Abstract
Sinonasal papilloma (SP), formerly Schneiderian papilloma, represents a rare group of benign epithelial neoplasms, most commonly identified in the sinonasal tract, while less frequently identified in the pharynx, lacrimal sac, and middle ear. Within temporal bone sinonasal-type papilloma (TBSP), there seems to be a much higher recurrence and malignant transformation risk than those identified in the sinonasal tract. Based on this clinical report and a review of the cases reported in the English literature, 49% of the 57 cases developed in the setting of concurrent or antecedent sinonasal or nasopharyngeal SP. There is an equal sex distribution (26 females and 31 males), with a broad age range (19–81 years) at presentation (median 56 years; average 54 years). Three patients had bilateral disease. Symptoms include a mass lesion with hearing loss, otitis media, otorrhea, otalgia, and tinnitus, among others. Inverted SP was identified in 42 patients, oncocytic SP in six, and exophytic SP in four (undefined in the remainder). Recurrence was identified in 38 of 49 patients with follow-up (78%), often with multiple recurrences over time, with carcinoma developing in the temporal bone in 19 patients (33%), with males developing carcinoma by a 1.7:1 ratio over females. Surgery was the treatment of choice (radical mastoidectomy) with 6 patients (10%) dead of disease (median 30 months, mean 38 months), while 47 patients were alive at last follow-up: 31 without disease (mean 33 months); 7 with locally recurrent disease (mean 20 months); 9 patients alive but with unknown disease status; and 4 patients without follow-up. In conclusion, TBSP is frequently identified in the setting of concurrent sinonasal tract disease, showing similar histologic features to sinonasal tract counterparts. There is no sex predilection, with patients most commonly presenting in the sixth decade of life. Recurrences are common, with carcinoma developing much more frequently than in sinonasal tract papilloma (33%), but recognizing that carcinoma may be documented in either or both anatomic sites. Overall outcome is excellent, with long term clinical follow-up warranted to manage recurrence or malignant transformation.
Keywords: Papilloma; Ear, middle; Temporal bone; Paranasal sinuses; Head and neck neoplasms; Mastoidectomy
Introduction
Sinonasal-type papilloma (SP), formerly Schneiderian papilloma, represents an uncommon group of benign epithelial neoplasms. These tumors have a tendency to recur, the ability to destroy surrounding structures, and a risk of malignant transformation. These tumors originate within the mucosal surfaces of the sinonasal tract, often showing a characteristic anatomic site of involvement that matches the histologic features. The three histologic subtypes of sinonasal papilloma are inverted and oncocytic types affecting predominantly the lateral nasal cavity and paranasal sinuses, and the exophytic type usually affecting the nasal septum, recognizing that overlap may be seen [1].
Tumors showing similar histological characteristics may occur less frequently in areas other than the sinonasal tract, including the pharynx, lacrimal sac and duct, and middle ear [2, 3]. Involvement of the middle ear and mastoid is explained by two possible mechanisms. The most common theory, considering the high frequency of concurrent sinonasal tract papillomas (49%), is direct extension from the sinonasal tract, usually via the eustachian tube. During embryologic development there may be ectopic migration of the ectodermally derived Schneiderian membrane to the endodermally derived mucosa of the upper aerodigestive tract, particularly of the tubotympanic recess, which becomes the tympanic cavity and the eustachian tumor [2, 4]. Isolated case reports and small series have suggested a higher recurrence and malignant transformation rate of temporal bone sinonasal-type papilloma (TBSP) compared to tumors of the sinonasal tract [2, 3, 5–10]. The purpose of this report is to present the clinicopathologic features of a new case to underscore the clinical spectrum of the disorder and set it within the context of a thorough review of the English literature on ear and temporal bone/mastoid bone sinonasal-type papilloma.
Materials and Methods
This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group relating to human subjects with appropriate informed consent and consent to publish.
A review of the English literature was based on a PubMed search from 1966 to 2021 with all 52 manuscripts of cases of ear, temporal bone, and/or mastoid Schneiderian papilloma (inverted, exophytic, or oncocytic types, including synonyms) and carcinoma ex Schneiderian papilloma evaluated [2–9, 11–49]. Search terms included: temporal bone, mastoid bone, middle ear, Eustachian tube, skull base/cranial cavity, external auditory canal, primary, secondary, multifocal, inverted papilloma, oncocytic papilloma, cylindrical cell papilloma, exophytic papilloma, Schneiderian papilloma, sinonasal papilloma, papillomatosis, squamous cell carcinoma, and malignant transformation. Cases were excluded if they did not contain clinical information (such as age, sex, anatomic site of involvement, surgery type performed), pathology descriptions, and/or histologic images to confirm the diagnosis [50, 51].
Clinical Case
A 53-year-old Caucasian male presented to the otorhinolaryngology service with a 25 year history of chronic sinusitis, nasal obstruction, and rhinorrhea. The patient experienced occasional epistaxis, nasal discharge, and anosmia. He was documented to have left lateral nasal cavity wall sinonasal papilloma, inverted type. By imaging, there was opacification of the left nasal cavity, bilateral maxillary sinuses, and left frontal, ethmoid, and sphenoid sinuses (Fig. 1). The papilloma was removed by bilateral endoscopic sinus surgery, with more papilloma on the left than the right. At the time of this surgery, 6 months prior to presentation, a left middle ear effusion was noted. The patient had a 37 pack-year history of cigarette use, and was a current smoker. His employment in the auto industry was also associated with wood-working exposure.
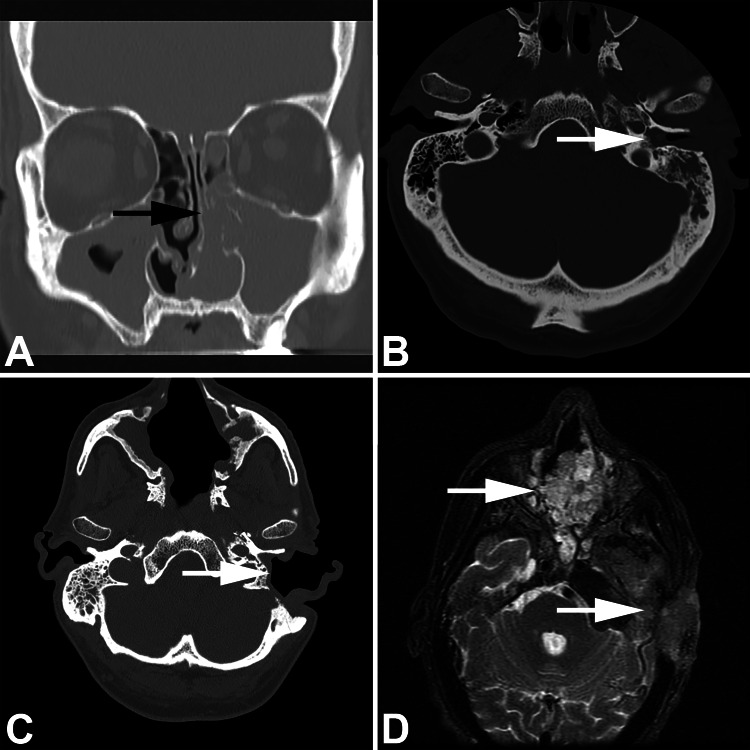
Fig. 1.
Imaging studies showing a coronal computed tomography with nasal cavity (black arrow) and maxillary sinus opacification by inverted papilloma; b middle ear (white arrow) and temporal bone opacified by papilloma; c post-surgical appearance of the ear (white arrow) and sinonasal tract; d malignant transformation identified in both the sinonasal tract (upper white arrow) and mastoid-temporal bone (lower white arrow)
About six months later he presented with ringing in the left ear with fullness and pain, requiring pain management. During placement of a drainage tube, an ear mass was identified. Concurrent endoscopy identified a papilloma at the eustachian tube orifice. CT demonstrated soft tissue opacification of middle ear and mastoid, focal tegmen, lateral mastoid cortex, and focal sigmoid sinus plate dehiscences (Fig. 1). A tympanomastoidectomy was performed, removing an inverted papilloma, histologically identical to the sinonasal tract tumor. Several months later, a bicraniofacial endoscopic approach with neurosurgery exploring the middle cranial fossa was performed to achieve a more thorough excision of both the sinonasal tract residual disease and disease in the ear, mastoid/temporal bone, and nasopharynx. At this time, histologic findings demonstrated well differentiated keratinizing squamous cell carcinoma (SCC) in the sinonasal tract, mastoid bone, and samples from the skull base. Consequently, postoperative radiation therapy (intensity-modulated radiation therapy technique to a total dose of 6600 cGy) was given to affected areas, with concurrent Cisplatin chemotherapy (100 mg/m2 once every 3 weeks for 3 weeks). Over the ensuing months, there was persistent disease in the mastoid-middle cranial fossa region and the development of metastatic disease to left neck lymph nodes. Several debulking and debridement procedures were performed to maintain skin hygiene. The tumor fungated out of the left ear and cheek, managed by debridement. The patient was managed for a pT4 pN2b M0 squamous cell carcinoma of the cranial fossa and mastoid (carcinoma ex sinonasal-type papilloma, inverted type), but with concurrent meningitis and mastoiditis, comfort measures and hospice care were eventually required. After 13 surgeries and 83 months of management, the patient died of disease.
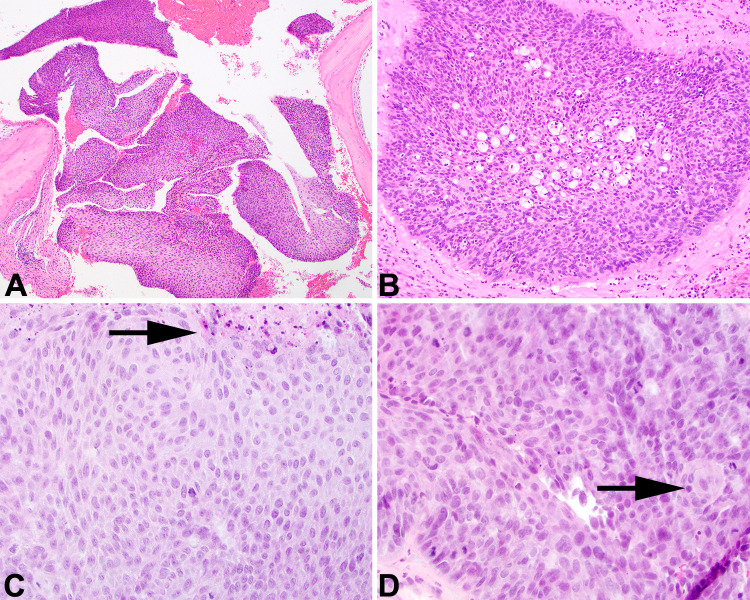
Histologically, the features of a sinonasal papilloma, inverted type (formerly Schneiderian papilloma) were identified, both in the sinonasal tract sample as well as in the ear and temporal bone specimen (Fig. 2). There was a markedly thick, inverted (endophytic) growth of nonkeratinizing transitional cells. The inverted areas were surrounded by a well-formed basement membrane without a destructive invasive growth. The cell borders were distinct, focally showing cleared cytoplasm. There was only minimal atypia. There were numerous intraepithelial microcysts containing macrophages, mucin, and cellular debris, accentuated along the luminal surface (Fig. 2). Inflammatory cells were sprinkled throughout the epithelium. In the initial material, there was no pleomorphism, no tumor necrosis, no increased or atypical mitoses, and no bone destructive invasion. However, in the subsequent resection that included the skull base, there was a well-developed SCC. There was no maturation of the process to the surface, cells were disorganized and streaming with a haphazard distribution (Fig. 2), with paradoxical maturation and keratin pearl formation. The cells were greatly enlarged with an increased nuclear to cytoplasmic ratio. Mitoses were increased and included atypical forms. Destructive infiltration into the adjacent structures was noted. Margins were not assessed, but tumor extended to the surfaces of the various samples submitted.
Fig. 2.
a Fragments of bone are seen adjacent to inverted papilloma in the middle ear. b Microabscesses are seen with a transitional-type epithelium in this area of an inverted papilloma. c Malignant transformation shows loss of maturation, tumor necrosis (black arrow), and loss of transepithelial elimination of neutrophils. d Paradoxical maturation and squamous pearl formation are seen, along with numerous mitoses, including atypical forms. There is streaming of the cells with loss of maturation
Discussion
Sinonasal papilloma (SP) were initially described by Dr. N. Ward in 1854 in Long Hospital Lancet, where he named them after Konrad Viktor Schneider, who in the 1600’s had shown the origin of nasal secretions from the ectodermal derived epithelium rather than from the central nervous system [28, 52]. However, current classification systems eschew eponyms, and so now sinonasal papilloma is the preferred term [1]. Sinonasal tract SP is uncommon, estimated to affect 2.3 patients per 100,000 population [53]. Involvement of the middle ear was documented by Stone, et al. [11] in 1987, with overall 57 cases reported in the English literature (including the present case) since that time (Table 1), where documentation is sufficient to warrant the diagnosis. Including the new case herein reported, TBSP occurred in 26 females and 31 males, with age range of 19–81 years, with a median age at presentation of 56 years (mean 54 years). While not reported in all cases, there was an equal distribution between the right (n = 27) and left (n = 21), with bilateral disease documented in three patients (no data reported in the remaining patients). Nearly all cases involved the middle ear most commonly, but concurrent disease in the external auditory canal, mastoid bone, eustachian tube, skull base, and sinonasal tract was common. As such, patients typically presented with a mass lesion. Other symptoms included hearing loss (n = 37) with conductive hearing loss more often than sensorineural; otorrhea (n = 26), otalgia (n = 12), tinnitus (n = 8), and otitis media (n = 8), along with other symptoms such as vertigo and nerve symptoms (facial nerve palsy). A ruptured membrane was documented otoscopically in 19 patients, with an intact membrane in 11 patients (not reported in the remaining cases). Symptoms were reported over a broad time range (1–360 months), with a median of 18 months (average 37 months). However, this finding may be distorted by the presence of concurrent or antecedent sinonasal tract disease, where the clinical findings may overshadow the ear and temporal bone findings. In 49% of cases, there was concurrent sinonasal tract disease or involvement of the nasopharynx. Specifically, the eustachian tube and/or orifice were commonly involved, and in all reported cases with secondary disease, it was ipsilateral, with only a few cases showing bilateral disease, but never exclusively contralateral. In the patients who presented with exclusive ear/temporal bone disease (as reported, n = 25), there were more females (n = 15) versus males (n = 10); the median age was younger (54 years versus 56 years), and the patients were less likely to develop carcinoma (5 of 26 developed carcinoma [19%] versus 14 of 28 [50%] in patients with concurrent sinonasal tract disease.
Table 1.
Patient information for papilloma of temporal bone and mastoid (literature review and current case)
| Author, year | Sex | Age | Side | Exact site | Number of recurrence | Papilloma category, with/without carcinoma | Carcinoma in temporal bone | Patient Status | Follow up in months |
|---|---|---|---|---|---|---|---|---|---|
| Stone, 1987 [11] | M | 55 | R | A, ME, E | Yes, 4 times | Inverted, with SCC | Yes | ANED | 18 |
| Altug, 1989 [12] | F | 64 | B | ME | Yes, 4 times | n/r | Yes | AWD | n/r |
| Kaddour, 1992 [13] | F | 77 | R | ME | Yes, twice | Transitional cell | No | AWD | 108 |
| Roberts, 1993 [1] | F | 19 | L | ME, E | No | Exophytic and inverted | No | ANED | 6 |
| Bold, 1995 [15] | F | 27 | n/r | ME | n/r | Inverted | No | n/r | n/r |
| Seshul, 1995 [16] | F | 31 | L | ME, S | Yes, 7 times | Inverted | Yes | AWD | 79 |
| Wenig, 1996 [2] | F | 31 | L | ME | Yes, 7 times in 180 months | Both inverted and oncocytic | No | ANED | 60 |
| F | 56 | L | ME | Yes, twice in 24 months | Both exophytic and endophytic | No | ANED | 144 | |
| F | 19 | L | ME | No | Oncocytic | No | ANED | 120 | |
| F | 57 | L | ME | Yes, twice in 72 months | Oncocytic | No | ANED | 84 | |
| F | 19 | L | ME | Yes | Oncocytic | No | ANED | 6 | |
| Jones, 1998 [4] | F | 35 | L | E | Yes, 3 times in 12 months | Papilloma | Yes | ANED | 14 |
| Vural, 1999 [17] | F | 44 | n/r | ME, S | Yes, once | Inverted | No | ANED | 96 |
| Chhetri, 2001 [18] | M | 26 | L | ME, E | Yes, once at 6 months | Papilloma | No | ANED | 14 |
| de Filippis, 2002 [9] | M | 58 | L | ME, S | No | Oncocytic | No | ANED | 8 |
| Pou, 2002 [5] | M | 54 | R | ME | Yes, 4 times | Inverted, with SCC | Yes | ANED | 11 |
| M | 81 | R | ME, S | Yes, once at 6 months | Inverted, with carcinoma in situ | No | DOD | 36 | |
| Blandamura, 2003 [19] | M | 54 | n/r | ME | n/r | Inverted | No | Alive | n/r |
| M | 58 | n/r | ME, S | n/r | Inverted | No | ANED | 14 | |
| Marioni, 2003 [20] | M | 54 | n/r | ME | n/r | Inverted | No | n/r | n/r |
| M | 58 | n/r | ME, S | n/r | Inverted | No | n/r | n/r | |
| Bui, 2004 [21] | F | n/r | R | ME, E | Yes, once at 72 months | Inverted | No | Dead | 72 |
| Mazlina, 2006 [6] | M | 54 | L | ME | Yes, once | Inverted, with SCC | Yes | Alive | n/r |
| de Menezes Santos Torres, 2007 [22] | F | 27 | R | ME, S | No | Papilloma | No | Alive | n/r |
| Acevedo-Henao, 2010 [23] | M | 63 | R | ME | Yes, 6 times | Inverted, with SCC | Yes | Alive | 12 |
| Ali, 2011 [24] | F | 42 | R | A, ME, S | n/r | Exophytic | n/r | Alive | n/r |
| Chrysovergis, 2011 [25] | M | 56 | R | E | No | Papilloma | No | ANED | 48 |
| Inoue, 2011 [26] | F | 53 | L | ME | No | Papilloma | No | ANED | 11 |
| Kainuma, 2011 [27] | M | 65 | L | N | Yes, once at 2 months | Inverted | No | ANED | 10 |
| Shen, 2011 [28] | M | 56 | L | ME, E, S | No | Papillary SCC in nasal cavity | No | ANED | 6 |
| Uchida, 2011 [29] | F | 52 | B | ME | Yes, once | Inverted | No | Alive | n/r |
| Zhou, 2011 [30] | M | 52 | L | ME, E | n/r | Inverted, with CIS | Yes | ANED | 8 |
| Lopes Barbosa, 2012 [31] | M | 46 | R | S | Yes, 9 times | Inverted | No | DOD | 2 |
| Dingle, 2012 [7] | M | 52 | B | ME, S | Yes, twice (2 and 6 months) | Inverted | Yes | AWD | n/r |
| Mitchell, 2012 [32] | F | 69 | R | A, ME, E | Yes, once | Inverted, with CIS | Yes | Alive | 36 |
| Miah, 2012 [33] | F | 73 | R | A, ME, S | Yes, once | Inverted, with SCC | Yes | ANED | 18 |
| Rubin, 2012 [34] | M | 73 | L | A, ME | Yes, 6 times | Inverted | No | ANED | 24 |
| Liu, 2013 [35] | F | 81 | R | ME, A | Yes, twice | Inverted, with papillary SCC, nonkeratinizing | Yes | Alive | 14 |
| van der Putten, 2013 [36] | F | 74 | R | ME | No | Exophytic | No | ANED | 60 |
| Carlson, 2015 [3] | M | 68 | R | ME, S, E | Yes, twice | Inverted with CIS | Yes | AWD | n/r |
| M | 65 | L | ME, S, E | No | Inverted with CIS | Yes | ANED | 18 | |
| M | 58 | R | ME, S, E | Yes, 7 times | Inverted | No | AWD | 3 | |
| M | 62 | R | ME | No | Inverted | No | ANED | 60 | |
| Schaefer, 2015 [8] | M | 46 | R | ME | No | Papilloma | No | Alive | 18 |
| Sharma, 2015 [37] | M | 67 | L | ME, S | Yes, twice | Inverted | No | Alive | n/r |
| Coca-Pelaz, 2016 [38] | M | 74 | L | A, S, ME | Yes, 3 times | Inverted | No | DOD | 24 |
| Nath, 2016 [39] | M | 60 | R | E, ME, S | Yes, twice | Inverted with CIS | Yes | n/r | n/r |
| Haywood, 2017 [40] | M | 44 | R | ME, E | Yes, twice | Inverted | No | Alive | 36 |
| Eui-Kyung, 2018 [41] | F | 39 | R | A, S | No | Inverted | No | Alive | 9 |
| Mummadi, 2018 [42] | M | 53 | R | ME, E | No | Inverted | No | ANED | 12 |
| Pla-Gil, 2018 [43] | M | 50 | R | A, ME, S | No | Inverted | No | ANED | 60 |
| Adams, 2019 [44] | F | 60 | L | ME | Yes, once | Inverted | No | Alive | n/r |
| Alghamdi, 2019 [45] | M | 76 | L | ME, S | Yes, once | Inverted, with SCC, nonkeratinizing | Yes | Alive | 48 |
| Bayindir, 2019 [46] | F | 77 | R | M, S | Yes, twice | Inverted, with CIS | Yes | DOD | 8 |
| Hasnaoui, 2019 [47] | M | 59 | L | ME | Yes, once | Inverted | No | Alive | 30 |
| Fu, 2020 [48] | F | 51 | R | A, ME, E, S | Yes, twice | Oncocytic | No | Alive | 20 |
| Marzouk, 2021 [49] | F | 68 | R | A, ME, S | Yes, once | Inverted with SCC | Yes | ANED | 17 |
| Current case | M | 53 | L | ME, S | Yes, 13 times over 83 months | Inverted, with SCC | Yes | DOD | 83 |
F female, M male, R right, L left, B bilateral, A external auditory canal, ME middle ear, E Eustachian tube, S mastoid, SCC squamous cell carcinoma, CIS carcinoma in situ, ANED alive with no evidence of disease, AWD alive with disease, DOD dead of disease, n/r not reported
With 49% of the reported cases developing in the setting of concurrent sinonasal tract or nasopharyngeal SP, it seems that direct extension via the eustachian tube is certainly a major consideration in disease development [11]. Still, with a slim majority exclusive of sinonasal tract disease, the embryological development of ectopic migration of the ectodermally derived Schneiderian membrane to the endodermally derived mucosa of the upper aerodigestive tract may account for ear and temporal bone development [2, 13]. The average length of follow-up for patients with concurrent sinonasal tract disease was 32.3 months, while 34.6 months for those without concurrent sinonasal tract disease, and thus follow-up time alone cannot be used to suggest a higher proportion showing sinonasal tract disease if followed for a longer duration. In the case herein presented, there is support for direct extension, but still both theories have good documentation.
Disease progression in the ear and temporal/mastoid bone shows a much higher recurrence risk and malignant transformation than for patients with sinonasal tract disease exclusively. Specifically, recurrence developed in 38 of 49 patients (78%) in whom follow-up information was available, usually over a variable time frame, but most were identified within 12 months of the original diagnosis (one case exceptionally developed 15 years after initial incomplete excision [2]). However, there was no comment on recurrence in many patients who were stated to be alive, but without disease status, or there was no reported data at all. As such, of those with reported data, 78% of patients developed recurrence that required additional management.
Further, while carcinoma (nearly all are squamous cell carcinoma of variable differentiation) may be seen to develop in the ear and temporal bone, sinonasal tract, or both sites, development of carcinoma in the ear and temporal bone was seen in 19 patients. Tumors were described as carcinoma in situ in several cases, but with limited sampling, lack of good orientation, and extension into skull base, middle cranial fossa, and other sites, it may be the tumor was really a SCC. Still, even when carcinoma is identified, there is a relatively good overall patient outcome. For the 57 patients reported (present case included), 6 patients had died with disease (median 30 months; mean 38 months); 47 patients were alive at last follow-up: 31 without disease (median 18 months; average 33 months); 7 with locally recurrent disease (median 6 months; mean 20 months); and 9 patients alive without disease status known; 4 patients were without follow-up. Male patients developed carcinoma at a 1.7:1 ratio over female patients, a finding that is different from sinonasal tract tumors [10]. Further, when exclusive ear and temporal bone disease is present, there is less chance of developing carcinoma, and when seen, females are less likely to develop carcinoma. In the vast majority of malignant cases reported, concurrent sinonasal tract carcinoma was also noted. Thus, if synchronous or metachronous ear/temporal bone disease is present in patients with malignant transformation in sinonasal tract tumors, it would be prudent to carefully exclude malignancy in the ear/temporal bone sites also. Thus, while recurrences and even carcinoma develop, long term follow-up is generally able to maintain control of the disease. Surgery is the mainstay of therapy, and the more aggressive the surgery performed initially, the less likely the patient was to develop recurrence: 67% recurrence with limited excisions; 10–13% with mastoidectomy/radical surgery [54].
The histological features of SP of the middle ear, mastoid, and temporal bone, are morphologically identical to those of the sinonasal tract. Still, the vast majority are inverted SP type (42 of 57 patients, 74%), with six oncocytic tumors, and four exophytic tumors (histologic type was not stated in several reports or was reported to be mixed, but histologic review of the printed pictures allowed cases to be more definitively classified). The inverted tumors may have a polypoid or papillary appearance macroscopically, but an inverted growth pattern histologically. There are multiple layers of transitional-squamous epithelium, occasionally showing surface ciliated epithelium and mucocytes, along with small collections of acute inflammatory cells. The cells had limited pleomorphism, with well-developed cell borders, but generally without frank keratinization. Mitoses were present but limited to the basal layers. Depending on the site of involvement, intimate association with bone, dura, or even nerves may be identified in otherwise histologically benign tumors. Six cases showed tall columnar stratified epithelium with oncocytically-altered epithelium, characteristic for oncocytic-type papilloma. An exophytic growth with surface keratinization was reported in four patients. If malignancy was identified, all of the reported cases were squamous cell carcinoma, identified by marked pleomorphism, increased mitoses (including atypical forms), tumor necrosis, and destructive infiltration into soft tissues and/or bone. All of the malignant cases reported have arisen from inverted papillomas rather than oncocytic or exophytic type tumors. When carcinoma was identified, it was not uncommon to employ radiation (n = 18) and/or chemotherapy (n = 2) after surgery, depending on disease extent and location [3, 5–7, 11, 12, 16, 23, 27, 28, 30, 33, 35, 39, 45, 46, 49, 55].
Testing by p16, in situ hybridization, and/or polymerase chain reaction techniques identifies HPV in 44% of cases [2–4, 7, 9, 14, 20, 27, 36, 40, 45]. However, many of the cocktails applied test only high risk types, and yet low risk HPV types are more commonly detected in sinonasal-type papilloma [56, 57]. As such, it does not seem that HPV detection or determination is critical to diagnosis or management.
These epithelial tumors have a very unique histology, but may still be confused with middle ear mixed epithelial neuroendocrine tumors (MeMeNET, formerly middle ear adenoma or neuroendocrine adenoma of the middle ear) and squamous cell carcinoma. The ribbon-like, glandular, and “infiltrative” appearance of MeMeNETs with a lack of inflammatory cells should aid in separation, while neuroendocrine markers are also helpful. A well-differentiated squamous cell carcinoma tends to show more pleomorphism, necrosis, and increased mitoses, along with destructive growth. Still, carcinoma may develop within SP, and so a careful review must be performed to exclude this possibility.
Conclusion
Sinonasal-type papillomas are rare tumors in the ear and temporal bone, with nearly 50% of patients documented to have concurrent sinonasal tract disease. Patients present over a broad age range, with a peak in the sixth decade without a sex predilection. Symptoms were non-specific and may relate to ear and temporal bone and/or sinonasal tract. Recurrences were common (78% of reported patients) with malignant transformation identified with greater frequency (33%) than in exclusively sinonasal tract sites (2–8%). The more radical and complete the initial surgery the less likelihood there was for tumor recurrence. Still, there is a good long term outcome for most patients, with close clinical follow-up advocated to manage recurrence or malignant transformation.
Funding
No external funding was obtained for this study.
Declarations
Conflict of interest
The author declares that he has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968) of Southern California Permanente Medical Group with appropriate consent. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
Informed Consent
No personally identifiable information is included in the imaging and pathology materials, with consent to publish.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunt JL, Bell D, Chiosea S, Sarioglu S, Richardson M, Lewis JS, et al. Tumours of the nasal cavity, paranasal sinuses and skull base: sinonasal papillomas. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al., editors. WHO classification of head and neck tumours. Lyon: IARC; 2017. pp. 28–31. [Google Scholar]
- 2.Wenig BM. Schneiderian-type mucosal papillomas of the middle ear and mastoid. Ann Otol Rhinol Laryngol. 1996;105:226–233. doi: 10.1177/000348949610500310. [DOI] [PubMed] [Google Scholar]
- 3.Carlson ML, Sweeney AD, Modest MC, Van Gompel JJ, Haynes DS, Neff BA. Inverting papilloma of the temporal bone: report of four new cases and systematic review of the literature. Laryngoscope. 2015;125:2576–2583. doi: 10.1002/lary.25359. [DOI] [PubMed] [Google Scholar]
- 4.Jones ME, Wackym PA, Said-Al-Naief N, Brandwein M, Shaari CM, Som PM, et al. Clinical and molecular pathology of aggressive Schneiderian papilloma involving the temporal bone. Head Neck. 1998;20:83–88. doi: 10.1002/(sici)1097-0347(199801)20:1<83::aid-hed14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Pou AM, Vrabec JT. Inverting papilloma of the temporal bone. Laryngoscope. 2002;112:140–142. doi: 10.1097/00005537-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Mazlina S, Shiraz MA, Hazim MY, Amran AR, Zulkarnaen AN, Wan Muhaizan WM. Sinonasal inverted papilloma with malignant transformation in the middle ear: a multicentric origin? J Laryngol Otol. 2006;120:597–599. doi: 10.1017/S0022215106001289. [DOI] [PubMed] [Google Scholar]
- 7.Dingle I, Stachiw N, Bartlett A, Lambert P. Bilateral inverted papilloma of the middle ear with intracranial involvement and malignant transformation: first reported case. Laryngoscope. 2012;122:1615–1619. doi: 10.1002/lary.23247. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer N, Chong J, Griffin A, Little A, Gochee P, Dixon N. Schneiderian-type papilloma of the middle ear: a review of the literature. Int Surg. 2015;100:989–993. doi: 10.9738/INTSURG-D-14-00242.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Filippis C, Marioni G, Tregnaghi A, Marino F, Gaio E, Staffieri A. Primary inverted papilloma of the middle ear and mastoid. Otol Neurotol. 2002;23:555–559. doi: 10.1097/00129492-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Nudell J, Chiosea S, Thompson LDR. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014 doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone DM, Berktold RE, Ranganathan C, Wiet RJ. Inverted papilloma of the middle ear and mastoid. Otolaryngol Head Neck Surg. 1987;97:416–418. doi: 10.1177/019459988709700416. [DOI] [PubMed] [Google Scholar]
- 12.Altug T, Sunar O, Bilgin H. Inverted papilloma. Apropos of a multicentric case. Rev Laryngol Otol Rhinol (Bord) 1989;110:299–301. [PubMed] [Google Scholar]
- 13.Kaddour HS, Woodhead CJ. Transitional papilloma of the middle ear. J Laryngol Otol. 1992;106:628–629. doi: 10.1017/s0022215100120377. [DOI] [PubMed] [Google Scholar]
- 14.Roberts WH, Dinges DL, Hanly MG. Inverted papilloma of the middle ear. Ann Otol Rhinol Laryngol. 1993;102:890–892. doi: 10.1177/000348949310201113. [DOI] [PubMed] [Google Scholar]
- 15.Bold EL, Wanamaker JR, Hughes GB, Rhee CK, Sebek BA, Kinney SE. Adenomatous lesions of the temporal bone immunohistochemical analysis and theories of histogenesis. Am J Otol. 1995;16:146–152. [PubMed] [Google Scholar]
- 16.Seshul MJ, Eby TL, Crowe DR, Peters GE. Nasal inverted papilloma with involvement of middle ear and mastoid. Arch Otolaryngol Head Neck Surg. 1995;121:1045–1048. doi: 10.1001/archotol.1995.01890090081016. [DOI] [PubMed] [Google Scholar]
- 17.Vural E, Suen JY, Hanna E. Intracranial extension of inverted papilloma: an unusual and potentially fatal complication. Head Neck. 1999;21:703–706. doi: 10.1002/(sici)1097-0347(199912)21:8<703::aid-hed4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Chhetri DK, Gajjar NA, Bhuta S, Andrews JC. Pathology forum. Quiz case 2. Schneiderian-type papilloma of the middle ear. Arch Otolaryngol Head Neck Surg. 2001;127(79):80–2. [PubMed] [Google Scholar]
- 19.Blandamura S, Marioni G, de Filippis C, Giacomelli L, Segato P, Staffieri A. Temporal bone and sinonasal inverted papilloma: the same pathological entity? Arch Otolaryngol Head Neck Surg. 2003;129:553–556. doi: 10.1001/archotol.129.5.553. [DOI] [PubMed] [Google Scholar]
- 20.Marioni G, Altavilla G, Busatto G, Blandamura S, De Filippis C, Staffieri A. Detection of human papillomavirus in temporal bone inverted papilloma by polymerase chain reaction. Acta Otolaryngol. 2003;123:367–371. doi: 10.1080/0036554021000028122. [DOI] [PubMed] [Google Scholar]
- 21.Bui M, Calès V, Barthelmé A, Deminière C, Darrouzet V. Nasal and auricular inverted papillomas. Ann Pathol. 2004;24:274–277. doi: 10.1016/s0242-6498(04)93965-x. [DOI] [PubMed] [Google Scholar]
- 22.de Santos Torres SM, Castro TW, Bento RF, Lessa HA. Middle ear papilloma. Braz J Otorhinolaryngol. 2007;73:431. doi: 10.1016/S1808-8694(15)30092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acevedo-Henao CM, Talagas M, Marianowski R, Pradier O. Recurrent inverted papilloma with intracranial and temporal fossa involvement: a case report and review of the literature. Cancer Radiother. 2010;14:202–205. doi: 10.1016/j.canrad.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Ali RB, Amin M, Hone S. Tinnitus as an unusual presentation of Schneiderian papillomatosis. Ir J Med Sci. 2011;180:597–599. doi: 10.1007/s11845-008-0268-2. [DOI] [PubMed] [Google Scholar]
- 25.Chrysovergis A, Paschalidis J, Michaels L, Bibas A. Nasopharyngeal cylindrical cell papilloma. J Laryngol Otol. 2011;125:86–88. doi: 10.1017/S0022215110002094. [DOI] [PubMed] [Google Scholar]
- 26.Inoue R, Kanazawa T, Morita M, Iino Y, Yamada S, Ishida T. Inverted papilloma of the middle ear. Otol Neurotol. 2011;32:e7–8. doi: 10.1097/MAO.0b013e3181d2f07e. [DOI] [PubMed] [Google Scholar]
- 27.Kainuma K, Kitoh R, Kenji S, Usami S. Inverted papilloma of the middle ear: a case report and review of the literature. Acta Otolaryngol. 2011;131:216–220. doi: 10.3109/00016489.2010.498025. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Baik F, Mafee MF, Peterson M, Nguyen QT. Inverting papilloma of the temporal bone: case report and meta-analysis of risk factors. Otol Neurotol. 2011;32:1124–1133. doi: 10.1097/MAO.0b013e31822a2b16. [DOI] [PubMed] [Google Scholar]
- 29.Uchida M, Fujita T, Okamoto K, Ueda M, Ushijima C, Dejima K. A case of Schneiderian middle-ear and sinonasal papilloma with intracranial complications. Nihon Jibiinkoka Gakkai Kaiho. 2011;114:768–773. doi: 10.3950/jibiinkoka.114.768. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Chen Z, Li H, Xing G. Primary temporal inverted papilloma with premalignant change. J Laryngol Otol. 2011;125:206–209. doi: 10.1017/S0022215110001829. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa JL, Pinheiro SD, Freitas MR, Nunes AA, Leite EB. Sinonasal inverted papilloma involving the middle ear and the mastoid. Braz J Otorhinolaryngol. 2012;78:122. doi: 10.5935/1808-8694.20120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell CA, Ebert CS, Buchman CA, Zanation AM. Combined transnasal/transtemporal management of the eustachian tube for middle ear inverted papilloma. Laryngoscope. 2012;122:1674–1678. doi: 10.1002/lary.23333. [DOI] [PubMed] [Google Scholar]
- 33.Miah MS, Crawford M, White SJ, Hussain SS. Malignant transformation from benign papillomatosis of the external auditory canal. Otol Neurotol. 2012;33:643–647. doi: 10.1097/MAO.0b013e31824b76d3. [DOI] [PubMed] [Google Scholar]
- 34.Rubin F, Badoual C, Moya-Plana A, Malinvaud D, Laccourreye O, Bonfils P. Inverted papilloma of the middle ear. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:207–210. doi: 10.1016/j.anorl.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu ZW, Walden A, Lee CA. Sinonasal inverted papilloma involving the temporal bone via the eustachian tube: case report. J Laryngol Otol. 2013;127:318–320. doi: 10.1017/S0022215113000042. [DOI] [PubMed] [Google Scholar]
- 36.van der Putten L, Bloemena E, Merkus P, Hensen EF. Schneiderian papilloma of the temporal bone. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma J, Goldenberg D, Crist H, McGinn J. Multifocal inverted papillomas in the head and neck. Ear Nose Throat J. 2015;94:E20–E23. [PubMed] [Google Scholar]
- 38.Coca-Pelaz A, Gomez-Martinez J, Vivanco-Allende B, Hermsen M, Llorente JL. Primary inverted papilloma of the middle ear with intracranial invasion. Head Neck. 2016;38:E105–E107. doi: 10.1002/hed.24329. [DOI] [PubMed] [Google Scholar]
- 39.Nath J, Das B. Primary inverted papilloma of middle ear and mastoid: a rare case report. J Clin Diagn Res. 2016;10:Xd01–Xd03. doi: 10.7860/JCDR/2016/18811.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haywood EB, Fuller C, Hill GW, 3rd, Olobatuyi F, Clark DW. Multifocal sinonasal inverted papilloma with middle ear involvement. Proc (Bayl Univ Med Cent) 2017;30:457–458. doi: 10.1080/08998280.2017.11930228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eui-Kyung G, Ilyoung C, Se-Joon O, Sung-Won C, Young-Keum K. A case of inverted papilloma of the mastoid cavity after cholesteatoma surgery. J Int Adv Otol. 2018;14:148–150. doi: 10.5152/iao.2018.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mummadi SM, Darr A, Hakim N, Din S, Bhimrao SK. A rare case of Schneiderian papilloma of the middle ear presenting with pulsatile tinnitus. Ann R Coll Surg Engl. 2018;100:e109–e111. doi: 10.1308/rcsann.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pla-Gil I, Morant Ventura A, Redondo Martinez J, Marco AJ. Inverted papilloma of middle ear and temporal bone. Acta Otorrinolaringol Esp. 2018;69:48–50. doi: 10.1016/j.otorri.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Adams M, Smith C, Hampton S. Isolated Schneiderian papilloma of the middle ear cleft. BMJ Case Rep. 2019;12:e228130. doi: 10.1136/bcr-2018-228130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alghamdi F, Aldohan N, Al-Otaibi S, Dababo M, Othman EO. Inverted Sinonasal papilloma involving the middle ear with evidence of squamous cell carcinoma. Cureus. 2019;11:e6176. doi: 10.7759/cureus.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayindir E, Hanege FM, Kalcioglu MT, Zenginkinet T, Celik S. A case of inverted papilloma originating from the middle ear and review of the literature. Case Rep Otolaryngol. 2019;2019:3041570. doi: 10.1155/2019/3041570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasnaoui M, Masmoudi M, Abdeljelil NB, Hmida NB, Driss N. A rare case of primary inverted papilloma of the middle ear. Pan Afr Med J. 2019;33:49. doi: 10.11604/pamj.2019.33.49.18065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu ZM, Zhao LP, Guo YY, Guan GF. A rare instance of primary oncocytic Schneiderian papilloma of middle ear and eustachian tube with a combined trans oto and nasal approach resection. J Craniofac Surg. 2020;31:504–506. doi: 10.1097/SCS.0000000000006136. [DOI] [PubMed] [Google Scholar]
- 49.Marzouk O, Brasch F, Todt I, Goon PKC, Sudhoff H. Malignant transformation of temporal bone Schneiderian papilloma associated with HPV-6. Case Rep Otolaryngol. 2021;2021:6684254. doi: 10.1155/2021/6684254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahali S, da Silva FB, Machado MC, da Silva DA, Reforeme OM, Cahali MB. Middle ear squamous papilloma: report of a case and literature review. Braz J Otorhinolaryngol. 2005;71:396–398. doi: 10.1016/S1808-8694(15)31344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avallone E, Raab P, Lenarz T, Willenborg KM. Temporal bone primary inverted papilloma—case report and review of the literature. Laryngorhinootologie. 2021;100:99–103. doi: 10.1055/a-1286-5059. [DOI] [PubMed] [Google Scholar]
- 52.Ward N. A mirror of the practice of medicine and surgery in the hospitals of London. Lond Hosp Lancet. 1854;2:480–482. [Google Scholar]
- 53.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wassef SN, Batra PS, Barnett S. Skull base inverted papilloma: a comprehensive review. ISRN Surg. 2012;2012:175903. doi: 10.5402/2012/175903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell EH, Diaz A, Yilmaz T, Roberts D, Levine N, DeMonte F, et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012;34:1372–1376. doi: 10.1002/hed.21940. [DOI] [PubMed] [Google Scholar]
- 56.Altavilla G, Staffieri A, Busatto G, Canesso A, Giacomelli L, Marioni G. Expression of p53, p16INK4A, pRb, p21WAF1/CIP1, p27KIP1, cyclin D1, Ki-67 and HPV DNA in sinonasal endophytic Schneiderian (inverted) papilloma. Acta Otolaryngol. 2009;129:1242–1249. doi: 10.3109/00016480802620647. [DOI] [PubMed] [Google Scholar]
- 57.Stoddard DG, Jr, Keeney MG, Gao G, Smith DI, Garcia JJ, O'Brien EK. Transcriptional activity of HPV in inverted papilloma demonstrated by in situ hybridization for E6/E7 mRNA. Otolaryngol Head Neck Surg. 2015;152:752–758. doi: 10.1177/0194599815571285. [DOI] [PubMed] [Google Scholar]