Abstract
Low-grade intraductal carcinoma is a rare neoplasia with an excellent prognosis, previously classified as low-grade cribriform cystadenocarcinoma and low-grade salivary duct carcinoma. The tumor mainly occurs in the parotid gland and presents a ductal phenotype and an intraductal/intracystic growth pattern. It resembles intraductal breast lesions such as atypical ductal hyperplasia, papillary and cribriform ductal carcinoma in situ. Despite its infrequency, discriminating low-grade intraductal carcinoma from other salivary gland tumors is crucial, especially because of its favorable prognosis. A 74-year-old woman with a history of neurofibromatosis underwent a superficial parotidectomy to remove a sharply demarcated multi-cystic mass, diagnosed as category 4 at FNAC. The histological examination revealed a demarcated but unencapsulated lesion composed of a bigger cyst surrounded by several smaller cysts, lined by a monolayer or bilayer epithelium alternated with a cribriform proliferation, characterized by “Roman-bridges”, with occasional micro-papillae. A myoepithelial component, with a basal disposition, was present, confirmed by intense staining for protein p63 and SMA. Immunohistochemical stains showed intense, strong uniform positivity for pan-cytokeratin, protein S100, and SOX10. The Ki67 proliferation index was low (< 10%). A diagnosis of Low-grade Intraductal Carcinoma (LGIC) of the parotid was made. We performed a literature search in PUBMED for “Intraductal carcinoma”, “Low-grade Intraductal Carcinoma”, “Cribriform Cystadenocarcinoma”, “Salivary Duct Carcinoma”, and “Low-Grade Salivary Duct Carcinoma”. We selected 17 papers published between 1983 and 2020; the most affected anatomical site was the parotid gland (77/90), followed by minor salivary glands (6/90), the intraparotid lymph nodes (3/90) and the submandibular gland (4/90). Their main histopathological features are reported in the paper. Here we present a case report and a review of scientific literature on this topic to provide some essential diagnostic tools to discriminate this rare entity.
Keywords: Low-grade intraductal carcinoma, Cribriform cystadenocarcinoma, Low-grade salivary duct carcinoma, Salivary gland, Parotid gland
Introduction
Low-grade intraductal carcinoma (LGIC), previously identified as low-grade cribriform cystadenocarcinoma or low-grade salivary duct carcinoma, is a rare salivary gland tumor. In about 80% of cases, it occurs in the parotid gland and rarely involves other anatomic sites [1]. LGIC displays histological features resembling atypical ductal hyperplasia or ductal carcinoma in situ of the breast [2]. It grossly appears as an unencapsulated uni- or multi-cystic lesion, microscopically characterized by an intra-cystic proliferation of neoplastic epithelial cells associated with a preserved layer of myoepithelial cells [3]. LGIC grows slowly, rarely develops metastases [4], and has never been associated with systemic diseases or cigarette smoking. Since its first description in 1996 by Delgado and colleagues, about 50 cases have been reported worldwide. Its incidence is low in Italian series, being as low as 2% of all salivary gland tumors [1].
Searching our pathology unit’s database from January 2000, using the terms “low-grade intraductal carcinoma”, “low-grade cribriform cystadenocarcinoma”, and “low-grade salivary duct carcinoma”, we found no cases that met the above criteria. Here we report an additional case of LGIC occurring in the left parotid gland of a 74-year-old woman affected by neurofibromatosis. The neoplasia presented morphological features generally considered an expression of aggressiveness, such as focal apocrine differentiation and invasive growth pattern. The patient is still alive and disease-free after 15 months from diagnosis.
Case Report
A 74-year-old woman was referred to the Maxillofacial Surgery Department of the University of Naples Federico II, Italy, because of a 6-month-history of left parotid region swelling. The patient was a heavy smoker and reported a history of arterial hypertension, neurofibromatosis, and previous resection of bladder cancer. The clinical examination revealed a well-circumscribed mass in the inferior portion of the left parotid gland near the mandibular angle and the presence of multiple neurofibromas on the skin surface. On palpation, the swelling was not painful and had a firm consistency. It was movable to the deeper tissue and to superficial skin that appeared normal for color and appearance. A CT scan (Fig. 1) of the head and neck region showed a non-homogeneous cystic formation of about 28 × 15 mm. Following an FNAC examination, the case was categorized as a neoplasm of uncertain malignant potential (diagnostic category 4, according to the Milan System) [5]. A superficial parotidectomy was performed, with the preservation of all facial nerve branches. The tumoral mass was removed “en bloc", and no complications were detected during the post-surgery follow-up (Fig. 2). The patient was discharged from hospital after 5 days.
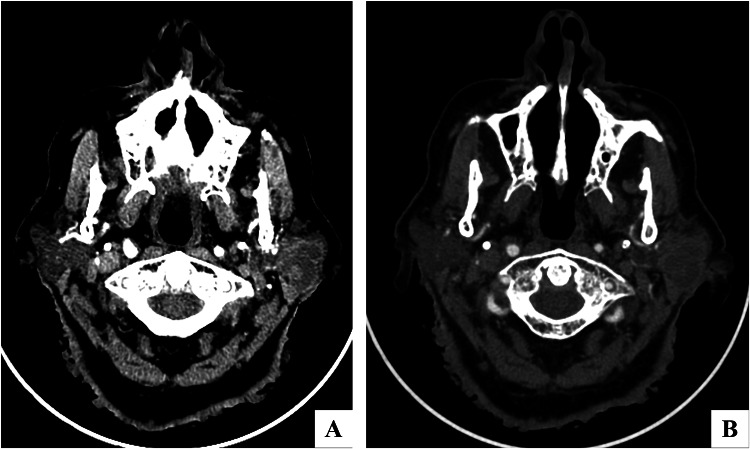
Fig. 1.
a, b Axial CT-Scan: A neoplasm alters the left parotid parenchyma and presents uneven density after enhancement (a)
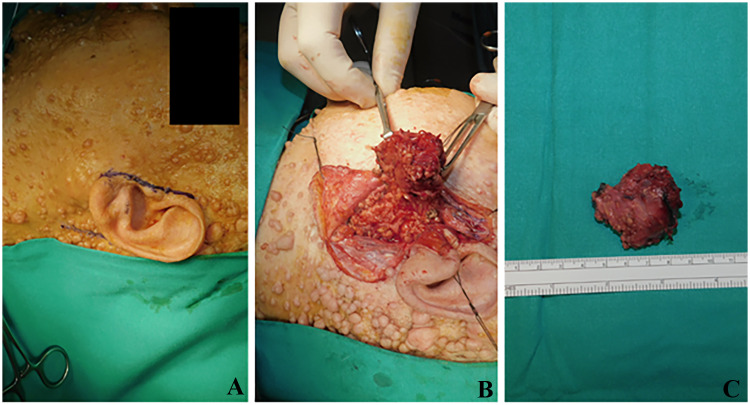
Fig. 2.
a A 74-year-old female affected by neurofibromatosis with a non-painful and non-tender swelling of the left parotid region; b superficial parotidectomy, with preservation of the integrity of all facial nerve branches was performed; c the superficial parotidectomy was partially occupied by a clearly demarcated multi-cystic mass of about 2.3 × 2.7 cm in diameter
The specimen consisted of a superficial parotidectomy partially occupied by a sharply demarcated multi-cystic mass of about 2.3 × 2.7 cm in diameter. Grossly, it showed cysts filled with a serous/mucoid/hemorrhagic fluid. No necrosis was macroscopically detected. The histologic examination showed a demarcated but unencapsulated lesion made up of a bigger cyst surrounded by several smaller cysts. Cysts were lined by a monolayer or bilayer epithelium alternated with a cribriform proliferation, characterized by “Roman-bridge” pattern with occasional micro-papillae. At high power magnification, it was possible to distinguish a neoplastic epithelial proliferation from a myoepithelial component, with a basal disposition. Neoplastic epithelial cells were small to medium-sized and presented mild atypia, eosinophilic cytoplasm, round/oval nuclei, and occasional prominent nucleoli (Fig. 3). Moreover, also apocrine differentiation and cytoplasmic microvacuoles (PAS and mucicarmine positive) were significant histologic features (Fig. 4). Mitotic activity was low, and atypical mitotic figures were not detected. We observed focal perineural invasion and a focal invasive growth pattern.
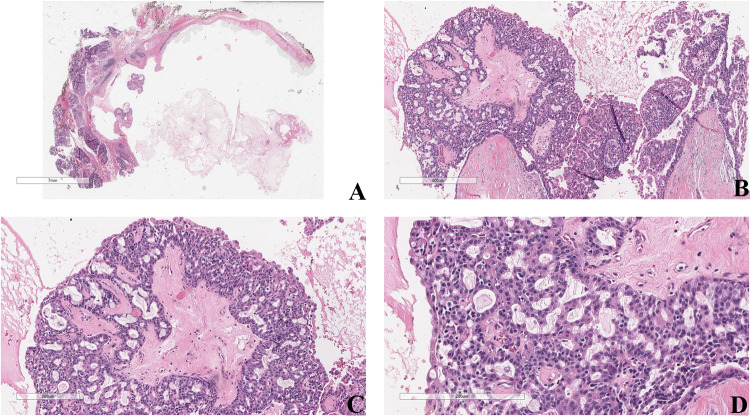
Fig. 3.
a The low-power field shows a discrete, well-circumscribed cyst filled with serous/mucous material (H&E, 2 ×,). b, c At higher magnification, the cyst is lined by a bilayered epithelium with rare islands of cribriform proliferation (H&E, 4 × and 10 ×, respectively). d Neoplastic cells present mild atypia and are small to medium-sized with eosinophilic cytoplasm, round/oval nuclei, and prominent nucleoli (H&E 20 ×)
Fig. 4.
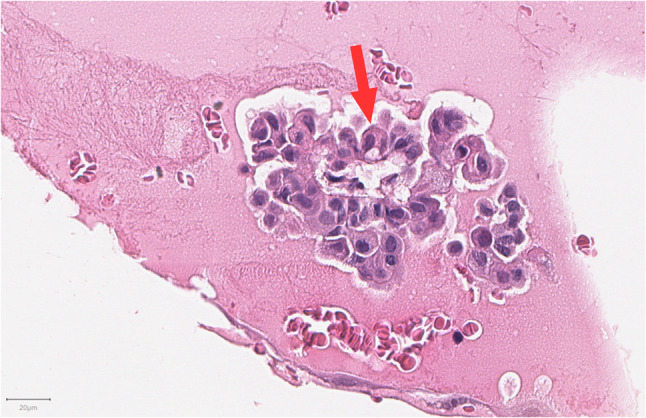
Apocrine cells show larger nuclei, variable nucleoli, abundant eosinophilic cytoplasm, and cytoplasmic microvacuoles (red arrow) (H&E, 20 ×)
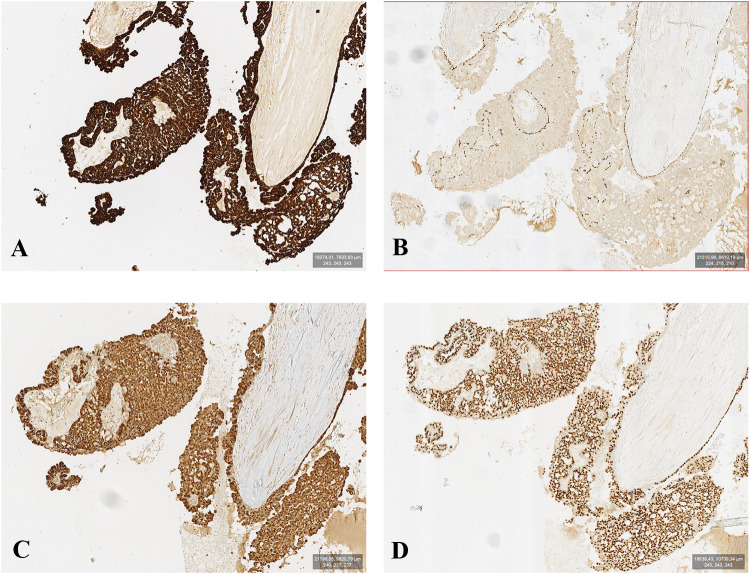
Immunohistochemical stains showed intense, strong uniform positivity for pan-cytokeratin, S100, and SOX10 (Fig. 5a,c,d). The presence of a non-neoplastic myoepithelial layer, rimming the cystic spaces, was confirmed by intense staining for protein p63 (Fig. 5b) and Smooth Muscle Actin (SMA) in this cellular population. Myoepithelial rim was lacking in the focal invasive component. The Ki-67 proliferation index was low (< 10%). Immunostaining for Androgen Receptor (AR), PSA, Gross Cystic Disease Fluid Protein 15(GCDFP-15) DOG-1 and Her-2 resulted negative.
Fig. 5.
The figure shows immunoreactivity for pan keratin (a) p63 (b); S100 (c), and SOX10 (d). Epithelial neoplastic cells present a strong reactivity for pan keratin, S100, and SOX10; p63 highlights myoepithelial cells with basal disposition (10 ×)
We concluded for a diagnosis of Low-grade Intraductal Carcinoma (LGIC). After 1-year follow-up, there was no evidence of recurrence or metastases.
Discussion
LGIC is an uncommon malignant tumor of the salivary glands that frequently occurs in the parotid gland. It involves intraparotid lymph nodes, submandibular glands, and, rarely, minor salivary glands [1]. We reviewed the scientific literature concerning Intraductal Carcinomas of Salivary Glands: Table 1 shows the morphological and clinicopathologic features of these tumors. Searching the PUBMED database for “Intraductal carcinoma”, “Low-grade Intraductal Carcinoma”, “Cribriform Cystadenocarcinoma”, “Salivary Duct Carcinoma”, and “Low-Grade Salivary Duct Carcinoma” we selected 17 papers published between 1983 and 2020. Table 2 summarizes the key features of the selected case series. The total number of patients included in the sorted series was 90, the median age ranged between 43 and 79 years old; 42 out of the 90 patients were female, 47 male and 1 unknown; the most affected anatomical site was the parotid gland (77/90), followed by minor salivary glands (6/90, 3 of the hard palate, 2 of the buccal mucosa and 1 of the tongue), intraparotid lymph nodes (3/90) and the submandibular gland (4/90). The described lesions ranged, in the largest diameter, between 1 and 4.5 cm. Invasive growth was described in 20 cases. Most of the reported cases showed cells with clear nuclei with dispersed chromatin and prominent nucleoli. The oncocytic appearance was also described. Apocrine features were frequently reported. The architecture of the reported lesions was mostly cystic with the presence of so-called “Roman-arches” or “Roman-bridges”. Some cases had a cribriform or papillary/micropapillary pattern of growth. Mitotic figures were absent or mostly few (frequently described as scattered). Except for three cases, the presence of myoepithelial cells was always reported in the analyzed cases. The term Intraductal Carcinoma was introduced by Chen in 1983 [6], but Delgado first described the tumor in 1996, reporting 10 cases of salivary gland lesions with histological features attributable to a low-grade counterpart of salivary duct carcinoma [7]. It had been named Low-Grade Salivary Duct Carcinoma and Low-Grade Cribriform Cystadenocarcinoma before being classified as Intraductal Carcinoma (IC) in the last edition of the WHO classification of Head and Neck Tumors [3]. The intraductal nature of the tumor is demonstrated by the presence of a preserved myoepithelial layer (highlighted by immunostaining for p63), surrounding the neoplastic epithelial component. Neoplastic cells show particular diffuse, co-expression of SOX10, cytokeratin 7, and S100 and are generally arranged in a cribriform, “Roman-bridge” or micropapillary intraductal/intra-cystic growth pattern. Based on the degree of cytological atypia and the number of mitotic figures, IC can be divided into Low-Grade Intraductal Carcinoma (LGIC), Intermediate Grade Intraductal Carcinoma, and High-Grade Intraductal carcinoma (HGIC) [3]. Moreover, IC has been recently further divided into two morphologic and immunophenotypic subtypes: intercalated duct type and apocrine type. Strong positivity for S100 and SOX-10, negativity for AR and mild atypia characterize the pure intercalated form. Instead, the apocrine type lacks S100 and SOX10 expression and shows a strong positivity for AR. It is composed of a pure apocrine population of cells with abundant eosinophilic granular cytoplasm, large nuclei with prominent nucleoli and apocrine snouts [8]. The last classification has been recently confirmed by molecular investigations, which revealed a particular association between NCOA4-RET gene fusion and the intercalated variant, and between TRIM27-RET gene fusion and the apocrine variant [2]. The focal invasive pattern of growth has also been reported and well-described [9], especially in the apocrine variant. However, in clinical practice, a clear distinction between these two variants is frequently difficult to make, and mixed/hybrid IC, with morphological, immunohistochemical, and genetic features of both intercalated and apocrine types, are reported [10]. Due to its analogy with atypical ductal hyperplasia and ductal carcinoma in situ of the breast, IC has been considered a preneoplastic lesion. In particular, Delgado first and other authors later investigated the relationship between IC, called low-grade salivary duct carcinoma at the time, and salivary duct carcinoma (SDC), assuming that they were opposite extremities of the same spectrum of salivary gland neoplasms [11]. However, they are currently considered different entities because of their morphological, immunohistochemical, and molecular features. Intercalated duct-type IC shows intense positivity for S100 and SOX-10, and negativity for AR and GCDFP-15. A continuous layer of p63 positive myoepithelial cells surrounds the tumor cells. Conversely, SDC is a high-grade neoplasia made up of pleomorphic apocrine cells, with S100 negative and AR/GCDFP15 positive immunophenotype. Despite the fact that this immunoprofile is shared with apocrine type IC, SDC is widely invasive and never rimmed by myoepithelial cells [12]. Moreover, the recent identification of RET rearrangements in ICs, but not in SDCs, has finally confirmed the clear distinction between these two neoplasms [2]. SDC has specific genomic alterations such as HER2-neu gene amplification and hotspot mutations of PIK3CA and HRAS. The latter two mutations were found in a rare form of ICs with apocrine features, high cellular grade and widespread invasion that could represent a precursor lesion of SDC [13, 14]. Thus, IC is a rare but widely debated neoplasia. It has a good prognosis, and therapy consists of surgical excision only. Therefore, it seems necessary to distinguish IC from other salivary gland neoplasms with more aggressive behavior but similar morphological features.
Table 1.
Morphological findings of salivary gland intraductal carcinomas reported in most important scientific papers published between 1983 and 2020
| Authors | Year | Number of cases | Median age | Gender | Location | Largest diameter (cm) | Cytology | Myoepithelial cells | Architectural pattern | Invasive growth pattern | Mitotic rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T.K. Chen [6] | 1983 | 1 | 60 | F | Minor salivary glands | Not reported | Epithelial cells with a small amount of cytoplasm and hyperchromatic nuclei | Not reported | Not reported | No | Frequent mitotic figures |
| R. Delgado et al. [7] | 1996 | 10 | 60 | 5 M, 5 F | 9 Parotid, 1 intraparotid lymph node | 1.1 (median value) | Apocrine/vacuolated, intercalated duct-like, nonspecific glandular | Present |
Cystic ducts with micropapillary projection Intraductal pseudo-cribriform cellular proliferation Ducts with architectural atypia |
1 case | Negligible |
| Y. Tatemoto et al. [15] | 1996 | 1 | 58 | F | Minor salivary glands | 1 | Tumor cells with eosinophilic cytoplasm and clear large nuclei | Not reported | Ductal structures with intraductal proliferation with cribriform pattern | No | Negligible |
| W. Cheuk et al. [16] | 2004 | 1 | 44 | F | Minor salivary glands | 1.2 | Tumor cells with eosinophilic cytoplasm and round/oval nuclei with finely dispersed chromatin and distinct nucleoli | Present | Intraductal/Intra-lobular proliferation with fenestrated or cribriform pattern | No | 1–2 mitotic figures/HPF |
| M. Brandwein-Gensler et al. [17] | 2004 | 16 | 64 | 7 M, 8 F, 1 unknown |
14 Parotid, 1 intraparotid lymph node, 1 submandibular gland |
Not reported |
Neoplastic ductal cells with finely dispersed chromatin and small nucleoli Ductal cells with apocrine-type microvacuoles and lipofuscin-like granules Oncocytoid cells in 2 cases |
Present | Cystic ducts and intraductal proliferation with micropapillary, cribriform (with occasional “Roman-bridge” formations) or solid pattern | 4 cases | Scattered mitotic figures in 2 cases |
| I. Weinreb et al. [9] | 2006 | 3 | 67 | 1 M, 2 F | Parotid | 2 (median value) | Tumor cells with deeply eosinophilic cytoplasm, nuclei with central eosinophilic nucleoli, apical apocrine snouts and cytoplasmic vacuoles | Present | Large cystic ducts with intraductal proliferation with "Roman-arches", cribriform, papillary or micropapillary architecture | 1 case | Scattered mitotic figures |
| I. Weinreb et al. [18] | 2011 | 1 | 59 | F | Intraparotid lymph node | 3.5 | Tumor cells with ovoid/round nuclei, inconspicuous nucleoli and granular, amphophilic or focally oncocytic cytoplasm and bubbly microvacuoles | Present | Nests and cystic spaces with cell proliferation with solid, cribriform, micropapillary and “Roman-bridge” architecture | No | No |
| L. Wang et al. [19] | 2013 | 2 | 53,5 | 1 M, 1 F | Parotid | 2.5 | Uniform tumor cells with round/oval nuclei with fine chromatin, prominent nucleoli and pale to amphophilic cytoplasm. Apocrine differentiation of cells in 1 case | Present | Large cystic or solid spaces with cribriform or micropapillary architecture | No | Negligible |
| S. Kokabu et al. [20] | 2015 | 1 | 56 | F | Minor salivary glands | 2 | Tumor cells with inconspicuous nuclear atypia | Present | Unilocular cyst with intracystic proliferation with tubular, cribriform or solid architecture | No | Presence of mitotic figures |
| N. Wakabayashi et al. [4] | 2017 | 1 | 51 | M | Parotid | 4.5 |
Tumor cells with mild atypia, low N/C ratio and intracytoplasmic mucin |
Not reported | Tumor cell proliferation with papillary, cribriform pattern | No | Negligible |
| T. Nishijima et al. [21] | 2016 | 1 | 75 | F | Parotid | 4 | Tumor cells with mild-to-moderate atypia, round to oval nuclei, dispersed chromatin, and distinct nucleoli | Present | Papillary-cystic proliferation of tumor cells with cribriform and papillary structure (lacking a fibro-vascular core) accompanied by prominent lymphoid stroma | No | 1–2 mitotic figures/HPF |
| M. Nakaguro et al. [22] | 2018 | 5 | 63 | 3 M, 2 F |
4 parotid, 1 submandibular gland |
3.3 | Tumor cells with medium-sized round nuclei with finely dispersed chromatin and conspicuous nucleoli, abundant granular eosinophilic cytoplasm | Present | Intraductal/Intra-cystic proliferation with papillary, fenestrated cribriform (with occasional “Roman-bridge” structures) and solid nest pattern | No | Few mitotic figures |
| F. Giovacchini et al. [1] | 2019 | 1 | 43 | F | Parotid | 1.6 | Small and monotonous tumor cells with mildly atypical nuclei and pale to eosinophilic cytoplasm | Present | Small nodules and dilated ducts with solid and cribriform pattern and cysts with comedo-like necrosis | No | Rare mitotic figures |
| A. Skálová et al. [2] | 2019 | 33 | 54 | 21 M, 12 F |
30 parotid, 1 submandibular, 2 minor salivary glands |
1.5 |
Luminal epithelial proliferation with multiple cystic, solid, cribriform or micropapillary pattern Small-medium sized tumor cells with indistinct cell borders, round/ovoid nuclei with dark condensed or finely dispersed chromatin and large pale to eosinophilic cytoplasm. Apocrine features in 6 cases |
Present | Luminal epithelial proliferation with multiple cystic, solid, cribriform, papillary or micropapillary pattern | 8 cases | Inconspicuous mitoses |
| H. Lu et al. [12] | 2019 | 1 | 79 | M | Parotid | 1.7 | Monomorphic tumor cells with round to oval nuclei with dispersed chromatin and scant cytoplasm with clear cell change. Apocrine tumor cells with large nuclei, hyperchromasia, variable nucleoli, and abundant eosinophilic cytoplasm with occasional apocrine snouts | Present | Nests or solid pattern with focal cribriform structures or large cystic spaces with cribriform and micropapillary intraductal proliferation | No | Scattered mitotic figures |
| J.A. Bishop et al. [13] | 2020 | 3 | 63 | 3 M | Parotid | 1.8 | Tumor cells with apocrine features with large round nuclei, prominent nucleoli, abundant granular eosinophilic cytoplasm and apical snouts | Present |
Large, dilated cystic spaces, with scattered smaller microcysts or cribriform nests adjacent to the dominant macrocysts with micropapillary or papillary or a “Roman-bridge”-like architecture |
No | 1–3 mitotic figures/ 10 HPF |
| Min-Shu Hsieh et al. [14] | 2020 | 9 | 70 | 4 M, 5F |
8 Parotid, 1 Submandibular gland |
3.3 (median value) |
Cuboidal tumor cells with amphophilic cytoplasm with low- to intermediate-grade features with oval nuclei, inconspicuous nucleoli, and fine chromatin Apocrine cells with eosinophilic cytoplasm, vesicular nuclei with occasionally prominent nucleoli, apical snouts, apocrine snouts, and decapitation secretions |
Present | Variable-sized cysts with micropapillary or filigreed epithelial tufts, cribriform, papillary, or solid patterns in variable proportions | 6 cases | Not reported |
Table 2.
Summary of clinicopathological features of salivary gland intraductal carcinomas reviewed in case series
| N° of selected papers | 17 | |
| Year of publication (range) | 1983–2020 | |
| N° of cases (total) | 90 | |
| Median age (years) | Min | 43 |
| Max | 79 | |
| Gender | F | 42 |
| M | 47 | |
| Unknown | 1 | |
| Anatomical site | Parotid gland | 77 |
| Minor salivary gland | 6 | |
| Intraparotid lymph node | 3 | |
| Submandibular gland | 4 | |
| Largest diameter (range) | Min | 1 cm |
| Max | 4.5 cm | |
| Tumors with invasive growth pattern | 20 |
The WHO Classification term “intraductal carcinoma” is confusing and, according to previous studies [2, 12, 12], it is feasible it will be changed again with a more appropriate definition that summarizes its origin from intercalated ducts, the intraductal or invasive nature of the lesion, and differences from other salivary gland neoplasms [2]. In our experience, this was the first case of intraductal carcinoma of salivary glands and, to the best of our knowledge, is the first case reported in a patient suffering from neurofibromatosis. Moreover, it is particular because of the combination of prominent histological and immunohistochemical features of intercalated duct type LGIC with focal apocrine differentiation and an invasive growth pattern, which complicate differential diagnosis. In our opinion, a reclassification of this pathological entity and a better definition of the morphological and molecular diagnostic parameters could make the diagnosis of this rare neoplasm more straightforward.
Acknowledgements
We wish to thank Prof. Antony Bridgewood of the Scientific Bureau of the University of Catania for language support.
Funding
POR Campania FESR 2014-2020 grant; “Technological Platform: eMORFORAD-Campania” 294 grant PG/2017/0623667.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest to disclose.
Informed Consent
Informed consent was obtained from patient for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniela Russo and Rosa Maria Di Crescenzo are co-first authors. These authors contributed equally to the work.
References
- 1.Giovacchini F, Bensi C, Belli S, Laurenti ME, Mandarano M, Paradiso D, et al. Low-grade intraductal carcinoma of salivary glands: a systematic review of this rare entity. J Oral Biol Craniofacial Res. 2019;9:96–110. doi: 10.1016/j.jobcr.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skálová A, Ptáková N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors. Am J Surg Pathol. 2019;43:1303–1313. doi: 10.1097/PAS.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JKC, Grandis JR, Takata TSP, El-Naggar AK, Chan JKC, Grandis JR, Takashi Takata PJS. WHO classification of head and neck tumours. Lyon: IARC; 2017. [Google Scholar]
- 4.Wakabayashi N, Umezawa H, Matsumoto NM, Endo Y, Naito Z, Ogawa R. Low-grade cribriform cystadenocarcinoma: a review of the literature and case report. Plast Reconstr Surg Glob Open. 2017;5:e1306. doi: 10.1097/GOX.0000000000001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faquin WC, Rossi ED, Baloch Z, Barkan GA, Foschini MP, Kurtycz DFI, et al. The Milan system for reporting salivary gland cytopathology. Cham: Springer; 2018. [DOI] [PubMed] [Google Scholar]
- 6.Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97:189–191. doi: 10.1017/S002221510009397X. [DOI] [PubMed] [Google Scholar]
- 7.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma: a distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–967. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018;42:442–452. doi: 10.1097/PAS.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordoñez B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–1021. doi: 10.1097/00000478-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Palicelli A. Intraductal carcinomas of the salivary glands: systematic review and classification of 93 published cases. Apmis. 2019;128:191–200. doi: 10.1111/apm.13009. [DOI] [PubMed] [Google Scholar]
- 11.Kuo Y-J, Weinreb I, Perez-Ordonez B. Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? Review of the literature. Head Neck Pathol. 2013;7:59–67. doi: 10.1007/s12105-013-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, Graham RP, Seethala R, Chute D. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2019;14:239–245. doi: 10.1007/s12105-018-0996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop JA, Gagan J, Krane JF, Jo VY. Low-grade apocrine intraductal carcinoma: expanding the morphologic and molecular spectrum of an enigmatic salivary gland tumor. Head Neck Pathol. 2020;14:869–875. doi: 10.1007/s12105-020-01128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh M-S, Lee Y-H, Jin Y-T, Kuo Y-J. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch. 2020;477:581–592. doi: 10.1007/s00428-020-02823-7. [DOI] [PubMed] [Google Scholar]
- 15.Tatemoto Y, Ohno A, Osaki T. Low malignant intraductal carcinoma of the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B. 1996;32:275–277. doi: 10.1016/0964-1955(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheuk W, Miliauskas JR, Chan JKC. Intraductal carcinoma of the oral cavity: a case report and a reappraisal of the concept of pure ductal carcinoma in situ in salivary duct carcinoma. Am J Surg Pathol. 2004;28:266–270. doi: 10.1097/00000478-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb I. Intraductal carcinoma of salivary gland (so-called low-grade cribriform cystadenocarcinoma) arising in an intraparotid lymph node. Head Neck Pathol. 2011;5:321–325. doi: 10.1007/s12105-011-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Liu Y, Lin X, Zhang D, Li Q, Qiu X, et al. Low-grade cribriform cystadenocarcinoma of salivary glands: report of two cases and review of the literature. Diagn Pathol. 2013;8:28. doi: 10.1186/1746-1596-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokabu S, Nojima J, Kayano H, Yoda T. Low-grade cribriform cystadenocarcinoma of the palatal gland: a case report. Oncol Lett. 2015;10:2453–2457. doi: 10.3892/ol.2015.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishijima T, Yamamoto H, Nakano T, Hatanaka Y, Taguchi K, Masuda M, et al. Low-grade intraductal carcinoma (low-grade cribriform cystadenocarcinoma) with tumor-associated lymphoid proliferation of parotid gland. Pathol Res Pract. 2017;213:706–709. doi: 10.1016/j.prp.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Nakaguro M, Urano M, Suzuki H, Yamada K, Sakaguchi A, Ogura K, et al. Low-grade intraductal carcinoma of the salivary gland with prominent oncocytic change: a newly described variant. Histopathology. 2018;73:314–320. doi: 10.1111/his.13517. [DOI] [PubMed] [Google Scholar]