Purpose of review
Chronic kidney disease-associated-pruritus (CKD-aP) is a common symptom in patients with end-stage kidney disease (ESKD) undergoing dialysis. CKD-aP typically occurs alongside other debilitating symptoms and may comprise so-called ‘symptom clusters’ which have synergistic effects that adversely impact patient health-related quality of life (HRQoL). Importantly, symptoms in a cluster may share a common biological mechanism. Here we review the clinical impact of CKD-aP and its association with other symptoms reported by dialysis patients. The clinical benefits of treating pruritus and its potential impact on other symptoms are also addressed.
Recent findings
Studies have shown CKD-aP significantly impairs HRQoL in patients with ESKD undergoing dialysis and is associated with adverse clinical outcomes, including increased risk of infections, hospitalizations, and mortality. Despite these negative effects, CKD-aP remains underrecognized and undertreated in clinical practice. CKD-aP is frequently associated with other symptoms, including disturbed sleep/poor sleep quality, anxiety, depression, and pain. Clinical studies of antipruritic therapies show that reduction of itch intensity may also alleviate other associated symptoms, such as poor sleep quality.
Summary
CKD-aP and its associated symptoms are inadequately managed in clinical practice. Greater understanding and awareness of CKD-aP and its surrounding symptom clusters in dialysis patients may improve their overall symptom management and HRQoL.
Keywords: chronic kidney disease, dialysis, pruritus, symptom cluster, unpleasant symptom
INTRODUCTION
People suffering from end-stage kidney disease (ESKD), also known as stage 5 chronic kidney disease (CKD), experience a high burden of symptoms, which markedly impair their physical and mental health-related quality of life (HRQoL) as well as their ability to perform everyday activities [1,2]. People with ESKD who require hemodialysis for renal replacement therapy value the reduction of their symptom burden over prolonged survival [3,4], illustrating the devastating impact these symptoms have on their lives. Hence, the proactive identification and alleviation of symptoms are critical to the holistic management of these individuals. CKD-associated pruritus (CKD-aP; previously known as uremic pruritus) is a frequent and burdensome symptom reported by patients with CKD, and often worsens as CKD progresses to ESKD requiring renal replacement therapy [5,6▪].
Symptom burden among people with ESKD on hemodialysis is high and symptom presentation is often complex. CKD-aP rarely occurs in isolation and is often a cause of distress to patients undergoing dialysis. In most cases, CKD-aP occurs in a constellation with other debilitating symptoms that have synergistic effects on one another and amplify patients’ suffering. When two or more symptoms co-occur and are related, they are known as a symptom cluster [7,8]. Because individual symptoms are related, they may share a common biological mechanism that can be targeted for intervention [9]. Thus, targeting one symptom in the cluster for intervention may have an effect on other symptoms in a given symptom cluster. However, symptom cluster science across the spectrum of CKD is in its infancy and more research is needed to untangle the complex relationships between symptoms and patient outcomes. Identification of symptom clusters among patients with ESKD undergoing dialysis may help inform symptom assessment, risk-stratification, and development of patient-centered interventions to reduce symptom burden, thereby reducing the negative impact on patient HRQoL [10▪].
Here, we review the clinical impact of CKD-aP on co-occurring symptoms and important patient outcomes, including HRQoL, risk for infections, hospitalizations and mortality, in patients with ESKD undergoing dialysis. The potential clinical benefits of alleviating CKD-aP and the potential effects on other symptoms will also be discussed.
Box 1.
no caption available
CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS: EPIDEMIOLOGY, PATHOPHYSIOLOGY, AND CLINICAL PRESENTATION
Although the prevalence estimates of CKD-aP in dialysis patients vary between epidemiological studies, patient-reported data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) indicate that approximately two-thirds of hemodialysis patients have CKD-aP, 37% of which experience moderate-to-severe symptoms [6▪]. Despite its high prevalence, CKD-aP is overlooked by healthcare professionals and under-reported by patients in clinical practice; 17% of patients always or nearly always bothered by itch do not report their symptoms to anyone [5,11].
The complex pathophysiology of chronic itching is not yet fully understood; however, it is likely to be a result of the release of pruritogens (e.g., histamine, prostaglandins, cytokines, neuropeptides, and proteases) by keratinocytes, immune cells, or neighboring neurons in the skin [12▪]. Chronic pruritus can be defined as an unpleasant sensation to skin leading to the desire to scratch, which persists for more than 6 months [13]. CKD-aP is a systemic condition characterized by pruritus related to CKD, which is unexplained by any other etiology [12▪]. Identification and diagnosis of CKD-aP are complicated by its variable clinical presentation. There is significant intra- and inter-patient variability with CKD-aP with respect to the severity and spatial distribution of pruritus over time. Pruritus intensity can range from sporadic mild discomfort to unrelenting and disturbing [14]. Although its distribution may be generalized, CKD-aP often presents in bilateral, symmetrical patterns or can be localized to specific areas of the body, most commonly the face, back or fistula arm [15]. Pruritus can occur at any time and its occurrence or intensity is unrelated to timing of dialysis sessions [5,16,17]. Many patients complain of worse symptoms at night when they may be more noticeable [5,18]. Observational studies evaluating the duration of CKD-aP show that pruritus typically persists for several years [19,20]. In contrast to other itch conditions with dermatological origin, CKD-aP is not associated with primary skin manifestations, although some patients may present with secondary scratch lesions [14]. However, while dry skin (xerosis) is not thought to be a primary cause of the condition, it frequently occurs with CKD-aP and can worsen the severity of itch [21].
The etiology of CKD-aP is not fully understood, but several possible mechanisms have been implicated in the generation of pruritus, including: (1) deposition of uremic toxins in the skin and subcutaneous tissues; (2) immune system dysregulation and inflammation; (3) peripheral neuropathy; (4) dysregulation of endogenous opioid system and; (5) alterations in the structure and function of the skin microbiome [12▪,14,22▪].
BURDEN OF CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS IN HEMODIALYSIS PATIENTS
Impact of chronic kidney disease-associated pruritus on clinical outcomes
CKD-aP is unpleasant and potentially debilitating for patients undergoing hemodialysis, and has been associated with worse clinical outcomes, including a greater risk of infection, hospitalizations, and mortality [6▪,23]. Data from DOPPS also showed that hemodialysis patients with extreme pruritus were more likely to withdraw from dialysis, miss scheduled dialysis sessions, and take a longer time to recover from dialysis treatment, compared with patients not bothered by pruritus [6▪]. Similarly, a large retrospective cohort study of US hemodialysis patients (n = 38,315) also observed a higher number of missed hemodialysis sessions, as well as increased medication use (intravenous [IV] antibiotics, erythropoietin-stimulating agents, and IV iron) among patients who self-reported severe pruritus as compared to those who were not bothered by pruritus [24].
Impact on general health status and quality of life
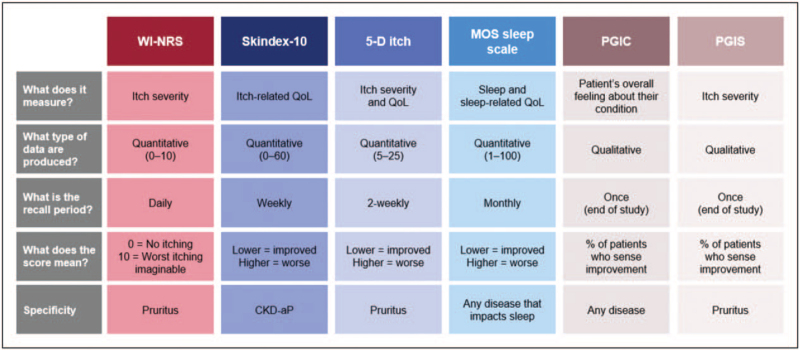
Many observational studies have shown CKD-aP is associated with reduced HRQoL for patients with ESKD undergoing hemodialysis [6▪,19,20,23,25–33]. Figure 1 provides an overview of patient-reported outcome instruments used to capture CKD-aP HRQoL, with validated measures described in Table 1. Among the largest epidemiological studies was a recent analysis of the international DOPPS patient cohort (Phases 4–6; 2009–2018) that included >23,000 hemodialysis patients from 21 countries, which evaluated the relationship between pruritus severity and patient-reported outcomes [6▪]. Itch intensity was assessed using the itch question from the self-reported Kidney Disease Quality of Life 36-item short-form survey (KDQOL-36), which asks patients the extent to which they were bothered by itchy skin during the past 4 weeks (5-category scale: ‘not at all’ to ‘extremely’). Health-related QoL was assessed using the 12-Item Short Form Health Survey (SF-12), a component of the KDQOL-36 that provides a generic measure of mental and physical HRQoL through a mental component scale (MCS) and physical component scale (PCS) scores (range: 0–100). Hemodialysis patients who reported being extremely bothered by pruritus had MCS and PCS scores 6.7 and 9.1 lower than patients not at all bothered by pruritus (the authors noted a 3- to 5-point difference was considered clinically relevant) [6▪]. Furthermore, longitudinal data from the German Epidemiological Hemodialysis Itch Study (GEHIS) which followed a cohort of hemodialysis patients with CKD-aP (N = 212) over a 4-year period showed that HRQoL (assessed by the SF-12 questionnaire) improved among those patients who no longer reported CKD-aP after 4 years [29].
FIGURE 1.
Overview of patient-reported outcome instruments used to capture chronic kidney disease-associated pruritus health-related quality of life. MOS, Medical Outcomes Study; PGIC, patient global impression of change; PGIS, patient global impression of severity; WI-NRS, worst itch numerical rating scale.
Table 1.
Patient-reported outcomes tools validated for use in the assessment of CKD-aP
| PRO tool | Symptom recall | Strength/limitations |
| Unidimensional | ||
| Visual Analogue Scale (VAS) | Worst itch in the previous 24 h | Simple and fast/highly subjective |
| Numeric Rating Scale (NRS) | Worst itch in the previous 24 h | Simple and fast/highly subjective |
| Verbal Rating Scale (VRS) | Worst itch in the previous 24 h | Simple and fast/highly subjective |
| Q-20 KDQOL-36 | Evaluation of the last 4 weeks | Used in large studies/not validated as a severity measure |
| Multidimensional | ||
| 5-D itch scale | 14 days | Validated to analyze the course of itch/time-consuming |
| Skindex-10 | 7 days | Validated to evaluate CKD-aP intensity/time-consuming |
| Self-assessed disease severity | At administration | Fast/less used in studies |
| Itch MOS | Previous week | Exclusively assesses sleep disturbance |
CKD-aP, chronic kidney disease-associated pruritus; MOS, Medical Outcomes Study.
Association between chronic kidney disease-associated pruritus and other symptoms
Potential symptom clusters including chronic kidney disease-associated pruritus
There is evidence that the symptoms experienced by patients with ESKD can occur in clusters. A ‘symptom cluster’ can be defined as two or more concurrent symptoms that are related to one another, may share a common cause, and occur independently of other symptom clusters [7]. A key concept of symptom cluster science is the assumption that alleviating one symptom can reduce the severity of other symptoms included in the cluster [8]. Identification of symptom clusters in ESKD may therefore help to inform individualized symptom management strategies, leading to improvements in HRQoL and other clinical outcomes.
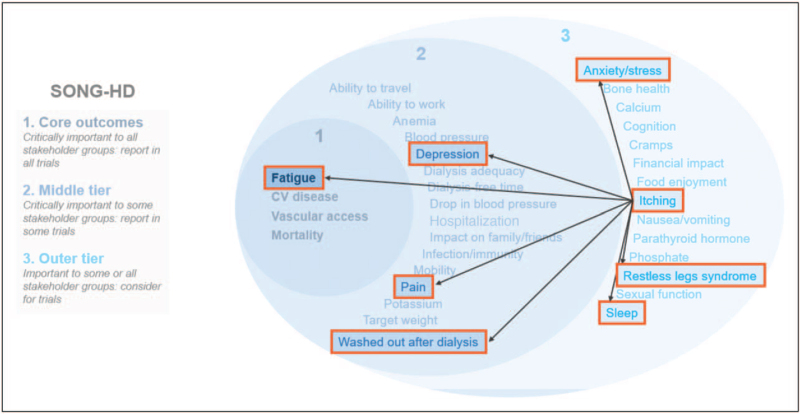
Although the application of symptom cluster science to CKD is still an emerging field, unlike oncology where it is well established, several published studies have attempted to identify symptom clusters in patients with ESKD undergoing dialysis [34,35▪,36–42,43▪]. The composition of symptom clusters identified by these studies has varied depending on the specific assessment tools and statistical methodologies applied [38]. Regarding symptom clusters, an important point is that many co-occurring symptoms in CKD/ESKD have not been considered in this way previously in nephrology. Thus, there is a huge opportunity to think about CKD/ESKD symptoms differently, using latent models to determine relationships between symptoms, with the idea that intervening on symptoms with shared biological underpinnings and have an impact on multiple symptoms in a given symptom cluster (e.g. pruritis/sleep disturbance/fatigue/pain). So far, little work has been done to establish the existence of symptom clusters in nephrology, despite evidence that CKD-aP has been found to co-occur with other common symptoms in patients with ESKD, including sleep disturbance, depression, pain, and restless legs syndrome, indicating that these may comprise a specific symptom cluster (Fig. 2).
FIGURE 2.
Potential symptom cluster for CKD-aP in patients with ESRD on hemodialysis. Figure adapted with permission from the SONG initiative (https://songinitiative.org/). CKD-aP, chronic kidney disease-associated pruritus; CV, cardiovascular; ESRD, end-stage kidney disease; SONG, Standardised Outcomes in Nephrology.
Sleep and fatigue
Many studies have reported a negative association between CKD-aP as well as sleep quality and quantity among patients with ESKD undergoing hemodialysis [6▪,19,20,23,28,33,44]. In the recent DOPPS analysis (Phases 4–6), poor sleep quality (defined as ≥3 nights in the past week of restless sleep as assessed by the sleep question from the Center for Epidemiologic Studies-Depression [CES-D] sleep questionnaire) was more commonly reported by patients bothered (vs not bothered) by pruritus. Furthermore, the prevalence of patients who reported feeling ‘washed out or drained’ was ≥2-fold greater for those with moderate-to-extreme pruritus than those not bothered by pruritus [6▪]. An earlier analysis of the DOPPS cohort (Phases 1 and 2; 1996–2004) observed that ∼45% of patients with moderate-to-severe pruritus reported poor sleep quality in comparison with 29% of patients with no or mild pruritus [23]. In this analysis, poor sleep quality was attributed to the higher rate of mortality (17%) in patients with pruritus vs those without pruritus [23].
The longitudinal prospective ITCH National Registry Study evaluating the natural history of CKD-aP in a cohort of US hemodialysis patients (N = 103) observed a significant association between pruritus intensity and sleep disruption caused by itching as assessed by two separate sleep measures (an adapted version of the Itch Medical Outcomes Study [MOS] sleep questionnaire and the sleep question from the Brief Itching Inventory [BII] questionnaire) [19].
The GEHIS study analyzed sleep data for 860 HD patients with and without CKD-aP. It showed the majority of patients with CKD-aP (54.5%) reported difficulty falling asleep more than once a week, whereas 48.3% complained of impaired sleep quality [20]. The authors reported that impairment in sleep quality was associated with the presence of pruritus, but not with pruritus severity [20].
Depression and anxiety
Several studies have also observed a positive association between CKD-aP and depression [6▪,28,31,45]. Analyses of the DOPPS observed that hemodialysis patients who were bothered by pruritus were more likely to have depressive symptoms (as evaluated by physician-diagnosed depression [Phases 1 and 2] [23] and self-reported CES-D score ≥10 [Phases 4–6]) [6▪]. A cross-sectional study of hemodialysis patients in Brazil (N = 980) also observed that patients who self-reported severe pruritus as assessed by the KDQOL-36 itch question also had higher CES-D scores than patients who reported mild or no pruritus [28]. A longitudinal study of 1799 Japanese patients from the J-DOPPS cohort (follow-up period: 0.5–2.5 years) found that patients with no or mild pruritus who self-reported symptoms of depression at baseline were more likely to develop severe pruritus in the future, indicating a potential link between these two symptoms [45]. The GEHIS showed that patients with pruritus had significantly higher anxiety scores than those without pruritus, as evaluated by the Hospital and Anxiety Depression Scale (HADS) but found no difference in the depression scores [20].
Other symptoms associated with chronic kidney disease-associated pruritus
The GEHIS study observed that, in addition to sleep disturbance, patients with CKD-aP were more likely to experience pain than those without CKD-aP, and that this also contributed toward their reduced HRQoL [31]. Restless legs syndrome and pruritus have been reported to frequently co-occur in patients with ESKD [46▪,47].
POTENTIAL IMPACT OF ALLEVIATING PRURITUS ON RELATED SYMPTOMS AND CLINICAL OUTCOMES
Data from interventional studies of antipruritic treatments show that reduction of pruritus intensity among patients with CKD-aP undergoing hemodialysis is associated with corresponding improvements in their HRQoL [48▪,49], and that reducing pruritus intensity can improve sleep quality of these patients.
Difelikefalin is a peripherally restricted, selective kappa opioid receptor (KOR) agonist, recently approved by the US Food and Drug Administration (FDA) for the treatment of moderate-to-severe pruritus associated with CKD in adult hemodialysis patients [50▪]. Difelikefalin is the first licensed treatment for CKD-aP except for nalfurafine in Japan. Phase 3 clinical trials have demonstrated that difelikefalin can significantly reduce pruritus intensity and improve itch-related QoL in patients with moderate-to-severe CKD-aP [51▪,52▪,53▪]. These studies also indicate that reduction in itch may also improve sleep quality. Studies involving gabapentin and other anti-itch medications are out of the scope of this review as they are nonlicensed treatments.
A 12-week, single-arm Phase 3 trial (Study 3105) found that treatment with IV difelikefalin was associated with improvements in patient-reported sleep quality questionnaire scores [52▪]. A post hoc analysis of this study subsequently showed these were strongly correlated (r = 0.78) with reductions in pruritus intensity at Week 12, as evaluated by changes in weekly mean of the patient-reported 24-h worst itch numerical rating scale (WI-NRS) [54▪]. Furthermore, an exploratory analysis of pooled data from the randomized, placebo-controlled, 12-week, Phase 3 KALM-1 and KALM-2 clinical trials of IV difelikefalin also reported a moderate correlation between reduction in itch intensity with difelikefalin as measured by WI-NRS and improvement in sleep quality as evaluated by the mean 5-D Itch questionnaire sleep disability question score [55▪].
A randomized, Phase 3, double-blind, placebo-controlled trial of centrally acting mu opioid receptor (MOR) antagonist/KOR agonist, nalbuphine, also showed that reductions in itch intensity with this treatment also resulted in improvements in sleep disruption in adult hemodialysis patients with CKD-aP [49].
Based on the study evidence described earlier showing that patients with severe CKD-aP are more likely to withdraw from dialysis than patients with mild or no symptoms [6▪,24]. Therefore, it is possible that alleviation of pruritus may increase patient satisfaction with dialysis and consequently improve their clinical outcomes.
TREATMENT OF CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS: CLINICAL EVIDENCE FOR DIFELIKEFALIN
Difelikefalin, a peripheral, selective KOR agonist, recently became the first therapy approved by the US FDA for the treatment of CKD-aP [50▪]. Difelikefalin is thought to treat pruritus through the activation of KORs on peripheral sensory neurons and immune cells [56].
The efficacy and safety of IV difelikefalin were evaluated by two similarly designed, randomized, placebo-controlled Phase 3 trials (KALM-1 and KALM-2) which together enrolled 851 adult patients with moderate-to-severe CKD-aP undergoing hemodialysis [48▪,53▪]. IV difelikefalin (0.5 μg/kg of dry body weight) or placebo was administered after hemodialysis sessions 3 times per week for 12 weeks. Both trials met their primary endpoints demonstrating that the proportion of patients achieving a clinically meaningful (≥3-point) reduction from baseline in daily 24-h WI-NRS scores was significantly greater with difelikefalin vs placebo at Week 12 (KALM-1: 50.0% vs 27.6%; KALM-2: 54.0% vs 42.2%) [48▪,53▪].
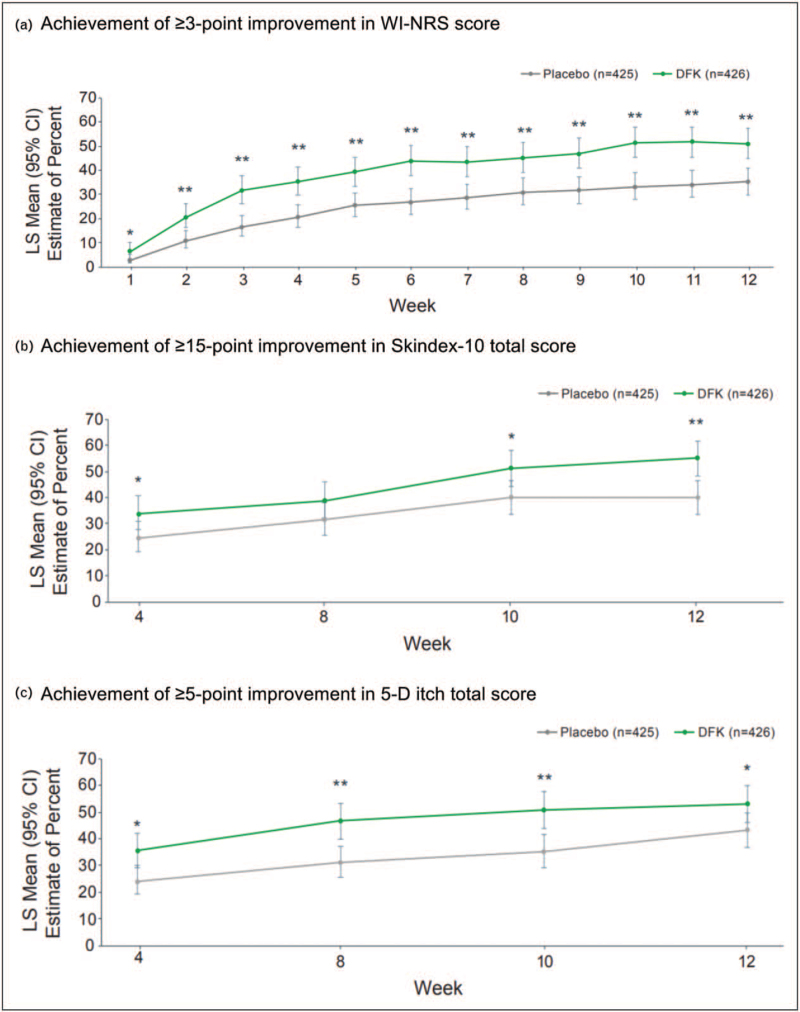
The efficacy of IV difelikefalin for itch reduction was also evaluated in a pooled analysis of the KALM-1 and KALM-2 trials that also assessed the effect of treatment on itch-related QoL, as evaluated by the Skindex-10 and 5-D itch scale questionnaires [57▪]. This pooled analysis showed that the achievement of ≥3-point WI-NRS improvement from baseline was significantly greater with difelikefalin vs placebo at all time points from Week 1 (Fig. 3a).
FIGURE 3.
Pooled analysis of KALM-1 and KALM-2 clinical trials: Efficacy of difelikefalin vs placebo for reduction of itch and clinically meaningful improvements in itch-related QoL (Skindex-10 and 5-D itch scale). (a) Achievement of ≥3-point improvement in WI-NRS score. (b) Achievement of ≥15-point improvement in Skindex-10 total score. (c) Achievement of ≥5-point improvement in 5-D itch total score. ∗P < 0.05, ∗∗P < 0.001 difelikefalin vs placebo. KALM-1 and -2, study title: A Study to Evaluate the Safety and Efficacy of CR845 in Hemodialysis Patients with Moderate-to-Severe Pruritus. CI, confidence interval; LS, least squares; QoL, quality of life; WI-NRS, worst itch numeric rating scale.
The proportion of patients who achieved a clinically meaningful improvement in total Skindex-10 score (≥15-point reduction) was significantly greater with difelikefalin vs placebo at Weeks 4, 10, and 12 (Fig. 3b), whereas those achieving clinically meaningful improvements in total 5-D itch score (≥5-point reduction) was significantly greater with difelikefalin vs placebo at all timepoints evaluated (Fig. 3c).
Both the KALM-1 and KALM-2 studies had open-label extension phases where eligible patients from the difelikefalin and placebo arms could receive treatment with difelikefalin for up to 52 weeks. A pooled analysis of KALM-1 and KALM-2 showed that IV difelikefalin maintained efficacy in terms of itch reduction and improvements in itch-related QoL over 1 year of treatment in the open-label extensions [58▪].
CONCLUSION
Despite the clear associations with poorer health outcomes and reduced HRQoL, CKD-aP remains under-appreciated, under-reported and under-diagnosed. Research focusing on single symptoms has led to significant advances in our understanding of CKD-aP but people with advanced kidney disease rarely present with just one; the future of research into symptom management needs to focus on evaluating the relationships between multiple symptoms, specific interventions and patient outcomes. Symptom clusters are those that share a common mechanism or etiology and whose inter-relationship leads to different outcomes when compared to each individual component symptom. With new treatment strategies on the horizon, this concept of symptom clusters represents an opportunity not just for the management of CKD-aP, but also for all of those associated symptoms which we know are important to our patients.
Acknowledgements
Editorial assistance for this article was provided by AXON Communications (London, United Kingdom).
Financial support and sponsorship
M.B.L. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number U01DK123787-S1. M.B.L. was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR018482. The content is solely the responsibility of the authors. The views expressed in this paper do not necessarily represent the views of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, the National Institute of Nursing Research, the Department of Health and Human Services, the Department of Veterans Affairs, or the government of the United States.
Editorial assistance for this article was funded by Vifor Pharma Ltd.
Conflicts of interest
R.S.A. reports none.
K.K-Z reports fees from Vifor, Kabi and AstraZeneca.
J.O.B. reports personal fees from Vifor Pharma Ltd outside the submitted work.
M.B.L. reports none.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013; 39:140–150. [DOI] [PubMed] [Google Scholar]
- 2.Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manag 2010; 39:477–485. [DOI] [PubMed] [Google Scholar]
- 3.Evangelidis N, Tong A, Manns B, et al. Developing a set of core outcomes for trials in hemodialysis: An International Delphi Survey. Am J Kidney Dis 2017; 70:464–475. [DOI] [PubMed] [Google Scholar]
- 4.Ramkumar N, Beddhu S, Eggers P, et al. Patient preferences for in-center intense hemodialysis. Hemodial Int 2005; 9:281–295. [DOI] [PubMed] [Google Scholar]
- 5.Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017; 12:2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2021; 3:42–53. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the clinical, dialysis-related, and patient-reported outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS) phases 4 to 6 (2009–2018). This article highlights the burden of CKD-aP.
- 7.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 2005; 28:270–282. quiz 283–284. [DOI] [PubMed] [Google Scholar]
- 8.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst 2017; 109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs 2012; 35:E1–E20. [DOI] [PubMed] [Google Scholar]
- 10▪.Lockwood MB, Lash JP, Pauls H, et al. Physical symptom cluster subgroups in chronic kidney disease. Nurs Res 2020; 69:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified symptom subgroups among patients with mild-to-moderate chronic kidney disease. Identification of symptom clusters may help inform symptom assessment, risk-stratification, and development of patient-centered interventions.
- 11.Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manag 2019; 58:578–586. e2. [DOI] [PubMed] [Google Scholar]
- 12▪.Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep 2020; 5:1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reviews the diagnosis, mangement and pathogenesis of CKD-aP. Recent clinical trials have expanded knowledge in this area.
- 13.Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007; 87:291–294. [DOI] [PubMed] [Google Scholar]
- 14.Mettang T, Kremer AE. Uremic pruritus. Kidney Int 2015; 87:685–691. [DOI] [PubMed] [Google Scholar]
- 15.Gilchrest BA, Stern RS, Steinman TI, et al. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol 1982; 118:154–156. [PubMed] [Google Scholar]
- 16.Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One 2013; 8:e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirazian S, Kline M, Sakhiya V, et al. Longitudinal predictors of uremic pruritus. J Ren Nutr 2013; 23:428–431. [DOI] [PubMed] [Google Scholar]
- 18.Merlino G, Gigli GL, Valente M. Sleep disturbances in dialysis patients. J Nephrol 2008; 21: (Suppl 13): S66–70. [PubMed] [Google Scholar]
- 19.Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5:1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss M, Mettang T, Tschulena U, et al. Prevalence of chronic itch and associated factors in haemodialysis patients: a representative cross-sectional study. Acta Derm Venereol 2015; 95:816–821. [DOI] [PubMed] [Google Scholar]
- 21.Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant 2004; 19:2709–2712. [DOI] [PubMed] [Google Scholar]
- 22▪.Kim HS, Yosipovitch G. The skin microbiota and itch: is there a link? J Clin Med 2020; 9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies alterations in the structure and function of the skin microbiome as one of the potential etiologies of CKD-aP.
- 23.Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21:3495–3505. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis 2013; 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayani K, Weiss M, Weisshaar E. Clinical findings and provision of care in haemodialysis patients with chronic itch: new results from the German Epidemiological Haemodialysis Itch Study. Acta Derm Venereol 2016; 96:361–366. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MK, Elshahid AR, El Baz TZ, et al. Impact of uraemic pruritus on quality of life among end stage renal disease patients on dialysis. J Clin Diagn Res 2016; 10:WC01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimata N, Fuller DS, Saito A, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int 2014; 18:657–667. [DOI] [PubMed] [Google Scholar]
- 28.Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res 2012; 21:603–612. [DOI] [PubMed] [Google Scholar]
- 29.Plewig N, Ofenloch R, Mettang T, Weisshaar E. The course of chronic itch in hemodialysis patients: results of a 4-year follow-up study of GEHIS (German Epidemiological Hemodialysis Itch Study). J Eur Acad Dermatol Venereol 2019; 33:1429–1435. [DOI] [PubMed] [Google Scholar]
- 30.Susel J, Batycka-Baran A, Reich A, Szepietowski JC. Uraemic pruritus markedly affects the quality of life and depressive symptoms in haemodialysis patients with end-stage renal disease. Acta Derm Venereol 2014; 94:276–281. [DOI] [PubMed] [Google Scholar]
- 31.Weiss M, Mettang T, Tschulena U, Weisshaar E. Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study). Qual Life Res 2016; 25:3097–3106. [DOI] [PubMed] [Google Scholar]
- 32.Satti MZ, Arshad D, Javed H, et al. Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus 2019; 11:e5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tessari G, Dalle Vedove C, Loschiavo C, et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J Nephrol 2009; 22:241–248. [PubMed] [Google Scholar]
- 34.Almutary H, Douglas C, Bonner A. Multidimensional symptom clusters: an exploratory factor analysis in advanced chronic kidney disease. J Adv Nurs 2016; 72:2389–2400. [DOI] [PubMed] [Google Scholar]
- 35▪.Chaiviboontham S, Phinitkhajorndech N, Tiansaard J. Symptom clusters in patients with end-stage renal disease undergoing hemodialysis. Int J Nephrol Renovasc Dis 2020; 13:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified eight symptom clusters in patients with ESKD undergoing dialysis: 1) gastrointestinal, 2) musculoskeletal and fluid volume, 3) neurological, 4) irritation of the mucous membranes and skin, 5) depression, 6) sleep disturbance, 7) sexual, 8) anemic.
- 36.Amro A, Waldum B, von der Lippe N, et al. Symptom clusters predict mortality among dialysis patients in Norway: a prospective observational cohort study. J Pain Symptom Manag 2015; 49:27–35. [DOI] [PubMed] [Google Scholar]
- 37.Amro A, Waldum B, Dammen T, et al. Symptom clusters in patients on dialysis and their association with quality-of-life outcomes. J Ren Care 2014; 40:23–33. [DOI] [PubMed] [Google Scholar]
- 38.Cao X, Tian L, Lin C. Symptom clusters in patients receiving haemodialysis: a systematic review of observational studies. J Clin Nurs 2017; 26:2545–2557. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez Sanchez D, Leiva-Santos JP, Cuesta-Vargas AI. Symptom burden clustering in chronic kidney disease stage 5. Clin Nurs Res 2019; 28:583–601. [DOI] [PubMed] [Google Scholar]
- 40.Shim HY, Cho MK. Factors influencing the quality of life of haemodialysis patients according to symptom cluster. J Clin Nurs 2018; 27:2132–2141. [DOI] [PubMed] [Google Scholar]
- 41.Thong MS, van Dijk S, Noordzij M, et al. Symptom clusters in incident dialysis patients: associations with clinical variables and quality of life. Nephrol Dial Transplant 2009; 24:225–230. [DOI] [PubMed] [Google Scholar]
- 42.Curtin RB, Bultman DC, Thomas-Hawkins C, et al. Hemodialysis patients’ symptom experiences: effects on physical and mental functioning. Nephrol Nurs J 2002; 29:562–574. 7-74; discussion 75, 98. [PubMed] [Google Scholar]
- 43▪.Ng MSN, So WKW, Wong CL, et al. Stability and impact of symptom clusters in patients with end-stage renal disease undergoing dialysis. J Pain Symptom Manag 2020; 59:67–76. [DOI] [PubMed] [Google Scholar]; This study examined changes in symptom clusters over time, identifying four symptom clusters: uremic, gastrointestinal, skin, and emotional. Symptom clusters had consistent negative effects on various aspects of patients’ well being.
- 44.Rehman IU, Chohan TA, Bukhsh A, Khan TM. Impact of pruritus on sleep quality of hemodialysis patients: a systematic review and meta-analysis. Medicina 2019; 55:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto Y, Hayashino Y, Yamazaki S, et al. Depressive symptoms predict the future risk of severe pruritus in haemodialysis patients: Japan Dialysis Outcomes and Practice Patterns Study. Br J Dermatol 2009; 161:384–389. [DOI] [PubMed] [Google Scholar]
- 46▪.Arzhan S, Roumelioti ME, Unruh ML. Itch and ache on dialysis: new approaches to manage uremic pruritus and restless legs. Blood Purif 2020; 49:222–227. [DOI] [PubMed] [Google Scholar]; This review identified recent findings supporting CKD-aP and restless legs sydrome as common but underdiagnosed conditions in ESRD patients on maintenance dialysis that are related to a decline in patients’ QOL and poor prognosis.
- 47.Scherer JS, Combs SA, Brennan F. Sleep disorders, restless legs syndrome, and uremic pruritus: diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis 2017; 69:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Fishbane S, Jamal A, Munera C, et al. A Phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med 2020; 382:222–232. [DOI] [PubMed] [Google Scholar]; This study demonstrated that difelikefalin reduced pruritus intensity among patients with CKD-aP undergoing hemodialysis and that reductions in itch intensity are associated with corresponding improvements in patients’ HRQoL.
- 49.Mathur VS, Kumar J, Crawford PW, et al. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol 2017; 46:450–458. [DOI] [PubMed] [Google Scholar]
- 50▪. KORSUVA (difelikefalin) injection for intravenous use. Highlights of Prescribing Information. Available at: https://korsuva.com/sites/g/files/brlbcj1286/files/2021-08/korsuva-prescribing-information.pdf. Accessed August 2021. [Google Scholar]; This identifies difelikefalin as recently becoming the first therapy approved by the US FDA for the treatment of CKD-aP.
- 51▪.Fishbane S, Mathur V, Germain MJ, et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep 2020; 5:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]; This Phase 3 clinical trial demonstrated that difelikefalin can significantly reduce pruritus intensity and improve sleep and itch-related QoL in patients with moderate-to-severe CKD-aP.
- 52▪. Weiner DE. An open-label, multicenter study to evaluate the safety and effectiveness of intravenous difelikefalin in patients with moderate-to-severe CKD–associated pruritus (CKD-aP) undergoing hemodialysis. Oral Presentation FC 022. ERA-EDTA Virtual Congress, June 5−8, 2021. [Google Scholar]; This Phase 3 clinical trial demonstrated that difelikefalin effectively reducted itch intensity and improved sleep quality and itch-related QoL at Week 12.
- 53▪. Wooldridge TD, Mccafferty K, Schoemig M, et al. Efficacy and Safety of Difelikefalin for Moderate-to-Severe Chronic Kidney Disease–Associated Pruritus: a Global Phase 3 Study in Hemodialysis Patients (KALM-2) [oral presentation]. Presented at: Annual Meeting of the American Society of Nephrology; October 20–25, 2021. [Google Scholar]; This Phase 3 clinical trial demonstrated that the proportion of patients achieving a clinically meaningful (≥3-point) reduction from baseline in daily 24-h WI-NRS scores was significantly greater with difelikefalin vs placebo at Week 12.
- 54▪. Weiner DE, Walpen S, Schaufler T, et al. Itch Reduction with Difelikefalin Correlates with Improved Sleep Quality in Hemodialysis Patients with Pruritus. Oral presentation at ASN Kidney Week 2021. [Google Scholar]; This post hoc analysis of a Phase 3 clinical study demonstrated that improvements in patient-reported sleep quality were were strongly correlated with reductions in pruritus intensity at Week 12.
- 55▪. Ahdoot R, Kalantar-Zadeh K, McCafferty K, et al. Improvement in sleep quality from reduction of itch intensity in patients with moderate-to-severe pruritus undergoing hemodialysis. Presented at the 11th World Congress on Itch 2021, October 22–23, 2021. [Google Scholar]; This post hoc analysis of pooled data from two randomized, placebo-controlled, Phase 3 clinical trials demonstrated a moderate correlation between reduction in itch intensity with difelikefalin as measured by WI-NRS and improvement sleep quality as evaluated by the mean 5-D Itch questionnaire sleep disability question score.
- 56.Albert-Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther 2016; 41:371–382. [DOI] [PubMed] [Google Scholar]
- 57▪. Topf J, Wen W, Munera C, et al. Efficacy of Difelikefalin in patients with moderate-to-severe chronic kidney disease–associated pruritus: pooled subgroup analysis of KALM-1 and KALM-2. Poster presentation at the National Kidney Foundation Spring Clinical Meeting, Virtual Meeting, April 6-10, 2021. 2021. [Google Scholar]; This pooled analysis from two randomized, placebo-controlled, Phase 3 clinical trials demonstrated that significantly more patients achieved a clinically relevent improvement in WI-NRS from baseline with difelikefalin vs placebo at all time points from Week 1.
- 58▪.Fishbane S, Wen W, Munera C, et al. Long-term safety and efficacy of difelikefalin in patients with chronic kidney disease–associated pruritus: analysis from KALM-1 and KALM-2. American Journal of Kidney Diseases 2021; 77:593–594. [Google Scholar]; This pooled analysis of the open-label extensions of two Phase 3 clinical studies demonstrated that difelikefalin maintained efficacy in terms of itch reduction and improvements in itch-related QoL over 1 year of treatment.