Abstract
Background
Neighborhood-level socioeconomic status (SES) is associated with health outcomes, including cardiovascular disease and diabetes, but these associations are rarely studied across large, diverse populations.
Methods
We used Ward’s Hierarchical clustering to define eight neighborhood clusters across North Carolina using 11 census-based indicators of SES, race, housing, and urbanicity and assigned 6992 cardiac catheterization patients at Duke University Hospital from 2001 to 2010 to clusters. We examined associations between clusters and coronary artery disease index > 23 (CAD), history of myocardial infarction, hypertension, and diabetes using logistic regression adjusted for age, race, sex, body mass index, region of North Carolina, distance to Duke University Hospital, and smoking status.
Results
Four clusters were urban, three rural, and one suburban higher-middle-SES (referent). We observed greater odds of myocardial infarction in all six clusters with lower or middle-SES. Odds of CAD were elevated in the rural cluster that was low-SES and plurality Black (OR 1.16, 95% CI 0.94-1.43) and in the rural cluster that was majority American Indian (OR 1.31, 95% CI 0.91-1.90). Odds of diabetes and hypertension were elevated in two urban and one rural low- and lower-middle SES clusters with large Black populations.
Conclusions
We observed higher prevalence of cardiovascular disease and diabetes in neighborhoods that were predominantly rural, low-SES, and non-White, highlighting the importance of public health and healthcare system outreach into these communities to promote cardiometabolic health and prevent and manage hypertension, diabetes and coronary artery disease.
Cardiovascular disease (CVD) often presents as coronary artery disease (CAD) and myocardial infarction (MI) and is the most common cause of death in the United States, accounting for 30.5% of all deaths 1. Similarly, important risk factors for CVD are increasingly common: an estimated 46% of Americans suffer from hypertension, and approximately 13.5% suffer from diabetes.1 , 2 Risks for CVD are disproportionately distributed between individuals and neighborhoods. Socioeconomic status (SES) is associated with CVD and diabetes, on both the individual3-5 and neighborhood6-12 levels. Additionally, demographic factors, including race, are associated with cardiometabolic outcomes, CVD and diabetes. Black Americans have a greater prevalence of hypertension and diabetes compared to White Americans. 1 , 2 American Indians suffer from higher prevalence of coronary heart disease and diabetes compared to White Americans.1 , 13 Although CVD incidence is declining in other populations, it may be increasing among some American Indian populations.13 However, epidemiologic literature rarely includes large numbers of American Indian and Black participants.
We hypothesized that a combination of SES factors (education, income, public assistance, unemployment, single-parent status, and occupation), combined with rurality/urbanicity, housing factors (owner-occupied housing and newer housing stock), and race may contribute to cardiometabolic outcomes. Our previous research categorized a three-county urban area of North Carolina (NC) into neighborhood clusters, based on distributions of SES characteristics, housing factors, and race14 , 15 and observed differences in prevalence of hypertension and diabetes by neighborhood cluster among cardiac catheterization patients.14 Since the previously studied three-county area was relatively urban, well-educated, and wealthy, the current study expanded our methods to the entire state of North Carolina, including urban, rural, and racially diverse areas. Our study population consisted of a diverse sample of cardiac catheterization patients. We examined associations between neighborhood cluster and the prevalence of significant hypertension, diabetes, CAD, and myocardial infarction. We hypothesized that neighborhood clusters that had low SES, large Black and American Indian populations, and rural areas would have elevated prevalence of cardiometabolic outcomes (CAD, MI, hypertension, and diabetes). This research is important to help identify communities that may be at higher risk of cardiometabolic outcomes and thus may benefit from public health and healthcare system outreach.
Materials and methods
Study population
We examined cardiometabolic outcomes among Catheterization Genetics (CATHGEN) participants: patients who underwent cardiac catheterization at Duke University Hospital between 2001 and 2010. All participants were medically stable and provided informed, written consent. This study was approved by the Duke University Institutional Review Board. Clinical, anthropometric, and demographic data were gathered from medical records and physician assessment as previously described.16 This study was funded by National Institutes of Health grants HL73042, HL36587, and HL095987; and Neurosciences Education and Research Foundation (Encinitas, CA). We included 6992 participants whose addresses were successfully geocoded and who resided in a neighborhood cluster (described below). If a participant received more than one cardiac catheterization, we used data from the first.
Exposure—neighborhood clusters
To define neighborhood clusters, we expanded methods used in our previous work to the entire state of North Carolina.14 , 15 We used data from the 2000 US Census (wwwcensus.gov) at the block group (BG) level. We used the following eleven socioeconomic and demographic variables (all are percent) to define residential clusters: Bachelor’s degree or more, income below the poverty level, households receiving public assistance income, unemployment, non-managerial occupation, single-parent housing, owner-occupied housing, house built within 5 years, Black householder, householder of other race (neither Black nor White), and urban environment.14 , 15 We defined urban environment as the percent of block group population that resided in an urban area (densely developed territory that contains ≥50,000 people) or urban cluster (densely developed territory that contains ≥2,500 people but <50,000 people).17 We excluded BGs with zero population or with more than 20% of the population residing in institutional (correctional facilities, nursing homes) or group (military housing, college dormitories) facilities, as demographics may not be representative of the general population. We used Ward’s hierarchical clustering (stats package, R version 3.3.0) method to establish eight residential clusters. Ward’s Hierarchical clustering method uses minimum within-cluster variance criteria to cluster BGs together based on the distribution of the eleven Census variables.14 , 18 , 19 We did not have any a priori specifications as to cluster characteristics, such as how many clusters were urban or rural. These clusters were sufficiently large and qualitatively distinct from each other. We performed multivariable analysis of variance (MANOVA) tests at each branch to ensure clusters were statistically distinct; all included clusters had P-values <.05.20 We mapped neighborhood clusters at the BG level using ArcGIS version 10.3 (ESRI). These neighborhood clusters are not necessarily spatially contiguous; widely separated metropolitan areas may be divided into similar collections of BGs with similar sociodemographic characteristics.
Outcomes
Outcomes of interest were: hypertension, diabetes, CAD, and MI at the time of catheterzation. Hypertension and diabetes status were based on medical records and physician assessment at the time of catheterization. Participants were classified as having CAD if they had a CAD index >23, determined from coronary angiography, which indicates at least 75% occlusion of at least one major epicardial artery and is commonly considered pathologic.21 , 22 Nearly all (6336 participants) had complete visualization of all coronary arteries and therefore had classification of CAD index. History of MI was defined as either medical record identification of MI or thrombolytic therapy, or referral for cardiac catheterization based on MI. Individual-level demographic and anthropometric information included sex, race, age, smoking status, and body mass index (BMI).
Statistical methods
We used logistic regression to examine the association between neighborhood cluster of residence and hypertension, diabetes, CAD, and MI; with each outcome modeled separately. Models were adjusted for a participant’s age, sex, race (White, Black, other), BMI, smoking status (yes or no, self-reported), region of NC, and distance between their residence and Duke University Hospital. We used the definition of region of NC (eight total, as shown on Figure 1) developed by the North Carolina Department of Public Instruction (https://dpi.nc.gov, accessed September 17, 2021). We compared the odds of cardiovascular outcomes by clusters, using the higher-middle-SES, suburban cluster (cluster 6) as the referent group. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
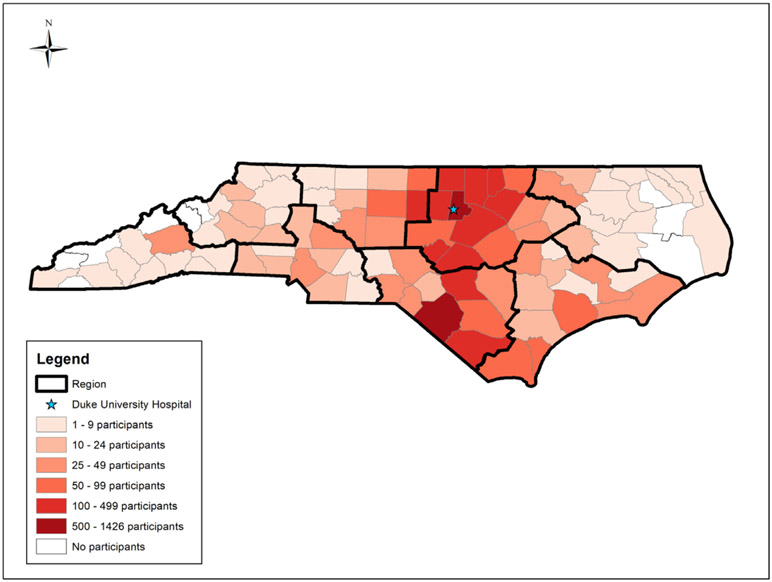
Figure 1.
Distribution of CATHGEN participants by county in NC and location of Duke University Hospital. Regions of NC are outlined in bold.
Sensitivity analysis
Exposure to air pollution may affect cardiovascular outcomes and may also be related to area of residence. Thus, we further adjusted models for annual average PM2.5 concentration. PM2.5 concentrations were estimated using a hybrid model; a neural network estimated PM2.5 concentrations at 1 × 1 km resolution and incorporated data from PM2.5 monitors, satellite data, meteorological data, and land use data.23 In order to determine whether any sociodemographic factor disproportionately influenced our results, we repeated the main analyses, using each of the 11 sociodemographic characteristics included in the clustering algorithm as alternative exposure metrics for all outcomes. Because race was a strong factor in determining clusters, we also conducted a race-stratified analysis among Black and White participants (there were too few participants of other races to generate stable models).
Results
Neighborhood clusters
North Carolina had a total of 5271 BGs; we excluded 164 BGs with no residents (n = 10) or with more than 20% of the population residing in institutional (correctional facilities, n = 59; nursing homes, n = 15; other, n = 6) or group housing facilities (college dormitories, n = 65; military barracks, n = 6; other, n = 8). Exclusion criteria were not mutually exclusive. We categorized the remaining 5107 BGs into eight distinct neighborhood clusters: four urban, one suburban, and three rural (relative to other clusters). Cluster numbers were assigned automatically during the clustering procedure and do not reflect any particular ranking or characteristic. Figure 2 shows the distribution of clusters across the state; Figure 3 shows proportions of each census variable across clusters, with the proportion of each census variable across North Carolina for reference. Figure S1 shows a circular dendrogram of block groups in NC, indicating which block groups clustered together.
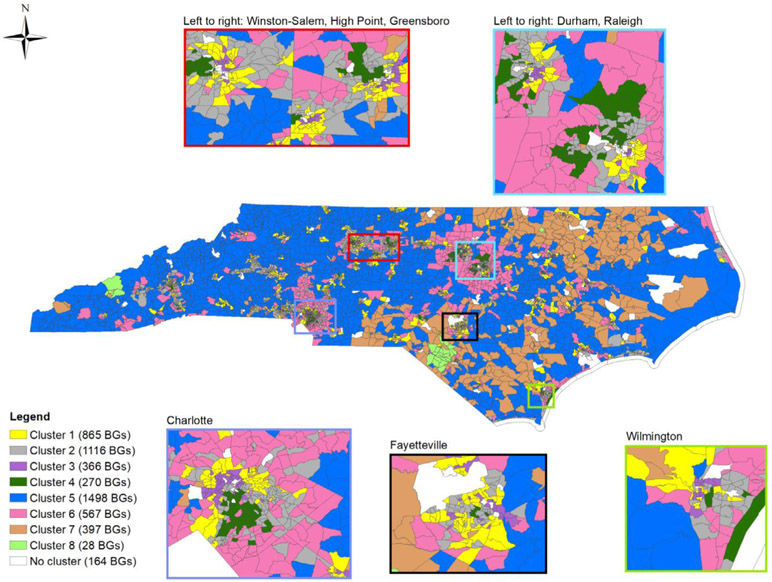
Figure 2.
Map showing eight neighborhood clusters* distributed across 5107 North Carolina block groups. Insets show cities with >75,000 population (2000). *Cluster descriptions are as follows: cluster 1 (lower-middle SES, urban, large Black population), cluster 2 (higher-middle SES, urban), cluster 3 (lower SES, urban, large Black population), cluster 4 (higher SES, urban, small Black population), cluster 5 (middle SES, rural, small Black population), cluster 6 (higher-middle SES, suburban, small Black population), cluster 7 (lower SES, rural, large Black population and population of other races), cluster 8 (lower SES, rural, large American Indian population).
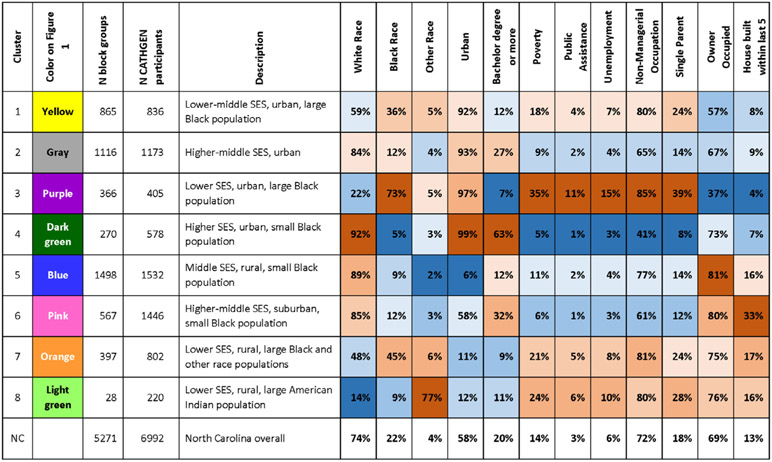
Figure 3.
Descriptions of the eight neighborhood clusters and mean % of 11 demographic variables across the neighborhood clusters, with total North Carolina distribution for comparison. Blue cells have lower values and red cells have higher values.
Clusters 1, 2, 3, and 4 were more than 90% urban (Figure 3) and include some suburban areas. Relative to other clusters, cluster 1 (n = 865 BGs, yellow in Figure 2) had lower-middle SES, a large Black population, and relatively low proportion owner-occupied housing and housing built within 5 years; cluster 2 (n = 1116 BGs, gray in Figure 2) was upper-middle SES with relatively low proportion owner-occupied housing and housing built within 5 years; cluster 3 (n = 366 BGs, purple in Figure 2), had lower SES, the greatest proportion Black population, and the lowest proportion of owner-occupied housing and housing built within 5 years; cluster 4 (n = 270 BGs, dark green in Figure 2), had higher SES, was the most urban and had the highest proportion owner occupied housing among urban clusters, but had the least proportion Black population and relatively low housing built within 5 years (Figure 3).
Relative to other clusters, cluster 6 (n = 567 BGs, pink in Figure 2) was higher-middle-SES mixed urban and rural (57% urban), was primarily located adjacent to cities, and had the highest proportion of housing built within 5 years (Figure 3). We refer to cluster 6 as suburban.
Relative to other clusters, clusters 5, 7, and 8 were rural (less than 15% urban). Cluster 5 (n = 1498 BGs, blue in Figure 2) was located throughout the state but was most prominent in western and central NC. Cluster 5 is the middle-SES rural cluster, with the greatest proportion of owner-occupied housing, the least proportion of other races, and least urban (Figure 3) relative to other clusters. Clusters 7 (n = 397 BGs, orange in Figure 2) and 8 (n = 28 BGs, light green in Figure 2), were lower-SES rural clusters with relatively high owner-occupied housing and housing built within 5 years. Cluster 7 was primarily located in eastern and central NC and had a relatively high Black population and population of other races. Cluster 8 had the greatest population of other races (77%); cluster 8 was located in areas with substantial American Indian populations (74% of householders in cluster 8 in the 2000 census self-identified as American Indian) (Figures 2 and 3).
Participants
We included 6992 participants; 124 participants lived in BGs that were excluded from the clustering analysis. Participants were most concentrated in central North Carolina counties (Figure 1). Among participants; 836 (12.0%) resided in cluster 1 (urban, lower-middle-SES); 1173 (16.8%) in cluster 2 (urban, higher-middle SES); 405 (5.8%) in cluster 3 (urban, lower SES); 578 (8.3%) in cluster 4 (urban, higher SES); 1532 (21.9%) in cluster 5 (rural, middle SES); 1446 in cluster 6 (suburban, higher-middle SES) (20.7%); 802 (11.5%) in cluster 7 (rural, lower SES, large Black population); and 220 (3.1%) in cluster 8 (rural, lower SES, large American Indian population) (Table 1). As expected, cluster 3 had the greatest proportion of Black participants (56.8%) and cluster 8 had the greatest proportion of American Indian participants (57.7%). Cluster 3 had the greatest proportion female (50.9%), greatest mean BMI (31.3 kg/m2), and greatest proportion diabetes (37.5%). Participants in cluster 4 were oldest (63.6 years) and had the least proportion female (33.7%), smokers (39.6%), mean BMI (28.7 kg/m2), and diabetes (20.2%). Participants in rural clusters lived furthest from Duke University Hospital, with a mean distance of >100 km in all rural clusters; participants in urban, higher SES cluster 4 lived nearest, with an average distance of 26 km.
Table.
Descriptive characteristics of North Carolina CATHGEN participants, by neighborhood cluster and overall (N = 6992)
| Cluster 1 (n = 836) |
Cluster 2 (n = 1173) |
Cluster 3 (n = 405) |
Cluster 4 (n = 578) |
Cluster 5 (n = 1532) |
Cluster 6 (n = 1446) |
Cluster 7 (n = 802) |
Cluster 8 (n = 220) |
Total (N = 6992) |
|
|---|---|---|---|---|---|---|---|---|---|
| n (%) or mean (SD) | |||||||||
| Outcomes | |||||||||
| CAD (n = 6336)* | 365 (59%) | 511 (50%) | 171 (46%) | 263 (50%) | 707 (51%) | 641 (49%) | 406 (54%) | 119 (59%) | 3183 (50%) |
| History of MI | 220 (26%) | 306 (26%) | 103 (25%) | 142 (25%) | 459 (30%) | 333 (23%) | 286 (36%) | 106 (48%) | 1955 (28%) |
| Diabetes | 278 (33%) | 316 (27%) | 152 (38%) | 117 (20%) | 422 (28%) | 388 (27%) | 268 (33%) | 70 (32%) | 2011 (29%) |
| Hypertension | 594 (71%) | 768 (65%) | 315 (78%) | 380 (66%) | 989 (65%) | 951 (66%) | 587 (73%) | 161 (73%) | 4745 (68%) |
| Covariates | |||||||||
| Female | 356 (43%) | 422 (36%) | 206 (51%) | 196 (34%) | 576 (38%) | 535 (37%) | 317 (40%) | 85 (39%) | 2693 (39%) |
| Race | |||||||||
| White | 493 (59%) | 942 (80%) | 152 (38%) | 492 (85%) | 1320 (86%) | 1200 (83%) | 508 (63%) | 61 (28%) | 5168 (74%) |
| Black | 320 (38%) | 197 (17%) | 230 (57%) | 63 (11%) | 57 (10%) | 200 (14%) | 203 (25%) | 21 (10%) | 1391 (20%) |
| American Indian | 7 (1%) | 2 (0.2%) | 15 (4%) | 1 (0.2%) | 15 (1%) | 7 (0.5%) | 71 (9%) | 127 (58%) | 245 (4%) |
| Other | 16 (2%) | 32 (3%) | 8 (2%) | 22 (4%) | 40 (3%) | 39 (3%) | 20 (3%) | 11 (5%) | 188 (3%) |
| History of smoking | 390 (47%) | 547 (47%) | 206 (51%) | 229 (40%) | 771 (50%) | 656 (45%) | 459 (57%) | 141 (64%) | 3399 (49%) |
| Age (years) | 60 (12) | 62 (12) | 59 (12) | 64 (12) | 60 (12) | 61 (12) | 59 (12) | 59 (13) | 61 (12) |
| BMI (kg/m2) | 31 (8) | 30 (7) | 31 (8) | 29 (6) | 30 (7) | 30 (7) | 31 (8) | 30 (7) | 30 (7) |
| Distance to Duke University Hospital (km) | 65 (59) | 73 (82) | 87 (70) | 26 (43) | 105 (75) | 54 (12) | 115 (51) | 147 (10) | 79 (72) |
CAD was assessed in 6336 participants; the remaining 656 participants had incomplete visualization of coronary arteries.Abbreviations: BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction.
Regression
We observed greater odds of cardiometabolic outcomes in clusters with lower to lower-middle SES and large non-White populations, compared to reference cluster 6 (suburban, higher-middle-SES, small non-White population) (Figure 4, Table S1). Among urban clusters, residents of cluster 1 (urban, relatively lower-middle-SES) had greater odds of MI (OR 1.25, 95% CI 1.02, 1.54) and diabetes (OR 1.14, 95% CI 0.94, 1.39). Residents of higher-middle SES cluster 2 had greater odds of MI (OR 1.18, 95% CI 0.98, 1.42). Residents of urban lower-SES areas (cluster 3) had greater odds of hypertension (OR 1.39, 95% CI 1.05, 1.84), diabetes (OR 1.19, 95% CI 0.92, 1.54) and MI (OR 1.19, 95% CI 0.90, 1.57). Residents of cluster 4 (urban higher-SES) had lower odds (0.74, 95% CI 0.58, 0.94) of diabetes.
Figure 4.
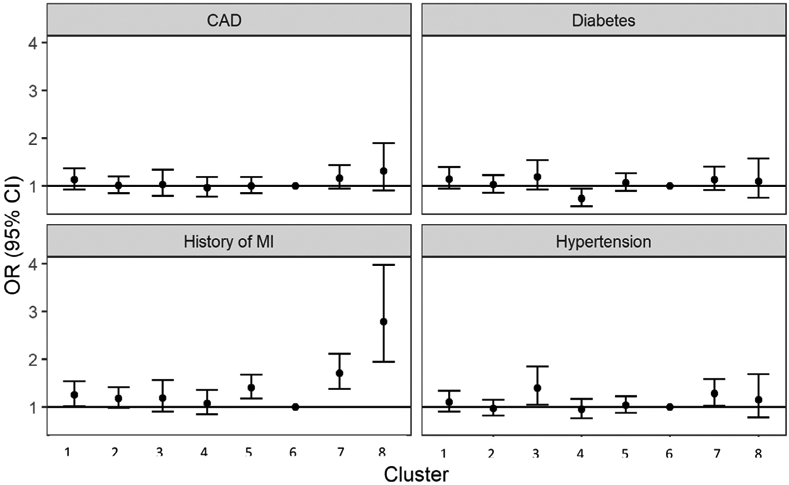
Results from logistic regression of residential neighborhood cluster on cardiometabolic outcomes.* *Adjusted for age, sex, race (White, Black, other), body mass index (BMI), smoking status, region of North Carolina, and distance to Duke University Hospital. Abbreviations: CAD, coronary artery disease; MI, myocardial infarction.
Among rural clusters, those who resided in rural, middle-SES cluster 5 had greater odds of MI (OR 1.40, 95% CI 1.18, 1.68) compared to suburban cluster 6. Residents of rural, lower-SES areas with a large Black population (cluster 7) had greater odds of all outcomes: hypertension (OR 1.28, 95% CI 1.03, 1.58), diabetes (OR 1.13, 95% CI 0.92, 1.40), CAD index >23 (OR 1.16, 95% CI 0.94, 1.43), and MI (OR 1.71, 95% CI 1.38, 2.11). Residents of rural, lower-SES areas with a large population who were neither Black nor White (cluster 8) had greater odds of CAD (1.31, 95% CI 0.91, 1.90) and MI (OR 2.78, 95% CI 1.94, 3.97).
Sensitivity analyses
Results examining hypertension, diabetes, or CAD did not substantially change after adjustment for PM2.5 (Table S1). However, associations between MI and urban clusters 1, 2, 3, and 4 were somewhat attenuated.
When examining associations between cluster indicators and outcomes, residence in BGs with greater poverty, public assistance, non-managerial occupation, and single-parent housing was associated with increased odds of all outcomes; and unemployment was associated with increased odds of all outcomes other than diabetes (Table S2). Residence in BGs with a greater percentage of Black residents was associated with greater odds of diabetes (OR 1.02, 95% CI 1.00, 1.05 per 10% increase) and hypertension (OR 1.04, 95% CI 1.01, 1.07 per 10% increase). Residence in BGs with a greater percentage of residents who were neither Black nor White was associated with greater odds of history of MI (OR 1.09, 95% CI 1.05, 1.14 per 10% increase), CAD (OR 1.03, 95% CI 0.99, 1.08 per 10% increase), and diabetes (OR 1.03, 95% CI 0.98, 1.08 per 10% increase). Residence in BGs with a greater percentage of residents with a Bachelor’s degree or more was associated with lower odds of all outcomes. Owner-occupied housing and urbanicity were not associated with outcomes.
In race-stratified regression models, associations between neighborhood cluster and CAD and hypertension were largely similar among White and Black participants (Table S3). Associations between neighborhood cluster and CAD, MI, and diabetes were generally more positive among White participants compared to Black participants, except in clusters 4 (high-SES, urban, low Black population) and 8 (lower-SES rural, large American Indian population), both of which had very few Black participants.
Discussion
We classified block groups across North Carolina into eight neighborhood clusters based on distribution of socioeconomic indicators, housing, urbanicity, and race at the time of the 2000 Census. We observed differences in odds of hypertension, diabetes, CAD, and MI, among cardiac catheterization patients residing in the different neighborhood clusters. Most notable were urban/rural disparities in greater odds of CAD and MI among rural clusters with relatively large Black (cluster 7) and American Indian (cluster 8) populations. Results were largely similar when stratified by individual-level race and after adjustment for indicators of air pollution exposure. Indicators of SES, housing, urbanicity, and race were generally not as strongly associated with outcomes as clusters were, indicating that the combination of these factors better predicts outcomes than each characteristic separately.
The lower-SES, predominately Black, urban cluster 3 had greater odds of hypertension, diabetes, and MI. The results for MI and hypertension were not substantially different by race within the clusters. These results align with previous studies showing associations between hypertension, diabetes, and CVD with neighborhood-level SES6-12. We observed similar associations between neighborhood SES and hypertension and diabetes in a previous CATHGEN study limited to a relatively small urban area14. Interestingly, we also observed lower odds of diabetes in the urban, predominately White, higher-SES cluster 4, indicating a continuum of neighborhood SES effects of diabetes. This result was consistent for White and Black participants, although there were few Black participants who lived in cluster 4.
We observed greater odds of MI in all clusters except urban, higher-SES cluster 4. The three rural clusters (clusters 5, 7, and 8) had the greatest odds. We observed higher odds of all outcomes among the rural, lower-SES cluster with a large Black population (cluster 7). Black Americans have previously been described as having high prevalence of hypertension and diabetes1 , 2, but this is rarely studied in rural settings. Rural/urban health disparities are evident in this study, with participants in all rural clusters having higher odds of MI than residents in the more urban cluster 6, and the rural lower-SES, predominately Black cluster 7 having higher odds of all outcomes compared to suburban, higher-middle-SES cluster 6. Notably, cluster 7 has similar SES and demographic indicators to cluster 1, with the exception of housing factors (which were not associated with most outcomes) and urbanicity, yet cluster 7 has much higher odds ratios for MI and hypertension.
Particularly striking is that the rural, low-SES neighborhood with a large (74%) American Indian population (cluster 8), had elevated odds of CAD and nearly 3 times the odds of MI compared to a suburban, higher-middle-SES cluster. Point estimates for associations between neighborhood cluster and hypertension in cluster 8 were similar to those in cluster 7; few participants (n = 220) in cluster 8 likely contributed to large confidence intervals. This cluster is unique in regard to the relatively large number of American Indian participants (n = 127), a group which is rarely included in hospital-based studies. Nearly all participants who resided in cluster 8 resided in or near Robeson County in southeastern NC. Results from this cluster may not be generalizable to other areas with large American Indian populations, including western NC. We are not aware of literature demonstrating greater prevalence of MI among those residing in areas with large American Indian populations. However, these results do align with prior studies suggesting higher prevalence of coronary heart disease among American Indian populations.1 , 13 This highlights the need to study urban/rural disparities in health, especially among non-White communities.10
In sensitivity analyses, most socioeconomic indicators were associated with outcomes in the expected direction, although associations were generally not as strong as those examining neighborhood clusters. The percentage of other races at the BG level was associated with MI. This corresponds to our finding of cluster 8 (with a large American Indian population) having the strongest observed association for MI. The percentage of Black race at the BG level was associated with hypertension and diabetes; this finding is consistent with prior data showing high prevalence of hypertension and diabetes among Black individuals.1 , 2 However, we did not observe substantial differences among Black and White participants in race-stratified analysis, indicating that racial distribution in a BG may be more important than an individual’s race in this study.
Limitations
This study has several limitations. It was conducted at a single site, Duke University Hospital, but participants were distributed across North Carolina. There is the possibility of selection bias by location and thus, by neighborhood cluster. To account for this, we adjusted for distance to Duke University Hospital and region of the state. Additionally, we do not have access to individual-level socioeconomic information or other important covariates—such as diet or physical activity—that may differ by cluster. Although our study had more American Indian participants than many hospital-based studies, this was still a fairly small sample and may not be representative. Data used in this study were collected from 2001 to 2010; these may not be reflective of current conditions. Our definitions of high or low distribution of Census characteristics is relative to clusters in this study and may not be generalizable to other areas with different distributions of underlying characteristics.
There are several different ways to define urban and rural. We used residence in a Census Bureau-defined urban area (densely developed territory that contains ≥50,000 people) or urban cluster (densely developed territory that contains ≥2,500 people but <50,000 people)17 to define urban. Those who did not live in an urban area or urban cluster were considered to live in a rural area. Neighborhood clusters generally were either very urban (>90% urban) or very non-urban (<15% urban). One cluster fell in between (57% urban) and was considered suburban. This relatively crude classification of urban or non-urban may not adequately distinguish between types of urban/suburban areas and rural/remote areas. This method of classification may not be adequate for larger cities and remote areas, more extreme than those found in NC.
In conclusion, we observed notable differences in the prevalence of hypertension, diabetes, severe CAD, and MI by neighborhood cluster among cardiac catheterization patients. We observed greater odds of hypertension, diabetes, and MI in an urban, relatively lower-SES, majority Black cluster compared to a suburban, relatively higher-middle-SES, majority White cluster. We also observed lower odds of diabetes in an urban, relatively higher-SES, majority White cluster. Residents of rural, relatively lower-SES areas with a large Black population had greater odds of all outcomes as compared to residents in suburban, relatively higher-middle-SES areas in NC. The most striking disparity was that participants living in a rural, relatively lower-SES, majority American Indian cluster had a 30% higher odds of significant CAD and nearly 3-fold greater odds of a previous MI (compared to study participants living in the suburban, higher-middle SES cluster). Areas characterized as rural or with high percentages of Black and American Indian populations are underrepresented in epidemiology studies. There is a need for future studies examining SES and cardiovascular outcomes to include diverse geographies and sociodemographic areas as well as non-White, populations to more effectively communities that will benefit from public health and healthcare system outreach to promote cardiometabolic health and prevent and manage hypertension, diabetes and coronary artery disease.
Supplementary Material
Acknowledgments
The authors acknowledge our funding sources, National Institutes of Health grants HL73042, HL36587, and HL095987; and Neurosciences Education and Research Foundation (Encinitas, CA). The authors also thank the participants and study staff of the CATHGEN study.
Footnotes
Disclaimer
This work does not necessarily represent the views or policy of the US EPA. Any mention of trade names does not constitute endorsement.
Disclosure
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2021.09.013.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Yang TP, Shavelle RM, et al. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol 2002;39:408–12. [DOI] [PubMed] [Google Scholar]
- 4.Min YI, Anugu P, Butler KR, et al. Cardiovascular disease burden and socioeconomic correlates: findings from the jackson heart study. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvin E, Parrinello CM, Sacks DB, Coresh J, Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010 trends in prevalence and control of diabetes in the United States. Ann Intern Med 2014;160:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark CR, Ommerborn MJ, Hickson DA, et al. Neighborhood disadvantage, neighborhood safety and cardiometabolic risk factors in African Americans: biosocial associations in the Jackson Heart study. PloS one 2013;8:e63254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey JA, Pollak J, Glymour MM, et al. Measures of SES for electronic health record-based research. Am J Prev Med 2018;54:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubowitz T, Ghosh-Dastidar M, Eibner C, et al. The women’s health initiative: the food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring) 2012;20:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein M, Diez Roux AV, Mujahid MS, et al. Unequal exposure or unequal vulnerability? Contributions of neighborhood conditions and cardiovascular risk factors to socioeconomic inequality in incident cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2018;187:1424–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137:2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Diez-Roux AV, Nieto FJ, Muntaner C. et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol 1997;146:48–63. [DOI] [PubMed] [Google Scholar]
- 13.Howard BV, Lee ET, et al. Rising tide of cardiovascular disease in American Indians. The strong heart study. Circulation 1999;99:2389–95. [DOI] [PubMed] [Google Scholar]
- 14.Mirowsky JE. Devlin RB. Diaz-Sanchez D, et al. A novel approach for measuring residential socioeconomic factors associated with cardiovascular and metabolic health. J Expo Sci Environ Epidemiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver AM, McGuinn L, Neas L. et al. Neighborhood sociodemographic effects on the associations between long-term PM2. 5 exposure and cardiovascular outcomes and diabetes mellitus. Environ Epidemiol 2019;3:e038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus WE, Granger CB, Sketch MH Jr. et al. A guide for a cardiovascular genomics biorepository: the CATHGEN experience. J Cardiovasc Transl Res 2015;8:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Census Bureau. 2010 Census Summary File 1 Technical Documentation. 2011. [Google Scholar]
- 18.Friedman HP, Rubin J. On some invariant criteria for grouping data. J Am Stat Assoc 1967;62:1159–78. [Google Scholar]
- 19.Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 1963;58:236–44. [Google Scholar]
- 20.Park PJ. Manjourides J, Bonetti M, Pagano M. A permutation test for determining significance of clusters with applications to spatial and gene expression data. Comput Stat Data Anal 2009;53:4290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bart BA, Shaw LK, McCants CB Jr, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997;30:1002–8. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002;39:210–18. [DOI] [PubMed] [Google Scholar]
- 23.Di Q, Kloog I, Koutrakis P, et al. Assessing PM2.5 exposures with high spatiotemporal resolution across the Continental United States. Environ Sci Technol 2016;50:4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.