Abstract
Ameloblastic fibro-odontoma (AFO) is a controversial, rare benign mixed odontogenic tumour that was re-defined as "developing odontoma" in the 2017 WHO classification arguing that once dental hard tissues form, it is programmed to transform into odontoma. However, AFO still remains unclear in terms of its nature. We aimed to analyze a large series of AFOs and compare it to a large series of odontomas (ODs) in an attempt to set cut-off diagnostic parameters between these entities and discuss latest updates on AFO histopathologic, clinical and molecular features. A total of 23 well-documented AFOs were analyzed versus 310 ODs focusing on the age of the patients and size of the lesions. For AFO, mean age was 9.4 ± 3.9 years (range 3–16 years) and mean size (greatest diameter) was 2.9 ± 1.5 cm (range 0.8–5.5 cm). For OD—mean age was 26.5 ± 15.6 years (range 3–81 years), mean size 1.9 ± 0.9 cm (range 1–5 cm). Receiver operating curve (ROC) showed that a cut-off age of 13.5 years and below [area under the curve (AUC) 0.902, 95%CI 0.859–0.945; p < 001; sensitivity 80%, specificity 87%] and a cut-off size of 2.1 cm and above are likely to be associated with AFO (AUC 0.7, 95%CI 0.574–0.827; p = 0.001; sensitivity 57%, specificity 77%). Thus, the combination of age and lesion size may be used to distinguish between lesions of a true neoplastic nature (i.e., AFO) and hamartomatous formation (i.e., OD). Further molecular and genetic specifications are needed to provide a better understanding on the pathogenesis of AFO in support of our suggestion and aid in an accurate classification of AFO.
Keywords: Ameloblastic fibro-odontoma, Odontoma, Odontogenic tumours, Classification, WHO
Introduction
Although more than 4 years have passed since the last World Health Organization (WHO) classification of Head and Neck Tumours, some complex issues remain unclear. Among these, ameloblastic fibro-odontoma (AFO) and ameloblastic fibro-dentinoma (AFD) which were 'removed' from the classification of odontogenic tumours as an entity and are being evaluated as developing odontomas. This last classification has emphasized that the appearance of hard tissue formation such as ‘dentin’ and ‘enamel’ is usually the first stage in maturation and more compatible with a developing odontoma [1]. These lesions are quite rare in the routine of even large departments of oral pathology, therefore, understanding their pathogenesis is complex.
The history of the WHO classification and definition of such lesions go back to the 1970s. The first WHO classification of odontogenic tumours was published in 1971 [2], followed by 1992 [3], 2005 [4] and 2017 [1] editions. Table 1 summarizes the descriptions of tumours composed of ameloblastic epithelium with/without dental hard tissues and odontomas according to each classification.
Table 1.
The list of odontogenic tumour types composed of ameloblastic epithelium and/or odontomas and their definitions according to each WHO classification
| 1971 WHO classification [2] | 1992 WHO classification [3] | 2005 WHO classification [4] | 2017 WHO classification [1] |
|---|---|---|---|
|
Ameloblastic fibroma: A neoplasm composed of proliferating odontogenic epithelium embedded in a cellular mesodermal tissue that resembles the dental papilla, but without the formation of odontoblasts Dentinoma: A very rare neoplasm composed of odontogenic epithelium and an immature connective tissue, and characterized by the formation of dysplastic dentine Ameloblastic fibro-odontoma: A neoplasm having the general features of an ameloblastic fibroma but containing dentine and enamel Odontoameloblastoma: A very rare neoplasm characterized by the presence of enamel, dentine and an odontogenic epithelium resembling that of an ameloblastoma both in structure and in behaviour Complex odontoma: A malformation in which all the dental tissues are represented, individual tissues being mainly well-formed but occurring in a more or less disorderly pattern Compound odontoma: A malformation in which all the dental tissues are represented in a more orderly pattern than in the complex odontoma, so that the lesion consists of many tooth-like structures. Most of these structures do not resemble morphologically the teeth of the normal dentition, but in each one enamel, dentine, cementum and pulp are arranged as in the normal tooth |
Ameloblastic fibroma and related lesions: Neoplasms composed of proliferating odontogenic epithelium embedded in a cellular ectomesenchymal tissue that resembles the dental papilla, and with varying degrees of inductive change and dental hard tissue formation Ameloblastic fibrodentinoma (dentinoma) and ameloblastic fibroodontoma: Lesions similar to ameloblastic fibroma, but also showing inductive changes that lead to the formationof dentine, and, ameloblastic fibro-odontoma, enamel as well Odontoameloblastoma: A very rare neoplasm which includes odontogenic ectomesenchyme, in addition to odontogenic epithelium that resembles an ameloblastoma both in structure and in behaviour. Because of the presence of odontogenic mesenchyme, inductive changes take place leading to the formation of dentine and enamel in parts of the tumour Complex odontoma: A malformation in which all the dental tissues are represented, individual tissues being mainly well forms but occurring in a more or less disorderly pattern Compound odontoma: A malformation in which all the dental tissues are represented in a more orderly pattern than in the complex odontoma, so that the lesion consists of many tooth-like structures. Most of these structures do not morphologically resemble the teeth of the normal dentition, but in each one enamel, dentine, cementum and pulp are arranged as in the normal tooth |
Ameloblastic fibroma (AF)/fibrodentinoma (AFD): AF consists of odontogenic ectomesenchyme resembling the dental papilla and epithelial strands and nests resembling dental lamina and enamel organ. No dental hard tissues are present. If there is dentin formation, the lesion is referred to as ameloblastic fibro-dentinoma Ameloblastic fibro-odontoma (AFO): AFO is a tumour, which has the histologic features of AF in conjunction with the presence of dentin and enamel Odontoma, complex type: Odontoma, complex type is a tumour-like malformation (hamartoma) in which enamel and dentin, and sometimes cementum, is present Odontoma, compound type A tumour like malformation (hamartoma)with varying numbers of tooth-like elements (odontoids) Odontoameloblastoma (OA): OA combines features of ameloblastoma with those of an odontoma |
Ameloblastic fibroma (AF): AF is a rare, benign, true mixed tumour composed of odontogenic ectomesenchyme resembling dental papilla and epithelial tissue resembling odontogenic epithelium, in which no dental hard tissues are present Odontoma: Odontomas are mixed epithelial and ectomesenchymal tumour-like malformations (hamartomas) composed of dental hard and soft tissues. They are subdivided into compound odontoma and complex odontoma |
AFO is quite a rare tumour with an estimated prevalence among oral biopsies of less than 1% and between 1 to 3% among odontogenic tumours [5, 6]. It usually occurs in patients younger than 20 years of age and has a propensity for the mandibular molar area [6].
In view of the 2017 WHO classification of AFO as a developing odontoma, we aimed to analyze a large series of AFO and compare it to a large series of odontomas (OD) for attempting to set cut-off diagnostic parameters and to discuss AFO relative to the latest updates on histopathologic, clinical, radiological and molecular features.
Material and Methods
Oral biopsy reports were retrieved and reviewed retrospectively using the archives of Oral and Maxillofacial Pathology Department of both centers, between 1971 and 2020. Cases diagnosed as AFO according to the 3rd WHO classification of odontogenic tumours [4] were revised and included only if they were well-documented. A large series of well-documented ODs submitted during the same period of time, was also collected. A total of 23 AFOs and 310 ODs were finally included in the study.
Regarding histopathologic features of AFO, cases showing the following criteria were included in the study:
Existence of distinct areas of ameloblastic fibroma (AF), including cords or islands of ameloblastic epithelium within ectomesenchyme that resembles the dental papilla; both components should be seen as a continuous solid structure without distinct lobular architecture
Dentine and enamel production should be present within the proliferating "ameloblastic fibroma-like" soft tissue
Items 1 and 2 should be intertwined throughout the lesion.
For statistical analysis, T-test was used to analyze differences between continuous variables and chi-square between categorical variables; age and gender of patients, size and location of lesions were analyzed by univariate and multivariate tests. The parameters with significant differences were further analyzed by receiver operating curve (ROC) for defining a potential discriminating cut-off between AFO and OD. Statistical tests were performed using the SPSS (version 23) software (IBM, Chicago, IL, USA). Statistical significance was set at p < 0.05.
This retrospective study was approved by the Research Ethics Committee of the Institutions (Numbers: 9703-12-SMC and 29624016-050.99- 1408-IU) in compliance with the Helsinki Declaration.
Results
A total of 23 AFOs and 310 ODs were included in the study; 160 of the ODs had been previously reported [7]. Table 2 summarizes the demographic and clinical features of AFO and OD cases. Figure 1 shows radiographic examples of these lesions.
Table 2.
Ameloblastic fibro-odontoma (AFO) and odontoma (OD)—demographic and clinical/radiological features
| AFO | OD | p-value | ||
|---|---|---|---|---|
| Age, years; mean ± SD, (range) | 9.4 ± 3.9 (3–16) | 26.5 ± 15.6 (3–81) | 0.008 | |
| Gender | Male (N, %) | 16, 69.6% | 160, 51.6% | > 0.05 |
| Female (N, %) | 7, 30.4% | 150, 49.4% | ||
| Male: Female | 2.3:1 | 1:1.1 | ||
| Location | Mandible (N, %) | 14, 60.9% (2 anterior, 12 posterior) | 193, 62.3% (59 anterior, 134 posterior) | > 0.05 |
| Anterior:Posterior | 1:6 | 1.8:1 | ||
| Maxilla (N, %) | 9, 39.1% (2 anterior, 7 posterior) | 117, 37.7% (75 anterior, 42 posterior) | 0.03 | |
| Anterior:Posterior | 1:3.5 | 1.8:1 | ||
| Size, greatest diameter, cm; mean ± SD, (range) | 2.9 ± 1.5 (0.8–5.5) | 1.9 ± 0.8 (1–5) | < 0.001 | |
Fig. 1.
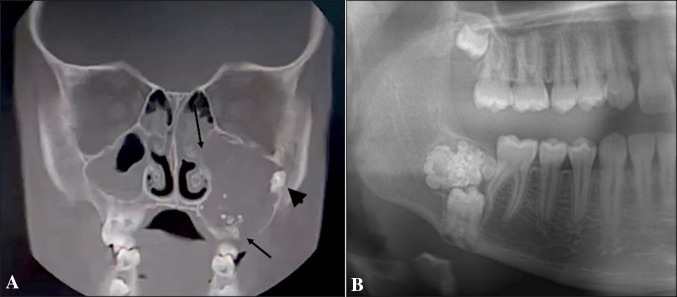
Radiographic examples of ameloblastic fibro-odontoma (AFO) and odontoma (OD). A Coronal plane cone-beam computed tomography of AFO. The lesion is radiolucent with scattered opacities in the left maxilla. It extends superiorly to the floor of the orbit and medially to the wall of the nasal cavity. Bony perforation is present (arrows) and involvement of unerupted second molar can be observed (arrow-head). B Cropped panoramic view of OD showing numerous tooth-like radio-opaque components and a radiolucent capsule in the right mandible. The lesion precludes eruption of the underneath molar
In regard to AFO, mean age was 9.4 ± 3.9 years, the male/female ratio was 2.3/1.0. The mandible/maxilla ratio was 1.55/1, the mean size of the lesions (greatest diameter) was 2.9 ± 1.5 cm. In addition, radiologically, AFOs were mixed radiolucent—radio-opaque, with the radio-opaque component being of varying size and intensity. Only five (21.7%) AFOs were multilocular (Fig. 2). Ten (43.5%) of the cases were symptomatic (swelling/facial asymmetry/pain) with an average age of 9.7 ± 4.3 years. In regard to OD, mean age was 26.5 ± 15.6 years, significantly higher than in AFO (p < 0.001), but male/female ratio (1.06/1) was similar to AFO (p > 0.05). The mandible/maxilla ratio was 1.65/1, similar to AFO (p > 0.05). Within jaws, in the maxilla there were 75 ODs in anterior and 42 in posterior and in the mandible—57 anterior and 136 in posterior areas. The mean size of ODs was 1.9 ± 0.8 cm, which was significantly lower than in AFO series (p = 0.008).
Fig. 2.
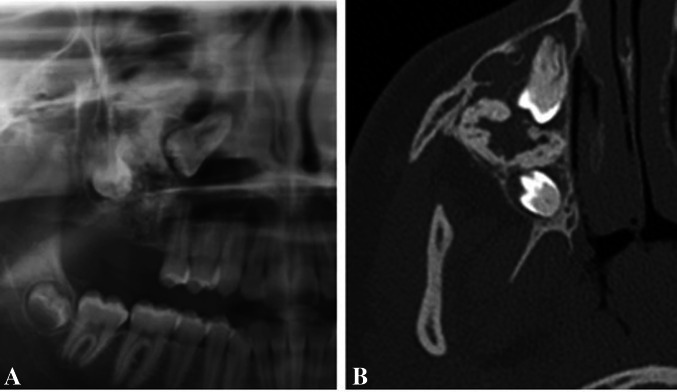
A Cropped panoramic view of an AFO showing a large, mixed radiolucent-radio-opaque lesion in the right maxilla. Margins are difficult to be defined. The first and second molars are involved and displaced. B Axial plane cone-beam computed tomography image highlights the extent of the lesion and its margins. A few bony septae denote the lesion a multilocular appearance
Univariate analysis showed that size of the lesions was positively influenced by type of lesion (β = 1.068, 95%CI 0.66–1.47; p < 0.001), negatively influenced by age [β = − 0.012, 95%CI (− 0.019)–(− 0.006); p < 0.001)], but was not affected by gender or location (p > 0.05).
Multivariate analysis showed that size of lesions was positively influenced by the interaction between age and type of lesion, so that in AFO the diameter of lesions has decreased with age less than in OD [β = − 0.111, 95%CI (− 0.019)–(− 0.202); p = 0.018)].
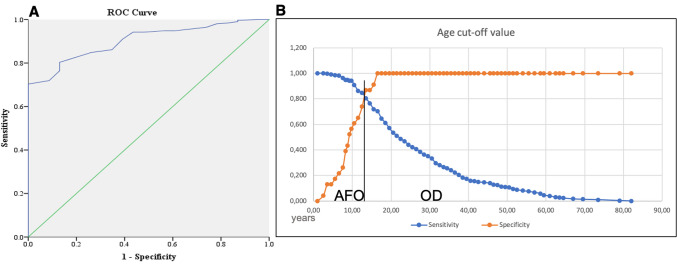
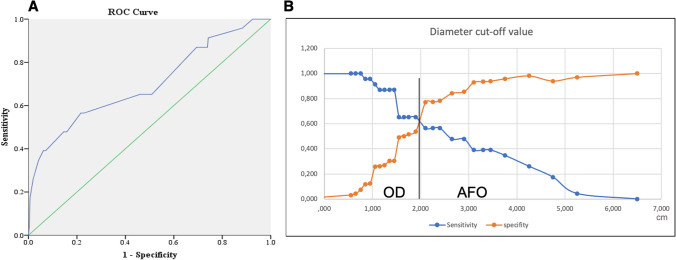
In light of the above, the ROC analysis was performed on variables of age (Fig. 3) and lesion size (Fig. 4). It was found that a cut-off age of 13.5 years and below [area under the curve (AUC) 0.902, 95%CI 0.859–0.945; p < 001; sensitivity 80%, specificity 87%] and a cut-off size of 2.1 cm and above are likely to be associated with AFO (AUC 0.7, 95%CI 0.574–0.827; p = 0.001; sensitivity 57%, specificity 77%).
Fig. 3.
Receiver operating curve (ROC) for age; A area under curve (AUC) – 0.902. B Plotted sensitivity versus specificity with cut-off value for discriminating ameloblastic fibro-odontoma (AFO) and odontoma (OD)
Fig. 4.
Receiver operating curve (ROC) for of lesion diameter; A area under curve (AUC) – 0.7. B Plotted sensitivity versus specificity with cut-off value for discriminating ameloblastic fibro-odontoma (AFO) and odontoma (OD)
Discussion
The status of AFO as a distinct entity has been revoked in the last WHO classification [1]. The histogenesis of these lesions has long been debated, and it is now thought that the great majority represent part of the spectrum of histological changes seen in a developing odontoma [8, 9]. We have presently analyzed a large series of AFOs, compared it to a large series of OD, and covered the literature in regard to current reviews, selected cases of remarkably massive AFOs and recent molecular findings.
The capacity of odontogenesis in the adult individual is programmed to cease with the establishment of the tooth buds of the permanent second and third molars [10]. However, in some circumstances, additional soft and hard dental tissues are being generated, recapitulating the embryonal series of reciprocal interactions between oral epithelium and adjacent neural crest-derived ectomesenchyme. The products of this ectopic odontogenesis vary in their ordered morphology from supernumerary teeth/compound odontomas to complex odontomas and partial and/or disorganized dental tissues, as seen in AFD, AFO and odonto-ameloblastoma [4], the last ceased to be a recognized entity in the 2017 WHO classification of odontogenic tumours [1].
The demographic and clinical features of our AFO series are compatible with the literature in terms of age (mean 9.4 ± 3.9 years, range 3–16 years), gender (M/F: 2.3/1.0) and localization (mandible predilection) [6, 11]. On the other hand, these results are not compatible with the 2005 WHO classification, which claimed that there is no predilection for either gender nor location [4]. Regarding the radiological features, our findings revealed that unilocular (78%) radiolucent lesions mixed with opacities of variable degrees was the most common presentation of AFO. These results are consistent with those reported in the large systematic review by Chrcanovic and Gomez [6].
There are published cases of massive maxillary AFOs which caused destruction of the sinus, facial disfigurement, perforated the cortical plates or extended to the orbital floor-pterygoid region [12, 13]. Another large mandibular AFO case, extending from the first molar to the coronoid process and the condylar neck with well-circumscribed borders and displacing the developing second molar tooth bud down to the inferior border of the mandible, was reported by Kirjavainen et al. [14]. An 11-year-old girl with a very large mixed tumour in the right maxillary sinus lesion with a missing first molar was also reported [15] and another left maxillary large swelling extended from the floor of the left maxillary sinus to the orbital floor, where the presence of an impacted tooth was described and root resorption of the upper left first molar, was also observed [16]. In light of these reports, it seems that some AFOs differ significantly from what would be the expected biological behavior of hamartomatous lesions (i.e., developing OD), by having a considerable potential for growing and causing considerable facial deformity and bone perforation. Furthermore, a malignant counterpart of AFO, the ameloblastic fibro-odontosarcoma, is a recognized entity with several well documented published cases [17, 18].
It is the authors' opinion that the major problem with AFO is that the quality of the literature is remarkedly poor and diagnostic criteria are not fully known. When the literature has been reviewed, it was observed that many AFO cases did not contain sufficient clinical and pathological information. Small AF-like areas (lobular structures and hypercellular areas only around the epithelium) near mature ODs should not be considered as AFO. In fact, these areas may represent areas of distorted dental papillae as a factor of the plane of sectioning. The 2017 WHO classification remarked that most lesions formerly designated as AFO probably represent immature stages of ODs [1], which is largely accepted, however, it should be emphasized that true AFOs merit to be a recognized separate entity. In a previous study of one of the authors (MST) on 160 cases of ODs [7], AF-like changes were observed in only 5 cases and patients were in the 3rd to 4th decades. On the other hand, in 7 ODs diagnosed in the 1st decade, areas of hard tissue production containing large amounts of enamel/dentin were observed. Therefore, the emerging question would be on the time interval that allows us to refer to AFOs as early/developing odontomas. According to our results, we can suggest that lesions diagnosed in patients younger than 13.5 years with a diameter larger than 2 cm are likely to be considered as true neoplasia rather than developing odontomas, especially in those cases which differential diagnosis cannot be made histopathologically. As ODs tend to be smaller lesions than AFOs, it would not be rational to assume that as "AFOs" mature their dimension tend to decrease.
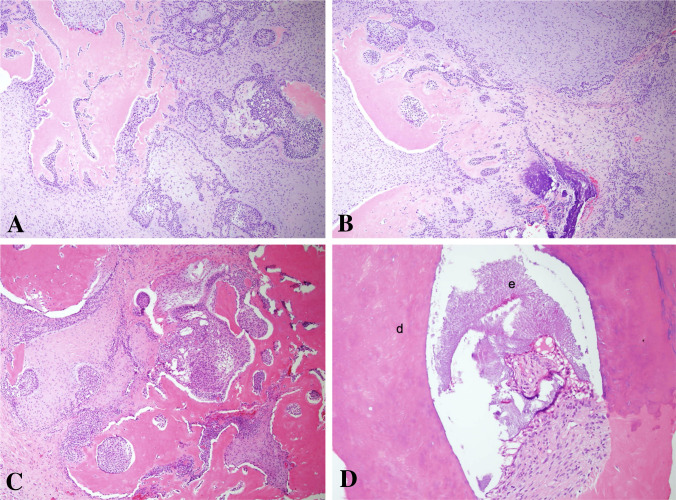
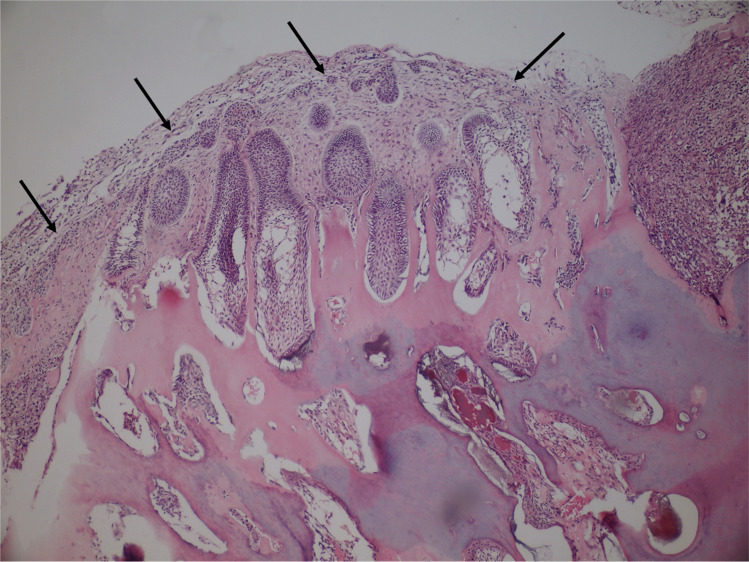
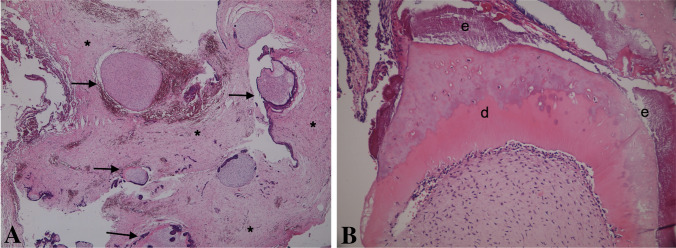
From a histopathological point of view, we may suggest that features favoring a diagnosis of AFO should consist of a very clear area of AF with narrow/"drum-stick"-like cords/islands of ameloblastic epithelium and ectomesenchyme resembling dental papilla. The stromal component should be hypercellular, evenly distributed among the epithelial components and lack any lobular architecture (Fig. 5A–C). Dentine and enamel production should occur randomly within the AF areas (Fig. 5D). In contrast, ODs usually show a compartmentalization, where large areas of hard tissue production are located in the center of the lesion, while the AF-like changes are observed at the periphery (Fig. 6). Even in cases where this zonation is less obvious, it should be noted that the epithelial component in ODs generally demonstrates distinct ameloblastic differentiation and the stromal component shows lobular arrangement and hypercellular areas being located mostly around the epithelial component. Presence of ghost cells, cyst-like structures, cementum, cords and strands of ameloblastic epithelium in a mature but loose connective tissue, most probably represent an OD rather than AFO. Developing compound ODs show several dysmorphic tooth germs in a loosely textured connective tissue with cords and islands of odontogenic epithelium and varying amounts of hard tissue might be observed (Fig. 7). This type of stroma differs from that of AFO, as the latter resembles embryonic tooth pulp due to the myxoid appearance and abundant stellate-shaped fibroblasts with long slender cytoplasmic extensions. Developing complex OD and AFO might sometimes be impossible to differentiate on histopathological grounds. Based on the findings of this study, we suggest the combination of age and lesion size be used to distinguish between lesions of a true neoplastic nature and hamartomatous formation. In those challenging cases in which neither histopathological features nor clinical/radiological findings can aid in reaching a definite diagnosis, it should be prudent to keep such cases in the spectrum of developing ODs.
Fig. 5.
Histopathologic finding of ameloblastic fibro-odontoma. A, B The soft tissue component of the tumor is similar to ameloblastic fibroma with a haphazard arrangement of hard tissue components, (H&E × 100). C Formation of disorganized tooth structures can be seen (H&E, × 100). D Higher magnification of these structures, dentin (d) and enamel matrix (e) (H&E, × 200)
Fig. 6.
Histopathologic finding of odontoma with ameloblastic fibroma-like areas (arrows) at the periphery. Please note the distinct ameloblastic differentiation of epithelial islands (H&E, × 100)
Fig. 7.
Histopathologic finding of developing odontoma. A Small structure of tooth germ like appearance (arrows) and loose connective tissue (asterisks) (H&E × 40). B Enamel (e) and dentin (d) production (H&E, × 200)
Hamartomas, like odontomas, are focal proliferation of cells and tissues typically found in the organ from which they arise [19]. Once thought to be a developmental malformation unworthy of the "oma" designation, many, in fact, have been recently found to harbor clonal chromosomal aberrations that are acquired through somatic mutations and on this basis are now considered neoplasms [20]. In other words, the line of demarcation between hamartomas and benign neoplasms is often unclear, since both lesions can be clonal. A hamartoma, however, contrary to a neoplasm, shows a self-limited growth [19, 20].
In this line, several studies have investigated the cellular and molecular engines that can lead to the generation of the ectopic teeth/dental tissues. It has been found that human ODs harbor putative post-natal mesenchymal stem cells with the ability to proliferate and differentiate into dental structures (enamel, dentin, cementum, pulp) [21]. Mesenchymal cells isolated from ODs expressed LHX8, an important factor in tooth morphogenesis, at a higher intensity than mesenchymal stem cells isolated from adult teeth, raising the possibility of its involvement in formation of ODs [22, 23]. In adult mice, up-regulation of the WNT/β-catenin pathway induced formation of ODs [24]. Furthermore, overexpression of IKKB, a factor for activation of the NF-kB family of transcriptor factors (concomitant to upregulation of β-catenin and downregulation of several tumour suppressor proteins, such as p53, p16 and p19) induced the formation of supernumerary teeth or even of an odontogenic tumour, equivalent with the human odonto-ameloblastoma [25]. Nestin, a marker of neuronal stem cells, is related to tooth development, especially to differentiation toward odontoblasts and dentin production. Normally, its expression becomes undetected by completion of the dentition. However, intense expression of nestin has been found in the ectomesenchymal tissues of ODs, AFD and AFO, particularly adjacent to odontogenic epithelium [26]. Lastly, on a genetic level, loss of heterozygosity (LOH) of tumour suppressor gene loci was investigated in AF, AFO and ameloblastic fibro-sarcoma (AFS). The most frequent LOH was found in relation to p53 and CHRNB1, however, the mean fractional allelic loss of the benign lesions (AF and AFO) was 22%, whereas of AFS—74.6%, implying that the different pattern and extent of LOH of tumour suppressor genes regulate changes in tumour behavior [27]. BRAF p.V600E mutation, initially known to characterize the epithelial component of ameloblastomas, was also identified in the mesenchymal component of AF (40%), AFD (50%), AFO (33%), and AFS (67%), but not in ODs [28]. Collectively, these findings suggest that at least a subset of AF, AFD and AFO are molecularly distinct from OD, and may represent distinct entities and be of a true neoplastic nature.
Classification of odontogenic tumours is challenging particularly in cases of histopathological overlaps, when separate small entities might "disappear" upon being aggregated into larger and less unique entities. It is well known that odontogenic tumours are derived from ectomesenchymal and/or epithelial tissues that constitute the tooth-forming apparatus. Like normal odontogenesis, the odontogenic tumours represent inductive interactions between odontogenic ectomesenchyme and epithelium [29, 30]. Therefore, it is inevitable that some histological features are overlapping, so it is necessary to interpret these important overlaps by combining them with clinical and radiological findings and to give the correct diagnosis.
In conclusion, in view of the findings of the present study and updated literature, we suggest to reconsider at least a part of the AFOs, especially those in patients younger than 13.5 years with lesions of 2.1 cm and larger in diameter, as representing true tumours rather than developing odontomas. Further molecular and genetic specifications are needed to provide a better understanding on the pathogenesis of AFO in support of our suggestion and aid in an accurate classification of AFO.
Funding
No funding obtained.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest to disclose.
Ethical Approval
This retrospective study was approved by the Research Ethics Committee of the Institutions (Numbers: 9703-12-SMC and 29624016-050.99- 1408-IU) in compliance with the Helsinki Declaration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg P, editors. WHO classification of head and neck tumours. Chapter 8: odontogenic and maxilofacial bone tumours. 4. Lyon: IARC; 2017. pp. 205–60. [Google Scholar]
- 2.Pindborg JJ, Kramer IR, editors. Histological typing of odontogenic tumours, jaw cysts, and allied lesions. International Histological Classification of Tumours, number 5. Geneva: World Health Organization; 1971. [Google Scholar]
- 3.Kramer IR, Pindborg JJ, Shear M, editors. Histological typing of odontogenic tumours. World Health Organization International Histological Classification of Tumours. 2. Berlin: Springer-Verlag; 1992. [Google Scholar]
- 4.Barnes L, Eveson J, Reichart P, Sidransky D, editors. WHO head and neck tumours. Chapter 6: odontogenic tumours. 3. Lyon: IARC; 2005. pp. 283–27. [Google Scholar]
- 5.Soluk-Tekkesin M, Cakarer S, Aksakalli N, Alatli C, Olgac V. New World Health Organization classification of odontogenic tumours: impact on the prevalence of odontogenic tumours and analysis of 1231 cases from Turkey. Br J Oral Maxillofac Surg. 2020;58(8):1017–1022. doi: 10.1016/j.bjoms.2020.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Chrcanovic BR, Gomez RS. Ameloblastic fibrodentinoma and ameloblastic fibro-odontoma: an updated systematic review of cases reported in the literature. J Oral Maxillofac Surg. 2017;75(7):1425–1437. doi: 10.1016/j.joms.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Soluk Tekkesin M, Pehlivan S, Olgac V, Aksakallı N, Alatli C. Clinical and histopathological investigation of odontomas: review of the literature and presentation of 160 cases. J Oral Maxillofac Surg. 2012;70(6):1358–1361. doi: 10.1016/j.joms.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472(3):331–39. doi: 10.1007/s00428-017-2182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JM, Vered M. Update from the 4th edition of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11(1):68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovorakova M, Lesot H, Peterka M, Peterkova R. Early development of the human dentition revisited. J Anat. 2018;233(2):135–145. doi: 10.1111/joa.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchner A, Kaffe I, Vered M. Clinical and radiological profile of ameloblastic fibro-odontoma: an update on an uncommon odontogenic tumor based on a critical analysis of 114 cases. Head Neck Pathol. 2013;7(1):54–63. doi: 10.1007/s12105-012-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piette EM, Tideman H, Wu PC. Massive maxillary ameloblastic fibro-odontoma: case repot with surgical management. J Oral Maxillofac Surg. 1990;48:526–530. doi: 10.1016/0278-2391(90)90247-Y. [DOI] [PubMed] [Google Scholar]
- 13.Hegde V, Hemavathy S. A massive ameloblastic fibro-odontoma of the maxilla. Indian J Dent Res. 2008;19:162–164. doi: 10.4103/0970-9290.40474. [DOI] [PubMed] [Google Scholar]
- 14.Kirjavainen A, Tuovinen V, Sándor GK. Large ameloblastic fibro-odontoma in a 7-year-old girl with analysis of 108 cases. Ann Maxillofac Surg. 2016;6(1):15–20. doi: 10.4103/2231-0746.186132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banihashem Rad SA, Mortazavi H, Eshghpour M, Salehinejad J, Shahakbari R. A large ameloblastic fibro-odontoma of the maxillary sinus. Iran J Otorhinolaryngol. 2014;26(75):111–114. [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuruki S, Kousuke K, Ichinokawa Y, Tsuruki T. A case of ameloblastic fibro-odontoma occupying the left maxillosinus which was enucleated by the Le Fort I osteotomy. J Oral Maxillofac Surg Med Pathol. 2015;27(3):380–384. doi: 10.1016/j.ajoms.2014.05.008. [DOI] [Google Scholar]
- 17.Howell RM, Burkes EJ., Jr Malignant transformation of ameloblastic fibro-odontoma to ameloblastic fibrosarcoma. Oral Surg Oral Med Oral Pathol. 1977;43(3):391–401. doi: 10.1016/0030-4220(77)90326-7. [DOI] [PubMed] [Google Scholar]
- 18.Chrcanovic BR, Gomez RS. Ameloblastic fibrodentinosarcoma and ameloblastic fibro-odontosarcoma: a systematic review. J Stomatol Oral Maxillofac Surg. 2018;119(5):401–406. doi: 10.1016/j.jormas.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Leiter Herrán F, Restrepo CS, Alvarez Gómez DI, Suby-Long T, Ocazionez D, Vargas D. Hamartomas from head to toe: an imaging overview. Br J Radiol. 2017;90(1071):20160607. doi: 10.1259/bjr.20160607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V, Abbas AK, Ater JC. Robbins&Cotran pathologic basis of disease chapter 7: neoplasia. 9. Amsterdam: Elsevier; 2015. p. 267, 473. [Google Scholar]
- 21.Song JS, Stefanik D, Damek-Poprawa M, Alawi F, Akintoye SO. Differentiation and regenerative capacities of human odontoma-derived mesenchymal cells. Differentiation. 2009;77(1):29–37. doi: 10.1016/j.diff.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabay RJ. An overview of molecular and genetic alterations in selected benign odontogenic disorders. Arch Pathol Lab Med. 2014;138(6):754–758. doi: 10.5858/arpa.2013-0057-SA. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Jeon SH, Park JY, Suh JD, Choung PH. Comparative study of LHX8 expression between odontoma and dental tissue-derived stem cells. J Oral Pathol Med. 2011;40(3):250–256. doi: 10.1111/j.1600-0714.2010.00970.x. [DOI] [PubMed] [Google Scholar]
- 24.Xavier GM, Patist AL, Healy C, Pagrut A, Carreno G, Sharpe PT, et al. Activated WNT signaling in postnatal SOX2-positive dental stem cells can drive odontoma formation. Sci Rep. 2015;5:14479. doi: 10.1038/srep14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page A, Bravo A, Suarez-Cabrera C, Sanchez-Baltasar R, Oteo M, Morcillo MA, et al. IKKβ overexpression together with a lack of tumour suppressor genes causes ameloblastic odontomas in mice. Int J Oral Sci. 2020;12(1):1. doi: 10.1038/s41368-019-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita S, Hideshima K, Ikeda T. Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. J Clin Pathol. 2006;59(3):240–245. doi: 10.1136/jcp.2004.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvão CF, Gomes CC, Diniz MG, Vargas PA, de Paula AM, Mosqueda-Taylor A, et al. Loss of heterozygosity (LOH) in tumour suppressor genes in benign and malignant mixed odontogenic tumours. J Oral Pathol Med. 2012;41(5):389–393. doi: 10.1111/j.1600-0714.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- 28.Coura BP, Bernardes VF, Ferreira de Sousa S, Diniz MG, Moreira RG, Benevenuto de Andrade BA, et al. Targeted next-generation sequencing and allele-specific quantitative PCR of laser capture microdissected samples uncover molecular differences in mixed odontogenic tumors. J Mol Diagn. 2020;22(12):1393–9. doi: 10.1016/j.jmoldx.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Nanci A. Ten cate’s oral histology: development, structure, and function. 7. St. Louis: Mosby, Elsevier; 2008. pp. 79–107. [Google Scholar]
- 30.Wright JM, Soluk Tekkesin M. Odontogenic tumors: where are we in 2017? J Istanb Univ Fac Dent. 2017;51(3 Suppl 1):S10–S30. doi: 10.17096/jiufd.52886. [DOI] [PMC free article] [PubMed] [Google Scholar]