Abstract
Küttner tumor is an uncommon cause of salivary gland enlargement that most frequently affects the submandibular gland. More recently it has been considered a manifestation of IgG4-related disease (IgG4-RD) and synonymous with chronic sclerosing sialadenitis (CSS). We present a series of cases to emphasize this clinical entity as a disease pattern and presentation that is separate from IgG4-RD. Retrospective case series of 3 patients with unilateral salivary gland enlargement, or “Küttner tumor,” histologically classified as “chronic sclerosing sialadenitis,” The clinical history, radiology reports, laboratory studies, and pathology slides were reviewed for each case. Radiology showed discrete unilateral mass-like lesions in all three cases. Immunohistochemistry showed reduced tissue IgG4-positive plasma cells in two cases and increased numbers in one case, but insufficient to diagnose IgG4-RD. Storiform fibrosis was not seen in all cases and did not coincide with increased IgG4-positive plasma cells. A systemic workup, including serum IgG4 levels in two cases, was normal. A brief review of the literature on the spectrum of salivary gland involvement by IgG4-RD is presented. Küttner tumor is not necessarily the same as chronic sclerosing sialadenitis and is not always associated with IgG4-related disease. This report includes the second documented case of Küttner tumor of the sublingual gland.
Keywords: IgG4-related disease, Salivary gland chronic inflammation, Küttner tumor, Submandibular sialadenitis, Sublingual gland sialadenitis, Sialadenitis, Chronic sclerosing sialadenitis
Introduction
IgG4-related disease (IgG4-RD) is a multi-organ immune-mediated disorder presenting most commonly with tumor-like enlargement of affected tissues. IgG4-RD can affect a number of systems but the orbit, salivary glands, and pancreaticobiliary system are frequently affected. The definitive diagnosis of IgG4-RD is based on clinic-pathologic correlation, including histopathologic examination of affected tissues showing increased tissue IgG4 plasma cells (IgG4 cell count > 10/hpf and IgG4/IgG ratio > 40%) along with characteristic storiform fibrosis and obliterative phlebitis [1, 2]. The clinicopathologic entity "Küttner tumor" was first elucidated by Hermann Küttner in 1896 [3], long before the initial characterization of IgG4-RD in 2003. Küttner tumor was originally described as a unilateral, discrete, painless, mass-forming lesion involving the submandibular gland. In the course of historical evolution, the histologic features of Küttner tumor came to be associated with the designation of chronic sclerosing sialadenitis (CSS). Histologically, CSS shows characteristic periductal sclerosis with dense lymphocytic inflammation, including lymphoid follicle formation, and acinar atrophy. Subsequently, Geyer and colleagues made the observation that CSS, particularly when accompanied by obliterative phlebitis, showed increased IgG4-positive plasma cells, and advocated that it was a part of IgG4-RD [4]. However, it remains unclear whether elevations in tissue IgG4-positive plasma cells are seen in all clinicopathologically defined Küttner tumor. Additionally, it is increasingly recognized that nonspecific increases in tissue IgG4-expressing plasma cells occur at a variety of body sites [5, 6]. In this context, we present three cases of clinical Küttner tumor with radiologic findings and histologic examination of affected tissue, including measurement of tissue IgG4 plasma cell levels.
Report of Cases
Case 1
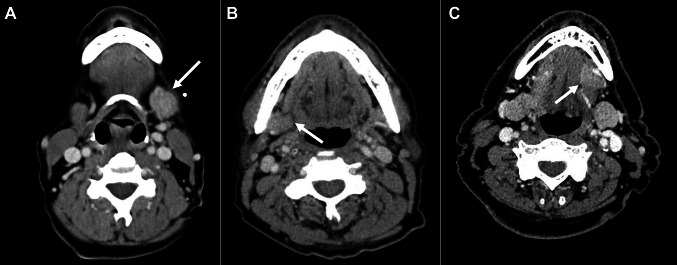
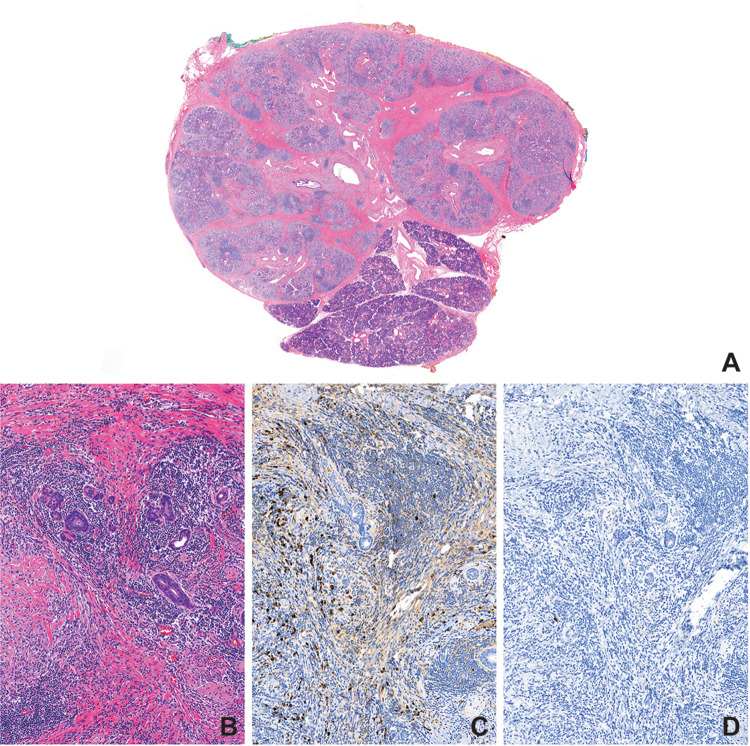
A 58-year-old woman presented to the otolaryngology clinic with painless, firm left submandibular swelling and dry mouth of 4 months duration. There were no constitutional symptoms. The patient, a former smoker who quit 6 years ago, had no personal or family history of rheumatologic disease. Her medical history was significant for asthma and hyperlipidemia. The patient was on albuterol and beclomethasone inhaler, montelukast (10 mg), meloxicam (15 mg), aspirin (81 mg) and atorvastatin (40 mg). On physical exam, the left submandibular gland measured 3 cm in diameter and was firm to palpation. Saliva could not be expressed from the gland. Facial nerve function was intact. ANA and erythrocyte sedimentation rate (ESR) were elevated (1:320 and 27 mm/h, respectively) but, SS-A, and SS-B antibody levels were within normal limits. CT scan showed slight enlargement of the left submandibular gland (Fig. 1a). Ultrasound of the mass showed several hypoechoic regions. Ultrasound-guided fine needle biopsy using a 25-guage needle was unsuccessful. Repeat procedure with 23-guage needle revealed lymphocytes and benign ductal cells consistent with sialadenitis. Due to concern for malignancy, surgical excision was recommended. Histopathology showed chronic inflammation and cellular interlobular fibrosis with areas with a storiform quality and prominent fibroblasts (Fig. 2b), with numerous plasma cells. IgG4 expression however was low (0–1/hpf in highest labeled area) (Fig. 2c, d). At post-operative follow-up, several months later, the patient reported complete resolution of symptoms other than mild xerostomia for which thrice daily pilocarpine was prescribed.
Fig. 1.
Computed tomography demonstrated diffuse, unilateral swelling and homogenous attenuation of the left submandibular gland in Case 1 (a), right submandibular gland in Case 2 (b), and left sublingual gland in Case 3 (c)
Fig. 2.
Küttner tumor may exhibit a range of histopathologic findings, from acellular interlobular fibrosis resulting in a discrete mass-forming lesion (a), to cellular interlobular fibrosis with storiform areas and chronic inflammation (b) consisting of numerous IgG-positive plasma cells (c) that did not stain positive for IgG4 by immunohistochemistry (d)
Case 2
A 76-year-old male developed painless right submandibular swelling of 3 months duration that was non-responsive to sialogogues and antibiotic therapy (amoxicillin/clavulanic acid). The patient was a never-smoker and there was no personal or family history of rheumatologic disease. His medical history was significant for hypothyroidism, atrial fibrillation, and obstructive sleep apnea. He was on apixaban (5 mg total/day) and levothyroxine (25 µg/day). Physical exam revealed an enlarged, firm, mobile, nontender right submandibular gland measuring approximately 1.5 cm. Saliva was expressible from bilateral submandibular glands. A CT scan showed a slightly enlarged right submandibular gland, normal parenchyma, and no evidence of sialolithiasis or sialadenitis (Fig. 1b). Ultrasound showed a 2.6 × 2.3 × 1.2 cm hypoechoic region with increased vascularity in the right submandibular gland abutting on homogenous submandibular tissue. MRI findings were concerning for primary tumor and a fine needle aspiration further heightened concern for a neoplasm with a diagnosis of basaloid epithelial neoplasm. The gland was therefore excised. Histopathology showed bland interlobular fibrosis (Fig. 2a) and moderate chronic inflammation; no storiform areas or cellular fibrosis were identified. The inflammatory infiltrate was rich in plasma cells, positive for both IgG and IgG4 on immunohistochemistry. The IgG4 count was 48/hpf and the IgG4/IgG ratio was ~ 30% in the highest labeled area. Additionally, foreign body giant cell reaction, surrounding calcific debris and polarizable crystals were identified, suggested early sialolith development. As other salivary glands did not appear affected, no further treatment was recommended.
Case 3
A 69-year-old asymptomatic male presented with a painless swelling of the left sublingual gland of 6 weeks duration that was recognized during a routine dental appointment. The patient was a smoker (1/2 pack per day for 23 years). There was no personal or family history of rheumatologic disease. His medical history was significant for hypertension and hyperlipidemia. The patient was on regular aspirin (81 mg), oral lisinopril and simvastatin. Examination of the oral cavity revealed a palpable, firm, nontender, submucosal mass in the region of the left sublingual gland. Saliva was readily expressed from the gland upon manipulation. CT scan revealed an enhancing mass, measuring 1.8 cm × 1.8 cm, arising from the floor of the mouth within the left sublingual space (Fig. 1c). No adjacent bony erosion or lymphadenopathy was identified, but malignancy could not be excluded. Fine needle aspiration showed benign salivary acini and fibrous tissue. The sublingual gland was excised, and histopathology showed dense fibrosis amidst salivary ducts and acini with preservation of lobular architecture. Immunohistochemistry showed scant plasma cells, predominantly negative for IgG4 (0–1/hpf in highest labeled area). Follow-up serum IgG4 levels were within normal limits. At a two-month post-operative follow-up the patient reported no recurrence of sublingual swelling or development of submandibular symptoms.
Case for Comparison
A 39-year-old woman presented for expert consultation after she underwent bilateral submandibular gland resections within a span of two years, following with a history of asymmetrical, bilateral hardening/swelling of her submandibular glands that progressed over the previous six years. Her serologic findings were negative for SS-A, and SS-B antibodies, and an ANA screen. Serum IgG4, ESR, and C-reactive protein (CRP) levels were within normal limits. There was an increased absolute eosinophil count (430/mm3 (n: 40–390/mm3). A review of the histopathology (of both glands) showed dense periductal fibrosis that was relatively bland, with a prominent eosinophilic infiltrate. Scattered periductal and periacinar dense lymphoplasmacytic aggregates were seen, with formation of germinal centers. On immunohistochemistry, performed on the right submandibular gland, the IgG4 count was 44/hpf and the IgG4/IgG ratio was 46.8% in the highest labeled area. A diagnosis of probable or possible IgG4-related disease was given.
A summary of the three cases (1–3) is presented in Table 1.
Table 1.
Summary of clinicopathologic findings for three cases (1–3) of unilateral, painless salivary gland enlargement
| Case | Clinical features | Laboratory findings | CT findings | Histopathology | IgG/IgG4 immunohistochemistry |
|---|---|---|---|---|---|
| 1 | 58-year-old female with painless, firm, left submandibular gland enlargement |
ANA elevated (1:320), ESR slightly elevated (27) IgG4, CRP, SS-A, SS-B within normal limits |
Slight enlargement of left submandibular gland | Chronic inflammation, numerous plasma cells, cellular storiform interlobular fibrosis | Positive for IgG + plasma cells; negative for IgG4 + plasma cells (0–1/hpf in highest area) |
| 2 | 76-year-old male with painless, firm, right submandibular gland enlargement | Not studied | Slightly enlarged right submandibular gland with normal parenchyma | Moderate chronic inflammation rich in plasma cells, interlobular dense fibrosis, foreign giant cell reaction to calcific debris and polarizable crystals | Positive for IgG + and IgG4 + plasma cells with highest IgG4 count—48/hpf and IgG4:IgG ratio ~ 30% |
| 3 | 69-year-old male with painless, firm, left sublingual gland enlargement | IgG4 within normal limits | Enhancing mass within the left sublingual space; no bony erosion or lymphadenopathy | Dense fibrosis with preservation of lobular architecture | Scant IgG + plasma cells; negative for IgG4 + plasma cells (0–1/hpf in highest area) |
Discussion
The differential diagnosis of submandibular gland enlargement is broad and includes lymphadenopathy, obstructive sialadenitis, infection, neoplasia, and immune-mediated conditions including IgG4-related disease (IgG4-RD) and Sjögren syndrome [7–9]. As these conditions may present with overlapping clinical, laboratory, radiographic, and histopathologic findings, an accurate diagnostic workup is essential for the provision of appropriate treatment [10]. Techniques like fine needle aspiration and flow cytometry are frontline and invaluable in the diagnosis of salivary gland tumors and lymphoproliferative neoplasms.
In 1896 Küttner described two patients with unilateral “hard [submandibular] swelling” in whom histopathologic examination revealed “the structure of the salivary gland is generally preserved…what is striking at first glance is a high degree of small cell infiltration and proliferation of connective tissue…” [3]. Though most commonly described in adults, the condition has rarely been reported in children [11]. The histopathologic correlate of the clinically diagnosed “Küttner tumor” came to be labeled chronic submandibular sialadenitis [12] and chronic sclerosing sialadenitis [13]. As the disease definition of IgG4-related disease began to crystallize in the 2000s and recognition of the entity grew, the presence of increased IgG4 plasma cells in sclerosing sialadenitis was initially described in Japanese patients [14, 15]. In 2010, Geyer and co-authors reported expression of high levels of tissue IgG4 plasma cells of patients with CSS and asserted that Küttner tumors are phenotypic expressions of IgG4-RD [4].
In the present series, all three patients presented with discrete, painless, unilateral, mass-like enlargement of the salivary gland with inflammatory histopathologic findings, thereby fulfilling criteria for diagnosis of Küttner tumor. Computed tomography findings of diffuse swelling and homogeneity were consistent with those previously described in Küttner tumor and IgG4-related disease of the head and neck [16, 17]. The pathologic findings, however, show a wide morphologic spectrum of inflammatory activity, ranging from cellular interlobular fibrosis with a storiform character with dense inflammation in Case 1 (Fig. 2b, c) to less inflamed and acellular interlobular fibrosis in Case 2 (Fig. 2a) and Case 3. Tissue infiltration by plasma cells was evident in Cases 1 and 2, but in neither were immunohistochemical parameters for diagnosis of IgG4-RD met. In Case 1, which exhibited storiform fibrosis, the IgG4-positive plasma cell count was not elevated (0–1 per high power field). Case 2, in contrast, exhibited bland collagen-rich fibrosis. The IgG4-positive plasma cell count was increased (48 IgG4-positive plasma cell/hpf), but the IgG4/IgG ratio was ~ 30%, less than that typically encountered in patients with IgG4-RD [2]. In comparing findings between cases without increased IgG4 and one with increased IgG4 plasma cells (Fig. 3), it is evident that there is significant overlap between the two, with the findings of the comparison case being most similar to those seen in Case 2. Furthermore, this highlights the importance of immunohistochemistry and IgG4 plasma cell quantification in arriving at the diagnosis.
Fig. 3.
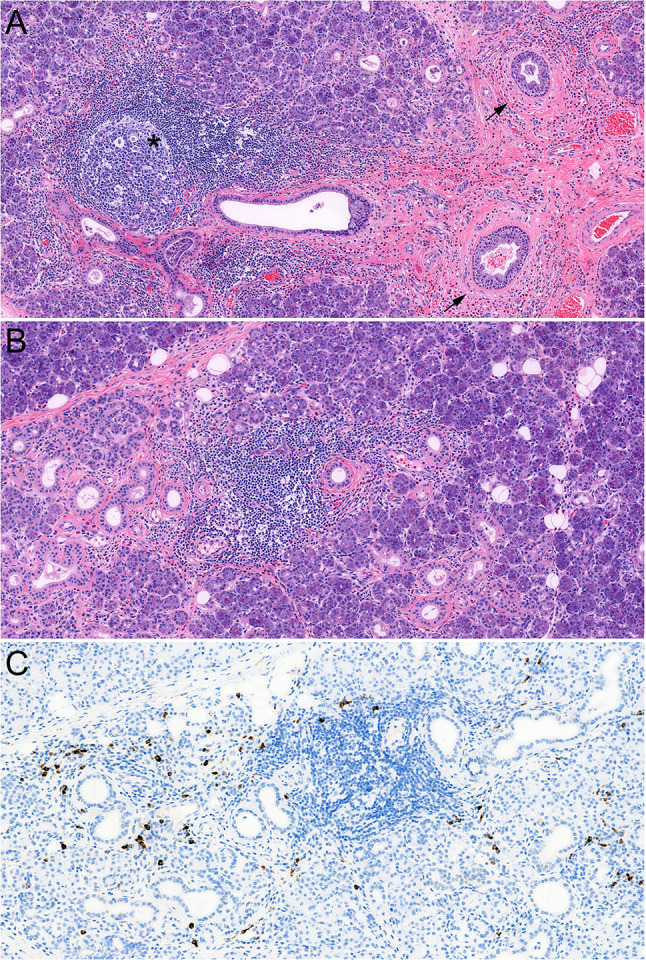
Histopathology of chronic sclerosing sialadenitis (CSS) consistent with probable/possible IgG4-related disease (comparison case, see text). There is a relatively cellular fibroinflammatory process with formation of concentric fibrosis around ducts (a, arrows) and lymphoplasmacytic inflammation with formation of germinal centers (a, asterisk). a Prominent eosinophilic infiltrate is seen in this case. b shows an acinar area with chronic inflammation and mild acinar atrophy and scattered eosinophils. c shows the same area with numerous IgG4-positive plasma cells
It is increasingly apparent that not all chronic inflammatory salivary gland enlargement is associated with histologic features of CSS, or increased levels of tissue IgG4-positive plasma cells even when features of CSS are present. In a retrospective histopathologic review of 51 cases of previously diagnosed submandibular CSS, Peuraharju et al.confirmed the diagnosis of CSS in only 34 instances, of which 8 were IgG4-positive. In addition, 4 of the 17 cases which lacked histologic features of CSS, designated “non-sclerosing chronic sialadenitis,” exhibited IgG4 positivity [18]. Harrison and Rodriguez-Justo examined 129 submandibular gland resections diagnosed as chronic submandibular sialadenitis; they found only 3 cases with elevated tissue IgG4-positive plasma cells, and these were not associated with storiform fibrosis or phlebitis [19]. Our findings align with those of both these studies, in that the histopathologic features of CSS occurred without IgG4-positive tissue plasma cell infiltration in Case 1, and in Case 2, even when IgG4-positive plasma cell infiltration was evident, parameters for diagnosis of IgG4-RD were still absent. In this context, it is noteworthy that in a study examining the presence of IgG4 plasma cells at multiple body sites, the head and neck, particularly the oral cavity, is the most common area to show a non-specific increase without the concomitant presence of other supporting histopathologic features of IgG4-RD [20].
In a retrospective series of 428 patients with clinically established IgG4-RD, Liu et al. found that in those with salivary gland involvement (IgG4-RD SG +) the disease was usually confined to the head and neck with frequent concomitant involvement of the lacrimal glands. Conversely, in the absence of salivary gland involvement (IgG4-RD SG-), the disease was more frequently systemic with involvement of multiple organs [21] Paradoxically, though, serum IgG4 levels and IgG4/IgG ratios were significantly higher in the IgG4-RD SG + group. None of the three patients in the present series had evidence for lacrimal gland involvement or elevated serum IgG4 levels.
While this study presents important information regarding the distinction between Küttner tumor and IgG4-related disease affecting the salivary glands, there are a few limitations. Firstly, this is a small series comprising three cases of Küttner tumor in the absence of IgG4-RD. Additionally, none of the three patients with isolated Küttner tumor were followed for a prolonged period of time. It is possible that one or more of them could develop localized or systemic IgG4-RD in the future. Nevertheless, these cases support our assertion that Küttner tumor, chronic sclerosing sialadenitis and IgG4-RD are not always one and the same. Küttner tumor comprises lesions that exhibit a range of inflammatory activity, some of which may fulfil the histopathologic criteria for CSS, while others may be classified as non-sclerosing chronic sialadenitis. A small cohort from either category may display elevated IgG4-positive tissue plasma cells, and an even smaller fraction might exhibit clinical and laboratory findings of systemic IgG4-RD.
In summary, Küttner tumor should be recognized as unilateral, painless salivary gland enlargement, consistent with Küttner’s original description. This entity may have numerous etiologies, one of which is IgG4-RD. Our cases lend support to the view that mass-forming lesions of the salivary glands with histopathologic features characteristic of CSS may not always be indicative of a widespread immune-mediated disease. This distinction of Küttner tumor as a clinicopathologic syndrome may beneficially impact patients who do not show signs or symptoms of systemic IgG4-RD and permit a more staged approach to clinical management of these lesions. Our findings highlight the need for a careful clinical and laboratory work-up of these patients. In the absence of systemic symptoms or elevated serum markers, systemic immunosuppressive treatment may not always be warranted. We highlight further that Küttner tumor may develop in the sublingual gland.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
Kathryn S. Marcus, BS: No conflict of interest to disclose; Henry T. Hoffman, MD: Research consultant for COOK Medical; contributing author for UpToDate; research consultant for IotaMotion®; Anand Rajan KD, MBBS: No conflict of interest to disclose.
Ethical Approval
The study was determined to meet the regulatory requirements for the protection of human subjects and approved by the University of Iowa Human Subjects Office/Institutional Review Board (IRB ID #: 201906740).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deshpande V, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 2.Wallace ZS, et al. The 2019 American College of Rheumatology/European League against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol. 2020;72(1):7–19. doi: 10.1002/art.41120. [DOI] [PubMed] [Google Scholar]
- 3.Küttner H. Uber entzundiche Tumoren der Submaaxillar-speicheldruse. Beiträge zur klinischen Chirurgie. 1896;15:815–834. [Google Scholar]
- 4.Geyer JT, et al. Chronic sclerosing sialadenitis (Kuttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34(2):202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 5.Verdijk RM, et al. Raised numbers of IgG4-positive plasma cells are a common histopathological finding in orbital xanthogranulomatous disease. Orbit. 2014;33(1):17–22. doi: 10.3109/01676830.2013.842252. [DOI] [PubMed] [Google Scholar]
- 6.Lehman JS, Smyrk TC, Pittelkow MR. Increased immunoglobulin (Ig) G4-positive plasma cell density and IgG4/IgG ratio are not specific for IgG4-related disease in the skin. Am J Clin Pathol. 2014;141(2):234–238. doi: 10.1309/AJCPTMWTCN04GSJH. [DOI] [PubMed] [Google Scholar]
- 7.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 8.Khosroshahi A, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688–1699. doi: 10.1002/art.39132. [DOI] [PubMed] [Google Scholar]
- 9.Rapidis AD, et al. Tumors of the submandibular gland: clinicopathologic analysis of 23 patients. J Oral Maxillofac Surg. 2004;62(10):1203–1208. doi: 10.1016/j.joms.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Hong X, et al. Differential diagnosis of IgG4-related sialadenitis, primary Sjogren syndrome, and chronic obstructive submandibular sialadenitis. Br J Oral Maxillofac Surg. 2017;55(2):179–184. doi: 10.1016/j.bjoms.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Melo JC, Kitsko D, Reyes-Múgica M. Pediatric chronic sclerosing sialadenitis: Küttner tumor. Pediatr Dev Pathol. 2012;15(2):165–169. doi: 10.2350/11-04-1023-OA.1. [DOI] [PubMed] [Google Scholar]
- 12.Harrison JD, Epivatianos A, Bhatia SN. Role of microliths in the aetiology of chronic submandibular sialadenitis: a clinicopathological investigation of 154 cases. Histopathology. 1997;31(3):237–251. doi: 10.1046/j.1365-2559.1997.2530856.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan JK. Kuttner tumor (chronic sclerosing sialadenitis) of the submandibular gland: an underrecognized entity. Adv Anat Pathol. 1998;5(4):239–251. doi: 10.1097/00125480-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa S, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner's tumor) Am J Surg Pathol. 2005;29(6):783–791. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- 15.Kamisawa T, et al. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6(1–2):132–137. doi: 10.1159/000090033. [DOI] [PubMed] [Google Scholar]
- 16.Fujita A, et al. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics. 2012;32(7):1945–1958. doi: 10.1148/rg.327125032. [DOI] [PubMed] [Google Scholar]
- 17.Katsura M, et al. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology. 2012;54(8):873–882. doi: 10.1007/s00234-012-1012-1. [DOI] [PubMed] [Google Scholar]
- 18.Peuraharju E, et al. Sclerosing sialadenitis of the submandibular gland is rarely an immunoglobulin G4-related disease in the Finnish population. Mod Pathol. 2019;33:551–559. doi: 10.1038/s41379-019-0395-5. [DOI] [PubMed] [Google Scholar]
- 19.Harrison JD, Rodriguez-Justo M. IgG4-related sialadenitis is rare: histopathological investigation of 129 cases of chronic submandibular sialadenitis. Histopathology. 2013;63(1):96–102. doi: 10.1111/his.12122. [DOI] [PubMed] [Google Scholar]
- 20.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64(3):237–243. doi: 10.1136/jcp.2010.085613. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Salivary gland involvement disparities in clinical characteristics of IgG4-related disease: a retrospective study of 428 patients. Rheumatology (Oxford) 2020;59(3):634–640. doi: 10.1093/rheumatology/kez280. [DOI] [PubMed] [Google Scholar]