Introduction
Our 2014 hypothesis, published in GENETICS, aimed to elucidate a set of traits associated with mammalian domestication, a phenomenon termed the “domestication syndrome.” Our explanation focused on a special group of cells found in embryos, the neural crest cells (NCCs) and we proposed that genetic changes affecting their development were at the root of vertebrate domestication. We now term this idea the “neural crest/domestication syndrome” (NCDS) hypothesis. In this issue of GENETICS, Johnsson et al. criticize our idea, arguing that it lacked a serious genetic foundation and claiming that, despite many citations, it has received little actual support from new findings.
In this reply to their critique, we do three things. First, we note some key facts about animal domestication that need to be recognized in any hypothesis about its genetics. Second, we explain the actual reasoning that led us to propose the NCDS hypothesis. (Johnsson et al. give an account of its genesis rather distant to our thinking.) Third, we briefly discuss some of the findings, not mentioned by them, that strongly support our idea and discuss how this hypothesis can be further tested. Finally, we mention points of agreement with Johnsson et al. but also note a few incorrect citations in their article.
Though we disagree with their main conclusions, we were glad to see their article. It is a serious discussion of our idea. We point out here, however, that our proposal has already passed one test of a worthwhile hypothesis—inspiring good research—and that it meets another, which is the ability to be falsified, as we will discuss.
Animal domestication is a multi-stage process
All traditional domesticated animals (such as dogs, cattle, sheep, goats, pigs, camels, horses, and chickens) began the process of domestication a long time ago, frequently millennia in the past (Francis 2015). Each of these species almost certainly experienced two semi-distinct stages in their domestication (Zeder 2015; Pendleton et al. 2018). The first would have involved an initial state of increased habituation to human presence, yielding both reduced fear and reduced reactive aggression, hence increased docility. This stage probably involved physiological changes at first, which eventually became genetically fixed, though early genetic changes might also have contributed. The second phase, a much longer “breed formation” stage, involved centuries to millennia of coexistence with humans, often accompanied by selection for various productivity properties (or later, in some species, various ornamental properties) plus, undoubtedly, some degree of natural selection in captivity. During this second stage, large numbers of genetic change would have taken place, overlaying the initial genetic changes. These two stages probably involved different sets of genetic change (Zeder 2015; Pendleton et al. 2018; Fitak et al. 2020), although the second often included further changes modifying behaviors, including increased docility. Indeed, what we are calling the “breed formation” stage would have involved multiple sub-stages for many domesticated species.
Our proposal concerns the early events when domestication was first established. In our hypothesis, this early stage was shared by all domesticated mammals and birds while later breed formation was often highly divergent between domesticated species, as when selecting for productivity traits such as wool production in sheep or high egg laying in chickens, or further diversity amongst breeds of the same species, for instance for beef vs milk production in different cattle breeds. This distinction between stages in domestication was implicit in our 2014 paper, when we mentioned “initial selection” for increased docility, but should have been stated explicitly. The basic idea, however, that the first events in domestication were different in character from the breed-formation phase is widely recognized; for a recent example, see Šimić et al. (2021).
Defining the term “domestication syndrome”
The term “domestication syndrome” has been applied for about four decades to a set of correlated changes in “domesticated” plants, namely crop plants. We use it to refer to a suite of changes in mammals and birds—but which probably occurs in vertebrates including fishes—that distinguish many different domesticated animals from their wild relatives. Johnsson et al. state that the expression “domestication syndrome” was first applied to animals by E.O. Price (Price 1984, 2002). We could find no use of this term, however, in either of the cited sources. Instead, Price referred to the “domesticated phenotype.”
Yet “syndrome” and “phenotype” are not strictly synonymous and therefore not interchangeable. Taber’s Medical Dictionary defines “syndrome” (from the Greek, “running together”) as “a group of symptoms and signs of disordered function related to one another by means of some anatomic, physiologic, or biochemical peculiarity.” Crucially, in common medical usage a patient can suffer from a syndrome without exhibiting all of the associated symptoms. Two examples illustrating this point are Down syndrome and Ehlers-Danlos syndrome (De Paepe and Malfait 2012; Bull 2020). “Syndrome” is thus a generic descriptive term, in which variability is an intrinsic aspect. In contrast, “phenotype,” when designating a mutationally altered property, generally refers to a smaller number of traits without great variability, apart from degrees of expressivity.
In our usage, the “domestication syndrome” refers to a set of unexpected physical differences that frequently show up in different domesticated mammals. The phenomenon was first described, though not named, by Charles Darwin in his two-volume study of domesticated animals and plants, Variation of Animals and Plants under Domestication (Darwin 1868). Darwin’s goal in his analysis of domesticated species was to derive the general mechanism of heredity from what had been learned about breeding domesticated species. Although his quest to understand the basis of heredity failed, his book launched the study of the hereditary basis of domestication (Wilkins et al. 2014).
The unexpected traits accompanying domestication in mammals that Darwin particularly focused on were: changes in coat color such as white and brown patches, smaller jaws (muzzles) and teeth, relatively smaller brains, floppy ears, curly tails, and altered female sexual cycles. The striking feature of these traits was that they had turned up independently in different domesticates. In contrast to the productivity traits that had been deliberately selected by humans during the long breed formation stage in different species, the traits of the domestication syndrome had seemingly appeared without deliberate selection. The inference Darwin drew was that they were unintended concomitants of domestication that had appeared when one selected for the domesticated state, a phenomenon he called “unconscious selection” which he attributed to the “mysterious laws of correlation.”
A further point is that none of these odd traits, seen in domesticates but not their wild brethren, are found in all the domesticated mammals. Indeed, different breeds within a given species can differ in some of these traits, e.g. floppy vs “prick” ears in different dog breeds, and even within breeds there can be variation in some of these traits, e.g. the size or presence of white patches. The crucial point, however, is that the same traits were often found in many different species of domesticated animals, having had entirely separate origins and with little reason to suspect their deliberate selection.
It is important to recognize that the idea of the “domestication syndrome” is not a hypothesis, as stated elsewhere by the same authors (Wright et al. 2020). Rather, it is a generalization from observations. Admittedly, our application of the word “syndrome” across species was unusual but, if anything, this suggests an even greater range of differences in these early domestication-associated traits. Indeed, some of the changes listed in our proposed syndrome are logically impossible in particular species, e.g. smaller teeth and floppy ears cannot be found in chickens since chickens lack teeth and pinnae entirely.
Nevertheless, Johnsson et al. claim that our hypothesis makes sense only if the domestication syndrome is “somewhat universal.” The same claim was made by Lord et al. (2019) but without the qualifier “somewhat.” However, we never believed nor stated that the domestication syndrome involved an identical set of altered traits. Table 1 of our 2014 paper listed which specific traits were associated with particular domesticated mammalian species, the clear implication being that other species did not exhibit those traits. A careful enumeration of these domestication-trait differences amongst mammals is given in Sánchez-Villagra et al. (2016) and the general point about such differences in different domesticates was further discussed in Wilkins (2017, 2019) as well as Zeder (2020); we return to it in a moment.
One last relevant point: to the best of our knowledge, the term “domestication syndrome” as applied to animals was first used in an earlier paper by one of us (RWW) and two other colleagues (Hare et al. 2012), then in our paper (Wilkins et al. 2014). A search for its frequency of usage, using the search engine “Dimensions,” shows a distinct inflection upwards in 2014–2015, an upward trend that continued through 2020 (data supplied on request). This suggests that our paper strongly stimulated interest in this phenomenon.
Tying the domestication syndrome to the neural crest
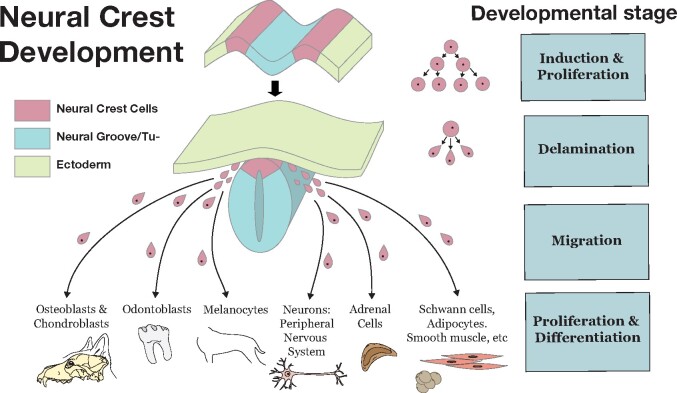
The question that motivated our paper was: “why these traits in particular?” Our starting point was realizing that many of the tissues involved in the traits of the domestication syndrome derive from the neural crest. These NCC-derived tissues include major parts of the jaws and teeth, pigmentation cells, components of the external ears, and cells involved in sympathetic responses and the sexual cycle (Hall 2000; Trainor 2014) (Figure 1). The domestication-related traits can all be interpreted as resulting from partial reduction in the number of differentiated cells (e.g. odontoblasts) that directly give rise to these structures which in turn can reflect the NCC numbers giving rise to them (reviewed in Schneider 2018). Such reductions could occur in multiple ways (see Figure 1), for example domesticated variants could have decreased NCC cell numbers or delayed NCC migration, or altered rates of proliferation or differentiation of the cells arriving at the target sites.
Figure 1.
Neural crest development. The figure illustrates the different developmental processes and stages at which the neural crest cell contribution to final target tissues could be modified by mutations. These range from: (1) initial induction and proliferation of NCCs; (2) delamination (the epithelial-to-mesenchymal transition), (3) migration from neural tube to final destination sites, and; (4) proliferation at the target destination, and differentiation into mature tissues. By the NCDS hypothesis, any of these could be altered to down-regulate final cell numbers from NCC at the different sites during development. It is very likely that different processes were affected by mutations in different NCC genes of different domesticated species.
The genetic corollary of this idea is that such reductions are caused by genetic alterations affecting NCCs, specifically variants that decrease their numbers or the frequency with which they gave rise to their various derived tissues. Indeed, many dozens of genes required for NCC formation or development have been identified and their roles within a NCC genetic regulatory network (GRN) have been elucidated by Bronner and her colleagues (see, for instance, Simões-Costa and Bronner 2015). Those genes can be provisionally designated as “neural crest cell genes” (NCC genes). However, as Johnsson et al. emphasize and as we were fully aware, all these genes are employed in multiple roles or stages of development involving other cell types. Such multi-functionality of individual gene products is termed “pleiotropy” or “pleiotropic usage.” We used “neural crest cell genes” as a shorthand term for genes required for NCC development, without intending to imply that they function only in that process.
The final element of our hypothesis concerns the possible connection between the physical traits of the domestication syndrome and the behavioral changes that constitute domesticated behavior. We proposed that it involved NCC gene mutations that produce pleiotropic effects on the physical traits of the domestication syndrome and these behaviors. We sketched one possible set of links as involving the hypothalamic–pituitary–adrenal (HPA) axis and the corticosteroids produced by the adrenal glands. Both sympathetic reactivity, controlled by the NCC-derived sympathetic ganglia, and endocrine release from the adrenal glands, partly derived from NCC, are known to be reduced and delayed in several domesticated species (Künzl and Sachser 1999; Albert et al. 2008; Trut et al. 2009; Suzuki et al. 2012). We suggested that such reduced activity led to increased docility—the trait at the heart of the initial domestication stage. In principle, such mutations could affect the HPA axis at any point. This is just one possibility since the NCCs are essential for formation of all neuroendocrine cells and many alterations in the neuroendocrine cells, for instance those that produce oxytocin, can affect social behavior.
One property that might seem problematical in terms of our hypothesis is the reduction in brain size seen in many domesticates (Kruska 1988, 2005) because NCCs do not participate directly in formation of the central nervous system. Nevertheless, experimental work in birds shows that cranial NCC play a crucial role in fostering forebrain development via their production of Fgf8 (Etchevers et al. 1999; Creuzet 2006), which we suggested might be relevant (Wilkins et al. 2014). In fact, cranial NCC produce several key ligands—SHH, Fgfs, Wnts—essential for the development of cranial nerves (La Mantia 2020). Whether these ligands produced by cranial NCC also affect growth and size of any brain regions is not known but seems likely. In short, the cranial NCC might indirectly affect brain size and, consequently, neural circuitry and behaviors. The profound effects of several neurocristopathies, such as Mowat-Wilson syndrome and Pitt-Hopkins syndrome, on various cognitive properties strongly suggests that cranial NCC have significant, if indirect, roles in brain development.
Johnsson et al. suggest that we presented little or no evidence of pleiotropic effects of mutations in NCC genes and imply that our genetic hypothesis was simply an extrapolation from pleiotropic expression of NCC genes. In reality, we did discuss pleiotropic effects of NCC gene mutants in our paper. Indeed, this material was central to the paper (Wilkins 2014, pp. 799–802) and dealt with the fact that partial loss-of-function of many of these genes produces phenotypes resembling some of the changes seen in domestication. Specifically, we cited the phenotypes produced when a null mutation of the gene is heterozygous with a wild-type allele, the kind of genotype symbolized as “−/+.” When the −/+ genotype shows one or more phenotypic effects instead of recessivity of the minus allele, it is termed a “haplo-insufficient genotype.” As Table 2 of our 2014 paper shows, many NCC genes show haplo-insufficiency. (For details, see the references in the table.) Indeed, many NCC genes encode transcriptional regulatory proteins, which frequently have pleiotropic expression and show haplo-insufficiency in “−/+” heterozygotes (Veitia, 2002). That table also indicated that interactive effects between NCC gene mutants frequently occur, a fact relevant to our proposal that domestication involves additive effects of multiple NCC gene mutations, a point to which we return shortly.
In contrast to such haplo-insufficient effects, homozygosity for null mutations of neural crest genes creates much stronger phenotypic effects, indeed causing severe genetic diseases, collectively known as “neurocristopathies” (Bolande 1974; reviewed in Vega-Lopez et al. 2018). Viable domesticated animals do not exhibit clinical-level neurocristopathies. We therefore proposed that, in domestication, only partial loss-of-function mutations of these genes, “hypomorphs” in developmental genetic terminology, were involved and that these states could be seen as “mild neurocristopathies.”
Why did we suggest that the domesticated condition involved cumulative effects of several such hypomorphs in different genes? The reason is that it is the simplest inference from the fox farm experiments of Dmitry Belyaev and his colleagues who selected for docility over many generations of outbred foxes. This occurred as a gradual process, such docility or “tameness” appearing to be a graded trait. The straightforward conclusion is that additive effects of different mutants, when combined in the same genome, were involved and that the more such mutations that come together in the same genome, the stronger the behavioral effect (reviewed in Belyaev 1979; Trut 1999). Significantly, the other phenotypic effects, the physical traits of the “domestication syndrome,” also became enhanced through this breeding for increasingly docile foxes (who showed increasing friendliness as well).
Though everyone agrees that Belyaev’s work was of major importance, his interpretation of it has recently come under fire from Lord et al. (2019), who argue that domestication had essentially already been achieved in the farm-bred foxes that were used as the initial stock in the Belyaev experiments. Indeed, the description and pictures in Lord et al. indicate that the farm-bred foxes used as Belyaev’s starting stock were already slightly less wild than wild foxes. Johnsson et al. make a similar claim to that of Lord et al. and cite the paper of Statham et al. (2011) as support. What Statham et al. actually said is that a degree of domestication had been established in those farm bred foxes over more than 50 years but that the Belyaev experiments took this state of domestication to a much higher level. We quote: “Mitochondrial DNA data together with historical records indicate two stages in the selection of domesticated foxes: an initial period of about 50 years of captive breeding in fur farms with conscious selection for fur quality and unconscious selection for behavior, followed by a second 50 years of intensive selection for tame behaviour carried out at the ICG in Novosibersk” (emphasis added) (Statham et al. 2011). A recent commentary from Trut makes the same point (Trut et al. 2020). This matter returns us to the idea that domestication needs to be seen as a process with stages or of degree, not as an all-or-none state.
The NCDS does not attempt to reduce all aspects of domestication to neural crest cell biology
To recap, we proposed that the general features of behavior seen in domesticated animals stem from multiple mutations in NCC genes with cumulative effects (Wilkins et al. 2014). Later, we pointed out that genetic variants in NCC genes would exert their effects through the NCC GRN (Wilkins 2019). (It is joint membership of genes within a GRN, and its hierarchical relationships between genes, that helps explain, in modern terms, Darwin’s “mysterious laws of correlation.”)
However, it is an over-simplification to speak of the GRN for NCCs. First, the cranial and trunk NCC differ in many of their properties, hence they must have distinct GRNs; indeed there are regional differences in NCC biology within the trunk NCC (La Mantia 2020). Second, given the disparate phylogenies of different domesticated species, each will have its own variant NCC GRNs, both cranial and trunk. That explains why, under our hypothesis, there can be no “universal” domestication syndrome of all mammals.
Johnsson et al. point out that for most complex phenotypic traits the genetic changes that produce them are usually not limited to one GRN, hence one should not expect all genetic changes affecting domestication to involve NCCs. We agree completely, which is why we never said that all of the properties of domesticated animals, even just the behavioral ones, stem from changes in NCC genes. Recall that our hypothesis focused on the initial genetic events in domestication. All the domesticated mammals with a long period of association with humans have experienced both deliberate selective breeding and the operation of natural selection while living in captivity. Accordingly, they will have accumulated genetic variants affecting their behavior during those periods. For example, most of the genetic differences underlying different cognitive abilities and behaviors in dog breeds must have arisen during the period of dog breed formation of the last 200 years or so (for details of those differences, see MacLean et al. 2019; Gnanadesikan et al. 2020). Hence, the existence of other genetic changes affecting developmental processes after the neural crest stage, in particular those affecting the developing neural system, are to be expected (Wilkins 2017). Amongst papers that report genetic variants associated with domestication affecting genes in the neural system, we note Carneiro et al. (2014), Montague et al. (2014), Kukekova et al. (2018), Pendleton et al. (2018), and O'Rourke and Boeckx (2020).
On testing the NCDS hypothesis
We were pleased to see the following comment in Johnsson et al.: “An upside of the neural crest cell hypothesis of domestication is that it has increased the interest in development amongst domestication researchers” (Johnsson et al. 2021). If a hypothesis promotes research and new thinking, it has real worth, independent of its ultimate fate. Indeed, several researchers have commented explicitly on the value and relevance of the NCDS hypothesis to their work. Those papers cover domestication in dogs (Pilot et al. 2016; Pendleton et al. 2018), cats (Montague et al. 2014), horses (Librado et al. 2017), rats (Singh et al. 2017), and Bengalese finches (Okanoya 2017).
Earlier, we reviewed genomic evidence for gene variants specific to domesticated lines as consistent with the NCDS hypothesis (Wilkins 2017). Johnsson et al. state that such evidence has to be treated with caution, however, because alleles picked up in such studies will tend to reflect more recent selective sweeps than the early ones our hypothesis predicts. We agree. Nevertheless, two of the genome studies identified NCC gene variants from older selective sweeps and yielded results that the authors believed were relevant to the early stages of domestication—and to our hypothesis. These were the reports of Pendleton et al. (2018) on dogs and Librado et al. (2017) on horses. Librado et al., indeed, extracted DNA from bones of ancient horses for their main analysis while Pendleton et al. analyzed DNA from three Neolithic dogs for comparison with that of contemporary “village dogs” and found some overlaps, confirming that the present day village dogs had some NCC variants from the Neolithic.
Furthermore, other kinds of evidence, not mentioned by Johnsson et al., support the NCDS hypothesis. For instance, there is a study of recent mouse commensal association with humans, in which signs of the domestication syndrome appeared spontaneously (Geiger et al. 2018). There is also an interesting pattern of changed DNA methylation in farm bred sea bass, including significant epigenetic changes in several NCC genes, which was interpreted by the authors as support for the NCDS (Anastasiadi and Piferrer 2019). Such epigenetic changes may precede similar-acting genetic changes in domestication (Trut et al. 2009; Wilkins 2019). Other articles have discussed applicability of the NCDS hypothesis to the controversial but increasingly accepted idea that Homo sapiens, modern humans, can be seen as “self-domesticated” animals (Theofanopolou et al. 2017; Zanella et al. 2019; Šimić et al. 2021). In particular, Zanella et al. (2019) found distinctive variants in a well-characterized NCC regulator gene necessarily for facial bone development that were distinctive to modern humans, not found in Neanderthals or Denisovans.
Nevertheless, all these findings are consistent with our hypothesis but do not constitute proof that alterations in NCC genes can promote domestication. The strongest experimental tests are those that attempt to falsify a hypothesis: an idea that survives such a test is a much stronger candidate for being a valid explanation (Popper 1959). In our 2014 paper, we discussed such possible tests but we will extend that discussion here, noting three approaches.
The first involves partial ablation of NCCs in embryos of animal species where the experimentally manipulated embryos would be allowed to develop to post-natal stages, allowing scoring of traits of domestication. This could involve physical ablation in embryos (Creuzet et al. 2006) or more tellingly, genetic ablation via hypomorphic knock-in mutations of NCC genes in species where this is possible, either by the older homologous recombination method or by CRISPR (Kumita et al. 2019; Wu et al. 2019) The most informative experiments would employ genetically engineered embryos from wild strains using surrogate mothers to bring them to term but such experiments are difficult. Instead, one could use lab strains in which any additional signs of domestication in the experimental animals could be detected. Perhaps even marmosets, Callithrix jacchus, a sociable if not domesticated primate, could be tested in this way since they are now known to be amenable to CRISPR modification (Kumita et al. 2019). Marmosets, in fact, seem to show some behavioral and physical signs of domestication (Ghazanfar et al. 2020). The prediction is that fostered embryos having hypomorphic knock-in mutations in NCC genes that somewhat diminish the number of NCCs arriving at key sites should give rise to juveniles or adults exhibiting further physical signs of the domestication syndrome. Controls could involve knock-in hypomorphic mutations of genes not suspected of any roles in domestication. If surviving postnatal offspring are raised with appropriate socialization as juveniles (a crucial component of domesticated behavior, see Scott 1962; Wilkins et al. 2014), the experimental animals only should also show exaggerated physiological or behavioral signs of domestication. These genetic manipulations should be carried out on NCC genes active in cranial NCCs since most of the traits of the domestication syndrome involve cranial traits (see Table 1 in Wilkins et al. 2014). A failure to see morphological or behavioral signs of domestication in the experimental offspring would falsify our hypothesis. Conversely, signs of domestication would strongly support it.
The second kind of test involves comparative studies, in domesticated vs wild strains of the same animal species, of NCC numbers during genesis, delamination, and migration in the embryo and at their final sites. The NCDS hypothesis predicts that the domesticated strains will show fewer cells derived from NCCs at their final sites than are found in the corresponding wild strains. Ideally, multiple domesticated and wild strains would be used for such comparisons. If there are no significant differences in those cell numbers between domesticated and wild strains, the hypothesis will have been falsified.
The third kind of test concerns genomic studies. The NCDS predicts that all domesticated strains will show NCC gene mutations that are not seen in the corresponding wild strains or, if the ancestral lines no longer exist, in sibling species that still exist in the wild. As noted above, Johnsson et al. point out that the existence of such genetic variants would not prove the hypothesis. Yet, a failure to find such loss-of-function mutations in domesticated strains would show important limitations to the generality of our idea. Crucially, the scan for mutations must include looking for variants in cis-regulatory elements of NCC genes, given how important such regulatory mutations are to so many evolutionary changes (Douglas and Hill 2014; Long et al. 2016). To date, they have been given relatively little attention in the published genomic domestication work. Not finding such partial loss-of-function cis-regulatory mutations in NCC genes of domesticated breeds would be a serious blow to the NCDS hypothesis.
Summary and conclusions
The scientific study of the animal domestication has a long history, beginning with Darwin’s opus on heredity published in 1868. In that same year, NCCs were first described by the distinguished German embryologist Wilhelm His (His 1868). Our 2014 article brought these two subjects together in the NCDS hypothesis. Here, we have addressed the critique of that idea by Johnsson et al. Their ultimate conclusion was that the “neural crest cell hypothesis is an explanation looking for a problem.” We have shown here that that is not true. The problem we addressed was the nature of the shared traits of the “domestication syndrome;” our hypothesis attempted to explain their common denominator.
In this reply, we have argued that the NCDS hypothesis has genuine explanatory power, is supported by much current evidence, and is testable. We hope that further tests will illuminate the domestication syndrome further and, in particular, how changes in the neuroendocrine system and in neural circuitry help shape domesticated animal social behaviors.
Acknowledgments
We are grateful to the editors of GENETICS for the opportunity to reply to Johnsson et al. and thank Rich Schneider, Nathalie Feiner, and two anonymous referees for helpful comments on an earlier draft of this article. We also thank Vladislav Nachev for doing the search for usage of the term “domestication syndrome.”
Conflicts of interest
None declared.
Literature cited
- Albert FW, Shchepina O, Winter C, Römpler H, Teupser D, et al. 2008. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm Behav. 53:413–421. [DOI] [PubMed] [Google Scholar]
- Anastasiadi D, Piferrer F.. 2019. Epimutations in developmental genes underlie the onset of domestication in farmed European sea bass. Mol Biol Evol. 36:2252–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev DK. 1979. Destabilizing selection as a factor in domestication. J Hered. 70:301–308. [DOI] [PubMed] [Google Scholar]
- Bolande RP. 1974. The neurocristopathies: a unifying concept of disease arising in neural crest maldevelopment. Hum. Pathol. 5:409–429. [DOI] [PubMed] [Google Scholar]
- Bull MJ. 2020. Down syndrome. N Engl J Med. 382:2344–2352. [DOI] [PubMed] [Google Scholar]
- Carneiro M, Rubin C-J, Di Palma F, Albert FW, Alfoldi J, et al. 2014. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science. 345:1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet SE, Martinez S, Le Douarin N.. 2006. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci USA. 103:14033–14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. 1868. The Variation in Animals and Plants under Domestication. London: John Murray. [Google Scholar]
- De Paepe A, Malfait F.. 2012. Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet. 82:1–11. [DOI] [PubMed] [Google Scholar]
- Douglas AT, Hill RE.. 2014. Variation in vertebrate cis-regulatory elements in evolution and disease. Transcription. 5:e288848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, Le Douarin NM.. 1999. Anterior cephalic neural crest is required for forebrain viability. Development. 126:3533–3543. [DOI] [PubMed] [Google Scholar]
- Fitak RR, Mohandesan E, Corander J, Yadamsuren A, Chuluunbat B, et al. 2020. Genomic signatures of domestication in Old World camels. Commun Biol. 3:316. doi:10-1038/s42003-020-1039.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RC. 2015. Domesticated: Evolution in a Man-Made World. New York: W.W. Norton & Company. [Google Scholar]
- Geiger M, Sanchez-Villagra MR, Lindholm AK.. 2018. A longitudinal study of phenotypic changes in early domestication of house mice. R Soc Open Sci. 5:172099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar A, Kelly LM, Takahashi DY, Winters S, Terrett R, et al. 2020. Domestication phenotype linked to vocal behavior in marmoset monkeys. Curr Biol. 30:5026–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanadesikan GE, Hare B, Snyder-Mackler N, Call J, Kaminski J, et al. 2020. Breed differences in dog cognition associated with brain-expressed genes and neurological functions. Integr Comp Biol. 60:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. 2000. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2:3–5. [DOI] [PubMed] [Google Scholar]
- Hare B, Wobber V, Wrangham RW.. 2012. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim Behav. 83:573–585. [Google Scholar]
- His W. 1868. Untersuchungen über die erste Anlage des Wirbelthierleibes. Die erste Entwicklung des Hünchens im Ei. Leipzig: F.C.W. Vogel.
- Johnsson M, Henricksen R, Wright D.. 2021. The neural crest cell hypothesis: no unified explanation for development. GENETICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruska D. 1988. Mammalian domestication and its effects on brain structure and behavior. In: Jerison HJ, Jerison I, editors. The Evolutionary Biology of Intelligence. Berlin: Springer-Verlag. p. 211–250. [Google Scholar]
- Kruska DC. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication and feralization. Brain Behav Evol. 65:73–108. [DOI] [PubMed] [Google Scholar]
- Kukekova AV, Johnson JL, Xiang X, Feng S, Liu S, et al. 2018. Red fox genome assembly identifies genomic regions associated with tame and aggressive behaviors. Nat Ecol Evol. doi:10.1038/s41559-018-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumita W, Sato K, Suzuki Y, Kurotaki Y, Harada T, et al. 2019. Efficient generation of knock-in and knock-out marmoset embryos via CRISPR/Cas 9 gene editing. Sci Rep. 9:12719. doi:10.1038/s41598-019-49110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzl C, Sachser N.. 1999. The behavioral endocrinology of domestication: a comparison between the domestic guinea pig (Cavia aperea f. porcellus) and its wild ancestor, the cavy (Cavia aperea). Horm Behav. 35:28–37. [DOI] [PubMed] [Google Scholar]
- La Mantia A-S. 2020. Why does the face predict the brain? Neural crest induction, craniofacial morphogenesis, and neural circuit development. Front Physiol. doi:10.3389/fphys.2020.610970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P, Gamba C, Gaunitz C, Der Sarkissian C, Pruvost M, et al. 2017. Ancient genomic changes associated with domestication of the horse. Science. 356:442–445. [DOI] [PubMed] [Google Scholar]
- Long HK, Prescott SL, Wysocka J.. 2016. Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell. 167:1170–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord KA, Larson G, Coppinger RP, Karlsson EKK.. 2019. The history of fox farm foxes undermines the animal domestication syndrome. Trends Ecol Evol. 35:125–136. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Snyder-Mackler N, von Holdt BM, Serpell JA.. 2019. Highly heritable and functionally relevant breed differences in dog behavior. Proc Biol Sci. 286:20190716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague MJ, Li G, Gandolfi B, Khan R, Aken BL, et al. 2014. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc Natl Acad Sci USA. 111:17230–17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K. 2017. Sexual communication and domestication may give rise to signal complexity necessary for the emergence of language: a hint from songbird studies. Psychon Bull Rev. 24:106–110. [DOI] [PubMed] [Google Scholar]
- O'Rourke T, Boeckx C.. 2020. Glutamate receptors in domestication and modern human evolution. Neurosci Biobehav Rev. 108:341–557. [DOI] [PubMed] [Google Scholar]
- Pendleton AL, Shen F, Taravella AM, Emery S, Veeramah KR, et al. 2018. Selective sweep analysis using village dogs highlights the pivotal role of the neural crest in dog domestication. BMC Biol. 16. doi:10.1186/5/2915-018-0535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot M, Malewski T, Moura AE, Grzybowski T, Oleński K, et al. 2016. Diversifying selection between pure-breed and free-breeding dogs inferred from genome-wide SNP analysis. G3 (Bethesda). 6:2285–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper K. 1959. The Logic of Scientific Discovery. Abingdon-on-Thames: Routledge. [Google Scholar]
- Price EO. 1984. Behavioral aspects of animal domestication. Q Rev Biol. 59:1–32. [Google Scholar]
- Price EO. 2002. Animal Domestication and Behavior. New York: CABI. [Google Scholar]
- Sánchez-Villagra MR, Geiger M, Schneider RA.. 2016. The taming of theneural crest: a developmental perspective on the origins of morphological covariation in domesticated animals. R Soc Open Sci. 3:160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA. 2018. Cellular control of time, size and shape in development and evolution. In: Hall BK, Moody S, editors. Cells in Evolutionary Biology: Translating Genotypes into Phenotypes—Past, Present and Future. Boca Rotan: CRC Press, Taylor & Francis Group. p. 167–212. [Google Scholar]
- Scott JP. 1962. Critical periods in behavioral development. Science. 138:949–958. [DOI] [PubMed] [Google Scholar]
- Šimić G, Vukić V, Kopić J, Krsnik Z, Hof PR.. 2021. Molecules, mechanisms and disorders of self-domestication: keys for understanding emotional and social communication from an evolutionary perspective. Biomolecules. 11. doi:10.3390/biom 11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Costa M, Bronner ME.. 2015. Establishing neural crest cell identity: a gene regulatory recipe. Development. 142:242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Albert FW, Plyusnina I, Trut L, Pääbo S, et al. 2017. Facial shape differences between rats selected for tame and aggressive behaviors. PLoS One. 12:e0175043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statham MJ, Trut LN, Sacks BN, Kharlamova AV, Oskina IN, et al. 2011. On the origin of a domesticated species: Identifying the parent population of Russian silver foxes (Vulpes vulpes). Biol J Linn Soc Lond. 103:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yamada H, Kobayashi T, Okanoya K.. 2012. Decreased fecal corticosterone levels due to domestication: a comparison between the white-backed munia (Lonchura striata) and its domesticated strain, the Bengalese finch (Lonchura striata var. domestica) with a suggestion for complex song evolution. J Exp Zool A Ecol Genet Physiol. 317:561–570. [DOI] [PubMed] [Google Scholar]
- Theofanopolou C, Gastaldon S, O’Rourke T, Samuels BD, et al. 2017. Self-domestication in Homo sapiens: insights from comparative genomics. PLoS One. doi:10.1371/journal.pone.0185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA (Editor). 2014. Neural Crest Cells: Evolution, Development and Disease. Amsterdam: Academic Press. [Google Scholar]
- Trut LN, Kharlamova AV, Herbeck YE.. 2020. Belyaev’s and PEI’s foxes: a far cry. Trends Ecol Evol. 35:649–651. [DOI] [PubMed] [Google Scholar]
- Trut LN, Oskina I, Kharlamova A.. 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays. 31:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trut LN. 1999. Early canid domestication: the farm fox experiment. Am Sci. 160.87: 160–168. [Google Scholar]
- Vega-Lopez GA, Cerrizuela S, Tribulo C, Aybar MJ.. 2018. Neurocristopathies: new insights 150 years after the neural crest discovery. Dev Biol. 444:S110–S143. [DOI] [PubMed] [Google Scholar]
- Veitia RA. 2002. Exploring the aetiology of haplo-insufficiency. Bioessays. 24:175–184. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Wrangham RW, Fitch WT.. 2014. The “domestication syndrome in mammals: a unified explanation based on neural crest cell behavior and genetics. GENETICS. 197:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AS. 2017. Revisiting two hypotheses on the “domestication syndrome” in light of genomic data. Vestn Vogis. 21:435–442. [Google Scholar]
- Wilkins AS. 2019. A striking example of developmental bias in an evolutionary process: the “domestication syndrome”. Evol Dev. 2019:1–11. [DOI] [PubMed] [Google Scholar]
- Wright D, Henriksen R, Johnsson M.. 2020. 2020 defining the domestication syndrome: comment on lord. Trends Ecol Evol. 35:1059–1060. [DOI] [PubMed] [Google Scholar]
- Wu N, Liu B, Du H, Zhao S, Li Y, et al. 2019. "The progress of CRISPR/Cas9-mediated gene editing in generating mouse/zebrafish models of human skeletal diseases. Comput Struct Biotechnol J. 17:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella M, Vitriolo A, Andirko A, Martins PT, Sturm S, et al. 2019. Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and underlying self-domestication. Sci Adv. 5:eaaw7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder MA, et al. 2012. Pathways to animal domestication. In: Gepts P, Famula TR, Bettinger RL, editors. Biodiversity in Agriculture: Domestication, Evolution, and Sustainability. Cambridge: Cambridge University Press. [Google Scholar]
- Zeder MA. 2015. Core questions in domestication research. Proc Natl Acad Sci USA. 112:3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder MA. 2020. Straw foxes: domestication syndrome evaluation comes up short. Trends Ecol Evol. 35:647–649. [DOI] [PubMed] [Google Scholar]