Abstract
Squamous cell carcinoma of the sinonasal tract is relatively rare and morphologically and genetically heterogeneous. We report the case of an adult male with a left sphenoid sinus mass. A biopsy revealed an undifferentiated carcinoma composed of sheets of epithelioid cells lacking keratinization and glandular formation. The tumor was associated with a prominent lymphoplasmacytic inflammatory infiltrate. Immunohistochemical staining demonstrated diffuse expression of pankeratin and p63; it was negative for p16. In addition, EBER was also negative. Morphologically the findings raised the possibility of non-keratinizing squamous cell carcinoma. RNA sequencing was undertaken to exclude the possibility of NUT carcinoma; interestingly, this revealed a novel ETV6-TNFRSF8 fusion transcript, which was independently confirmed by fluorescence in situ hybridization. The current case is illustrative because it broadens our understanding of the molecular pathogenesis of non-keratinizing squamous cell carcinoma and adds to the diversity of ETV6-rearranged malignancies.
Electronic supplementary material
The online version of this article (10.1007/s12105-020-01249-6) contains supplementary material, which is available to authorized users.
Keywords: Sinonasal carcinoma, ETV6, TNFRSF8
Introduction
Squamous cell carcinoma arising within the sinonasal tract is relatively uncommon and is currently divided by the World Health Organization into: keratinizing, non-keratinizing, spindle cell (sarcomatoid), and lymphoepithelial subtypes [1]. Risk factors vary from environmental exposures to infectious aetiologies (i.e., human papillomavirus and Epstein-Barr virus). The underlying molecular attributes tend to overlap with tumors at other anatomic sites, which mostly exhibit complex karyotypes and aneuploidy with frequent loss of TP53 and CDKN2A/B tumor suppressor genes [2–6]. In contrast to salivary gland neoplasms, chromosomal translocations are relatively uncommon in squamous cell carcinoma. Herein we report the case of an adult male with a poorly differentiated carcinoma of the sphenoid sinus harboring a novel ETV6-TNFRSF8 gene fusion.
Case Report
A 66-year-old male presented with diplopia and mild proptosis with no sinonasal symptoms. CT and MRI imaging showed an infiltrative mass in the base of skull centered along the left sphenoid sinus and extending into the left cavernous sinus and sella, which prompted an endoscopic sphenoidotomy and biopsy. Microscopic examination revealed a neoplasm composed of sheets of epithelioid cells with a vague fascicular-storiform pattern (Fig. 1a–c). The cytoplasm was abundant and eosinophilic and lacked overt keratinization. The nuclei were ovoid with mild-moderate pleomorphism and prominent small nucleoli; mitotic activity was conspicuous (Fig. 1d). There was a brisk lymphoplasmacytic inflammatory infiltrate and occasional neutrophils (Fig. 1c, d). Immunohistochemical staining demonstrated the tumor was diffusely positive for keratin (AE1/AE3), p40 and p63; it was negative for p16, CD30, CD45, CD56, and S100; INI-1 was intact (Fig. 2). In situ hybridization was negative for Epstein-Barr virus-encoded small RNAs (EBER) (not shown). Based on the findings, the differential diagnosis included non-keratinizing squamous cell carcinoma, NUT carcinoma, adamantinoma-like Ewing sarcoma, and adenoid cystic carcinoma. Targeted RNA sequencing was performed which excluded the presence of NUTM1, EWSR1, FUS, MYB, MYBL1 and NFIB rearrangement, amongst others (Illumina TruSight RNA fusion panel; San Diego, CA). Surprisingly, it revealed a novel ETV6-TNFRSF8 fusion product involving ETV6 (exon 5 of 8; NCBI Reference Sequence: NM_001987.4) and TNFRSF8 (exon 13 of 15; NM_001243.4). Independent validation of this event by fluorescence in situ hybridization (FISH)—using custom probes from bacterial artificial chromosomes (Supplementary Table 1)—confirmed the presence of ETV6 rearrangement (Fig. 3). The molecular findings therefore suggest a novel form of non-keratinizing squamous cell carcinoma.
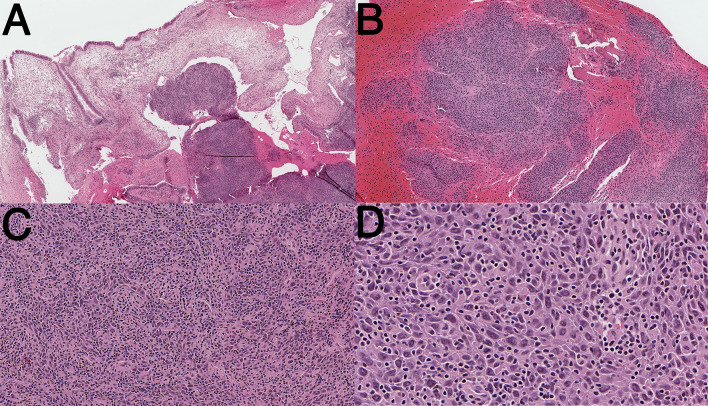
Fig. 1.
Representative photomicrographs of ETV6-TNFRSF8 associated carcinoma (Hematoxylin and Eosin). a Low-power magnification showing respiratory-type mucosa overlying neoplasm. b Intermediate power magnification demonstrating infiltration of bone. c Intermediate magnification showing sheets of epithelioid to spindled cells with a prominent lymphoplasmacytic inflammatory infiltrate. d High power magnification highlighting moderate nuclear atypia and numerous mitotic figures. There is no evidence of overt keratinization, or glandular formation
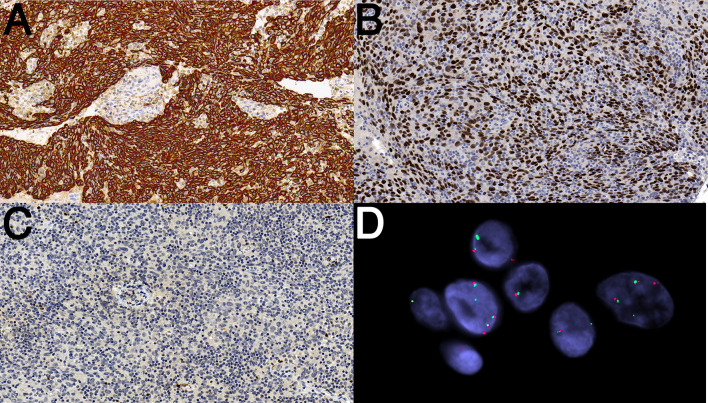
Fig. 2.
Immunohistochemical staining and fluorescence in situ hybridization of ETV6-TNFRSF8 associated carcinoma. a Diffuse cytoplasmic immunostaining for pan-cytokeratin. b Diffuse nuclear immunostaining for p63. c The tumor is negative for p16. d Florescence in situ hybridization showing unbalanced ETV6 gene rearrangement with deletion of the centromeric signal (red), in cells with 2n, 3n, and 4n copies (polysomic) of ETV6 (red, centromeric; green, telomeric)
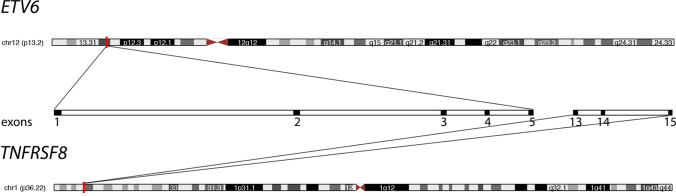
Fig. 3.
Diagrammatic representation of ETV6-TNFRSF8 fusion transcript. The gene location is highlighted by red band on each chromosome (http://genome.ucsc.edu) [31]. The expanded view in the center of the image depicts the spatial relationship of the exons for each gene
Staging revealed T4N2M0 disease. The patient received induction chemotherapy followed by concurrent chemoradiotherapy with high-dose cisplatin. There was no evidence of recurrence at the 1-year follow-up.
Discussion
In contrast to salivary gland neoplasms, oncogenic fusion genes are relatively uncommon in squamous carcinoma of the head and neck. A notable exception includes NUT carcinoma—characterized by NUTM1-rearrangement—which may represent an aggressive subtype of squamous cell carcinoma [6]. More recently, squamous cell carcinomas with other gene fusions have been reported in the literature. For example, the DEK-AFF2 fusion gene in a subset of non-keratinizing squamous cell carcinoma of the middle ear and temporal bone [7, 8]. These neoplasms can be morphologically undifferentiated, necessitating immunohistochemistry to highlight evidence of squamous differentiation. Herein we describe another carcinoma, arising at the base of skull, which harbored a novel ETV6-TNFRSF8 fusion product.
ETV6 encodes ETS variant transcription factor 6, which is normally involved in hematopoiesis through the regulation of stem cells and maintenance of vascular networks [9]. ETV6 fusions with various partner genes are well-documented in hematologic malignancies; however, ETV6 rearrangements in solid tumors are increasingly recognized [9]. The ETV6-NTRK3 fusion gene has been demonstrated in secretory carcinoma of the breast [10], salivary gland [11], thyroid gland [12, 13] and skin [14] as well as in low-grade sinonasal adenocarcinoma [15, 16], infantile fibrosarcoma [17], congenital mesoblastic nephroma [18], ALK-negative inflammatory myofibroblastic tumor [19], gastrointestinal stromal tumor [20], radiation-associated papillary thyroid carcinoma [21], pediatric high grade glioma [22] and melanocytic neoplasms such as Spitz tumors [23]. Alternate ETV6 fusion partners have also been described. These include ALK in epithelioid fibrous histiocytoma [24] as well as RET, MET and MAML3 in secretory carcinoma of the salivary glands [25, 26]. To the best of our knowledge, TNFRSF8 has not previously been reported as an ETV6 fusion partner.
The TNFRSF8 gene is a member of the tumor necrosis factor receptor superfamily. It encodes for CD30 protein, a well-known marker of Hodgkin and anaplastic large cell lymphoma [27]. CD30 interacts with its ligand, CD30L, to activate NF-κB, MAP kinase, and Akt signaling pathways [27, 28]. Depending on the specific cell type and costimulatory signals involved, signal transduction can either promote cell proliferation and survival or promote apoptosis and cell cycle arrest [28, 29]. Rare cases of lung adenocarcinoma and breast carcinoma harboring TNFRSF8- PLEKHG5 and TNFRSF8-STARD13 fusions, respectively, have been reported in the literature [30].
The tumor in our patient was morphologically undifferentiated and characterized by sheets of epithelioid cells lacking keratinization. Immunohistochemical stains revealed squamous differentiation. Interestingly, the tumor contained a brisk lymphoplasmacytic infiltrate, raising the possibility of lymphoepithelial carcinoma; however, EBER was negative. Targeted RNA sequencing was performed to exclude the possibility of NUT carcinoma, amongst other considerations; surprisingly, it revealed a novel ETV6-TNFRSF8 fusion gene. While the presence of ETV6-rearrangement in a tumor at this location raises the possibility of secretory carcinoma or a low-grade sinonasal adenocarcinoma with ETV6 rearrangement, these tumors can be readily differentiated based on their distinctive morphologies and immunophenotypes. Based on its undifferentiated morphology and immunohistochemical evidence supporting squamous differentiation, this tumor is believed to be best classified as an undifferentiated carcinoma given the information available to date. This fusion product is potentially diagnostically relevant; however, at present, it does not appear to have direct therapeutic relevance.
In summary, we report an undifferentiated carcinoma arising in the sphenoid sinus that harbored a novel ETV6-TNFRSF8 fusion gene. This finding expands the spectrum of neoplasms characterized by ETV6-rearrangement. Further studies are necessary to determine the prevalence of this finding, and ascertain whether this is restricted to carcinoma of the sinonasal tract.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 13 kb)
Funding
Panov 2 Research Fund.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P. WHO Classification of head and neck tumours. 4. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26(6):1216–23. doi: 10.1093/annonc/mdv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–41. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–65. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 7.Todorovic E, Truong T, Eskander A, et al. Middle ear and temporal bone nonkeratinizing squamous cell carcinomas with DEK-AFF2 fusion: an emerging entity. Am J Surg Pathol. 2020;44(9):1244–50. doi: 10.1097/PAS.0000000000001498. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Lee KW, Srivastava RM, et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med. 2019;25(5):767–75. doi: 10.1038/s41591-019-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas A, Rajesh Y, Mitra P, Mandal M. ETV6 gene aberrations in non-haematological malignancies: a review highlighting ETV6 associated fusion genes in solid tumors. Biochim Biophys Acta Rev Cancer. 2020;1874:188389. doi: 10.1016/j.bbcan.2020.188389. [DOI] [PubMed] [Google Scholar]
- 10.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2(5):367–76. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 11.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 12.Dogan S, Wang L, Ptashkin RN, et al. Mammary analog secretory carcinoma of the thyroid gland: a primary thyroid adenocarcinoma harboring ETV6-NTRK3 fusion. Mod Pathol. 2016;29(9):985–95. doi: 10.1038/modpathol.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dettloff J, Seethala RR, Stevens TM, et al. Mammary analog secretory carcinoma (MASC) involving the thyroid gland: a report of the first 3 cases. Head Neck Pathol. 2017;11(2):124–30. doi: 10.1007/s12105-016-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin SM, Beattie A, Ling X, Jennings LJ, Guitart J. Primary cutaneous mammary analog secretory carcinoma with ETV6-NTRK3 translocation. Am J Dermatopathol. 2016;38(11):842–5. doi: 10.1097/DAD.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen S, Skálová A, Agaimy A, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. 2017;41(11):1552–60. doi: 10.1097/PAS.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen S, Kiss K, Melchior LC, Laco J. The ETV6-RET gene fusion is found in ETV6-rearranged low-grade sinonasal adenocarcinoma without NTRK3 involvement. Am J Surg Pathol. 2018;42(7):985–8. doi: 10.1097/PAS.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 17.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18(2):184–7. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 18.Anderson J, Gibson S, Sebire NJ. Expression of ETV6-NTRK in classical, cellular and mixed subtypes of congenital mesoblastic nephroma. Histopathology. 2006;48(6):748–53. doi: 10.1111/j.1365-2559.2006.02400.x. [DOI] [PubMed] [Google Scholar]
- 19.Alassiri AH, Ali RH, Shen Y, et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am J Surg Pathol. 2016;40(8):1051–61. doi: 10.1097/PAS.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 20.Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. 2016;238(4):543–9. doi: 10.1002/path.4677. [DOI] [PubMed] [Google Scholar]
- 21.Leeman-Neill RJ, Kelly LM, Liu P, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu T, Wang H, Huang X, et al. Gene fusion in malignant glioma: an emerging target for next-generation personalized treatment. Transl Oncol. 2018;11(3):609–18. doi: 10.1016/j.tranon.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh I, Tee MK, Botton T, et al. NTRK3 kinase fusions in Spitz tumours. J Pathol. 2016;240(3):282–90. doi: 10.1002/path.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson BC, Swanson D, Charames GS, Fletcher CD, Hornick JL. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31(5):753–62. doi: 10.1038/modpathol.2017.191. [DOI] [PubMed] [Google Scholar]
- 25.Skalova A, Vanecek T, Martinek P, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation: report of 10 cases. Am J Surg Pathol. 2018;42(2):234–46. doi: 10.1097/PAS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 26.Guilmette J, Dias-Santagata D, Nosé V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–8. doi: 10.1016/j.humpath.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 27.So T, Ishii N. The TNF-TNFR family of co-signal molecules. Adv Exp Med Biol. 2019;1189:53–84. doi: 10.1007/978-981-32-9717-3_3. [DOI] [PubMed] [Google Scholar]
- 28.Ward-Kavanagh LK, Lin WW, Šedý JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44(5):1005–19. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oflazoglu E, Grewal IS, Gerber H. Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Adv Exp Med Biol. 2009;647:174–85. doi: 10.1007/978-0-387-89520-8_12. [DOI] [PubMed] [Google Scholar]
- 30.Yoshihara K, Wang Q, Torres-Garcia W, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015;34(37):4845–54. doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 13 kb)