data, particularly for S100A12, may have limited our ability to fully explore our study aims. Both calprotectin and S100A12 also have a high degree of intraindividual and interindividual variability in measurement which may limit meaningful association with social, demographic and clinical factors of interest.11,21,36 Furthermore, the results of this study are preliminary and further studies are necessary to determine clinical significance. Our research and previous research documenting the relationship between social and demographic factors and infant health7,9 suggests that clinicians caring for VLBW infants should recognize that non-clinical risk factors such as infant race and socioeconomic status may contribute to preterm infant health outcomes.
CONCLUSION
This study offers several valuable insights regarding inflammatory processes in VLBW infants over the first six weeks of life. Social determinants such as race and socioeconomic status, in conjunction with a variety of clinical factors, may be determinants of intestinal inflammation. Future studies should continue investigation into the effects of social and environmental factors on VLBW health outcomes in NICU-hospitalized infants and prioritize identification of interventions that may reduce health disparities.
Funding Source:
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of health under NINR RO1NRO4019-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acronyms/Abbreviations:
- VLBW
Very low birth weight
- NEC
Necrotizing enterocolitis
- GLMM
General linear mixed model
- GA
Gestational age
- SES
Socioeconomic status
- BW
Birthweight
- MOM
Mother’s own milk
- NICU
Neonatal intensive care unit
Footnotes
Potential Conflicts of Interest: Dr. Parker, who is a Section Editor for Advances in Neonatal Care and the coauthor and mentor to the primary author, was not involved in the editorial review or decision to publish this article. The entire process from submission, referee assignment, and editorial decisions was handled by other members of the editorial team for the journal.
REFERENCE LIST
- 1.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. The Journal of Maternal-Fetal & Neonatal Medicine. 2011;24(1):25–31. [DOI] [PubMed] [Google Scholar]
- 2.Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. Journal of the American College of Surgeons. 2014;218(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 3.Bazacliu C, Neu J. Necrotizing enterocolitis: long term complications. Current pediatric reviews. 2019;15(2):115–124. [DOI] [PubMed] [Google Scholar]
- 4.Giurgescu C, Engeland CG, Templin TN, Zenk SN, Koenig MD, Garfield L. Racial discrimination predicts greater systemic inflammation in pregnant African American women. Applied Nursing Research. 2016;32:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2018. NCHS Data Brief. 2019(346):1–8. [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final data for 2018. National Vital Statistics Reports. Vol 68. Hyattsville, MD: National Center for Health Statistics; 2019. [PubMed] [Google Scholar]
- 7.Jammeh ML, Adibe OO, Tracy ET, et al. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. Journal of Perinatology. 2018;38(10):1386–1390. [DOI] [PubMed] [Google Scholar]
- 8.La Pine TR, Jackson JC, Bennett FC. Outcome of infants weighing less than 800 grams at birth: 15 years’ experience. Obstetrical & gynecological survey. 1996;51(4):218–219. [PubMed] [Google Scholar]
- 9.Anderson JG, Baer RJ, Partridge JC, et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics. 2016;138(1):e20154434. [DOI] [PubMed] [Google Scholar]
- 10.Morse SB, Wu SS, Ma C, Ariet M, Resnick M, Roth J. Racial and gender differences in the viability of extremely low birth weight infants: a population-based study. Pediatrics. 2006;117(1):e106. [DOI] [PubMed] [Google Scholar]
- 11.Däbritz J, Jenke A, Wirth S, Foell D. Fecal phagocyte-specific S100A12 for diagnosing necrotizing enterocolitis. The Journal of pediatrics. 2012;161(6):1059–1064. [DOI] [PubMed] [Google Scholar]
- 12.Campeotto F, Kalach N, Lapillonne A, Butel MJ, Dupont C, Kapel N. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatrica. 2007;96(10):1531–1533. [DOI] [PubMed] [Google Scholar]
- 13.Rougé C, Butel M-J, Piloquet H, et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS One. 2010;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefsson S, Bunn SK, Domellöf M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44(4):407–413. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94(4):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang H-J, Park JH, Kim CS, Lee SL, Lee WM. Amino acid-based formula in premature infants with feeding intolerance: comparison of fecal calprotectin level. Pediatr Gastroenterol Hepatol Nutr. 2018;21(3):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aydemir O, Aydemir C, Sarikabadayi YU, et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. The Journal of Maternal-Fetal & Neonatal Medicine. 2012;25(11):2237–2241. [DOI] [PubMed] [Google Scholar]
- 18.Däbritz J, Foell D, Wirth S, Jenke A. Fecal S100A12: Identifying intestinal distress in very-low-birth-weight infants. Journal of Pediatric Gastroenterology and Nutrition. 2013;57(2). [DOI] [PubMed] [Google Scholar]
- 19.Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS pathogens. 2009;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groer M, Ashmeade T, Louis-Jacques A, Beckstead J, Ji M. Relationships of feeding and mother’s own milk with fecal calprotectin levels in preterm infants. Breastfeed Med. May 2016;11:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campeotto F, Baldassarre M, Butel MJ, et al. Fecal calprotectin: cutoff values for identifying intestinal distress in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48(4):507–510. [DOI] [PubMed] [Google Scholar]
- 22.Parker LA, Weaver M, Murgas Torrazza RJ, et al. Effect of gastric residual evaluation on enteral intake in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2019;173(6):534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. The Journal of pediatrics. 2001;138(1):92–100. [DOI] [PubMed] [Google Scholar]
- 24.Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- 25.Olsson U Confidence intervals for the mean of a log-normal distribution. Journal of Statistics Education. 2005;13. [Google Scholar]
- 26.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin Perinatol. 2017;41(8):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy S, Xu J, Kochanek K, Arias E, Betzaida T-V. Deaths: final data for 2018. National Vital Statistics Reports. 2020;69(13). [PubMed] [Google Scholar]
- 28.Yunis K, Beydoun H, Khogali M, Alameh M, Tamim H. Low socioeconomic status and neonatal outcomes in an urban population in a developing country. The Journal of Maternal-Fetal & Neonatal Medicine. 2003;14(5):338–343. [DOI] [PubMed] [Google Scholar]
- 29.Bohanon FJ, Nunez Lopez O, Adhikari D, et al. Race, income and insurance status affect neonatal sepsis mortality and healthcare resource utilization. The Pediatric infectious disease journal. 2018;37(7):e178–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller GE, Culhane J, Grobman W, et al. Mothers’ childhood hardship forecasts adverse pregnancy outcomes: Role of inflammatory, lifestyle, and psychosocial pathways. Brain, Behavior, and Immunity. 2017;65:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitsiou-Tzeli S, Tzetis M. Maternal epigenetics and fetal and neonatal growth. Current Opinion in Endocrinology, Diabetes and Obesity. 2017;24(1). [DOI] [PubMed] [Google Scholar]
- 32.Cong X, Xu W, Janton S, et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One. 2016;11(4):e0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho TTB, Groer MW, Kane B, et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatric research. 2019;85(3):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laforgia N, Baldassarre ME, Pontrelli G, et al. Calprotectin levels in meconium. Acta Paediatrica. 2003;92(4):463–466. [DOI] [PubMed] [Google Scholar]
- 35.Zoppelli L, Güttel C, Bittrich H-J, Andrée C, Wirth S, Jenke A. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology. 2012;102(1):68–74. [DOI] [PubMed] [Google Scholar]
- 36.Nakayuenyongsuk W, Christofferson M, Stevenson DK, Sylvester K, Lee HC, Park KT. Point-of-care fecal calprotectin monitoring in preterm infants at risk for necrotizing enterocolitis. The Journal of pediatrics. 2018;196:98–103. [DOI] [PubMed] [Google Scholar]


