Abstract
There have been significant improvements in the technology available for treating extensive burns in the past decade. This case presents two unique, skin replacement technologies that were used to treat an 86% surface area flame burn in a pediatric patient. A temporary dermal replacement, known as “Novosorb™ Biodegradable Temporizing Matrix” was first used to stabilize the burn injury and remained in place for approximately 3 months. Given the large burn size and lack of available donor skin for grafting, a permanent skin replacement product known as “Self-Assembled Skin Substitute (SASS)” was then utilized to cover the burns. SASS is a novel technology that was developed to replace skin as an autologous skin graft and is currently available in Canada through a clinical trial for major burns. Ultimately, the concurrent use of these two technologies allowed for the unprecedented survival of a child following an extensive and life-threatening burn injury.
Extensive burn injuries in pediatric patients are a rarity in the developed world and survival primarily depends on replacing skin loss to prevent infection.1–3 Advances in acute wound care for managing extensive burns include the use of dermal replacements and engineered skin equivalents which allow for the closure of the open inflammatory wounds and the eventual epithelial replacement to restore homeostasis.4–6 The following case report illustrates the introduction of a dermal replacement, “Novosorb™ Biodegradable Temporizing Matrix” (BTM; Polynovo, Adelaide, Australia), which was effectively used to cover a large burn surface area for a prolonged period. In addition, this case describes the successful use of a novel, permanent skin replacement product known as “Self-Assembled Skin Substitute (SASS)” that was used to address the lack of donor sites encountered in this extensive burn injury. Given that the options available to cover extensive burn cases such as this are limited, this case was reviewed to provide timely information regarding the utility of novel skin replacement technologies.
CASE PRESENTATION
A previously healthy, 8-year-old boy sustained an unwitnessed flame burn to 86% of his body surface area (BSA) sparing only his scalp and portions of his lower legs, from an outdoor fire used to burn brush. Following the injury, the patient was urgently transferred to an American Burn Association-verified pediatric burn center via the provincial emergency triage agency in Ontario, Canada. All of the burned areas were full thickness wounds which required surgical removal and reconstruction. Fluid resuscitation, central line placement, pain/sedation management, nutritional/metabolic, and wound care protocols were instituted according to clinical practice guidelines for treating a major burn. Intubation for burn size, anticipated swelling, large fluid volume replacement, and pain control were also initiated. Testosterone was utilized as a metabolic agent,7,8 which commenced after admission at a dose of 25 mg IM q2weeks until all wounds closed and the patient was metabolically stable by weight and indirect calorimetry. The patient remained in the pediatric intensive care unit for 6 months with medical care comanaged by the Burn Team and pediatric intensivists.
The only available donor sites for skin grafting were small patches of unburned skin on the patient’s back (1.5% BSA), scalp and upper forehead/face (5% BSA), and legs below the knees (7.5% BSA) excluding the skin posteriorly below the knees which was also burned. Surgical planning for skin coverage included excision of all burns over the first 10 days postadmission in a series of major operations and covering the excised burned areas with cryopreserved human allograft skin. More specifically, excision of full thickness burns to the chest, abdomen, back, and buttocks with allografting was performed 2 days postadmission. In the following days, the patient’s face, neck, arms, hands, and legs were debrided. All areas were subsequently allografted. Integra® (Life Sciences Corp, NJ) was applied to both arms but had to be replaced (due to infection) with allograft before autograft was available. The initial donor site harvest was used to provide sheet grafts to the hands and face with the remaining autograft being widely meshed for the limbs. A meek technique was also used to cover the entire chest and arms with approximately 65% graft take and required regrafting to close the trunk and limbs with widely meshed autograft (3:1).
Due to inadequate donor site skin availability, BTM, a temporary dermal replacement, was accessed using the Health Canada Special Access Program which allows clinicians to access therapies that are not currently available in Canada. BTM was chosen over other existing dermal replacement products due to its documented resistance to infection,9,10 the treating burn surgeon’s familiarity with the product on a similar extensive pediatric burn case, and its ease of handling reported by other burn experts. BTM was utilized at 10 days postadmission and was used to cover the patient’s back, buttocks, abdomen, chest, and upper neck (35% of the patient’s BSA). The BTM remained in place for approximately 3 months while waiting for donor skin to become available, with the exception of a small section to the upper thighs (6% BSA) which was successfully replaced with another application of BTM 1 week after application due to loose areas which were clinically concerning as sites of potential wound infection.
Given the large size of the burn and the lack of available donor sites, the treating burn surgeon liaised with researchers at the University of Laval in Québec, Canada to obtain access to a permanent skin replacement product, SASS, through a clinical research trial (ClinicalTrials.gov identifier: NCT02350205). Informed consent was obtained from the patient’s guardian prior to commencing participation in the research study. Consent for photography and video for this publication was also obtained from the patient’s guardian.
In order to produce the SASS grafts, a biopsy of skin (1 cm by 3 cm in length from the dorsum of the patient’s unburned foot) was sent to the University of Laval laboratory for processing at 10 days postburn. The SASS grafts were received approximately 6 weeks later and were used to cover the patient’s entire back by placing it on top of the BTM at approximately 3.5 months postadmission.
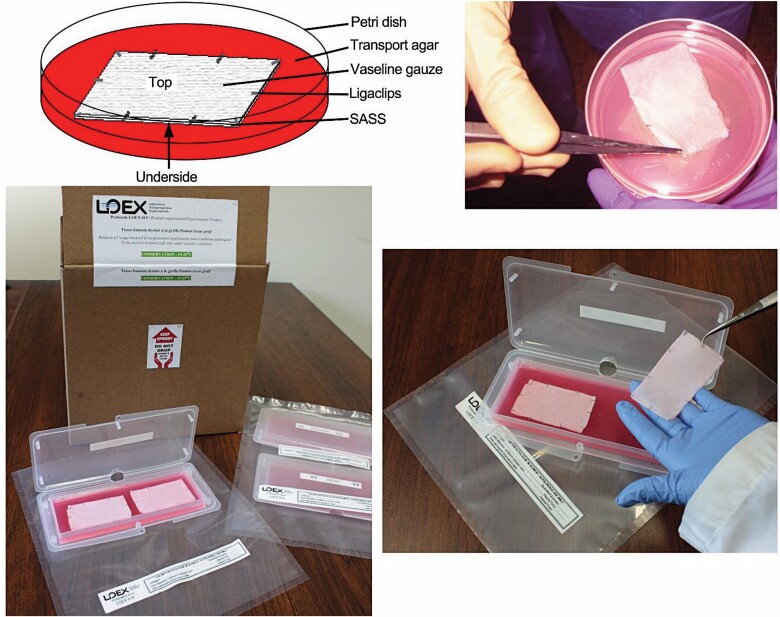
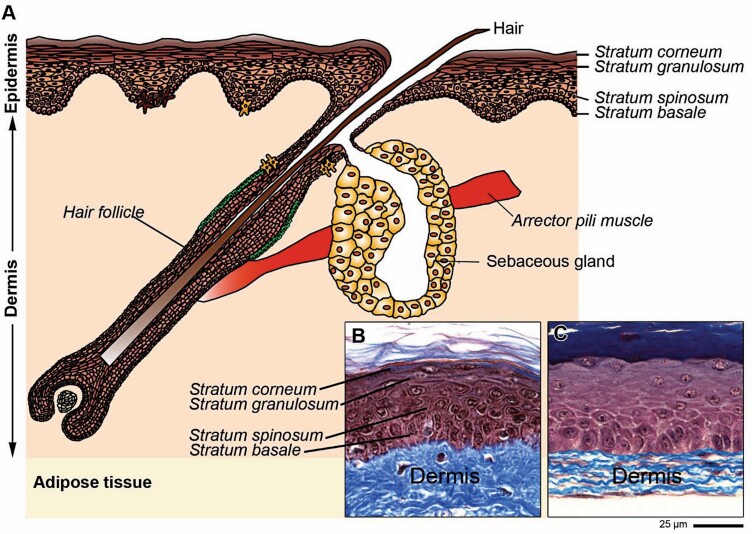
SASS is a skin graft which contains living cells, produced in the laboratory by cell culture techniques (Figures 1 and 2) and is composed of two layers: an epidermis formed by 25 to 50 million living epidermal cells per SASS forming a stratified epithelium, and a dermis formed by 2 to 7 million living fibroblasts that secrete and organize natural collagen-rich extracellular matrix4,11 (Figure 2). The mechanism of action of SASS is to replace skin as an autologous skin graft.
Figure 1.
Composition and appearance of the SASS grafts ready for shipment in Petri dishes and in cocoon boxes. The SASS is clipped to a Vaseline® gauze in order to facilitate handling and placement on the burn wound. SASS, Self-Assembled Skin Substitute.
Figure 2.
Schematic representation of human skin (A). Histological aspects of normal human skin (B) and of SASS (C). Staining: Masson’s Trichrome. SASS, Self-Assembled Skin Substitute.
The included video illustrates the intact vascularized matrix, the delamination necessary on the day of surgery to allow for skin coverage, and the application of the SASS grafts. The SASS grafts were treated as per normal protocol for autologous skin grafts with a nonadherent dressing and examined 7 days later. The graft take was 99.5% with a few minor open seams which closed without requirement for additional operative care. The remaining BTM on the patient’s legs, neck, and upper chest area was grafted with autologous skin grafts from the existing donor sites over 6 months, with final grafting completed at 8 months postadmission.
Ten months postadmission, the patient was discharged to a pediatric rehabilitation facility with the specific goals of increasing independence of activities of daily care and fully independent ambulation before transfer to his home. At the 10-month assessment, the skin on the patient’s back matured without evidence of hypertrophic scar formation and was assigned a score of 3/13 on the Vancouver Scar Scale, which is consistent with mature scar characteristics. Although the scar appeared hypopigmented, its texture was soft and mobile with a smooth contour, and the patient did not experience any pruritus.
DISCUSSION
This case of an extensive, 86% BSA full thickness burn injury in an 8-year-old child highlights the combined use of Canadian research technology, SASS, with the first Canadian case of using a new temporary dermal matrix, BTM. These technologies allowed for the unprecedented survival of a child following an extensive life-threatening burn injury.
The mortality rate for an 8-year-old with an 86% BSA burn is high, largely due to the lack of available donor skin for grafting.3,12 The only solution to this problem involves stabilizing the burn injury by removing the burned skin and replacing it with temporary biological or synthetic coverage until the patient’s own skin is available for surgical transplantation in the form of autologous skin grafts. Biological replacement with human allograft skin requires surgical reoperation for removal and increased blood loss and is therefore associated with increased risks of mortality in an already extensively burned patient.13,14 Alternatively, synthetic dermal replacements are widely available and have been used routinely for more than 15 years.15 However, their use is limited by the risks of infection while waiting for the wounds to become vascularized.16
In this case, BTM was used to cover a large surface area of burned skin until donor skin became available. This is the first case in Canada to use BTM which was primarily chosen due its recognized resistance to infection relative to existing dermal matrixes.9,10 This property is essential, as the excised burn wounds take weeks to vascularize and adhere to the membrane.17 It is during this juncture that dermal replacements can fail.16 BTM is entirely synthetic and biodegradable and for this reason, does not pose the same threat to colonization. Moreover, the product has been previously used to treat extensive burn wounds.18–20
Aside from a single reapplication, the BTM remained vascularized and stayed in place for months while the outer layer was gradually removed, and native skin was used to gradually delaminate the membrane and ultimately graft over 35% BSA. In addition to this novel technology, the patient was also treated with a novel, Canadian skin replacement technology known as SASS. This tissue engineered skin was successfully applied to the matrix and covered 20% of the BSA in a single step, with 99.5% graft take and very favorable scarring characteristics compared to the areas grafted with donor skin. This skin construct added positive survival benefit by closing a large surface area of the patient’s burn injury. Although epidermal sheets have been used in extensive burn injuries since the 1970s,21,22 they can be unstable and often require replacement with native autografts. However, because SASS grafts are constructed with both epidermal and dermal layers, their stability is increased.4,11 Moreover, this skin substitute favors the formation of an epithelial stem cell niche which allows for long term skin regeneration after grafting.23
The application of SASS grafts to other forms of trauma is a natural extension of the technology. Other nontraumatic forms of serious skin disease, specifically, epidermolysis bullosa,24 are also under investigation with the goal of growing a skin equivalent in the laboratory becoming a reality. In addition, the temporary matrix used in this burn case is also applicable to other forms of trauma, particularly where the tissues are unstable and prone to infection (eg, fasciitis or ulcers25), and offers the ability to close wounds early and improve survival. Although this case study was not designed to compare the scarring of SASS and BTM treated areas with native skin grafts, the scars on the patient’s back are of better quality compared to the scars on the hands and face which were grafted with donor skin earlier in the acute course to prioritize treatment of these important functional areas. More specifically, the scars on the patient’s hands and face appear erythematous with stiffer, raised areas which are expected to improve over time with natural scar maturation and ongoing rehabilitation (eg, pressure garments, scar massage, etc.).
The technologies described in this case of extensive burn injury are applicable to other forms of trauma and highlight the fact that advanced technologies that can save lives are now available.
SUPPLEMENTARY DATA
Supplementary data is available at Journal of Burn Care & Research online.
Video 1. The stepwise process of using BTM and SASS to treat a patient with an extensive burn injury. In addition, the progress of the patient from the initial burn injury to 8 months postgrafting is depicted.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the contributions of Ed Graubart, the Health Canada Special Access Program, and Dr. François A. Auger and the LOEX personnel involved in the SASS trial. The authors are also grateful for the hundreds of healthcare providers at the Hospital for Sick Children who actively cared for this patient (Burn Team, Pediatric Intensive Care Team, and operating room staff).
Funding: Funding support was provided by CIHR and the Stem Cell Network.
Conflict of interest statement. None declared.
REFERENCES
- 1. Chipp E, Milner CS, Blackburn AV. Sepsis in burns: a review of current practice and future therapies. Ann Plast Surg 2010;65:228–36. [DOI] [PubMed] [Google Scholar]
- 2. Lachiewicz AM, Hauck CG, Weber DJ, Cairns BA, van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clin Infect Dis 2017;65:2130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheridan RL, Remensnyder JP, Schnitzer JJ, Schulz JT, Ryan CM, Tompkins RG. Current expectations for survival in pediatric burns. Arch Pediatr Adolesc Med 2000;154:245–9. [DOI] [PubMed] [Google Scholar]
- 4. Larouche D, Cantin-Warren L, Desgagné Met al. Improved methods to produce tissue-engineered skin substitutes suitable for the permanent closure of full-thickness skin injuries. Biores Open Access 2016;5:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li A, Dearman BL, Crompton KE, Moore TG, Greenwood JE. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J Burn Care Res 2009;30:717–28. [DOI] [PubMed] [Google Scholar]
- 6. Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen Med 2021;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007;246:351–60; discussion 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller JT, Btaiche IF. Oxandrolone in pediatric patients with severe thermal burn injury. Ann Pharmacother 2008;42:1310–5. [DOI] [PubMed] [Google Scholar]
- 9. Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra® dermal regeneration template in a porcine wound model. J Burn Care Res 2012;33:163–73. [DOI] [PubMed] [Google Scholar]
- 10. Sangji NF, Levin JM, Friedstat JSet al. 475 acute and reconstructive burn surgery with a bilaminate polyurethane skin substitute: a case series. J Burn Care Res 2019;40:S211–2. [Google Scholar]
- 11. Germain L, Larouche D, Nedelec Bet al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: a case series of 14 severely burned patients indicating clinical effectiveness. Eur Cell Mater 2018;36:128–41. [DOI] [PubMed] [Google Scholar]
- 12. Wolf SE, Rose JK, Desai MH, Mileski JP, Barrow RE, Herndon DN. Mortality determinants in massive pediatric burns. An analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness). Ann Surg 1997;225:554–65; discussion 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farny B, Fontaine M, Latarjet J, Poupelin JC, Voulliaume D, Ravat F. Estimation of blood loss during adult burn surgery. Burns 2018;44:1496–501. [DOI] [PubMed] [Google Scholar]
- 14. Housinger TA, Lang D, Warden GD. A prospective study of blood loss with excisional therapy in pediatric burn patients. J Trauma 1993;34:262–3. [DOI] [PubMed] [Google Scholar]
- 15. Chua AW, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shahin H, Elmasry M, Steinvall I, Söberg F, El-Serafi A. Vascularization is the next challenge for skin tissue engineering as a solution for burn management. Burns Trauma 2020;8:tkaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen 2014;22:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Concannon E, Coghlan P, DamKat Thomas Let al. Biodegradable temporizing matrix reconstruction of complex perineal burn wound: a case report. J Burn Care Res 2021. doi: 10.1093/jbcr/irab073. [DOI] [PubMed] [Google Scholar]
- 19. Greenwood JE, Wagstaff MJ, Rooke M, Caplash Y. Reconstruction of extensive calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty 2016;16:e17. [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Velamuri S, Hassouba Met al. 473 The application of biodegradable temporizing matrix in burn reconstructive surgery: preliminary results of 36 cases. J Burn Care Res 2019;40:S209–10. [Google Scholar]
- 21. O Connor NE, Mulliken JB, Banks-Schlegel Set al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981;1:75–8. [PubMed] [Google Scholar]
- 22. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. [DOI] [PubMed] [Google Scholar]
- 23. Cortez Ghio S, Larouche D, Doucet EJet al. The role of cultured autologous bilayered skin substitutes as epithelial stem cell niches after grafting: a systematic review of clinical studies. Burns Open 2021;5:56–66. [Google Scholar]
- 24. Dakiw Piaceski A, Larouche D, Ghani Ket al. Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa. Eur Cell Mater 2018;35:73–86. [DOI] [PubMed] [Google Scholar]
- 25. Boa O, Cloutier CB, Genest Het al. Prospective study on the treatment of lower-extremity chronic venous and mixed ulcers using tissue-engineered skin substitute made by the self-assembly approach. Adv Skin Wound Care 2013;26:400–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.