Abstract
People living with burn injury often report temperature sensitivity. However, its epidemiology and associations with health-related quality of life (HRQOL) are unknown. We aimed to characterize temperature sensitivity and determine its impact on HRQOL to inform patient education after recovery from burn injury. We used the multicenter, longitudinal Burn Model System National Database to assess temperature sensitivity at 6, 12, and 24 months after burn injury. Chi-square and Kruskal–Wallis tests determined differences in patient and injury characteristics. Multivariable, multilevel generalized linear regression models determined the association of temperature sensitivity with Satisfaction with Life (SWL) scale scores and Veterans RAND 12 (VR-12) physical and mental health summary component (MCS) scores. The cohort comprised 637 participants. Two thirds (66%) experienced temperature sensitivity. They had larger burns (12% TBSA, interquartile range [IQR] 4–30 vs 5% TBSA, IQR 2–15; P < .0001), required more grafting (5% TBSA, IQR 1–19 vs 2% TBSA, IQR 0–6; P < .0001), and had higher intensity of pruritus at discharge (11% severe vs 5% severe; P = .002). After adjusting for confounding variables, temperature sensitivity was strongly associated with lower SWL (odds ratio [OR] −3.2, 95% confidence interval [CI] −5.2, −1.1) and MCS (OR −4.0, 95% CI −6.9, −1.2) at 6 months. Temperature sensitivity decreased over time (43% at discharge, 4% at 24 months) and was not associated with poorer HRQOL at 12 and 24 months. Temperature sensitivity is common after burn injury and associated with worse SWL and MCS during the first year after injury. However, temperature sensitivity seems to improve and be less intrusive over time.
Burn injury causes pain and altered sensation acutely and during recovery, including peripheral and central sensitization, loss of descending modulation, hyperalgesia, allodynia, postburn pruritis, and temperature sensitivity.1–5 Temperature sensitivity is thought to be caused by two general mechanisms: 1) loss of vasodilation, sweating, and piloerection capabilities in injured skin and scar and 2) disordered nerve signaling and hypersensitivity. The former challenges patients’ recoveries by lowering their abilities to compensate for heat stress.6,7 The latter can limit patients’ abilities to tolerate extremes of temperature and be triggered by moving air, contact with objects and liquids, or low ambient climates. Studies of temperature sensitivity have reported that sensory alterations postburn occur for both heat and cold sensations and induce significant discomfort.2,8
Temperature intolerance and sensitivity experiences vary dramatically. The experiences of two burn survivor-collaborators illustrate this. One burn survivor experienced a 45% TBSA burn injury on her left arm and hand, chest, back, legs, and face as a young woman. Her first temperature sensitivity experience was in the hospital. She recalls that one of her rooms had an air-conditioning vent directed toward her, which made her uncomfortably cold. In response, she burrowed under blankets, only to become quickly overheated. Being comfortable in a narrow range of ambient temperatures remained a hallmark of her experience. She was discharged from the hospital in the summer and was constantly “overheating.” Compounding the sense of overheating, she could feel her scars flush when hot, particularly those on her face, and stay red longer than other parts of her face even after she had cooled down. Some social situations exacerbated the warmth of the flushing. These two symptoms together—the overheating and exaggerated flushing—challenged her re-integration into her “normal life.” She was overheated and emotionally overwhelmed in crowded rooms, which increased the frequency of her flashbacks of the heat from the fire that injured her. It was more comfortable to stay in a “little bubble” than venture out and experience heat symptoms. As a result, she became more socially isolated. Her physical, emotional, and social function was depressed because of heat intolerance. However, as the months passed, her symptoms improved and she learned to cope and adapt to her environment. Years out from her injury, she has only occasional temperature sensitivity and thrives as an artist and educator (Box 1).
Box 1. Experiences of heat intolerance and cold sensitivity from people living with burn injuries.

Burn Survivor 1: I experienced a 45% TBSA burn on my arms, chest, back, legs, and face. After discharge in the summer, I returned to a hot, dry climate. I often felt hot and was easy to overheat, both indoors and outdoors. I had a very narrow range of temperatures where I felt comfortable, around the 60’s to 70’s. I tried to volunteer 3–4 months after my injury, but it was difficult because I wasn’t able to control the temperature in the building. Similarly, when I was able to start going out again with friends and returned to school on a busy campus, I couldn’t handle being in a crowded space because the physical heat of all the people was overwhelming. Therefore, I stayed home more often and became more socially isolated. The heat intolerance decreased most significantly over the first one and a half years after my injury. After that, I could tolerate a wider range of temperatures more comfortably. But even now, I still notice that my left arm overheats quickly compared to my right arm, which was less injured. Heat intolerance contributed to my inability and stress around reentering life; it was one piece among many that made it physically and mentally difficult to return to “normal.”

Burn Survivor 2: I had a 65–70% TBSA burn on my arms, legs, buttocks, hands, face, and back. Before my burn injury, I was very active physically. I worked in construction and was active in the life of rural Alaska—fishing, hunting, and traveling in the Arctic country. At discharge, my biggest concern was getting back to my pre-injury life. Upon my return to Alaska, I stepped off the plane to a wind chill of −20°F. The scarring all over my body tightened up immediately. The wind hitting my face was unbearable, and I had to wear a facemask for months to tolerate being outside. Burn scars that were exposed to cold temperatures—my face and hands in particular—began to dry, crack and bleed. However, I have learned how to overcome the omnipresent cold—it was something I had to do. I worked at exposing myself to it daily. It took at least a year before I noticed I could go outside without covering my face and other burn injuries.
Another burn survivor experienced a 70% TBSA burn injury on his arms and hands, legs, buttocks, back, and face. Before his injury, Chris lived in a remote community in Alaska. His identity was, in part, entwined with creating with his hands and practicing subsistence living—hunting, fishing, and providing for his family and community. His first recollection of temperature sensitivity was the feeling of the scars on his legs immediately constricting when he got off the plane in Alaska returning home from the hospital. It was −28°C and the windchill was −45°C. The cold-induced tightening of his scars, particularly those on his legs, hands, and face, significantly limited his physical function. He wore a face mask for many months to avoid uncomfortable air movement across it. Unable to immediately “get back to normal life” in rural Alaska, he temporarily shifted his work to be focused on more indoor activities. He and his wife built and ran a food truck and restaurant, which served as steppingstones to more physical and outdoor work. With predominantly extremity scars that are unable to sweat when he became hot, he would experience profuse truncal sweating with warm indoor temperatures and while sleeping. Like Grace, Chris also learned how to cope and adapt in the months and early years after his injury. He now can climb into a bush plane, jump into a fishing boat, and manage a snowmobile even in the coldest of temperatures (Box 1).
These experiences are specifically unique, but generally shared among people living with burn injury. Heat sensitivity has been repeatedly identified by the Burn Specific Health Scale—Brief as common, often severe, and negatively associated with health-related quality of life (HRQOL).3,4,9 Although several reports have described heat intolerance in the setting of exercise among survivors of a large burn injury,10,11 no report has characterized the prevalence or natural history of temperature sensitivity among people living with burn injury or correlated it other HRQOL measures.
To address this gap, we sought to describe the epidemiology of temperature sensitivity after burn injury and determine its associations with satisfaction with life, physical health, and mental health using validated HRQOL measures. We hypothesized that temperature sensitivity is frequently experienced by people living with burn injury and that it negatively affects physical and mental health, and thus be associated with overall lower satisfaction with life when compared to those who do not experience temperature sensitivity. Our findings might facilitate patient education for patients who are experiencing temperature sensitivity and identify opportunities to improve HRQOL.
METHODS
Study Design and Study Population
We used the National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model Systems (BMS) National Database12 to perform a retrospective cohort study using prospectively collected data. The BMS National Database is a longitudinal repository of data from participants who are living with burn injury from burn centers across the United States since 1994. Patients enrolled in BMS and included in this study met one of the following enrollment criteria:
18 to 64 years of age with a burn injury ≥20% TBSA with surgical intervention;
65 years of age with a burn injury ≥10% TBSA with surgical intervention;
18 years of age with a burn injury to their face/neck, hands, or feet with surgical intervention; or
18 years of age with a high-voltage electrical burn injury with surgical intervention.
The BMS National Database contains demographic, injury, and recovery data elements, as well as patient-reported outcome measures. BMS participants are surveyed at hospital discharge and 6 ± 2, 12 ± 3, and 24 ± 6 months after their injury.13 Participants were recruited at four different burn centers participating in the BMS National Database across the states of Washington, Texas, and Massachusetts. For this study, we extracted observations of participants from 2015 to 2020 as temperature sensitivity data collection began in 2015. Surveys are administered in person, over telephone, by mail, or via a web-based platform depending on participant preference, and symptoms were asked about in real time to minimize the possibility of recall bias.
Participatory Research Strategy
Members of the Northwest Regional Burn Model System (NWRBMS) Community Advisory Board and people living with burn injury who participated in focus group discussions with NWRBMS investigators and Alaska-based clinicians reported that temperature sensitivity was commonly experienced and that providers were unable to give them concrete guidance on what to expect as they recover. These stakeholders encouraged us to investigate temperature sensitivity using the BMS National Database. We used the focus groups and their markedly different testimonies to develop our conceptual framework and analytical models. We also collaborated with individuals who reported temperature sensitivity after burn injury to assist in interpreting the results and editing the manuscript and who will support dissemination efforts to the burn community.
Exposure and Covariables
Self-reported temperature sensitivity at survey time points was our key exposure. BMS participants were instructed to reflect on their current health at the time of the survey and asked if they experienced “difficulty in cold environments” and if they experienced “difficulty in hot environments” with answer choices including “yes,” “no,” and “I don’t know” at each survey interval. Participants were given the choice to “refuse” the question. Participants were categorized as endorsing temperature sensitivity if they answered “yes” to either the cold or heat intolerance question. They were categorized as having no temperature sensitivity if they answered “no” to both cold and heat intolerance.
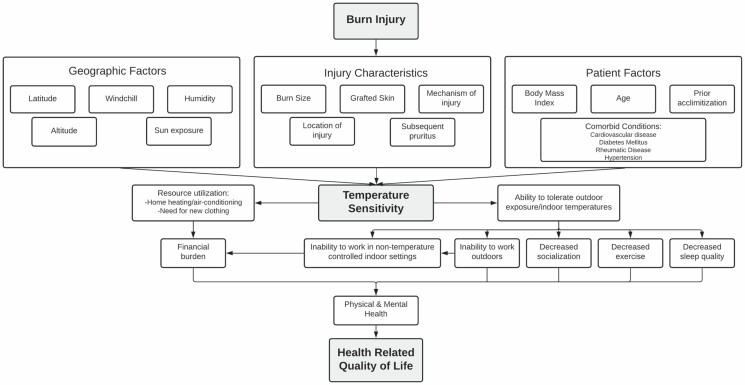
Several covariables were used to describe participants, their injuries, and their recovery experiences based on literature, discussion among our focus groups, and the conceptual framework revised with our participant collaborators (Figure 1). Demographic characteristics included study site, sex, age, race, ethnicity, and body mass index (BMI).3,4 Injury characteristics included burn size (ie, %TBSA affected), graft %TBSA, etiology, anatomical location, need for amputation, presence/intensity of pruritus at discharge, and neuropathy (ie, pins/needles/numbness in scars). The anatomical burn location was characterized as either in a commonly exposed location (ie, head, face, neck, forearm, and hand) or in a generally unexposed location. We included other conditions that potentially incur disability and medical comorbid conditions associated with thermal dysregulation of extremities, including those associated with cardiovascular disease and neuropathy including high blood pressure, history of myocardial infarction, and diabetes mellitus.14 We also included comorbid psychiatric diseases (eg, alcohol use disorder, affective disorders, anxiety, and psychosis) given their documented associations with mental health, satisfaction with life, and associations between psychologic stress and increased core body temperature.15–18
Figure 1.
A conceptual model relating burn injury to the development of temperature sensitivity and subsequent impact on health-related quality of life.
Outcomes
We assessed three separate outcomes. The Veterans RAND 12 (VR-12), derived from the Veterans RAND 36-Item Health Survey, was used to measure physical component summary (PCS) and mental component summary (MCS) scores. The VR-12, validated in diverse populations and used in many studies of people living with burn injury,19,20 is comprised of 12 items that assess functional physical and mental health. PCS and MCS scores ranging between 0 and 100 with a lower score indicating a lower HRQOL are standardized using a t-score transformation and normalized to a U.S. population mean score of 50 with a standard deviation of 10.21 Overall satisfaction with life was assessed using the validated and commonly used Satisfaction with Life (SWL) scale.19,20,22–25 SWL comprises five questions, each of which are scored from 1 to 7 with the possible range of total scores from 5 to 35. A score of 20 represents the neutral point on the scale with lower scores indicating lower satisfaction with life and higher scores indicating higher satisfaction with life.
Statistical Analysis
Missing data (10%) were similar between temperature sensitivity groups. As key variables did not differ for participants with missing temperature sensitivity data, a complete case analysis was performed. Continuous variables were summarized as medians (and interquartile range) and compared using Kruskal–Wallis tests. Categorical variables were summarized as number (and percent) and compared using χ 2 analysis. For each comparison, P values were reported and considered significant if P < .05.
The associations between temperature sensitivity and each of the three HRQOL measures over the follow-up period were assessed using multivariable, multilevel random effects regression with robust standard errors to account for heteroskedasticity. The impact of temperature sensitivity for each follow-up period was assessed using multivariable, multilevel generalized linear regression modeling. The models included participant and injury characteristics that were found to be significantly different between our populations of interest or important in the conceptual model (eg, study site, burn size, graft percentage, location of burn, pruritus, amputation, preinjury MCS score), and those potential disabilities and comorbid conditions associated with neuropathic pain, poor peripheral circulation, and microvascular disease. To account for multiple hypothesis testing, P values were considered significant if P < .016.
RESULTS
Cohort Description
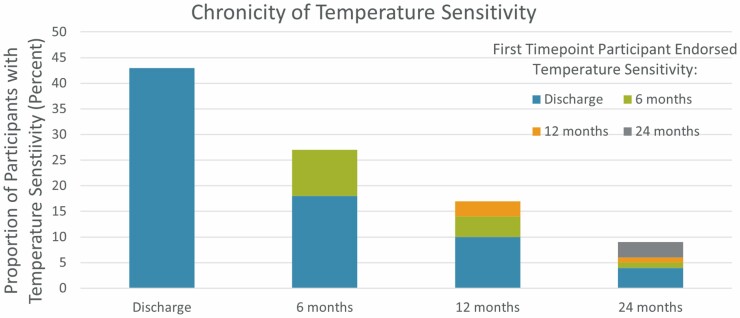
From 2015 to 2020, 711 participants enrolled in the multicenter BMS National Database. Of these, 637 participants provided responses to the temperature sensitivity items and were included in our analysis. Within our study population, 418 (66%) of participants experienced temperature sensitivity at any point during the study. Temperature sensitivity was most prevalent at discharge with 43% of our participants endorsing this symptom, which persisted at 6 months for 18% of our participants, at 12 months for 10%, and at 24 months for 4% (Figure 2). Only 10% of participants first endorsed temperature sensitivity at 6 months and 3% first endorsed temperature sensitivity at 12 months or 24 months.
Figure 2.
Chronicity of temperature sensitivity. Incidence of temperature sensitivity is given at discharge and for each follow-up interval as a percent of the total study population (y-axis). Color corresponds to the follow-up time point that the participant first endorsed temperature sensitivity and the persistence of temperature sensitivity at subsequent follow-up visits. 43% of participants endorsed temperature sensitivity on discharge with 18% continuing to endorse at 6 months postinjury.
Characteristics of Participants With Temperature Sensitivity
Within our study population, most were male (69%). The median age was 47 (IQR 32–58) and BMI was 27 (IQR 24–31). Most of our study population was non-Hispanic white (66%). Median burn size was 10% TBSA (IQR 3–25) with a median graft size of 3% TSBA (IQR 1–14). The most common etiology was flame burn (56%) and most involved exposed body locations (82%). More than three quarters of participants (82%) endorsed postburn pruritus at the site of injury at discharge, with the intensity predominantly reported to be mild (37%) or moderate (31%). A third of all participants endorsed both cold and heat sensitivity (34%); 17% endorsed only heat sensitivity and 15% endorsed only cold sensitivity. Heat sensitivity was most frequent at participants treated at the Texas sites while cold sensitivity was most frequent at the Massachusetts site. The Washington site, with a more temperate climate, had about even numbers of cold and heat sensitivity.
When comparing those who endorsed temperature sensitivity to those who did not, significant differences were found among injury characteristics (Table 1). Participants who endorsed temperature sensitivity had larger burns (12% vs 5%, P < .0001), required more grafting (5% vs 2%, P < .0001), and had injuries located more frequently in exposed areas (84% vs 76%, P = .002). Participants who endorsed temperature sensitivity also more frequently experienced postburn pruritus at discharge (77% vs 63%, P = .004) and with higher intensity (severe: 11% vs 5%, unbearable: 3% vs 1%, P = .002). Participants who endorsed temperature sensitivity also had a higher prevalence of depression (6% vs 5%, P = .042) and twice as commonly reported experiencing anxiety (4% vs 2%, P = .019) prior to their injuries.
Table 1.
Patient and injury characteristics
| Temperature Sensitivity | ||||
|---|---|---|---|---|
| Yes (n = 418) | No (n = 219) | Missing (n = 74) | P | |
| Sex | .389 | |||
| Male | 283 (68%) | 157 (72%) | 56 (76%) | |
| Female | 135 (32%) | 62 (28%) | 18 (24%) | |
| Age | 46 (32–56) | 48 (34–59) | 43 (32–56) | .1332 |
| Race | .639 | |||
| White | 335 (80%) | 171 (78%) | 57 (77%) | |
| Black | 43 (10%) | 22 (10%) | 7 (9%) | |
| Asian | 9 (2%) | 4 (2%) | 0 (0%) | |
| American Indian/Alaskan Native | 9 (2%) | 3 (1%) | 0 (0%) | |
| Native Hawaiian or Other Pacific Islander | 1 (1%) | 2 (1%) | 0 (0%) | |
| More than one race | 2 (1%) | 3 (1%) | 0 (0%) | |
| Other | 10 (2%) | 8 (4%) | 3 (4%) | |
| Ethnicity | .77 | |||
| Hispanic/Latino | 69 (17%) | 33 (15%) | 8 (11%) | |
| Non-Hispanic/Latino | 342 (82%) | 175 (80%) | 54 (73%) | |
| BMI | 27 (23–31) | 27 (24–31) | 26 (23–30) | .37 |
| Comorbidities* | ||||
| High blood pressure | 19 (5%) | 22 (10%) | 7 (9%) | .766 |
| Diabetes mellitus | 17 (4%) | 12 (5%) | 4 (5%) | .214 |
| History of myocardial infarction | 2 (0.5%) | 3 (1%) | 1 (1%) | .707 |
| Rheumatic disease | 1 (1%) | 0 (0%) | 0 (0%) | .299 |
| Alcohol addiction | 12 (3%) | 5 (2%) | 4 (5%) | .054 |
| Depression | 20 (6%) | 10 (5%) | 2 (3%) | .042* |
| Anxiety | 16 (4%) | 6 (2%) | 3 (4%) | .019* |
| Bipolar disorder | 5 (1%) | 2 (1%) | 3 (4%) | .216 |
| Burn size (%TBSA) | 12 (4–30) | 5 (2–15) | 5 (2–15) | .0001* |
| Graft size (%TBSA) | 5 (1–19) | 2 (0–6) | 1 (0–5) | .0001* |
| Etiology | .184 | |||
| Fire/flame | 247 (59%) | 109 (50%) | 35 (47%) | |
| Scald | 47 (11%) | 37 (17%) | 13 (18%) | |
| Contact | 23 (6%) | 18 (8%) | 7 (10%) | |
| Grease | 47 (11%) | 34 (16%) | 8 (11%) | |
| Tar | 4 (1%) | 1 (1%) | 1 (1%) | |
| Chemical | 10 (2%) | 3 (1%) | 4 (5%) | |
| Electricity | 26 (6%) | 9 (4%) | 2 (3%) | |
| UV light | 1 (1%) | 0 (0%) | 0 (0%) | |
| Flash | 10 (2%) | 5 (2%) | 3 (4%) | |
| Other | 3 (1%) | 3 (1%) | 1 (1%) | |
| Exposed burn (face, forearm, hand) | .002* | |||
| Yes | 353 (84%) | 167 (76%) | 51 (69%) | |
| No | 65 (16%) | 52 (24%) | 23 (31%) | |
| Pruritus at discharge | .004* | |||
| Present | 320 (77%) | 138 (63%) | 8 (11%) | |
| Absent | 56 (13%) | 46 (21%) | 2 (3%) | |
| Intensity of pruritus at discharge | .002* | |||
| Mild | 127 (30%) | 78 (36%) | 2 (3%) | |
| Moderate | 128 (31%) | 46 (21%) | 2 (3%) | |
| Severe | 48 (11%) | 11 (5%) | 0 (0%) | |
| Unbearable | 14 (3%) | 1 (1%) | 0 (0%) | |
| Amputation due to burn | 31 (7%) | 7 (3%) | 4 (5%) | .031* |
| Cold sensitivity* | 97 (15%) | |||
| Heat sensitivity* | 109 (17%) | |||
| Both* | 212 (34%) | |||
| Site | Texas | Washington | Massachusetts | .001* |
| Cold sensitivity | 26 (17%) | 30 (20%) | 34 (32%) | |
| Heat sensitivity | 51 (33%) | 34 (23%) | 23 (21%) | |
| Both | 76 (50%) | 86 (57%) | 50 (47%) | |
Patient and injury characteristics for patients who experience temperature sensitivity within 24 months of initial burn injury. Values are given as count (percent) for categorical variables and median (interquartile range) for continuous variables. Those missing data on temperature sensitivity are included as a separate group to determine the potential for bias in missing data. Testing for significant difference between those who experienced temperature sensitivity and those who did not was completed for categorical variables using chi-square testing and for continuous variables using Kruskal–Wallis testing.
*Percent of the total study population.
*Indicates significance of P < .05.
Temperature Sensitivity and VR-12 and SWL Scale
There were no differences in preinjury PCS and SWL between those who endorsed temperature sensitivity and those who did not. However, participants who endorsed temperature sensitivity had a lower preinjury MCS score (endorsed temperature sensitivity: median VR-12 MCS 56 [IQR 47–61], did not endorse temperature sensitivity: median VR-12 MCS 59 [IQR 50–63], P = .0037; Table 2). At all time points, participants who endorsed temperature sensitivity had statistically significant lower median VR-12 PCS, MCS, and SWL scores compared to those who did not endorse temperature sensitivity.
Table 2.
Median quality of life measures
| Temperature Sensitivity | P | ||
|---|---|---|---|
| Preinjury measures | Yes (n = 418) | No (n = 219) | |
| Satisfaction with life | 28 (20–33) | 29 (23–34) | .0922 |
| VR-12 mental health component score | 56 (47–61) | 59 (50–63) | .0037* |
| VR-12 physical health component score | 54 (45–56) | 55 (48–56) | .1287 |
| 6-month follow-up | Yes (n = 197) | No (n = 211) | |
| Satisfaction with life | 22 (14–30) | 28 (22–30) | .0001* |
| VR-12 mental health component score | 51 (38–60) | 59 (52–62) | .0001* |
| VR-12 physical health component score | 41 (32–49) | 53 (44–56) | .0001* |
| 12-month follow-up | Yes (n = 172) | No (n = 166) | |
| Satisfaction with life | 22 (15–29) | 29 (24–31) | .0001* |
| VR-12 mental health component score | 52 (42–60) | 59 (53–62) | .0001* |
| VR-12 physical health component score | 44 (34–53) | 54 (45–56) | .0001* |
| 24-month follow-up | Yes (n = 110) | No (n = 95) | |
| Satisfaction with life | 24 (17–30) | 30 (26–34) | .0001* |
| VR-12 mental health component score | 56 (40–60) | 59 (55–63) | .0001* |
| VR-12 physical health component score | 46 (38–54) | 55 (50–56) | .0001* |
Outcome scores including satisfaction with life, VR-12 mental health component score, and VR-12 physical health component score by each follow-up year and by temperature sensitivity category. Scores are presented as median (interquartile range) with Kruskal–Wallis testing used to determine significance. Temperature sensitivity and outcome scores were reassessed at each follow-up interval.
*Indicates significance of P < .05.
Random effects regression models including confounders of burn size, graft size, location of burn, intensity of pruritus, amputation, preinjury MCS score, and comorbid health conditions demonstrated significant and negative independent association between temperature sensitivity and SWL scores (coefficient −2.7 [95% CI −4.0, −1.4], P < .001) and VR-12 MCS (coefficient −2.8 [95% CI −4.6, −1.1], P = .002; Table 3). VR-12 PCS score was not significantly different between the two groups. When analyzing the impact of temperature sensitivity at each time interval, linear regression models demonstrated significant and negative independent association between temperature sensitivity and SWL (coefficient −3.2 [95% CI −5.2, −1.1], P = .003) and VR-12 MCS (coefficient −4.0 [95% CI −6.9, −1.2], P = .006) at 6-month follow-up, but not at 12 and 24 months after injury (Table 4).
Table 3.
Random effects regression modeling of temperature sensitivity with quality-of-life measures
| Outcome | Coefficient (95% CI) | P |
|---|---|---|
| Satisfaction with life | −2.7 (−4.0, −1.4) | <.001* |
| VR-12 mental health component score | −2.8 (−4.6, −1.1) | .002* |
| VR-12 physical health component score | 0.5 (−1.1, 2.1) | .533 |
The regression model considers the fluctuation of temperature sensitivity and outcome measures over time and accounts for patient and injury characteristics that were determined to be significantly different within our population (burn size, graft percentage, location of burn, intensity of pruritus, amputation, preinjury MCS), comorbid conditions that were determined to be significantly different within our population (depression and anxiety) along with the review of system components that may affect the quality of life (hearing loss, change in voice, vision problem, eyelid problem, excessive tearing of eyes, memory difficulty, difficulty with thought processing, numbness/pins/burning in burn scar, numb/pins/burn hands or feet, trouble with balance, joint pain). The model demonstrates that temperature sensitivity is independently associated with lower satisfaction with life and VR-12 mental health composite scores. A P value of <.017 indicates significance to account for multiple hypothesis testing.
*Indicates significance of P < .017.
Table 4.
Multivariable linear regression of temperature sensitivity with quality-of-life measures
| Coefficient (95% CI) | P | |
|---|---|---|
| 6-month follow-up | ||
| Satisfaction with life | −3.2 (−5.2, −1.1) | .003* |
| VR-12 mental health component score | −4.0 (−6.9, −1.2) | .006* |
| VR-12 physical health component score | −0.1 (−2.7, 2.4) | .911 |
| 12-month follow-up | ||
| Satisfaction with life | −2.1 (−4.1, −0.1) | .042 |
| VR-12 mental health component score | −1.5 (−4.4, 1.4) | .319 |
| VR-12 physical health component score | −1.8 (−4.6, 0.9) | .181 |
| 24-month follow-up | ||
| Satisfaction with life | −3.1 (−6.4, 0.3) | .073 |
| VR-12 mental health component score | −5.3 (−9.6, −0.9) | .018 |
| VR-12 physical health component score | −2.1 (−6.4, 2.2) | .328 |
Linear regression model comparing outcome measures from those with temperature sensitivity to those without. The regression model includes patient and injury characteristics that were determined to be significantly different within our population (burn size, graft percentage, location of burn, intensity of pruritus, amputation, preinjury MCS), comorbid conditions that were determined to be significantly different within our population (depression and anxiety) along with the review of system components that may affect the quality of life (hearing loss, change in voice, vision problem, eyelid problem, excessive tearing of eyes, memory difficulty, difficulty with thought processing, numbness/pins/burning in burn scar, numb/pins/burn hands or feet, trouble with balance, joint pain). A P value of <.017 indicates significance to account for multiple hypothesis testing.
*Indicates significance of P < .017.
Discussion
There are no estimates of temperature sensitivity among the general U.S. population to compare with our findings. The Cold and Health in Northern Sweden (CHINS) study reported that 4% of the randomly sampled population met the criteria for cold sensitivity, which is much lower than reported by our participants (65% experienced any temperature sensitivity and 49% experienced cold sensitivity).26 Most participants in our database endorsed this symptom at discharge; however, some participants developed the symptom at 6, 12, or 24 months after injury. This observation corroborates previous reports that scar and grafted skin have diminished thermoregulatory function even months and years after the injury.6,7 Importantly, the prevalence of temperature sensitivity decreased with time, affecting only 4% of participants after 24 months of follow-up, which is consistent with the baseline population prevalence of temperature sensitivity found by the CHINS study. This finding may allow providers to counsel burn patients who experience temperature sensitivity, and reassure them that with time, most patients return to their preinjury temperature experiences.
Several injury characteristics were significantly different between those who reported temperature sensitivity after their injury and those who did not. Larger burns were associated with temperature sensitivity, which corroborates findings that more severe burn injuries have a larger impact on thermal dysregulation due to a greater area with impaired dermal blood flow, impaired peripheral vasodilation capabilities, piloerection, and impaired sweating.10,11,27 Those with larger grafts were also more likely to experience temperature sensitivity, which may be a product of extreme thermal dysregulation of grafted skin or donor sites.6,7 Burn injuries that affected exposed locations (eg, head, face, neck, forearm, or hands) were also associated with temperature sensitivity, perhaps due to these locations being more susceptible to convection, sunlight, and contact exposures. These characteristics present potential risk factors or indicators of developing temperature sensitivity after burn injury and may help to predict which patients are at higher risk for experiencing this symptom. In addition to identifying those who may benefit from targeted patient education about temperature sensitivity, these characteristics can support vocational rehabilitation counseling among patients and their employers. In some cases, particularly those with manual labor jobs or those who require work outside or in extremes of temperature, vocational rehabilitation specialists, burn providers, and/or primary care providers may need to work with employers to ensure that patients receive sufficient adaptations that facilitate comfort and safety while returning to work (eg, work gloves, breaks to thermoregulate, sunshade, adequate access to hydration).
The presence and intensity of postburn pruritus at discharge were also associated with temperature sensitivity. Pruritus is modulated through pruriceptive signaling and nerve-related neuropathic pathways closely intertwined with neuropathic pain among people living with burn injury.28 The Transient Receptor Potential channels are one common link between temperature sensitivity and neuropathic pain, as these channels respond to both temperature and mechanical stimuli and promote painful sensations via nociceptive activation.29 Sensory alterations occur after burns secondary to injury to the peripheral nervous system associated with the dermis and epidermis and the pilosebaceous unit; the systems that sense pain, itch, and temperature are similarly affected.2,30,31 Therefore, it is not surprising that the presence of postburn pruritus was associated with intolerance to heat or cold temperatures. Extending this relationship, neuropathic pain and itch are often treated with medications that act either peripherally or centrally (eg, gabapentinoids, serotonin–norepinephrine reuptake inhibitors, centrally acting beta-agonists, ondansetron). In animal models, after induction of peripheral and central nerve injury, gabapentin effectively decreased thermal sensitivity, showing potential as a treatment for temperature sensitivity after burn injury.32,33 Additionally, cognitive behavioral therapy that focuses on cognitive restructuring, relaxation techniques, de-catastrophizing, sleep hygiene, anxiety management, and time- or quota-based activity pacing has been shown to reduce the symptoms of chronic and neuropathic pain.34,35 A shared mechanistic relationship between temperature sensitivity and neuropathic pain/itch may facilitate co-management of pain, pruritis, and temperature sensitivity.28,36,37
Our 6-month HRQOL data particularly suggest that temperature sensitivity may have a dramatic impact on patient-reported health outcomes in the short term. After accounting for injury characteristics and comorbidities known to affect SWL and VR-12 MCS and PCS scores, SWL and VR-12 MCS scores remained significantly lower among those who experienced temperature sensitivity compared to those who did not at the 6-month follow-up interval. There was no evidence that this relationship persisted at the 12-month and 24-month intervals. These findings suggest that although temperature sensitivity may be distressing early after injury, the symptom may decrease in intensity or participants learned to cope with it and adapt to their working environments and lifestyles over time.
This study has several limitations that should be considered while interpreting the results. Although we collected data at multiple time points, our data are not sufficiently granular to address the quality of sensations, intensity, frequency, and duration. For example, we cannot differentiate between temperature sensitivity and temperature intolerance, the former indicating a heightened awareness of temperature and the latter indicating distress from temperatures that would otherwise be felt as normal. Moving forward, it would be valuable to assess temperature sensitivity with the same granularity of detail that we use for pain and postburn pruritis, which may allow us to better fit adaptations. Although we used multilevel modeling that included burn center location, the database did not allow us to incorporate seasonal or geographic effects (eg, latitude, altitude, humidity) on participant temperature sensitivity. It is possible that weather and temperature at the time of follow-up influenced participants’ responses. Furthermore, participants living in more extremes of climates (eg, Alaska, Texas, New England) may experience greater temperature sensitivity for longer durations than those living in more temperate regions. Many participants did not respond to survey items about the occupation. As a result, we were not able to assess the associations between occupation types and settings and temperature sensitivity. We also excluded pediatric patients from this analysis, and so cannot extrapolate these findings for that age group. Finally, the retrospective nature of our study limits our ability to draw causal conclusions or include mechanistic measurements, such as specific biomarker measurements of stress, into our study.
Conclusions
People living with burn injuries experience a higher prevalence of temperature sensitivity than the general population, and these symptoms have an adverse effect on satisfaction with life and mental well-being during the first year after injury. However, most participants experienced an improvement in temperature sensitivity with time and the symptom became less of a factor contributing to HRQOL in the long term. These findings highlight the importance of detecting and managing temperature sensitivity while people transition to their home environments, return to work, and adjust to community living. Patients should be systematically screened for temperature sensitivity and future effort should be placed in developing educational tools and support for symptomatic patients and care providers (Box 2).
Box 2. Tips that can be provided to patients about how one can manage heat intolerance and cold sensitivity.
Burn Survivor 1: Tips for heat sensitivity
Wear light synthetic material and multiple layers to be more in control of your temperature.
Use cool packs and damp cloths to adjust your temperature quickly, if needed.
Work with a therapist to learn how to use relaxation, meditation, and visualization. As an example, I learned to visualize my body where I felt most comfortable and free—swimming in the perfect temperature water.
Focus on a pursuit to take your mind off all the challenges. For me, that pursuit is making art; it is a form of meditation and self-expression. It gives me a sense of control and calm. I can enter a flow state while creating art—that is empowering for recovery.
Burn Survivor 2: Tips for cold sensitivity
Wear a light synthetic material as a base layer to wick sweat away. The wind cuts right through it, so dress in layers, which also allows you to change your temperature when needed.
Apply moisturizing and topical cream barriers. These acted as an occlusive barrier against the wind chill and helped with the dryness and tightening of contractures and grafts.
Cover up sensitive and exposed areas, like the hands, neck, and face until you are comfortable to have them in the environment. I used a fleece balaclava and thick gloves.
Discuss your comfort and safety needs with your employer. You will need space and time to care for your wounds, apply moisturizer, change layers, and warm up. It takes time to be able to work as you once did.
Create goals for recovery. I trained for a marathon post-hospital, and I felt that regular exercise acclimated my body to the high body temperatures associated with physical activity.
Conflict of interest statement. No conflicts of interest are stated for the authors involved.
Funding: This study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) grants #90DPBU0004 and #90DPBU0001. J.O. received funding through an NIH-T32 grant for fellowship training (National Institute of Health (NIH) T32 GM1212190). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, HHS, and do not assume endorsement by the Federal Government.
References
- 1. Rowan MP, Cancio LC, Elster EAet al. Burn wound healing and treatment: review and advancements. Crit Care 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tirado-Esteban A, Seoane JL, Serracanta Domènech J, Aguilera-Sáez J, Barret JP. Sensory alteration patterns in burned patients. Burns 2020;46:1729–36. [DOI] [PubMed] [Google Scholar]
- 3. Spronk I, Legemate C, Oen I, van Loey N, Polinder S, van Baar M. Health related quality of life in adults after burn injuries: a systematic review. PLoS One 2018;13:e0197507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie B, Xiao SC, Zhu SH, Xia ZF. Evaluation of long term health-related quality of life in extensive burns: a 12-year experience in a burn center. Burns 2012;38:348–55. [DOI] [PubMed] [Google Scholar]
- 5. Carrougher GJ, Martinez EM, McMullen KSet al. Pruritus in adult burn survivors: postburn prevalence and risk factors associated with increased intensity. J Burn Care Res 2013;34:94–101. [DOI] [PubMed] [Google Scholar]
- 6. Davis SL, Shibasaki M, Low DAet al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res 2009;30:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis SL, Shibasaki M, Low DAet al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res 2007;28:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malenfant A, Forget R, Amsel R, Papillon J, Frigon JY, Choinière M. Tactile, thermal and pain sensibility in burned patients with and without chronic pain and paresthesia problems. Pain 1998;77:241–51. [DOI] [PubMed] [Google Scholar]
- 9. Chin TL, Carrougher GJ, Amtmann Det al. Trends 10 years after burn injury: a burn model system national database study. Burns 2018;44:1882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cramer MN, Moralez G, Huang MU, Kouda K, Poh PYS, Crandall CG. Exercise thermoregulation with a simulated burn injury: impact of air temperature. Med Sci Sports Exerc 2020;52:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganio MS, Schlader ZJ, Pearson Jet al. Nongrafted skin area best predicts exercise core temperature responses in burned humans. Med Sci Sports Exerc 2015;47:2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goverman J, Mathews K, Holavanahalli RKet al. The National Institute on Disability, Independent Living, and Rehabilitation Research burn model system: twenty years of contributions to clinical service and research. J Burn Care Res 2017;38:e240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BMS—guidelines for collection of follow-up data. Accessed 23 April 2019; http://burndata.washington.edu/sites/burndata/files/files/105BMS%20-%20Guidelines%20for%20Collection%20of%20Follow-up%20Data2015-12–21.pdf
- 14. Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ 2010;182:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Musich S, Wang SS, Hawkins K, Yeh CS. The impact of loneliness on quality of life and patient satisfaction among older, sicker adults. Gerontol Geriatr Med 2015;1:2333721415582119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding K, Slate M, Yang J. History of co-occurring disorders and current mental health status among homeless veterans. BMC Public Health 2018;18:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kristensen JK, Vestergaard DG, Swartling Cet al. Association of primary hyperhidrosis with depression and anxiety: a systematic review. Acta Derm Venereol 2020;100:adv00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oka T. Psychogenic fever: how psychological stress affects body temperature in the clinical population. Temperature (Austin) 2015;2:368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stockly OR, Wolfe AE, Carrougher GJet al. Inhalation injury is associated with long-term employment outcomes in the burn population: findings from a cross-sectional examination of the burn model system national database. PLoS One 2020;15:e0239556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheckter CC, Carrougher GJ, McMullen Ket al. Evaluation of patient-reported outcomes in burn survivors undergoing reconstructive surgery in the rehabilitative period. Plast Reconstr Surg 2020;146:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selim AJ, Rogers W, Fleishman JAet al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res 2009;18:43–52. [DOI] [PubMed] [Google Scholar]
- 22. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess 1985;49:71–5. [DOI] [PubMed] [Google Scholar]
- 23. Amtmann D, Bocell FD, Bamer Aet al. Psychometric properties of the satisfaction with life scale in people with traumatic brain, spinal cord, or burn injury: a National Institute on Disability, Independent Living, and Rehabilitation Research model system study. Assessment 2019;26:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amtmann D, Bocell FD, McMullen Ket al. Satisfaction with life over time in people with burn injury: a National Institute on Disability, Independent Living, and Rehabilitation Research burn model system study. Arch Phys Med Rehabil 2020;101:S63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goverman J, Mathews K, Nadler Det al. Satisfaction with life after burn: a burn model system national database study. Burns 2016;42:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stjernbrandt A, Carlsson D, Pettersson H, Liljelind I, Nilsson T, Wahlström J. Cold sensitivity and associated factors: a nested case-control study performed in Northern Sweden. Int Arch Occup Environ Health 2018;91:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilmore DW, Mason AD Jr, Johnson DW, Pruitt BA Jr. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol 1975;38:593–7. [DOI] [PubMed] [Google Scholar]
- 28. Steinhoff M, Schmelz M, Szabó IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol 2018;17:709–20. [DOI] [PubMed] [Google Scholar]
- 29. Marwaha L, Bansal Y, Singh R, Saroj P, Bhandari R, Kuhad A. TRP channels: potential drug target for neuropathic pain. Inflammopharmacology 2016;24:305–17. [DOI] [PubMed] [Google Scholar]
- 30. Palanivelu V, Maghami S, Wallace HJ, Wijeratne D, Wood FM, Fear MW. Loss of Type A neuronal cells in the dorsal root ganglion after a non-severe full-thickness burn injury in a rodent model. Burns 2018;44:1792–800. [DOI] [PubMed] [Google Scholar]
- 31. Hodge BD, Sanvictores T, Brodell RT. Anatomy, skin sweat glands. In: StatPearls. StatPearls Publishing; 2020; accessed 19 January 2021. http://www.ncbi.nlm.nih.gov/books/NBK482278/ [PubMed] [Google Scholar]
- 32. Yezierski RP, Green M, Murphy K, Vierck CJ. Effects of gabapentin on thermal sensitivity following spinal nerve ligation or spinal cord compression. Behav Pharmacol 2013;24:598–609. [DOI] [PubMed] [Google Scholar]
- 33. Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J Neurochem 2007;100:1289–99. [DOI] [PubMed] [Google Scholar]
- 34. Wiechman SA. Psychosocial recovery, pain, and itch after burn injuries. Phys Med Rehabil Clin N Am 2011;22:327–45, vii. [DOI] [PubMed] [Google Scholar]
- 35. Romanowski KS, Carson J, Pape Ket al. American burn association guidelines on the management of acute pain in the adult burn patient: a review of the literature, a compilation of expert opinion and next steps. J Burn Care Res 2020;41:1152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaul I, Amin A, Rosenberg M, Rosenberg L, Meyer WJ 3rd. Use of gabapentin and pregabalin for pruritus and neuropathic pain associated with major burn injury: a retrospective chart review. Burns 2018;44:414–22. [DOI] [PubMed] [Google Scholar]
- 37. Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol 2016;75:619–25.e6. [DOI] [PubMed] [Google Scholar]